The Long-Term Pannexin 1 Ablation Produces Structural and Functional Modifications in Hippocampal Neurons
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Genotyping
2.3. Electrophysiology
2.4. Golgi Staining
2.5. Electron Microscopy
2.6. Subcellular Fractionation-Synaptoneurosome Isolation
2.7. Western Blotting
2.8. Quantification of F-actin Staining
2.9. F-actin/G-actin Assay
2.10. Rho GTPases Pull-Down Assay
2.11. Primary Neuronal Culture and Transfection
2.12. Transfection and Phalloidin Staining in Cultured Neurons
2.13. Statistics
3. Results
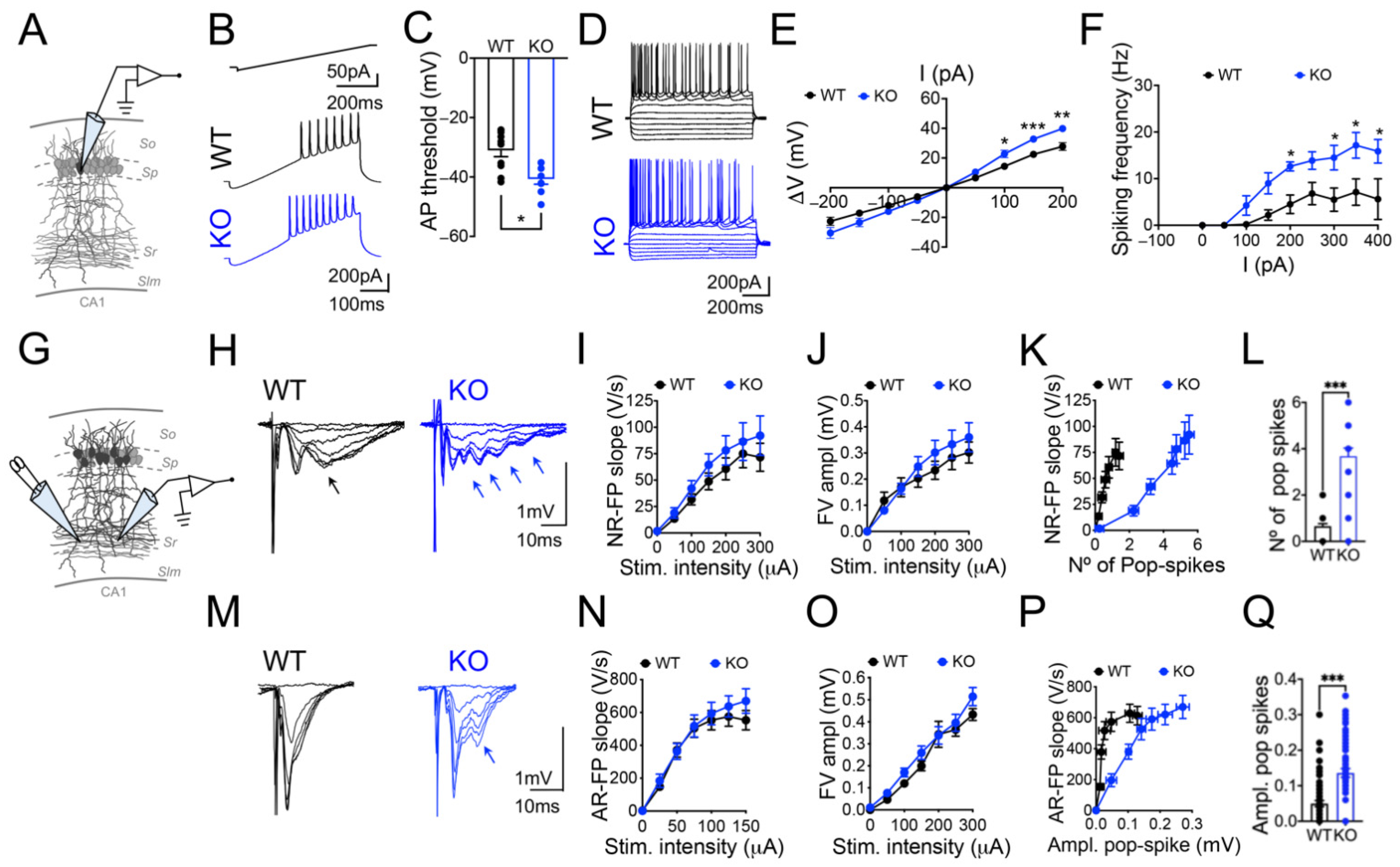
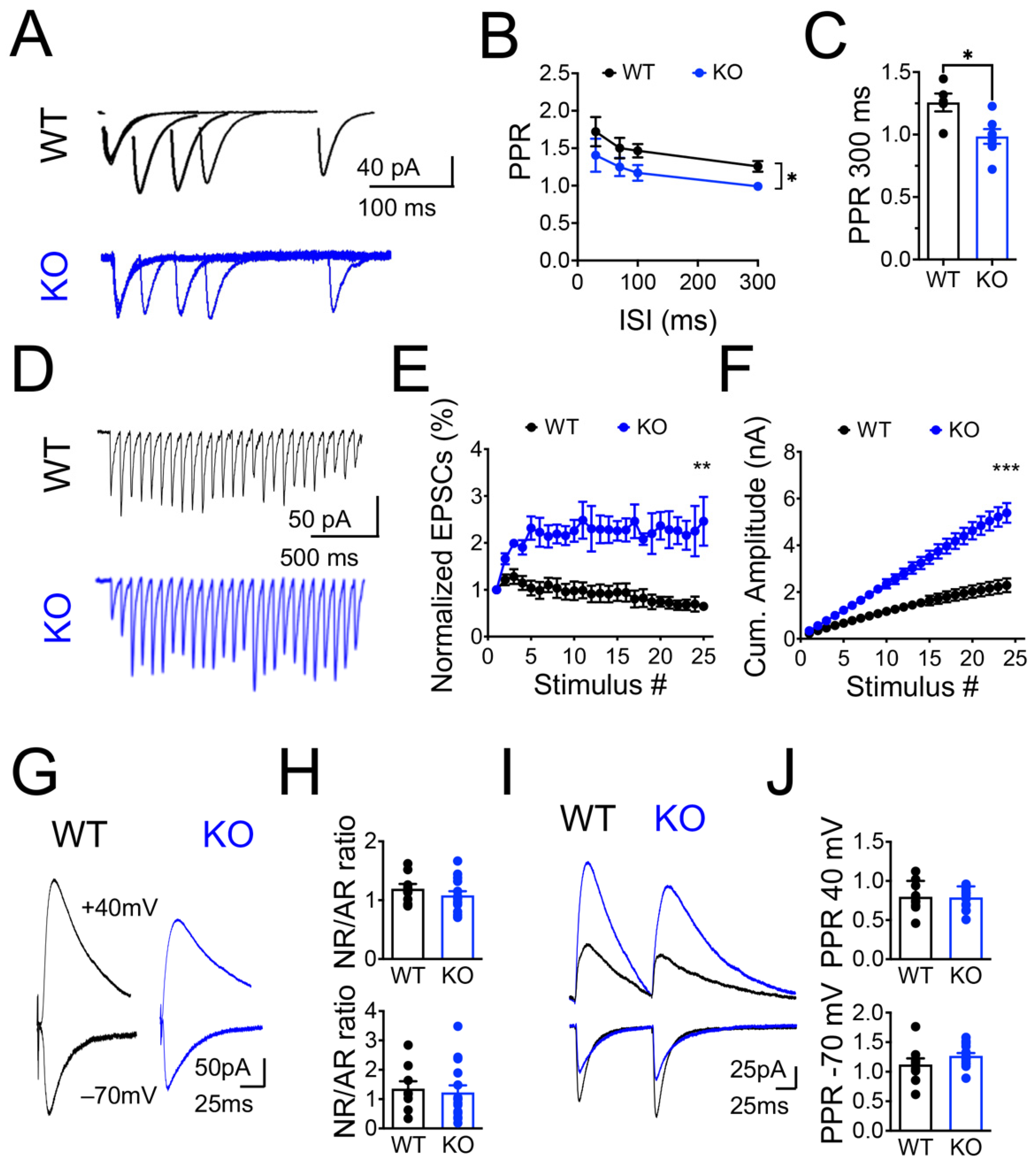
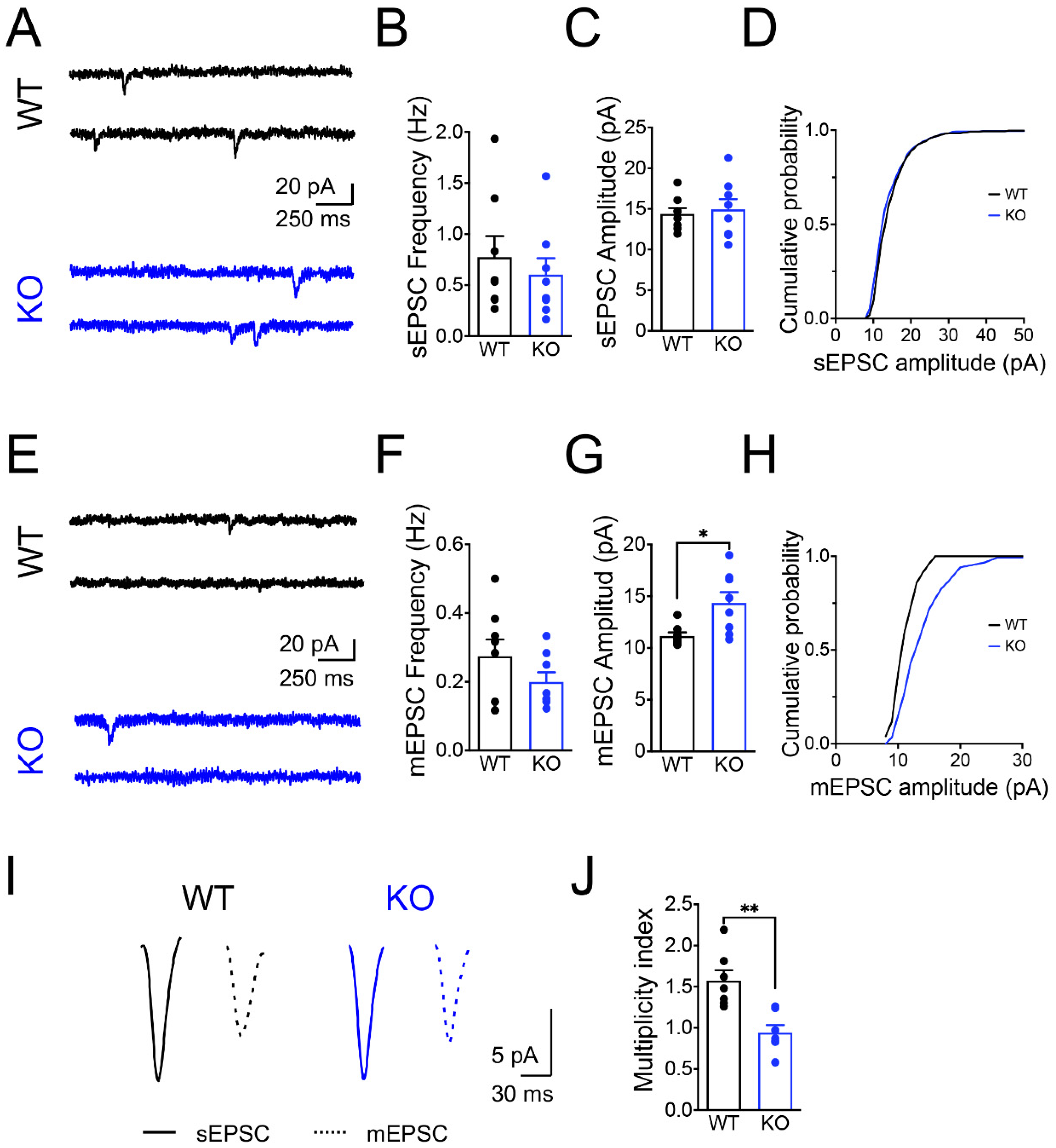
3.1. Panx1 Ablation Increases Neural Excitability without Affecting the Spontaneous Glutamate Release
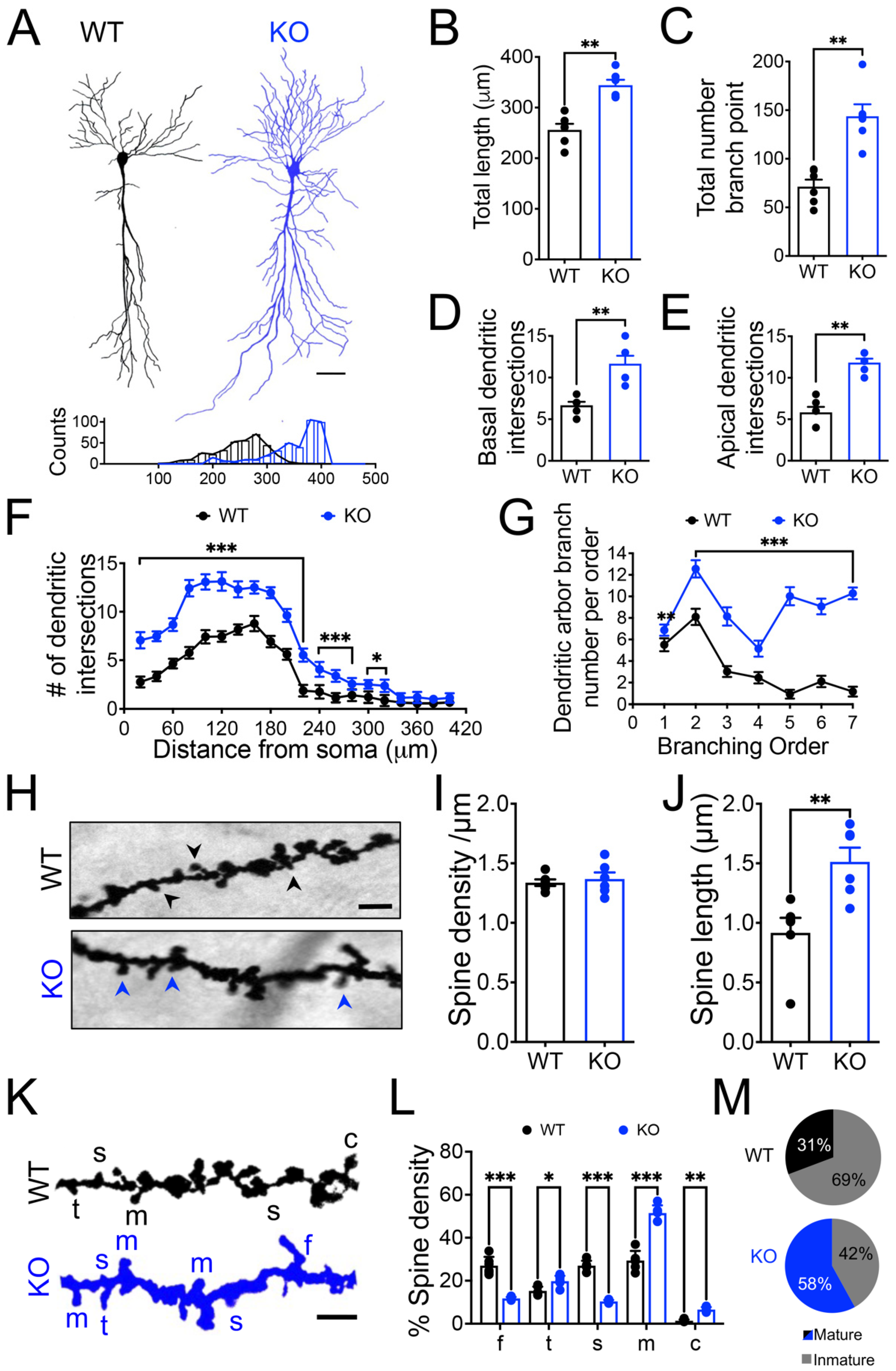
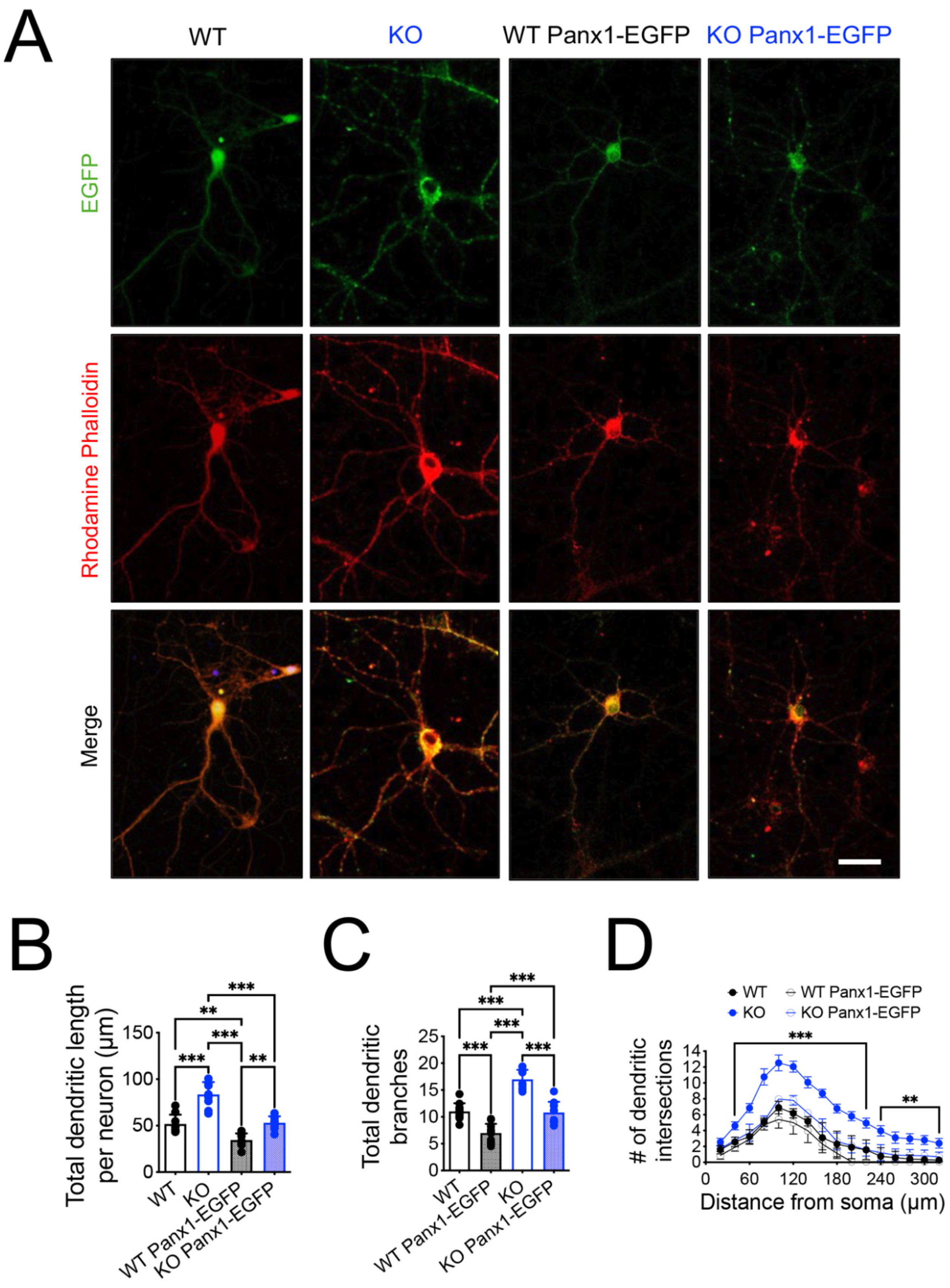
3.2. Increased Dendritic Arborization and Dendritic Spine Maturity of Hippocampal CA1 Pyramidal Neurons of Panx1-KO Mice
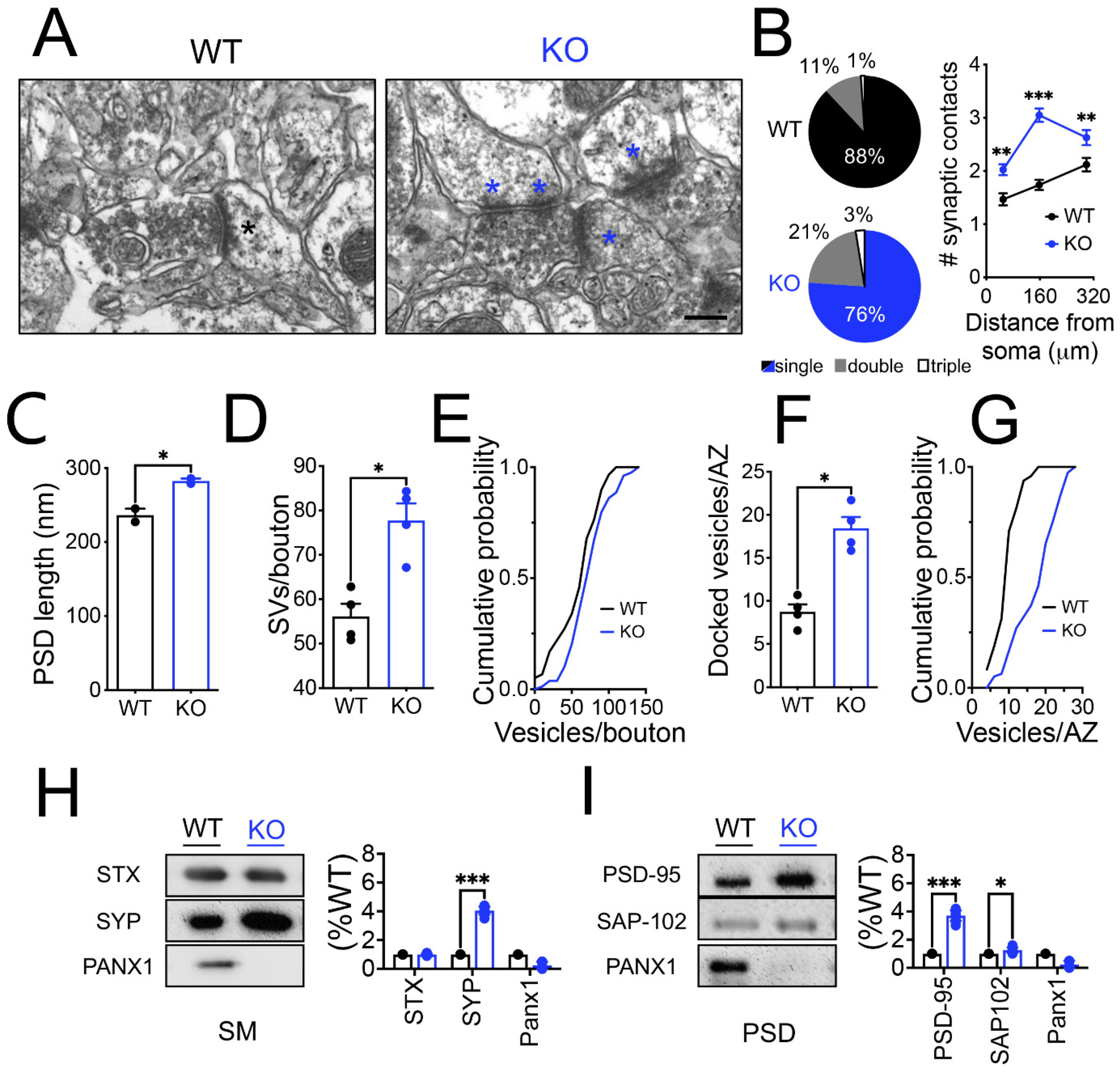
3.3. Structural and Molecular Remodeling of the Synapses in Hippocampal CA1 Pyramidal Neurons from Panx1-KO Animals
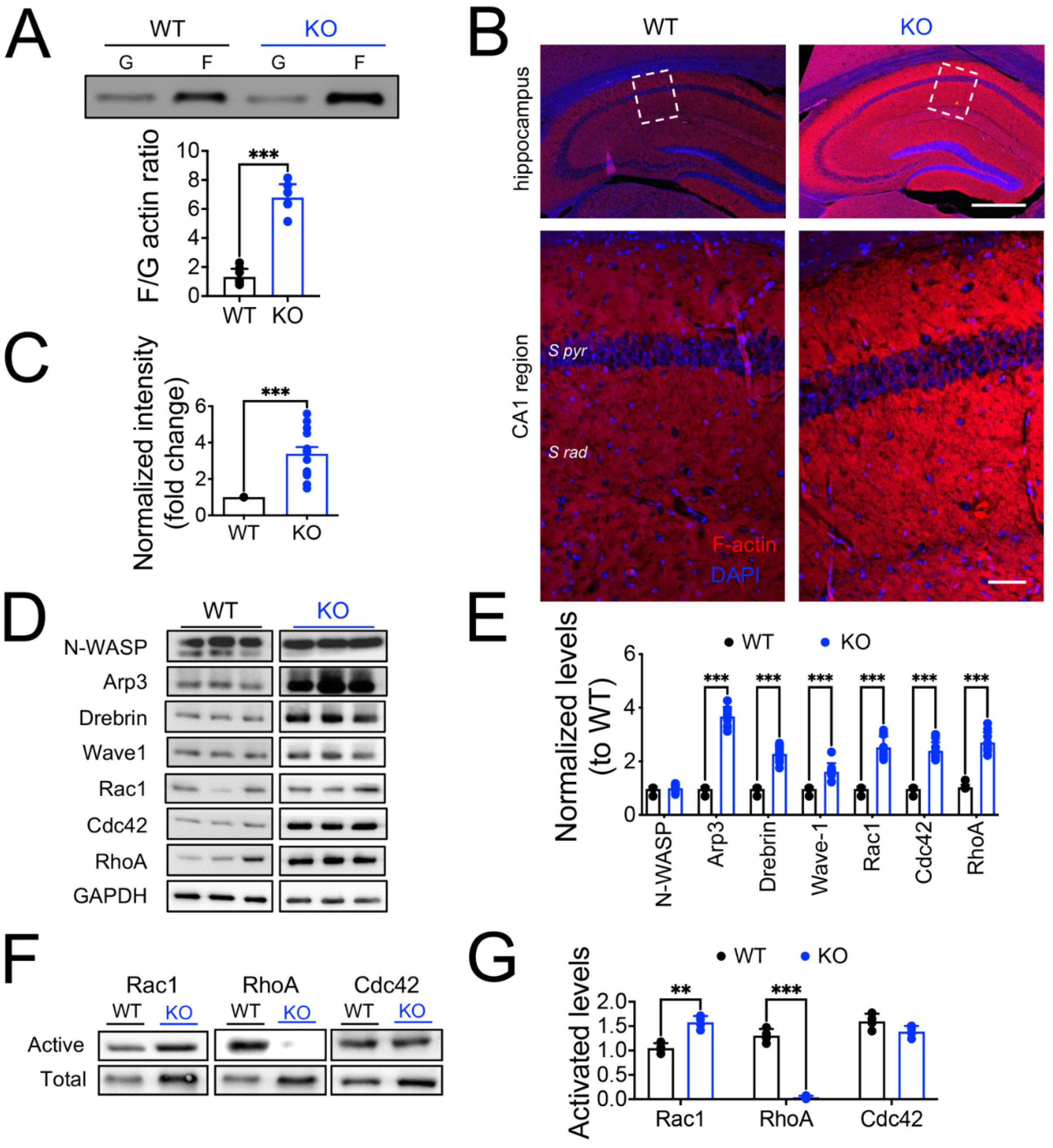
3.4. The lack of Panx1 Promotes F-actin Polymerization in Hippocampal Neurons via the Activation of Rac1 and Repression of the RhoA GTPases
4. Discussion
4.1. The Ablation of Panx1 Perturbs Neuronal Excitability without Affecting the Spontaneous Release of Glutamate
4.2. The Release Probability and RRP of Synaptic Vesicles Are Increased in Panx1-KO Neurons
4.3. Long-Term Panx1 Ablation Affects Structural Connectivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Z.; He, Z.; Maksaev, G.; Bitter, R.M.; Rau, M.; Fitzpatrick, J.A.J.; Yuan, P. Cryo-EM structures of the ATP release channel pannexin 1. Nat. Struct. Mol. Biol. 2020, 27, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Michalski, K.; Syrjanen, J.L.; Henze, E.; Kumpf, J.; Furukawa, H.; Kawate, T. The Cryo-EM structure of pannexin 1 reveals unique motifs for ion selection and inhibition. eLife 2020, 9, e56114. [Google Scholar] [CrossRef]
- Ruan, Z.; Orozco, I.J.; Du, J.; Lu, W. Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature 2020, 584, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Decrock, E.; De Bock, M.; Wang, N.; Bultynck, G.; Giaume, C.; Naus, C.C.; Green, C.R.; Leybaert, L. Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology? Cell Mol. Life Sci. 2015, 72, 2823–2851. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.; Chever, O.; Rouach, N. Connexons and pannexons: Newcomers in neurophysiology. Front. Cell. Neurosci. 2014, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ambrosi, C.; Qiu, F.; Jackson, D.G.; Sosinsky, G.; Dahl, G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci. Signal. 2014, 7, ra69. [Google Scholar] [CrossRef]
- Ma, W.; Compan, V.; Zheng, W.; Martin, E.; North, R.A.; Verkhratsky, A.; Surprenant, A. Pannexin 1 forms an anion-selective channel. Pflug. Arch. 2012, 463, 585–592. [Google Scholar] [CrossRef]
- Nomura, T.; Taruno, A.; Shiraishi, M.; Nakahari, T.; Inui, T.; Sokabe, M.; Eaton, D.C.; Marunaka, Y. Current-direction/amplitude-dependent single channel gating kinetics of mouse pannexin 1 channel: A new concept for gating kinetics. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Dahl, G. Pannexin1: A multifunction and multiconductance and/or permeability membrane channel. Am. J. Physiol. Cell Physiol. 2018, 315, C290–C299. [Google Scholar] [CrossRef]
- Romanov, R.A.; Bystrova, M.F.; Rogachevskaya, O.A.; Sadovnikov, V.B.; Shestopalov, V.I.; Kolesnikov, S.S. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J. Cell Sci. 2012, 125, 5514–5523. [Google Scholar] [CrossRef]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Narahari, A.K.; Kreutzberger, A.J.; Gaete, P.S.; Chiu, Y.H.; Leonhardt, S.A.; Medina, C.B.; Jin, X.; Oleniacz, P.W.; Kiessling, V.; Barrett, P.Q.; et al. ATP and large signaling metabolites flux through caspase-activated Pannexin 1 channels. eLife 2021, 10, e64787. [Google Scholar] [CrossRef]
- Thompson, R.J.; Jackson, M.F.; Olah, M.E.; Rungta, R.L.; Hines, D.J.; Beazely, M.A.; MacDonald, J.F.; MacVicar, B.A. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 2008, 322, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Lopatar, J.; Dale, N.; Frenguelli, B.G. Pannexin-1-mediated ATP release from area CA3 drives mGlu5-dependent neuronal oscillations. Neuropharmacology 2015, 93, 219–228. [Google Scholar] [CrossRef]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef] [PubMed]
- Locovei, S.; Bao, L.; Dahl, G. Pannexin 1 in erythrocytes: Function without a gap. Proc. Natl. Acad. Sci. USA 2006, 103, 7655–7659. [Google Scholar] [CrossRef]
- Thompson, R.J.; Zhou, N.; MacVicar, B.A. Ischemia opens neuronal gap junction hemichannels. Science 2006, 312, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Weilinger, N.L.; Lohman, A.W.; Rakai, B.D.; Ma, E.M.; Bialecki, J.; Maslieieva, V.; Rilea, T.; Bandet, M.V.; Ikuta, N.T.; Scott, L.; et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 2016, 19, 432–442. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef]
- Billaud, M.; Lohman, A.W.; Straub, A.C.; Looft-Wilson, R.; Johnstone, S.R.; Araj, C.A.; Best, A.K.; Chekeni, F.B.; Ravichandran, K.S.; Penuela, S.; et al. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circ. Res. 2011, 109, 80–85. [Google Scholar] [CrossRef]
- Maldifassi, M.C.; Momboisse, F.; Guerra, M.J.; Vielma, A.H.; Maripillan, J.; Baez-Matus, X.; Flores-Munoz, C.; Cadiz, B.; Schmachtenberg, O.; Martinez, A.D.; et al. The interplay between alpha7 nicotinic acetylcholine receptors, pannexin-1 channels and P2X7 receptors elicit exocytosis in chromaffin cells. J. Neurochem. 2021, 157, 1789–1808. [Google Scholar] [CrossRef] [PubMed]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Shestopalov, V.I.; Slepak, V.Z. Molecular pathways of pannexin1-mediated neurotoxicity. Front. Physiol. 2014, 5, 23. [Google Scholar] [CrossRef]
- Wei, R.; Wang, J.; Xu, Y.; Yin, B.; He, F.; Du, Y.; Peng, G.; Luo, B. Probenecid protects against cerebral ischemia/reperfusion injury by inhibiting lysosomal and inflammatory damage in rats. Neuroscience 2015, 301, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Bargiotas, P.; Krenz, A.; Hormuzdi, S.G.; Ridder, D.A.; Herb, A.; Barakat, W.; Penuela, S.; von Engelhardt, J.; Monyer, H.; Schwaninger, M. Pannexins in ischemia-induced neurodegeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 20772–20777. [Google Scholar] [CrossRef] [PubMed]
- Aquilino, M.S.; Whyte-Fagundes, P.; Zoidl, G.; Carlen, P.L. Pannexin-1 channels in epilepsy. Neurosci. Lett. 2019, 695, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zang, Z.; He, J.; Chen, X.; Yu, S.; Pei, Y.; Hou, Z.; An, N.; Yang, H.; Zhang, C.; et al. Expression of pannexin 1 and 2 in cortical lesions from intractable epilepsy patients with focal cortical dysplasia. Oncotarget 2017, 8, 6883–6895. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Blauwblomme, T.; Moulard, J.; Chever, O.; Vasile, F.; Guinard, E.; Le Bert, M.; Couillin, I.; Pallud, J.; Capelle, L.; et al. Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci. Transl. Med. 2018, 10, eaar3796. [Google Scholar] [CrossRef]
- Flores-Munoz, C.; Gomez, B.; Mery, E.; Mujica, P.; Gajardo, I.; Cordova, C.; Lopez-Espindola, D.; Duran-Aniotz, C.; Hetz, C.; Munoz, P.; et al. Acute Pannexin 1 Blockade Mitigates Early Synaptic Plasticity Defects in a Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2020, 14, 46. [Google Scholar] [CrossRef]
- Ardiles, A.O.; Flores-Munoz, C.; Toro-Ayala, G.; Cardenas, A.M.; Palacios, A.G.; Munoz, P.; Fuenzalida, M.; Saez, J.C.; Martinez, A.D. Pannexin 1 regulates bidirectional hippocampal synaptic plasticity in adult mice. Front. Cell. Neurosci. 2014, 8, 326. [Google Scholar] [CrossRef]
- Prochnow, N.; Abdulazim, A.; Kurtenbach, S.; Wildforster, V.; Dvoriantchikova, G.; Hanske, J.; Petrasch-Parwez, E.; Shestopalov, V.I.; Dermietzel, R.; Manahan-Vaughan, D.; et al. Pannexin1 stabilizes synaptic plasticity and is needed for learning. PLoS ONE 2012, 7, e51767. [Google Scholar] [CrossRef]
- Sanchez-Arias, J.C.; Liu, M.; Choi, C.S.W.; Ebert, S.N.; Brown, C.E.; Swayne, L.A. Pannexin 1 regulates network ensembles and dendritic spine development in cortical neurons. eNeuro 2019. [Google Scholar] [CrossRef]
- Wicki-Stordeur, L.E.; Swayne, L.A. Panx1 regulates neural stem and progenitor cell behaviours associated with cytoskeletal dynamics and interacts with multiple cytoskeletal elements. Cell Commun. Signal. 2013, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.H.; Kandel, E.R. Structural changes accompanying memory storage. Annu. Rev. Physiol. 1993, 55, 397–426. [Google Scholar] [CrossRef]
- Ardiles, A.O.; Grabrucker, A.M.; Scholl, F.G.; Rudenko, G.; Borsello, T. Molecular and Cellular Mechanisms of Synaptopathies. Neural Plast. 2017, 2017, 2643943. [Google Scholar] [CrossRef]
- Gipson, C.D.; Olive, M.F. Structural and functional plasticity of dendritic spines-root or result of behavior? Genes Brain Behav. 2017, 16, 101–117. [Google Scholar] [CrossRef]
- Turrigiano, G. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 2012, 4, a005736. [Google Scholar] [CrossRef]
- Desai, N.S. Homeostatic plasticity in the CNS: Synaptic and intrinsic forms. J. Physiol. 2003, 97, 391–402. [Google Scholar] [CrossRef]
- Lee, H.K.; Kirkwood, A. Mechanisms of Homeostatic Synaptic Plasticity in vivo. Front. Cell. Neurosci. 2019, 13, 520. [Google Scholar] [CrossRef]
- Davis, G.W. Homeostatic signaling and the stabilization of neural function. Neuron 2013, 80, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Linden, D.J. The other side of the engram: Experience-driven changes in neuronal intrinsic excitability. Nat. Rev. Neurosci. 2003, 4, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Pozo, K.; Goda, Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 2010, 66, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Rafael, A.; Cairus, A.; Tizzoni, M.; Abudara, V.; Vitureira, N. Glial ATP and Large Pore Channels Modulate Synaptic Strength in Response to Chronic Inactivity. Mol. Neurobiol. 2020, 57, 2856–2869. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, F.; Hernandez, V.H.; Crispino, G.; Seydel, A.; Ortolano, S.; Roper, S.D.; Kessaris, N.; Richardson, W.; Rickheit, G.; Filippov, M.A.; et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA 2008, 105, 18770–18775. [Google Scholar] [CrossRef]
- Schneggenburger, R.; Meyer, A.C.; Neher, E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron 1999, 23, 399–409. [Google Scholar] [CrossRef]
- Fioravante, D.; Regehr, W.G. Short-term forms of presynaptic plasticity. Curr. Opin. Neurobiol. 2011, 21, 269–274. [Google Scholar] [CrossRef]
- Moulder, K.L.; Mennerick, S. Reluctant vesicles contribute to the total readily releasable pool in glutamatergic hippocampal neurons. J. Neurosci. 2005, 25, 3842–3850. [Google Scholar] [CrossRef]
- Fuenzalida, M.; Aliaga, E.; Olivares, V.; Roncagliolo, M.; Bonansco, C. Developmental increase of asynchronic glutamate release from hippocampal synapses in mutant taiep rat. Synapse 2009, 63, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Hsia, A.Y.; Malenka, R.C.; Nicoll, R.A. Development of excitatory circuitry in the hippocampus. J. Neurophysiol. 1998, 79, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Risher, W.C.; Ustunkaya, T.; Singh Alvarado, J.; Eroglu, C. Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS ONE 2014, 9, e107591. [Google Scholar] [CrossRef]
- Gajardo, I.; Salazar, C.S.; Lopez-Espindola, D.; Estay, C.; Flores-Munoz, C.; Elgueta, C.; Gonzalez-Jamett, A.M.; Martinez, A.D.; Munoz, P.; Ardiles, A.O. Lack of Pannexin 1 Alters Synaptic GluN2 Subunit Composition and Spatial Reversal Learning in Mice. Front. Mol. Neurosci. 2018, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Ardiles, A.O.; Tapia-Rojas, C.C.; Mandal, M.; Alexandre, F.; Kirkwood, A.; Inestrosa, N.C.; Palacios, A.G. Postsynaptic dysfunction is associated with spatial and object recognition memory loss in a natural model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2012, 109, 13835–13840. [Google Scholar] [CrossRef]
- Gonzalez-Jamett, A.M.; Baez-Matus, X.; Olivares, M.J.; Hinostroza, F.; Guerra-Fernandez, M.J.; Vasquez-Navarrete, J.; Bui, M.T.; Guicheney, P.; Romero, N.B.; Bevilacqua, J.A.; et al. Dynamin-2 mutations linked to Centronuclear Myopathy impair actin-dependent trafficking in muscle cells. Sci. Rep. 2017, 7, 4580. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, G.M., 3rd; Lee, S.H.; Singh, D.; Yuan, Y.; Ng, Y.G.; Reichardt, L.F.; Arikkath, J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 2012, 7, 1741–1754. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Regehr, W.G. Short-term presynaptic plasticity. Cold Spring Harb. Perspect. Biol. 2012, 4, a005702. [Google Scholar] [CrossRef] [PubMed]
- Kaeser, P.S.; Deng, L.; Chavez, A.E.; Liu, X.; Castillo, P.E.; Sudhof, T.C. ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron 2009, 64, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Schneggenburger, R.; Sakaba, T.; Neher, E. Vesicle pools and short-term synaptic depression: Lessons from a large synapse. Trends Neurosci. 2002, 25, 206–212. [Google Scholar] [CrossRef]
- Huang, Y.; Grinspan, J.B.; Abrams, C.K.; Scherer, S.S. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 2007, 55, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Zoidl, G.; Petrasch-Parwez, E.; Ray, A.; Meier, C.; Bunse, S.; Habbes, H.W.; Dahl, G.; Dermietzel, R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 2007, 146, 9–16. [Google Scholar] [CrossRef]
- Ichikawa, M.; Muramoto, K.; Kobayashi, K.; Kawahara, M.; Kuroda, Y. Formation and maturation of synapses in primary cultures of rat cerebral cortical cells: An electron microscopic study. Neurosci. Res. 1993, 16, 95–103. [Google Scholar] [CrossRef]
- Grabrucker, A.; Vaida, B.; Bockmann, J.; Boeckers, T.M. Synaptogenesis of hippocampal neurons in primary cell culture. Cell Tissue Res. 2009, 338, 333–341. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Pine, J.; Potter, S.M. An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neurosci. 2006, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Chiappalone, M.; Bove, M.; Vato, A.; Tedesco, M.; Martinoia, S. Dissociated cortical networks show spontaneously correlated activity patterns during in vitro development. Brain Res. 2006, 1093, 41–53. [Google Scholar] [CrossRef]
- Verstraelen, P.; Van Dyck, M.; Verschuuren, M.; Kashikar, N.D.; Nuydens, R.; Timmermans, J.P.; De Vos, W.H. Image-Based Profiling of Synaptic Connectivity in Primary Neuronal Cell Culture. Front. Neurosci. 2018, 12, 389. [Google Scholar] [CrossRef]
- Faulstich, H.; Zobeley, S.; Rinnerthaler, G.; Small, J.V. Fluorescent phallotoxins as probes for filamentous actin. J. Muscle Res. Cell Motil. 1988, 9, 370–383. [Google Scholar] [CrossRef]
- Dillon, C.; Goda, Y. The actin cytoskeleton: Integrating form and function at the synapse. Annu. Rev. Neurosci. 2005, 28, 25–55. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Nobes, C.D. Rho GTPases: Molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R Soc. Lond. B Biol. Sci. 2000, 355, 965–970. [Google Scholar] [CrossRef]
- Duman, J.G.; Mulherkar, S.; Tu, Y.K.; Cheng, J.X.; Tolias, K.F. Mechanisms for spatiotemporal regulation of Rho-GTPase signaling at synapses. Neurosci. Lett. 2015, 601, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Tolias, K.F.; Duman, J.G.; Um, K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog. Neurobiol. 2011, 94, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.K.; Patil, C.S.; Jackson, M.F. Pannexin-1 in the CNS: Emerging concepts in health and disease. J. Neurochem. 2020, 154, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Bialecki, J.; Werner, A.; Weilinger, N.L.; Tucker, C.M.; Vecchiarelli, H.A.; Egana, J.; Mendizabal-Zubiaga, J.; Grandes, P.; Hill, M.N.; Thompson, R.J. Suppression of Presynaptic Glutamate Release by Postsynaptic Metabotropic NMDA Receptor Signalling to Pannexin-1. J. Neurosci. 2020, 40, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.F.; Veliskova, J.; Patel, N.K.; Lutz, S.E.; Caille, D.; Charollais, A.; Meda, P.; Scemes, E. Targeting pannexin1 improves seizure outcome. PLoS ONE 2011, 6, e25178. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Bashashati, M.; Hirota, S.A.; Gui, X.; Roberts, J.A.; MacDonald, J.A.; Muruve, D.A.; McKay, D.M.; Beck, P.L.; Mawe, G.M.; et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 2012, 18, 600–604. [Google Scholar] [CrossRef]
- Abitbol, J.M.; Kelly, J.J.; Barr, K.; Schormans, A.L.; Laird, D.W.; Allman, B.L. Differential effects of pannexins on noise-induced hearing loss. Biochem. J. 2016, 473, 4665–4680. [Google Scholar] [CrossRef]
- Abitbol, J.M.; O’Donnell, B.L.; Wakefield, C.B.; Jewlal, E.; Kelly, J.J.; Barr, K.; Willmore, K.E.; Allman, B.L.; Penuela, S. Double deletion of Panx1 and Panx3 affects skin and bone but not hearing. J. Mol. Med. 2019, 97, 723–736. [Google Scholar] [CrossRef]
- Schmidt-Hieber, C.; Jonas, P.; Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 2004, 429, 184–187. [Google Scholar] [CrossRef]
- Desai, N.S.; Rutherford, L.C.; Turrigiano, G.G. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci. 1999, 2, 515–520. [Google Scholar] [CrossRef]
- Wefelmeyer, W.; Puhl, C.J.; Burrone, J. Homeostatic Plasticity of Subcellular Neuronal Structures: From Inputs to Outputs. Trends Neurosci. 2016, 39, 656–667. [Google Scholar] [CrossRef]
- Morgan, P.J.; Bourboulou, R.; Filippi, C.; Koenig-Gambini, J.; Epsztein, J. Kv1.1 contributes to a rapid homeostatic plasticity of intrinsic excitability in CA1 pyramidal neurons in vivo. eLife 2019, 8, e49915. [Google Scholar] [CrossRef]
- Hoffman, D.A.; Magee, J.C.; Colbert, C.M.; Johnston, D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 1997, 387, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, Y.; Nerbonne, J.M. IA channels: Diverse regulatory mechanisms. Neuroscientist 2014, 20, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Gasselin, C.; Inglebert, Y.; Ankri, N.; Debanne, D. Plasticity of intrinsic excitability during LTD is mediated by bidirectional changes in h-channel activity. Sci. Rep. 2017, 7, 14418. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Hanff, J.; Bean, B.P. Persistent sodium current drives conditional pacemaking in CA1 pyramidal neurons under muscarinic stimulation. J. Neurosci. 2013, 33, 15011–15021. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, R. Potassium channels. FEBS Lett. 2003, 555, 62–65. [Google Scholar] [CrossRef]
- Johnston, J.; Forsythe, I.D.; Kopp-Scheinpflug, C. Going native: Voltage-gated potassium channels controlling neuronal excitability. J. Physiol. 2010, 588, 3187–3200. [Google Scholar] [CrossRef]
- Goutierre, M.; Al Awabdh, S.; Donneger, F.; Francois, E.; Gomez-Dominguez, D.; Irinopoulou, T.; de la Prida, L.M.; Poncer, J.C. KCC2 Regulates Neuronal Excitability and Hippocampal Activity via Interaction with Task-3 Channels. Cell Rep. 2019, 28, 91–103.e107. [Google Scholar] [CrossRef]
- Sudkamp, N.; Shchyglo, O.; Manahan-Vaughan, D. Absence of Pannexin 1 Stabilizes Hippocampal Excitability After Intracerebral Treatment with Abeta (1–42) and Prevents LTP Deficits in Middle-Aged Mice. Front. Aging Neurosci. 2021, 13, 591735. [Google Scholar] [CrossRef]
- Kavalali, E.T. The mechanisms and functions of spontaneous neurotransmitter release. Nat. Rev. Neurosci. 2015, 16, 5–16. [Google Scholar] [CrossRef]
- Kaeser, P.S.; Regehr, W.G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 2014, 76, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Weilinger, N.L.; Tang, P.L.; Thompson, R.J. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J. Neurosci. 2012, 32, 12579–12588. [Google Scholar] [CrossRef] [PubMed]
- Spruston, N. Pyramidal neurons: Dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008, 9, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 1999, 9, 343–348. [Google Scholar] [CrossRef]
- Arellano, J.I.; Benavides-Piccione, R.; Defelipe, J.; Yuste, R. Ultrastructure of dendritic spines: Correlation between synaptic and spine morphologies. Front. Neurosci. 2007, 1, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Schikorski, T.; Stevens, C.F. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 1997, 17, 5858–5867. [Google Scholar] [CrossRef]
- Wicki-Stordeur, L.E.; Dzugalo, A.D.; Swansburg, R.M.; Suits, J.M.; Swayne, L.A. Pannexin 1 regulates postnatal neural stem and progenitor cell proliferation. Neural Dev. 2012, 7, 11. [Google Scholar] [CrossRef]
- Bhalla-Gehi, R.; Penuela, S.; Churko, J.M.; Shao, Q.; Laird, D.W. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J. Biol. Chem. 2010, 285, 9147–9160. [Google Scholar] [CrossRef]
- Racz, B.; Weinberg, R.J. Organization of the Arp2/3 complex in hippocampal spines. J. Neurosci. 2008, 28, 5654–5659. [Google Scholar] [CrossRef]
- Bruzzone, R.; Hormuzdi, S.G.; Barbe, M.T.; Herb, A.; Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13644–13649. [Google Scholar] [CrossRef]
- Lee, E.; Chung, W.S. Glial Control of Synapse Number in Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Stogsdill, J.A.; Eroglu, C. The interplay between neurons and glia in synapse development and plasticity. Curr. Opin. Neurobiol. 2017, 42, 1–8. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Tsaneva-Atanasova, K.; Burgo, A.; Galli, T.; Holcman, D. Quantifying neurite growth mediated by interactions among secretory vesicles, microtubules, and actin networks. Biophys. J. 2009, 96, 840–857. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, A.; Minden, A.; Yuste, R. Regulation of dendritic spine morphology by the rho family of small GTPases: Antagonistic roles of Rac and Rho. Cereb Cortex 2000, 10, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.Y.; Harms, M.B.; Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 2000, 20, 5329–5338. [Google Scholar] [CrossRef]
- Pilpel, Y.; Segal, M. Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur. J. Neurosci. 2004, 19, 3151–3164. [Google Scholar] [CrossRef]
- Wegner, A.M.; Nebhan, C.A.; Hu, L.; Majumdar, D.; Meier, K.M.; Weaver, A.M.; Webb, D.J. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J. Biol. Chem. 2008, 283, 15912–15920. [Google Scholar] [CrossRef]
- Sturner, T.; Tatarnikova, A.; Mueller, J.; Schaffran, B.; Cuntz, H.; Zhang, Y.; Nemethova, M.; Bogdan, S.; Small, V.; Tavosanis, G. Transient localization of the Arp2/3 complex initiates neuronal dendrite branching in vivo. Development 2019, 146, dev171397. [Google Scholar] [CrossRef]
- Bobo-Jimenez, V.; Delgado-Esteban, M.; Angibaud, J.; Sanchez-Moran, I.; de la Fuente, A.; Yajeya, J.; Nagerl, U.V.; Castillo, J.; Bolanos, J.P.; Almeida, A. APC/C(Cdh1)-Rock2 pathway controls dendritic integrity and memory. Proc. Natl. Acad. Sci. USA 2017, 114, 4513–4518. [Google Scholar] [CrossRef]
- Hotulainen, P.; Llano, O.; Smirnov, S.; Tanhuanpaa, K.; Faix, J.; Rivera, C.; Lappalainen, P. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J. Cell Biol. 2009, 185, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Medina, C.B.; Doyle, C.A.; Zhou, M.; Narahari, A.K.; Sandilos, J.K.; Gonye, E.C.; Gao, H.-Y.; Guo, S.Y.; Parlak, M.; et al. Deacetylation as a receptor-regulated direct activation switch for pannexin channels. Nat. Commun. 2021, 12, 4482. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Vidal, L.; Okada, S.F.; Sesma, J.I.; Kreda, S.M.; van Heusden, C.A.; Zhu, Y.; Jones, L.C.; O’Neal, W.K.; Penuela, S.; Laird, D.W.; et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 2011, 286, 26277–26286. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Y.; Qu, H.L.; Dai, Y.J.; Wang, Q.; Ling, Z.M.; Su, W.F.; Zhao, Y.Y.; Shen, W.X.; Chen, G. Pannexin 1, a large-pore membrane channel, contributes to hypotonicity-induced ATP release in Schwann cells. Neural Regen. Res. 2021, 16, 899–904. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Linseman, D.A. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell. Neurosci. 2014, 8, 314. [Google Scholar] [CrossRef]
- Mulherkar, S.; Tolias, K.F. RhoA-ROCK Signaling as a Therapeutic Target in Traumatic Brain Injury. Cells 2020, 9, 245. [Google Scholar] [CrossRef]
- Salloum, G.; Jaafar, L.; El-Sibai, M. Rho A and Rac1: Antagonists moving forward. Tissue Cell 2020, 65, 101364. [Google Scholar] [CrossRef]
- Dong, Z.; Bai, Y.; Wu, X.; Li, H.; Gong, B.; Howland, J.G.; Huang, Y.; He, W.; Li, T.; Wang, Y.T. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology 2013, 64, 65–73. [Google Scholar] [CrossRef]
- Mills, F.; Bartlett, T.E.; Dissing-Olesen, L.; Wisniewska, M.B.; Kuznicki, J.; Macvicar, B.A.; Wang, Y.T.; Bamji, S.X. Cognitive flexibility and long-term depression (LTD) are impaired following beta-catenin stabilization in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 8631–8636. [Google Scholar] [CrossRef]
- Nicholls, R.E.; Alarcon, J.M.; Malleret, G.; Carroll, R.C.; Grody, M.; Vronskaya, S.; Kandel, E.R. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron 2008, 58, 104–117. [Google Scholar] [CrossRef]

| WT | KO | Mann–Whitney Test | |
|---|---|---|---|
| (n = 10) | (n = 8) | p-value | |
| Vm (mV) | −71.30 ± 2.83 | −68.11 ± 1.42 | 0.9655 |
| Rin (MΩ) | 124.64 ± 10.14 | 170.15 ± 16.90 | * 0.0248 |
| Cm (pF) | 81.09 ± 8.12 | 77.09 ± 9.16 | 0.8286 |
| τ | 9.83 ± 0.97 | 12.92 ± 1.65 | 0.1011 |
| Number of AP | 5.44 ± 1.18 | 8.75 ± 1.30 | 0.0779 |
| Spiking frequency200pA | 4.9 ± 2.24 | 12.62 ± 0.96 | * 0.0328 |
| Spiking frequency250pA | 6.4 ± 2.19 | 14 ± 1.82 | * 0.0204 |
| Spiking frequency300pA | 5.5 ± 2.23 | 14.5 ± 2.63 | * 0.0247 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Muñoz, C.; García-Rojas, F.; Pérez, M.A.; Santander, O.; Mery, E.; Ordenes, S.; Illanes-González, J.; López-Espíndola, D.; González-Jamett, A.M.; Fuenzalida, M.; et al. The Long-Term Pannexin 1 Ablation Produces Structural and Functional Modifications in Hippocampal Neurons. Cells 2022, 11, 3646. https://doi.org/10.3390/cells11223646
Flores-Muñoz C, García-Rojas F, Pérez MA, Santander O, Mery E, Ordenes S, Illanes-González J, López-Espíndola D, González-Jamett AM, Fuenzalida M, et al. The Long-Term Pannexin 1 Ablation Produces Structural and Functional Modifications in Hippocampal Neurons. Cells. 2022; 11(22):3646. https://doi.org/10.3390/cells11223646
Chicago/Turabian StyleFlores-Muñoz, Carolina, Francisca García-Rojas, Miguel A. Pérez, Odra Santander, Elena Mery, Stefany Ordenes, Javiera Illanes-González, Daniela López-Espíndola, Arlek M. González-Jamett, Marco Fuenzalida, and et al. 2022. "The Long-Term Pannexin 1 Ablation Produces Structural and Functional Modifications in Hippocampal Neurons" Cells 11, no. 22: 3646. https://doi.org/10.3390/cells11223646
APA StyleFlores-Muñoz, C., García-Rojas, F., Pérez, M. A., Santander, O., Mery, E., Ordenes, S., Illanes-González, J., López-Espíndola, D., González-Jamett, A. M., Fuenzalida, M., Martínez, A. D., & Ardiles, Á. O. (2022). The Long-Term Pannexin 1 Ablation Produces Structural and Functional Modifications in Hippocampal Neurons. Cells, 11(22), 3646. https://doi.org/10.3390/cells11223646








