Effects and Mechanisms of Exosomes from Different Sources in Cerebral Ischemia
Abstract
1. Introduction
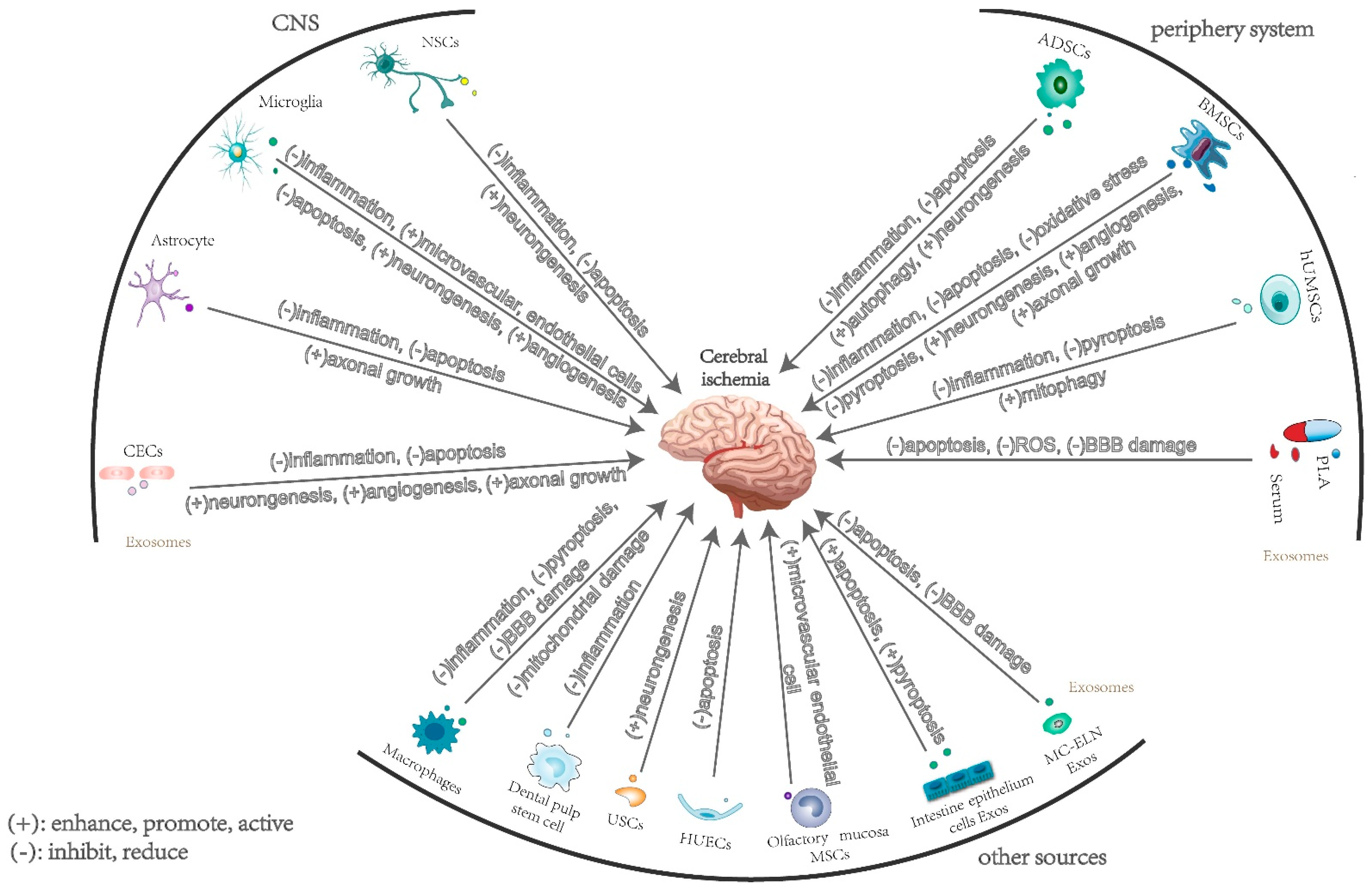
2. The Effects and Mechanisms of Exosomes Derived from Central Nervous System Cells in Cerebral Ischemia
2.1. Neuron-Derived Exosomes
2.2. Microglia-Derived Exosomes
2.3. Astrocyte-Derived Exosomes
2.4. Brain Microvascular Endothelial Cell-Derived Exosomes
3. The Effects and Mechanisms of Exosomes Derived from Peripheral Cells in Cerebral Ischemia
3.1. Mesenchymal Stem-Cell-Derived Exosomes
3.1.1. Bone Marrow Mesenchymal Stem-Cell-Derived Exosomes
3.1.2. Adipose-Derived Mesenchymal Stem Cells
3.1.3. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes
3.2. Plasma and Serum Exosomes
4. The Effects and Mechanisms of Exosomes Derived from Other Sources in Cerebral Ischemia
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Shin, T.H.; Lee, D.Y.; Basith, S.; Manavalan, B.; Paik, M.J.; Rybinnik, I.; Mouradian, M.M.; Ahn, J.H.; Lee, G. Metabolome Changes in Cerebral Ischemia. Cells 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.I.; de la Parra, J.; Garcia-Culebras, A.; Ballesteros, I.; Lizasoain, I.; Moro, M.A. The Kynurenine Pathway in the Acute and Chronic Phases of Cerebral Ischemia. Curr. Pharm. Des. 2016, 22, 1060–1073. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.R.; Willing, A. Enhancing endogenous capacity to repair a stroke-damaged brain: An evolving field for stroke research. Prog. Neurobiol. 2018, 163–164, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Tan, S.; Xia, L.; Yi, P.; Han, Y.; Tang, L.; Pan, Q.; Tian, Y.; Rao, S.; Oyang, L.; Liang, J.; et al. Exosomal miRNAs in tumor microenvironment. J. Exp. Clin. Cancer Res. 2020, 39, 67. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-M.; Feng, Y.-S.; Tan, Z.-X.; Xing, Y.; Dong, F.; Zhang, F. The role of exosomes in stroke. Mol. Biol. Rep. 2020, 47, 6217–6228. [Google Scholar] [CrossRef]
- de Abreu, R.C.; Fernandes, H.; da Costa Martins, P.A.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 2020, 17, 685–697. [Google Scholar] [CrossRef]
- Aminzadeh, M.A.; Rogers, R.G.; Fournier, M.; Tobin, R.E.; Guan, X.; Childers, M.K.; Andres, A.M.; Taylor, D.J.; Ibrahim, A.; Ding, X.; et al. Exosome-Mediated Benefits of Cell Therapy in Mouse and Human Models of Duchenne Muscular Dystrophy. Stem Cell Rep. 2018, 10, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, H.; Jia, L.; Lyu, J.; Sun, Y.; Yu, H.; Li, H.; Liu, W.; Weng, Y.; Yu, W. Exosomes Mediate Hippocampal and Cortical Neuronal Injury Induced by Hepatic Ischemia-Reperfusion Injury through Activating Pyroptosis in Rats. Oxid. Med. Cell Longev. 2019, 2019, 3753485. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, W.; Ye, J.; Wang, Y. Potential Role of Exosomes in Ischemic Stroke Treatment. Biomolecules 2022, 12, 115. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Li, Y.; Peng, N.; Liu, Q.; Qiu, D.; Cho, J.; Borlongan, C.V.; Yu, G. Exosomes Derived From Mesenchymal Stem Cells Pretreated With Ischemic Rat Heart Extracts Promote Angiogenesis via the Delivery of DMBT1. Cell Transpl. 2022, 31, 9636897221102898. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Cao, L.; He, C.; Ye, Q.; Liang, R.; You, W.; Zhang, H.; Wu, J.; Ye, J.; Tannous, B.A.; et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics 2021, 11, 6507–6521. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cheng, C.; Wei, Y.; Yang, F.; Li, G. The Role of Exosomes in Inflammatory Diseases and Tumor-Related Inflammation. Cells 2022, 11, 1005. [Google Scholar] [CrossRef]
- Li, J.Y.; Li, Q.Q.; Sheng, R. The role and therapeutic potential of exosomes in ischemic stroke. Neurochem. Int. 2021, 151, 105194. [Google Scholar] [CrossRef]
- Yang, D.; Li, Z.; Gao, G.; Li, X.; Liao, Z.; Wang, Y.; Li, W.; Zhange, Y.; Liu, W. Combined Analysis of Surface Protein Profile and microRNA Expression Profile of Exosomes Derived from Brain Microvascular Endothelial Cells in Early Cerebral Ischemia. ACS Omega 2021, 6, 22410–22421. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, X.; Shu, K. Cell-Derived Exosomes as Therapeutic Strategies and Exosome-Derived microRNAs as Biomarkers for Traumatic Brain Injury. J. Clin. Med. 2022, 11, 3223. [Google Scholar] [CrossRef]
- Davidson, S.M.; Riquelme, J.A.; Zheng, Y.; Vicencio, J.M.; Lavandero, S.; Yellon, D.M. Endothelial cells release cardioprotective exosomes that may contribute to ischaemic preconditioning. Sci. Rep. 2018, 8, 15885. [Google Scholar] [CrossRef] [PubMed]
- Zagrean, A.M.; Hermann, D.M.; Opris, I.; Zagrean, L.; Popa-Wagner, A. Multicellular Crosstalk Between Exosomes and the Neurovascular Unit After Cerebral Ischemia. Therapeutic Implications. Front. Neurosci. 2018, 12, 811. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog. Neurobiol. 2019, 173, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kos, A.; Loohuis, N.O.; Meinhardt, J.; van Bokhoven, H.; Kaplan, B.B.; Martens, G.J.; Aschrafi, A. MicroRNA-181 promotes synaptogenesis and attenuates axonal outgrowth in cortical neurons. Cell Mol. Life Sci. 2016, 73, 3555–3567. [Google Scholar] [CrossRef]
- Luo, H.; Ye, G.; Liu, Y.; Huang, D.; Luo, Q.; Chen, W.; Qi, Z. miR-150-3p enhances neuroprotective effects of neural stem cell exosomes after hypoxic-ischemic brain injury by targeting CASP2. Neurosci. Lett. 2022, 779, 136635. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Chen, R.; Miao, J.; Wang, L.; Cui, L.; Ji, H.; Liu, Y. Cortical Neuron-Derived Exosomal MicroRNA-181c-3p Inhibits Neuroinflammation by Downregulating CXCL1 in Astrocytes of a Rat Model with Ischemic Brain Injury. Neuroimmunomodulation 2019, 26, 217–233. [Google Scholar] [CrossRef]
- De La Rosa-Prieto, C.; Laterza, C.; Gonzalez-Ramos, A.; Wattananit, S.; Ge, R.; Lindvall, O.; Tornero, D.; Kokaia, Z. Stroke alters behavior of human skin-derived neural progenitors after transplantation adjacent to neurogenic area in rat brain. Stem Cell Res. Ther. 2017, 8, 59. [Google Scholar] [CrossRef]
- Hermanto, Y.; Sunohara, T.; Faried, A.; Takagi, Y.; Takahashi, J.; Maki, T.; Miyamoto, S. Transplantation of feeder-free human induced pluripotent stem cell-derived cortical neuron progenitors in adult male Wistar rats with focal brain ischemia. J. Neurosci. Res. 2018, 96, 863–874. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Li, W.-Y.; Zhu, Q.-B.; Jin, L.-Y.; Yang, Y.; Xu, X.-Y. Exosomes derived from human induced pluripotent stem cell-derived neural progenitor cells protect neuronal function under ischemic conditions. Neural. Regen. Res. 2021, 16, 2064–2070. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Xu, Y.; He, M.; Yang, J.; Li, J.; Li, Y.; Ao, G.; Cheng, J.; Jia, J. The cystathionine β-synthase/hydrogen sulfide pathway contributes to microglia-mediated neuroinflammation following cerebral ischemia. Brain Behav. Immun. 2017, 66, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Zhao, S.; Zhang, H.; Cai, W.; Cai, M.; Ji, X.; Leak, R.K.; Gao, Y.; Chen, J.; et al. Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia. Stroke 2016, 47, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, P.; Liu, S.; Jin, Z.; Li, D.; Zhao, H.; Zhu, X.; Shu, C.; Yan, D.; Dong, Z. IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv. Clin. Exp. Med. 2019, 28, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cai, G.; Liu, K.; Zhuang, Z.; Jia, K.; Pei, S.; Wang, X.; Wang, H.; Xu, S.; Cui, C.; et al. Microglia exosomal miRNA-137 attenuates ischemic brain injury through targeting Notch1. Aging 2021, 13, 4079–4095. [Google Scholar] [CrossRef]
- Zang, J.; Wu, Y.; Su, X.; Zhang, T.; Tang, X.; Ma, D.; Li, Y.; Lui, Y.; Weng, Z.; Liu, X.; et al. Inhibition of PDE1-B by Vinpocetine Regulates Microglial Exosomes and Polarization Through Enhancing Autophagic Flux for Neuroprotection Against Ischemic Stroke. Front. Cell. Dev. Biol. 2020, 8, 616590. [Google Scholar] [CrossRef]
- Xie, L.; Zhao, H.; Wang, Y.; Chen, Z. Exosomal shuttled miR-424-5p from ischemic preconditioned microglia mediates cerebral endothelial cell injury through negatively regulation of FGF2/STAT3 pathway. Exp. Neurol. 2020, 333, 113411. [Google Scholar] [CrossRef]
- Ravi, K.; Paidas, M.J.; Saad, A.; Jayakumar, A.R. Astrocytes in rare neurological conditions: Morphological and functional considerations. J. Comp. Neurol. 2021, 529, 2676–2705. [Google Scholar] [CrossRef]
- Kang, C.; Avery, L. To be or not to be, the level of autophagy is the question: Dual roles of autophagy in the survival response to starvation. Autophagy 2008, 4, 82–84. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp. Cell Res. 2019, 382, 111474. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Zhu, Z.; Feng, J.; Chen, L. Exosome-Shuttled circSHOC2 from IPASs Regulates Neuronal Autophagy and Ameliorates Ischemic Brain Injury via the miR-7670-3p/SIRT1 Axis. Mol. Ther. Nucleic Acids 2020, 22, 657–672. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Su, M.; Wang, X.; Xie, C. Exosomal microRNA-22-3p alleviates cerebral ischemic injury by modulating KDM6B/BMP2/BMF axis. Stem Cell Res. Ther. 2021, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, Y.; Sun, Y. Astrocyte-derived exosomes carry microRNA-17-5p to protect neonatal rats from hypoxic-ischemic brain damage via inhibiting BNIP-2 expression. Neurotoxicology 2021, 83, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. Astrocyte-Derived Exosomes Treated With a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D2 Synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cao, H.; Xie, Y.; Zhang, Y.; Du, M.; Xu, X.; Ye, R.; Liu, X. Exosome-shuttled miR-92b-3p from ischemic preconditioned astrocytes protects neurons against oxygen and glucose deprivation. Brain Res. 2019, 1717, 66–73. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, P.; Hong, T.; Wang, Y.; Liu, N.; He, B.; Zou, S.; Ren, D.; Duan, J.; Zhoa, L.; et al. Astrocytes-derived exosomes induce neuronal recovery after traumatic brain injury via delivering gap junction alpha 1-20 k. J. Tissue Eng. Regen. Med. 2020, 14, 412–423. [Google Scholar] [CrossRef]
- Xiao, B.; Chai, Y.; Lv, S.; Ye, M.; Wu, M.; Xie, L.; Fan, Y.; Zhu, X.; Gao, Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int. J. Mol. Med. 2017, 40, 1201–1209. [Google Scholar] [CrossRef]
- Gao, B.; Zhou, S.; Sun, C.; Cheng, D.; Zhang, Y.; Li, X.; Zhang, L.; Zhao, J.; Xu, D.; Bai, Y. Brain Endothelial Cell-Derived Exosomes Induce Neuroplasticity in Rats with Ischemia/Reperfusion Injury. ACS Chem. Neurosci. 2020, 11, 2201–2213. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Chopp, M.; Li, C.; Kemper, A.; Liu, X.; Wang, X.; Zhang, L.; Zhang, Z.G. Ischemic Cerebral Endothelial Cell-Derived Exosomes Promote Axonal Growth. Stroke 2020, 51, 3701–3712. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, B.; Sun, C.; Bai, Y.; Cheng, D.; Zhang, Y.; Li, X.; Zhao, J.; Xu, D. Vascular Endothelial Cell-derived Exosomes Protect Neural Stem Cells Against Ischemia/reperfusion Injury. Neuroscience 2020, 441, 184–196. [Google Scholar] [CrossRef]
- Huang, R.; Cheng, T.; Lai, X. Mechanism of ischemic brain injury repair by endothelial progenitor cell-derived exosomes. Mol. Med. Rep. 2022, 26, 269. [Google Scholar] [CrossRef]
- Caplan, A.I. MSCs: The Sentinel and Safe-Guards of Injury. J. Cell Physiol. 2016, 231, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, X.; Wang, Y.; Cheng, W.; Zuo, X.; Tang, W.; Huang, W. MSCs-derived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Mol. Med. 2021, 27, 67. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Liu, Z.; Buller, B.; Li, Y.; Golembieski, W.; Gan, X.; Wang, F.; Lu, M.; Ali, M.M.; Zhang, Z.G.; et al. MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J. Cereb. Blood Flow Metab. 2021, 41, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Wang, Y.; Lan, Q.; Wu, W.; Li, Z.; Ma, X.; Yu, L. Exosomes Derived from Mesenchymal Stem Cells Ameliorate Hypoxia/Reoxygenation-Injured ECs via Transferring MicroRNA-126. Stem Cells Int. 2019, 2019, 2831756. [Google Scholar] [CrossRef]
- Pan, Q.; Kuang, X.; Cai, S.; Wang, X.; Du, D.; Wang, J.; Wang, Y.; Chen, Y.; Bihl, J.; Chen, Y.; et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res. Ther. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Wu, Y.; Fang, Y.; Hong, B.; Dai, D.; Zhou, Y.; Liu, J.; Li, Q. Bone marrow mesenchymal stem cells-derived exosomal microRNA-124-3p attenuates hypoxic-ischemic brain damage through depressing tumor necrosis factor receptor associated factor 6 in newborn rats. Bioengineered 2022, 13, 3194–3206. [Google Scholar] [CrossRef]
- Gan, C.; Ouyang, F. Exosomes Released from Bone-Marrow Stem Cells Ameliorate Hippocampal Neuronal Injury Through transferring miR-455-3p. J. Stroke Cerebrovasc. Dis. 2022, 31, 106142. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Ye, Z.; Lin, H. Neuroprotective effect of exosomes derived from bone marrow mesenchymal stem cells via activating TGR5 and suppressing apoptosis. Biochem. Biophys. Res. Commun. 2022, 593, 13–19. [Google Scholar] [CrossRef]
- Li, X.; Bi, T.; Yang, S. Exosomal microRNA-150-5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll-like receptor 5. Bioengineered 2022, 13, 3030–3043. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, J.; Shen, Q.; Li, M.; Peng, Y. Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J. Neuroinflamm. 2018, 15, 242. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Liu, H.; Zhu, R.; He, H.; Zhou, Y.; Zhang, Y.; Li, C.; Liang, D.; Zeng, Q.; et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp. Neurol. 2021, 341, 113700. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhou, Y.; Liang, D.; He, H.; Liu, X.; Zhu, R.; Zhang, M.; Luo, X.; Wang, Y.; Huang, G. Exosomes Secreted From Bone Marrow Mesenchymal Stem Cells Attenuate Oxygen-Glucose Deprivation/Reoxygenation-Induced Pyroptosis in PC12 Cells by Promoting AMPK-Dependent Autophagic Flux. Front. Cell Neurosci. 2020, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Wang, Y.; Zhao, D.; Sun, C.; Zhou, S.; Xu, D.; Zhao, J. Exosomes Derived from CXCR4-Overexpressing BMSC Promoted Activation of Microvascular Endothelial Cells in Cerebral Ischemia/Reperfusion Injury. Neural Plast. 2020, 2020, 8814239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gan, Y.; Xu, G.; Yin, G.; Liu, D. MSCs-Derived Exosomes Attenuate Acute Brain Injury and Inhibit Microglial Inflammation by Reversing CysLT2R-ERK1/2 Mediated Microglia M1 Polarization. Neurochem. Res. 2020, 45, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Dumbrava, D.A.; Surugiu, R.; Börger, V.; Ruscu, M.; Tertel, T.; Giebel, B.; Hermann, D.M.; Popa-Wagner, A. Mesenchymal stromal cell-derived small extracellular vesicles promote neurological recovery and brain remodeling after distal middle cerebral artery occlusion in aged rats. Geroscience 2022, 44, 293–310. [Google Scholar] [CrossRef]
- Wang, C.; Börger, V.; Yusuf, A.M.; Tertel, T.; Stambouli, O.; Murke, F.; Freund, N.; Kleinschnitz, C.; Herz, J.; Gunzer, M.; et al. Postischemic Neuroprotection Associated With Anti-Inflammatory Effects by Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles in Aged Mice. Stroke 2022, 53, e14–e18. [Google Scholar] [CrossRef]
- Xu, R.; Bai, Y.; Min, S.; Xu, X.; Tang, T.; Ju, S. In vivo Monitoring and Assessment of Exogenous Mesenchymal Stem Cell-Derived Exosomes in Mice with Ischemic Stroke by Molecular Imaging. Int. J. Nanomed. 2020, 15, 9011–9023. [Google Scholar] [CrossRef]
- Guo, X.-F.; Gu, S.-S.; Wang, J.; Sun, H.; Zhang, Y.-J.; Yu, P.-F.; Zhang, J.-S.; Jiang, L. Protective effect of mesenchymal stem cell-derived exosomal treatment of hippocampal neurons against oxygen-glucose deprivation/reperfusion-induced injury. World J. Emerg. Med. 2022, 13, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, F.; Zhang, Y.; Liu, Y.; Zhang, D. Buyang Huanwu Decoction (BYHWD) Enhances Angiogenic Effect of Mesenchymal Stem Cell by Upregulating VEGF Expression After Focal Cerebral Ischemia. J. Mol. Neurosci. 2015, 56, 898–906. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.J.; Kang, L.; Kim, Y.J.; Kang, M.K.; Kim, J.; Ryu, J.H.; Hyeon, T.; Yoon, B.-W.; Ko, S.-B.; et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials 2020, 243, 119942. [Google Scholar] [CrossRef]
- Huang, X.; Ding, J.; Li, Y.; Liu, W.; Ji, J.; Wang, H.; Wang, X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp. Cell. Res. 2018, 371, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tu, Z.; Yang, D.; Hu, M.; Zhou, L.; Li, Q.; Yu, B.; Hou, S. Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization. Neurosci. Lett. 2022, 769, 136389. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Tang, H.; Luo, S.; Lv, Y.; Liang, D.; Kang, X.; Hong, W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 2019, 11, 780–792. [Google Scholar]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, X.; Zhang, R.; Xie, Y.; Feng, Z.; Li, F.; Han, J.; Sun, H.; Ouyang, Q.; Hua, S.; et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 2021, 13, 3060–3079. [Google Scholar] [CrossRef]
- Hu, Z.; Yuan, Y.; Zhang, X.; Lu, Y.; Dong, N.; Jiang, X.; Xu, J.; Zheng, D. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Attenuate Oxygen-Glucose Deprivation/Reperfusion-Induced Microglial Pyroptosis by Promoting FOXO3a-Dependent Mitophagy. Oxid. Med. Cell. Longev. 2021, 2021, 6219715. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Chen, Q.; Ding, H.; Li, H. MicroRNA-342-3p loaded by human umbilical cord mesenchymal stem cells-derived exosomes attenuates deep vein thrombosis by downregulating EDNRA. J. Thromb. Thrombolysis 2022, 54, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, R.; Shi, Y.; Liang, J.; Zhao, L. Plasma exosomes protect against cerebral ischemia/reperfusion injury via exosomal HSP70 mediated suppression of ROS. Life Sci. 2020, 256, 117987. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Pan, J.; Li, F.; Zhao, L.; Shi, Y. A novel brain targeted plasma exosomes enhance the neuroprotective efficacy of edaravone in ischemic stroke. IET Nanobiotechnol. 2021, 15, 107–116. [Google Scholar] [CrossRef]
- Wang, K.; Ru, J.; Zhang, H.; Chen, J.; Lin, X.; Lin, Z.; Wen, M.; Huang, L.; Ni, H.; Zhuge, Q.; et al. Melatonin Enhances the Therapeutic Effect of Plasma Exosomes Against Cerebral Ischemia-Induced Pyroptosis Through the TLR4/NF-κB Pathway. Front. Neurosci. 2020, 14, 848. [Google Scholar] [CrossRef]
- Yang, T.; He, R.; Li, G.; Liang, J.; Zhao, L.; Zhao, X.; Li, L.; Wang, P. Growth arrest and DNA damage-inducible protein 34 (GADD34) contributes to cerebral ischemic injury and can be detected in plasma exosomes. Neurosci. Lett. 2021, 758, 136004. [Google Scholar] [CrossRef] [PubMed]
- Bubak, A.N.; Coughlan, C.; Posey, J.; Saviola, A.J.; Niemeyer, C.S.; Lewis, S.W.R.; Lopez, S.B.; Solano, A.; Tyring, S.K.; Delaney, C.; et al. Zoster-associated Prothrombotic Plasma Exosomes and Increased Stroke Risk. J. Infect Dis. 2022, jiac405. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, N.; Chang, Z.; Gao, Y.; Bao, M.; Xie, Y.; Xu, W.; Liu, X.; Jiang, S.; Liu, Y.; et al. Exosomal MicroRNA-126 from RIPC Serum Is Involved in Hypoxia Tolerance in SH-SY5Y Cells by Downregulating DNMT3B. Mol. Ther. Nucleic. Acids 2020, 20, 649–660. [Google Scholar] [CrossRef]
- Ye, Z.; Hu, J.; Xu, H.; Sun, B.; Jin, Y.; Zhang, Y.; Zhang, J. Serum Exosomal microRNA-27-3p Aggravates Cerebral Injury and Inflammation in Patients with Acute Cerebral Infarction by Targeting PPARγ. Inflammation 2021, 44, 1035–1048. [Google Scholar] [CrossRef]
- Wang, X.; Han, C.; Jia, Y.; Wang, J.; Ge, W.; Duan, L. Proteomic Profiling of Exosomes From Hemorrhagic Moyamoya Disease and Dysfunction of Mitochondria in Endothelial Cells. Stroke 2021, 52, 3351–3361. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, R.; Wang, P.; Shi, Y.; Zhao, L.; Liang, J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater. Sci. 2019, 7, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Su, C.; Qi, Y.; Liang, J.; Zhao, L.; Shi, Y. Brain-targeted heptapeptide-loaded exosomes attenuated ischemia-reperfusion injury by promoting the transfer of healthy mitochondria from astrocytes to neurons. J. Nanobiotechnol. 2022, 20, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, L.; Shi, Y.; Liang, J. Edaravone-Loaded Macrophage-Derived Exosomes Enhance Neuroprotection in the Rat Permanent Middle Cerebral Artery Occlusion Model of Stroke. Mol. Pharm. 2020, 17, 3192–3201. [Google Scholar] [CrossRef]
- He, R.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-laden exosomes target ischemic brain tissue and alleviate cerebral ischemia-reperfusion injury by inhibiting ROS-mediated mitochondrial apoptosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111314. [Google Scholar] [CrossRef]
- Xiao, T.; Qu, H.; Zeng, Z.; Li, C.; Wan, J. Exosomes from M2-polarized macrophages relieve oxygen/glucose deprivation/normalization-induced neuronal injury by activating the Nrf2/HO-1 signaling. Arch. Biochem. Biophys. 2022, 721, 109193. [Google Scholar] [CrossRef]
- Yue, K.-Y.; Zhang, P.-R.; Zheng, M.-H.; Cao, X.-L.; Cao, Y.; Zhang, Y.-Z.; Wu, H.-N.; Lu, Z.-H.; Liang, L.; Xiang, X.-F.; et al. Neurons can upregulate Cav-1 to increase intake of endothelial cells-derived extracellular vesicles that attenuate apoptosis via miR-1290. Cell Death Dis. 2019, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, L.; He, Y.; Li, R.; Xiang, Y.; Xing, Z.; Li, Y.; Albashari, A.A.; Liao, X.; Zhang, K.; et al. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 2021, 54, e13093. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zhang, G.; Xia, Y.; Zhu, Q.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Yang, Y.; Wang, Y.; et al. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J. Cell Mol. Med. 2020, 24, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xu, X.; Zhang, M.; Song, X. Human urine-derived stem cell-derived exosomal miR-21-5p promotes neurogenesis to attenuate Rett syndrome via the EPha4/TEK axis. Lab. Investig. 2021, 101, 824–836. [Google Scholar] [CrossRef]
- Ge, L.; Xun, C.; Li, W.; Jin, S.; Liu, Z.; Zhuo, Y.; Da Duan, D.; Hu, Z.; Chen, P.; Lu, M. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J. Nanobiotechnol. 2021, 19, 380. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Huang, C.-C.; Chien, L.-H.; Lin, M.-T.; Chang, C.-P.; Lin, H.-J.; Chio, C.-C. Ischemia/reperfusion injured intestinal epithelial cells cause cortical neuron death by releasing exosomal microRNAs associated with apoptosis, necroptosis, and pyroptosis. Sci. Rep. 2020, 10, 14409. [Google Scholar] [CrossRef]
- Cai, H.; Huang, L.-Y.; Hong, R.; Song, J.-X.; Guo, X.-J.; Zhou, W.; Hu, Z.-L.; Wang, W.; Wang, Y.-L.; Shen, J.-G.; et al. Momordica charantia Exosome-Like Nanoparticles Exert Neuroprotective Effects Against Ischemic Brain Injury via Inhibiting Matrix Metalloproteinase 9 and Activating the AKT/GSK3β Signaling Pathway. Front. Pharmacol. 2022, 13, 908830. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Liu, T.; Wang, Y.; Wei, X.; Qi, S.; Gu, B. Effects of Momordica charantia exosomes on platelet activation, adhesion, and aggregation. Blood Coagul. Fibrinolysis 2022, 33, 372–380. [Google Scholar] [CrossRef]

| Source of Exosomes | Component | Mechanism | Function | Experiment Type | Reference |
|---|---|---|---|---|---|
| NSCs | Transferred into ischemic cells, and repair tissue | Reduce injury and protect neurons | In vitro | [34] | |
| NSCs | miR-150-3p | Inhibit CASP2 signaling pathway | Anti-apoptosis, promote neurogenesis | In vitro and in vivo | [35] |
| Cortical neuron | miR-181c-3p | Inhibit the expression CXCL1 | Anti-inflammation | In vitro | [36] |
| NPC-EVs | Anti-inflammation, suppressing cell apoptosis | In vitro and in vivo | [26] | ||
| iPSC-NPCs | PTEN/AKT signaling pathway and neurite outgrowth | Promote neurogenesis | In vitro | [39] | |
| Microglia | Inhibition of PDE1-D | Anti-inflammatory, enhance autophagic flux | In vitro and in vivo | [45] | |
| Microglia | miR-424-5p | Inhibit STAT3 pathway | Injure microvascular endothelial cells | In vitro and in vivo | [46] |
| M2 microglia | miR-124 | Target USP14 | Attenuate neuronal apoptosis and promote neurogenesis | In vitro | [16] |
| M2 microglia | miR-137 | Target gene Notch1 | Attenuate neuronal apoptosis | In vitro and in vivo | [44] |
| M2 microglia | miR-26a | Promote angiogenesis | In vitro and in vivo | [43] | |
| Astrocyte | Inhibit autophagy | In vitro and in vivo | [49] | ||
| Astrocyte | circular RNA circSHOC2 | Regulate autophagy and miR-7670-3p/SIRT1 | Suppress neuronal apoptosis | In vitro and in vivo | [50] |
| Astrocyte | miR-22-3p | Suppress KDM6B-mediated effects on the BMP2/BMF axis | Enhance neuron viability, anti-apoptosis | In vitro and in vivo | [51] |
| Astrocyte | miR-17-5p | Anti-apoptosis, anti-oxidation, anti-inflammation | In vitro and in vivo | [52] | |
| Astrocytes | Axonal neuronal outgrowth | In vitro and in vivo | [53] | ||
| Astrocytes | miR-92b-3p | Attenuated neuron death and apoptosis | In vitro | [54] | |
| Astrocyte | Deliver GJA1 | Anti-apoptosis | In vitro and in vivo | [55] | |
| ECs | Anti-apoptosis and promote neurogenesis | In vitro and in vivo | [56] | ||
| ECs | miR-126-3p | Anti-apoptosis and increase neurite outgrowth | In vivo | [57] | |
| CECs | miRNAs | Target RhoA | Facilitate axonal growth | In vitro | [58] |
| VECs | Anti-apoptosis and promote neurogenesis | In vitro and in vivo | [59] | ||
| EPCs | Inhibit apoptosis and promote angiogenesis | In vivo | [60] |
| Source of Exosomes | Component | Mechanism | Function | Experiment Type | Reference |
|---|---|---|---|---|---|
| BMSCs | miR-26a-5p | Downregulate CDK6 | Inhibit microglia apoptosis, reduce inflammation | In vitro and in vivo | [62] |
| BMSCs | miR-17-92 | Downregulate gene PTEN, activate the PI3K/Akt/mTOR pathway | Increase axonal extension and axonal myelination | In vivo | [63] |
| BMSCs | miR-126 | PI3K/Akt/eNOS pathway | Promote angiogenesis, downregulate caspase-3 of ECs | In vitro | [64] |
| BMSCs | miR-132-3p | PI3K/Akt/eNOS pathway | Ameliorate endothelial apoptosis and oxidative stress | In vitro and in vivo | [65] |
| BMSCs | miR-124-3p | Target TRAF6 | Suppress oxidative stress and reduce neuronal apoptosis | In vivo | [66] |
| BMSCs | miR-455-3p | Target PDCD7 | Anti-apoptosis | In vitro and in vivo | [67] |
| BMSCs | Target TGR5 | Anti-apoptosis | In vitro and in vivo | [68] | |
| BMSCs | miR-150-5p | Reduce TLR5 expression | Repress inflammation, block neuron apoptosis | In vivo | [69] |
| BMSCs | promote microglial polarization toward M2 | Inhibit NLRP3 inflammasome-mediated inflammation and pyroptosis | In vitro and in vivo | [71] | |
| BMSCs | Promote AMPK-dependent autophagic flux | Ameliorate NLRP3 inflammasome-mediated pyroptosis | In vitro | [72] | |
| BMSCs | Wnt-3a/β-catenin pathway | Anti-apoptotic, facilitate the proliferation and tube formation of MSCs | In vitro and in vivo | [73] | |
| BMSCs | Promote the differentiation of microglia to the M2 | Reverse CysLT2R-ERK1/2′s effect, reduce inflammation | In vitro and in vivo | [74] | |
| BMSCs | Increase peri-infarct angiogenesis | In vivo | [75] | ||
| BMSCs | Decrease the infiltrates of inflammatory cells | Anti-inflammation | In vivo | [76] | |
| BMSCs | Reduce the expression of IL-1β | Facilitate angiogenesis and neurogenesis | In vivo | [77] | |
| BMSCs | Alleviate oxidative stress and dysregulation of mitochondrial function | In vitro | [78] | ||
| BMSCs | miRNA and VEGF | Promote angiogenesis | In vitro and in vivo | [79] | |
| BMSCs | Inhibit inflammation and apoptosis, as well as promote angiogenesis | In vivo | [80] | ||
| ADSCs | Increase PEDF content | Activate autophagy and suppress neuronal apoptosis | In vitro and in vivo | [81] | |
| ADSCs | circ-Rps5 | MiR-124-3p overexpression or SIRT7 downregulation | Attenuate inflammation | In vitro and in vivo | [82] |
| ADSCs | miR-126 | Enhance neurogenesis and inhibit neuroinflammation | In vitro and in vivo | [83] | |
| hUMSCs | miR-146a-5p | Suppress IRAK1/TRAF6 pathway | Anti-neuroinflammation | In vitro and in vivo | [85] |
| hUMSCs | Toll-like receptor 4 signaling of BV-2 microglia | Reduce microglia-mediated neuroinflammation | In vitro and in vivo | [84] | |
| hUMSCs | Increase FOXO3a expression | Enhance mitophagy, attenuate pyroptosis | In vitro | [86] | |
| hUMSCs | Upregulate miR-342-3p and downregulate endothelin A receptor expression | Alleviate DVT | In vitro and in vivo | [87] | |
| PLA | HSP70 | Reduces ROS, apoptosis, and BBB damage | In vitro | [88] | |
| PLA | Interaction between transferrin and transferrin receptor | Reduces ROS generation | In vivo | [89] | |
| PLA | TLR4/NF-κB signaling pathway | Enhance plasma exosome against inflammatory responses and pyroptosis | In vivo | [90] | |
| PLA | Dephosphorylation of eIF2α and phosphorylation of p53 | Induce neuronal apoptosis | In vivo | [91] | |
| HZ PLA | Form platelet-leukocyte aggregates | Human | [92] | ||
| RIPC serum | miR-126 | Downregulate DNMTs3B | Reduce SH-SY5Y cells injure | In vivo | [93] |
| Serum exosomes | miR-27-3p | Target PPARγ | Promote inflammation, thereby aggravating ACI | In vitro and in vivo | [94] |
| MMD serum | Promote neuroblastoma cells proliferation | In vivo | [95] |
| Source of Exosomes | Component | Mechanism | Function | Experiment Type | Reference |
|---|---|---|---|---|---|
| Macrophages | Modulating microglial polarity | Anti-inflammation | In vitro and in vivo | [96] | |
| Macrophages | Drp1-Fis1 interaction | Reduce mitochondrial damage in astrocytes | In vitro and in vivo | [97] | |
| Macrophage | Targets neuronal cells and microglia | Reduce inflammation response | In vivo | [98] | |
| Macrophages | Downregulating ROS | Protect BBB and antagonize neuronal apoptosis | In vitro and in vivo | [99] | |
| M2 macrophages | Activate Nrf2/HO-1 signaling pathway | Inhibit ROS, protect HT22 neurons | In vitro | [100] | |
| Dental pulp stem cell | Inhibit HMGB1/TLR4/MyD88/NF-κB pathway | Anti-inflammation | In vitro and in vivo | [102] | |
| USCs | MiR-26a/HDAC6 axis | Promote neurogenesis | In vitro and in vivo | [103] | |
| USCs | miR-21-5p | EPha4/TEK axis | Promote neurogenesis | In vitro and in vivo | [104] |
| Olfactory mucosa MSCs | miR-612-TP53-HIF-1α-VEGF axis | Promote the formation of HBMECs | In vitro and in vivo | [105] | |
| HUECs | miR-1290 | Cav-1 upregulates intake of HUECs-EVs | Attenuates apoptosis | In vitro and in vivo | [101] |
| Intestinal epithelium cells | miRNA | Promote apoptosis, necroptosis, and/or pyroptosis of cortical neurons | In vitro | [106] | |
| MC-ELNs | AKT/GSK3β signaling pathway | Protect BBB and anti-apoptosis | In vivo | [107] | |
| MCEs | Inhibit platelet activation, aggregation, adhesion, and HCT116 cells migration | In vitro and in vivo | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, R.; Zeng, X.; Yan, H.; Huang, X.; Deng, C. Effects and Mechanisms of Exosomes from Different Sources in Cerebral Ischemia. Cells 2022, 11, 3623. https://doi.org/10.3390/cells11223623
Xie R, Zeng X, Yan H, Huang X, Deng C. Effects and Mechanisms of Exosomes from Different Sources in Cerebral Ischemia. Cells. 2022; 11(22):3623. https://doi.org/10.3390/cells11223623
Chicago/Turabian StyleXie, Ruoxi, Xinbing Zeng, Huan Yan, Xiaoping Huang, and Changqing Deng. 2022. "Effects and Mechanisms of Exosomes from Different Sources in Cerebral Ischemia" Cells 11, no. 22: 3623. https://doi.org/10.3390/cells11223623
APA StyleXie, R., Zeng, X., Yan, H., Huang, X., & Deng, C. (2022). Effects and Mechanisms of Exosomes from Different Sources in Cerebral Ischemia. Cells, 11(22), 3623. https://doi.org/10.3390/cells11223623





