Prevalence and Prognostic Relevance of Homologous Recombination Repair Gene Mutations in Uterine Serous Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry (IHC) Analysis
2.3. Genomic DNA Isolation
2.4. Next-Generation Sequencing Library Preparation and Sequencing
2.5. Variant Classification and Analysis of Homologous Recombination Repair Gene Mutations
2.6. Statistical Analysis
3. Results
3.1. Clinicopathologic Characteristics
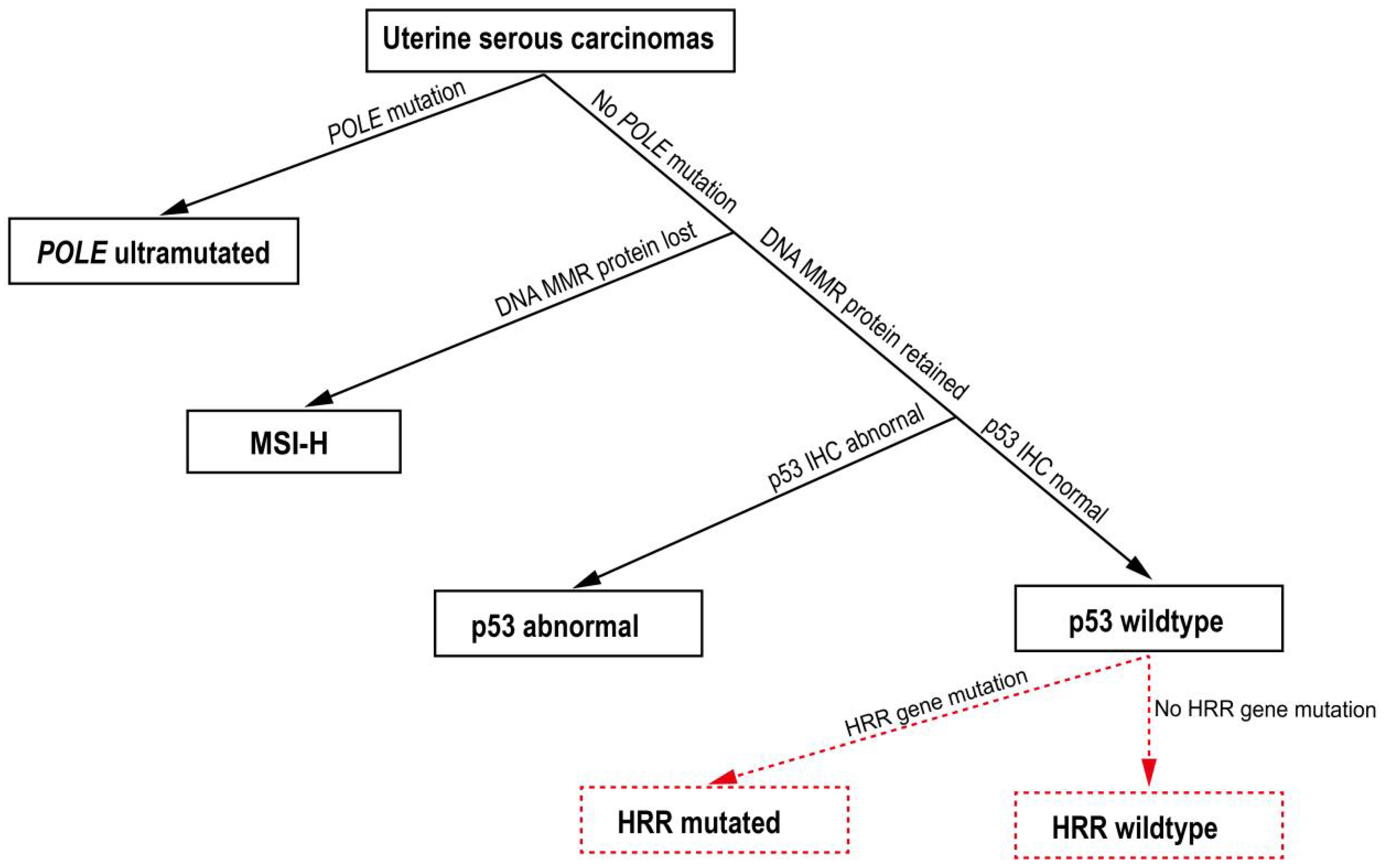
3.2. HRR Gene Mutation Profile in USC Patients
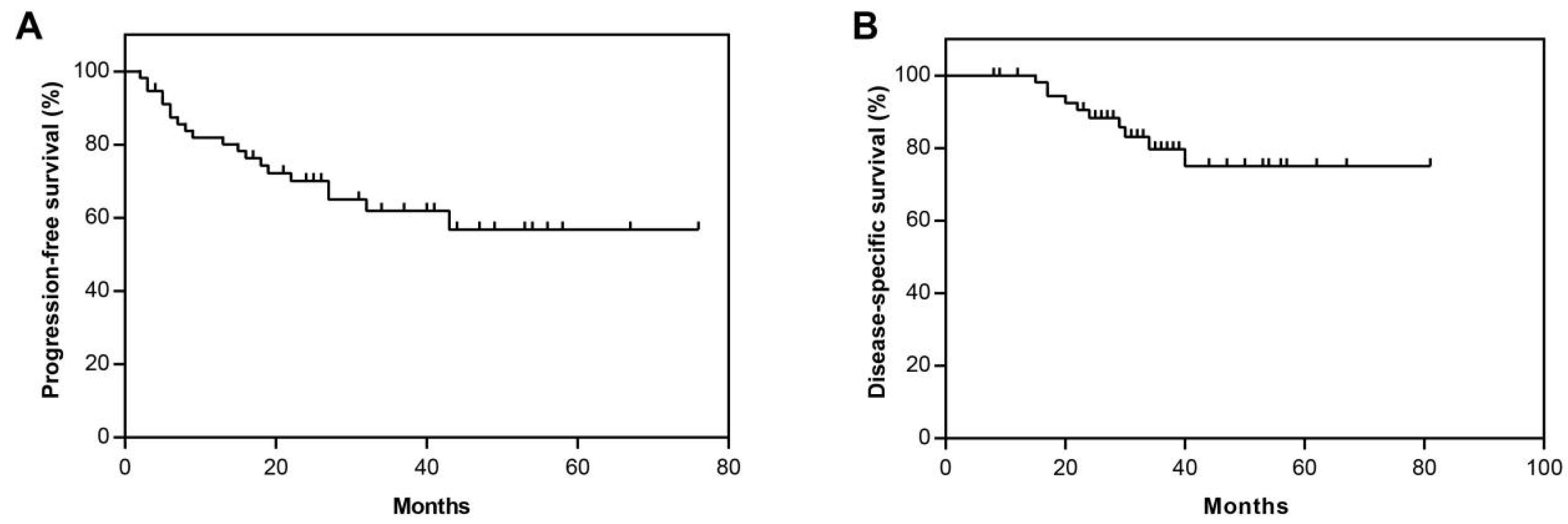
3.3. Overall Clinical Outcomes in Study Cohort
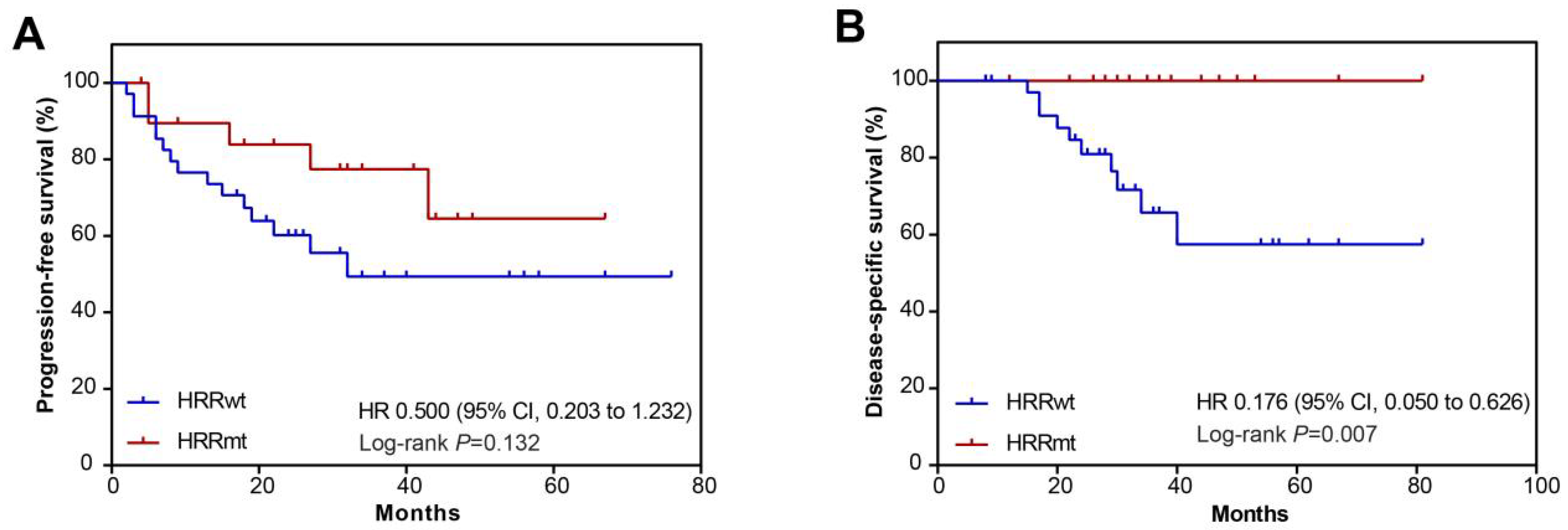
3.4. Association between HRR Gene Mutations and Prognosis
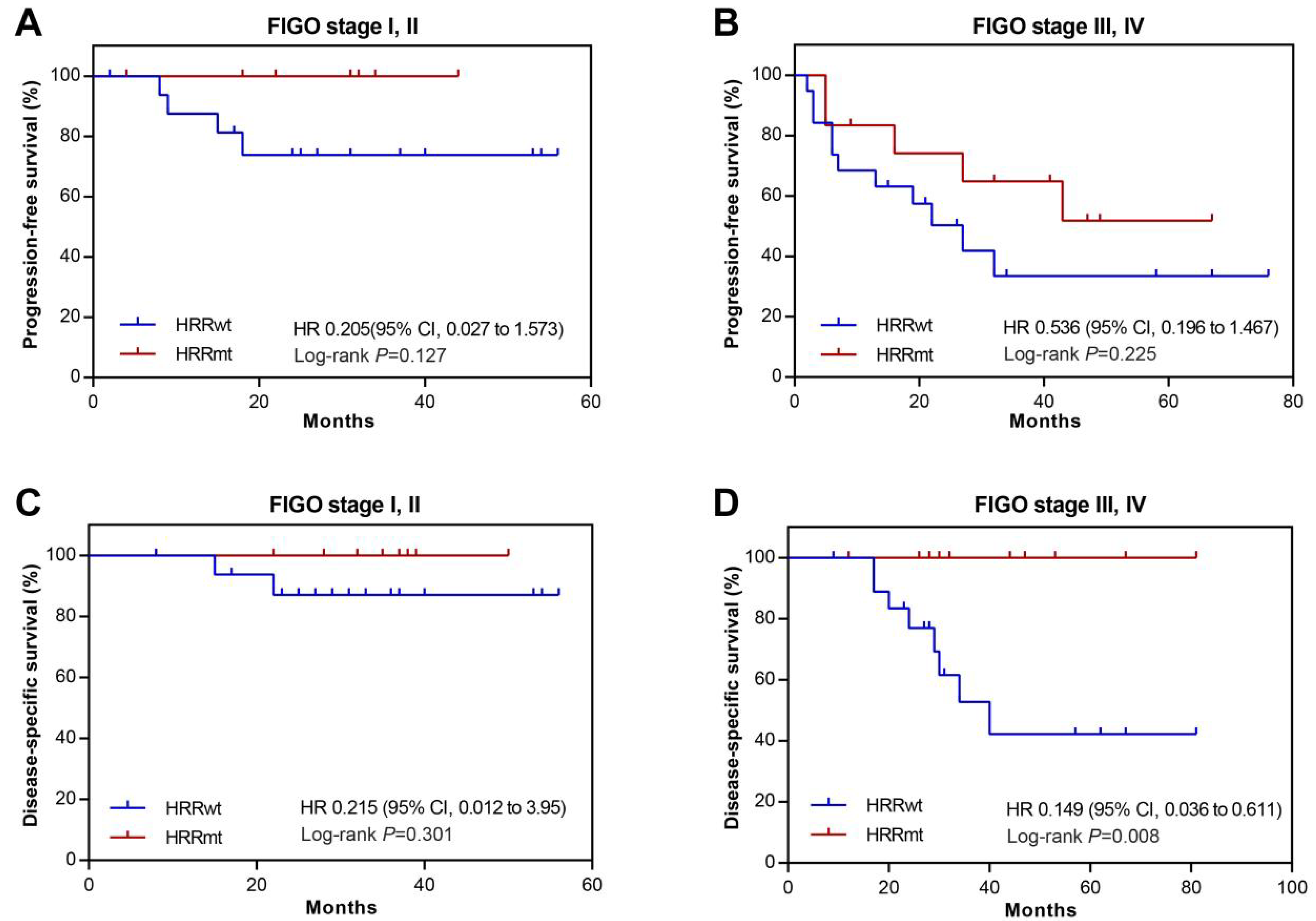
3.5. Association between HRR Gene Mutations and Prognosis in USC Patients with Abnormal p53 Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Novelty and Impact Statements
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhang, L.; Kwan, S.Y.; Wong, K.K.; Solaman, P.T.; Lu, K.H.; Mok, S.C. Pathogenesis and Clinical Management of Uterine Serous Carcinoma. Cancers 2020, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Tran, K.; Richardson, M.; Darcy, K.; Tiao, C.; Hamilton, C.; Maxwell, L.; Mann, A.; Cohen, J.; Kapp, D.; et al. 41 Increase in uterine serous carcinoma: Will it surpass uterine endometrioid cancer? A population analysis of 720,984 uterine cancer patients. Int. J. Gynecol. Cancer 2020, 30, A27–A28. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jurgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms (Version 1.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 17 October 2022).
- Ashley, C.W.; Da Cruz Paula, A.; Kumar, R.; Mandelker, D.; Pei, X.; Riaz, N.; Reis-Filho, J.S.; Weigelt, B. Analysis of mutational signatures in primary and metastatic endometrial cancer reveals distinct patterns of DNA repair defects and shifts during tumor progression. Gynecol. Oncol. 2019, 152, 11–19. [Google Scholar] [CrossRef]
- de Jonge, M.M.; Mooyaart, A.L.; Vreeswijk, M.P.; de Kroon, C.D.; van Wezel, T.; van Asperen, C.J.; Smit, V.T.; Dekkers, O.M.; Bosse, T. Linking uterine serous carcinoma to BRCA1/2-associated cancer syndrome: A meta-analysis and case report. Eur. J. Cancer 2017, 72, 215–225. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmana, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef]
- Oza, A.M.; Tinker, A.V.; Oaknin, A.; Shapira-Frommer, R.; McNeish, I.A.; Swisher, E.M.; Ray-Coquard, I.; Bell-McGuinn, K.; Coleman, R.L.; O′Malley, D.M.; et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol. Oncol. 2017, 147, 267–275. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O′Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef]
- McCabe, N.; Turner, N.C.; Lord, C.J.; Kluzek, K.; Bialkowska, A.; Swift, S.; Giavara, S.; O′Connor, M.J.; Tutt, A.N.; Zdzienicka, M.Z.; et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006, 66, 8109–8115. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Miller, R.E.; Leary, A.; Scott, C.L.; Serra, V.; Lord, C.J.; Bowtell, D.; Chang, D.K.; Garsed, D.W.; Jonkers, J.; Ledermann, J.A.; et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann. Oncol. 2020, 31, 1606–1622. [Google Scholar] [CrossRef]
- Dong, L.; Jin, X.; Wang, W.; Ye, Q.; Li, W.; Shi, S.; Guo, L.; Ying, J.; Zou, S. Distinct clinical phenotype and genetic testing strategy for Lynch syndrome in China based on a large colorectal cancer cohort. Int. J. Cancer 2020, 146, 3077–3086. [Google Scholar] [CrossRef]
- Kobel, M.; Ronnett, B.; Singh, N.; Soslow, R.; Gilks, C.; McCluggage, W. Interpretation of P53 Immunohistochemistry in Endometrial Carcinomas: Toward Increased Reproducibility. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S123–S131. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Van der Auwera, G.; O′Connor, B.D. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra, 1st ed.; O′Reilly Media: Sebastopol, CA, USA, 2020. [Google Scholar]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang le, L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Bratman, S.V.; Stehr, H.; Lee, L.J.; Liu, C.L.; Diehn, M.; Alizadeh, A.A. FACTERA: A practical method for the discovery of genomic rearrangements at breakpoint resolution. Bioinformatics 2014, 30, 3390–3393. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Toh, M.; Ngeow, J. Homologous Recombination Deficiency: Cancer Predispositions and Treatment Implications. Oncologist 2021, 26, e1526–e1537. [Google Scholar] [CrossRef]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018, 2018, PO.17.00286. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef]
- de Jonge, M.M.; Auguste, A.; van Wijk, L.M.; Schouten, P.C.; Meijers, M.; Ter Haar, N.T.; Smit, V.; Nout, R.A.; Glaire, M.A.; Church, D.N.; et al. Frequent Homologous Recombination Deficiency in High-grade Endometrial Carcinomas. Clin. Cancer Res. 2019, 25, 1087–1097. [Google Scholar] [CrossRef]
- Jonsson, J.M.; Baath, M.; Bjornheden, I.; Sahin, I.D.; Masback, A.; Hedenfalk, I. Homologous Recombination Repair Mechanisms in Serous Endometrial Cancer. Cancers 2021, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Wallbillich, J.J.; Morris, R.T.; Ali-Fehmi, R. Comparing mutation frequencies for homologous recombination genes in uterine serous and high-grade serous ovarian carcinomas: A case for homologous recombination deficiency testing in uterine serous carcinoma. Gynecol. Oncol. 2020, 159, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.; Mileshkin, L.; Mackay, H.; Leary, A.; Nijman, H.; Singh, N.; Pollock, P.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]


| HRRmt | HRRwt | Total | |
|---|---|---|---|
| Total | 22 (36.7%) | 38 (63.3%) | 60 |
| Age, years | |||
| Mean ± SD | 58 ± 1.9 | 61 ± 1.1 | 59.6 ± 0.9 |
| Tumor | |||
| Primary | 22 (100%) | 38 (100%) | 60 (100%) |
| Recurrent | 0 (0%) | 0 (0%) | 0 (0%) |
| FIGO stage | |||
| I | 7 (32%) | 12 (32%) | 19 (32%) |
| II | 2 (9%) | 5 (13%) | 7 (12%) |
| III | 10 (45%) | 16 (42%) | 26 (43%) |
| IV | 3 (14%) | 5 (13%) | 8 (13%) |
| LVSI | |||
| YES | 10 (45%) | 20 (53%) | 30 (50%) |
| NO | 12 (55%) | 18 (47%) | 30 (50%) |
| dMMR | |||
| YES | 1 (5%) | 1 (3%) | 2 (3%) |
| NO | 21 (95%) | 37 (97%) | 58 (97%) |
| Abnormal p53 IHC | |||
| YES | 18 (82%) | 33 (87%) | 51 (85%) |
| NO | 4 (18%) | 5 (13%) | 9 (15%) |
| POLE mutation | |||
| YES | 1 (5%) | 1 (3%) | 2 (3%) |
| NO | 21 (95%) | 37 (97%) | 58 (97%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Wang, T.; Li, N.; Yao, H.; Ying, J.; Wu, L.; Yuan, G. Prevalence and Prognostic Relevance of Homologous Recombination Repair Gene Mutations in Uterine Serous Carcinoma. Cells 2022, 11, 3563. https://doi.org/10.3390/cells11223563
Dong L, Wang T, Li N, Yao H, Ying J, Wu L, Yuan G. Prevalence and Prognostic Relevance of Homologous Recombination Repair Gene Mutations in Uterine Serous Carcinoma. Cells. 2022; 11(22):3563. https://doi.org/10.3390/cells11223563
Chicago/Turabian StyleDong, Lin, Tingting Wang, Ning Li, Hongwen Yao, Jianming Ying, Lingying Wu, and Guangwen Yuan. 2022. "Prevalence and Prognostic Relevance of Homologous Recombination Repair Gene Mutations in Uterine Serous Carcinoma" Cells 11, no. 22: 3563. https://doi.org/10.3390/cells11223563
APA StyleDong, L., Wang, T., Li, N., Yao, H., Ying, J., Wu, L., & Yuan, G. (2022). Prevalence and Prognostic Relevance of Homologous Recombination Repair Gene Mutations in Uterine Serous Carcinoma. Cells, 11(22), 3563. https://doi.org/10.3390/cells11223563








