Mexico: A Landscape of Viroid Origin and Epidemiological Relevance of Endemic Species
Abstract
1. A Landscape of Viroid Biodiversity in Mexico
2. Mexican Viroid Species: Economic Impact, Disease Relevance, and Spread
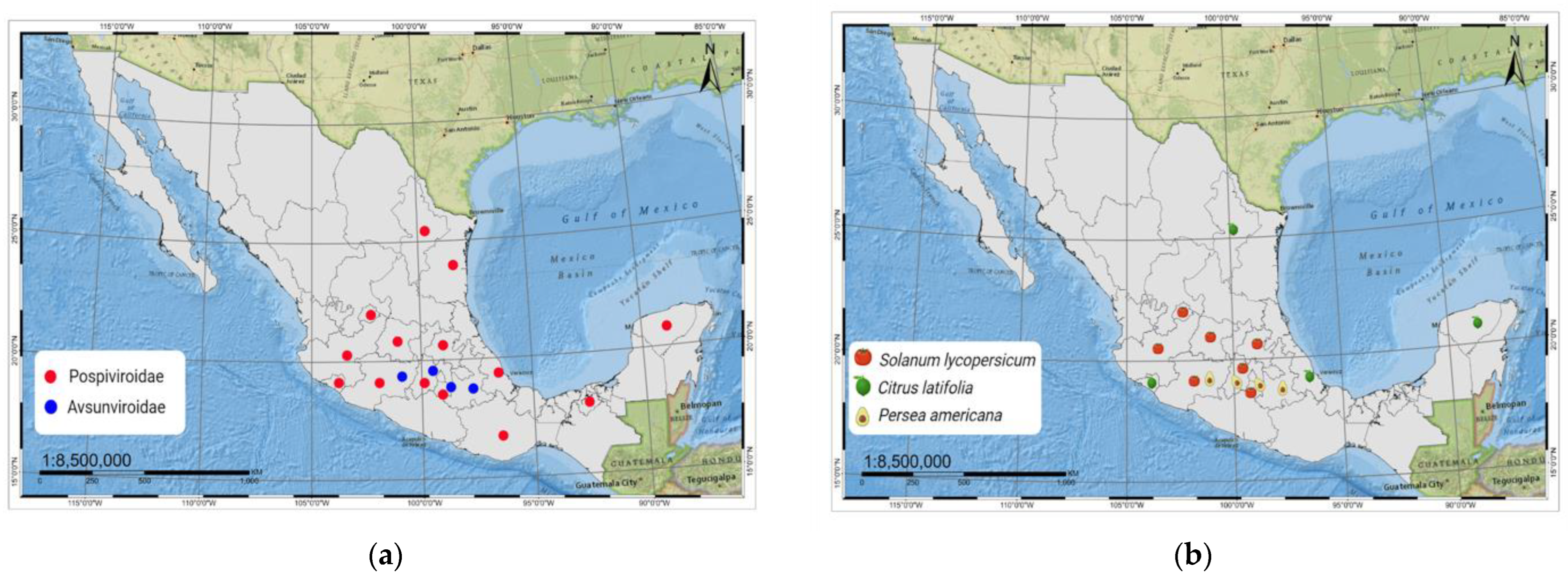
2.1. Pospiviroidae Species Reported in Mexico
2.2. Avsunviroidae Species
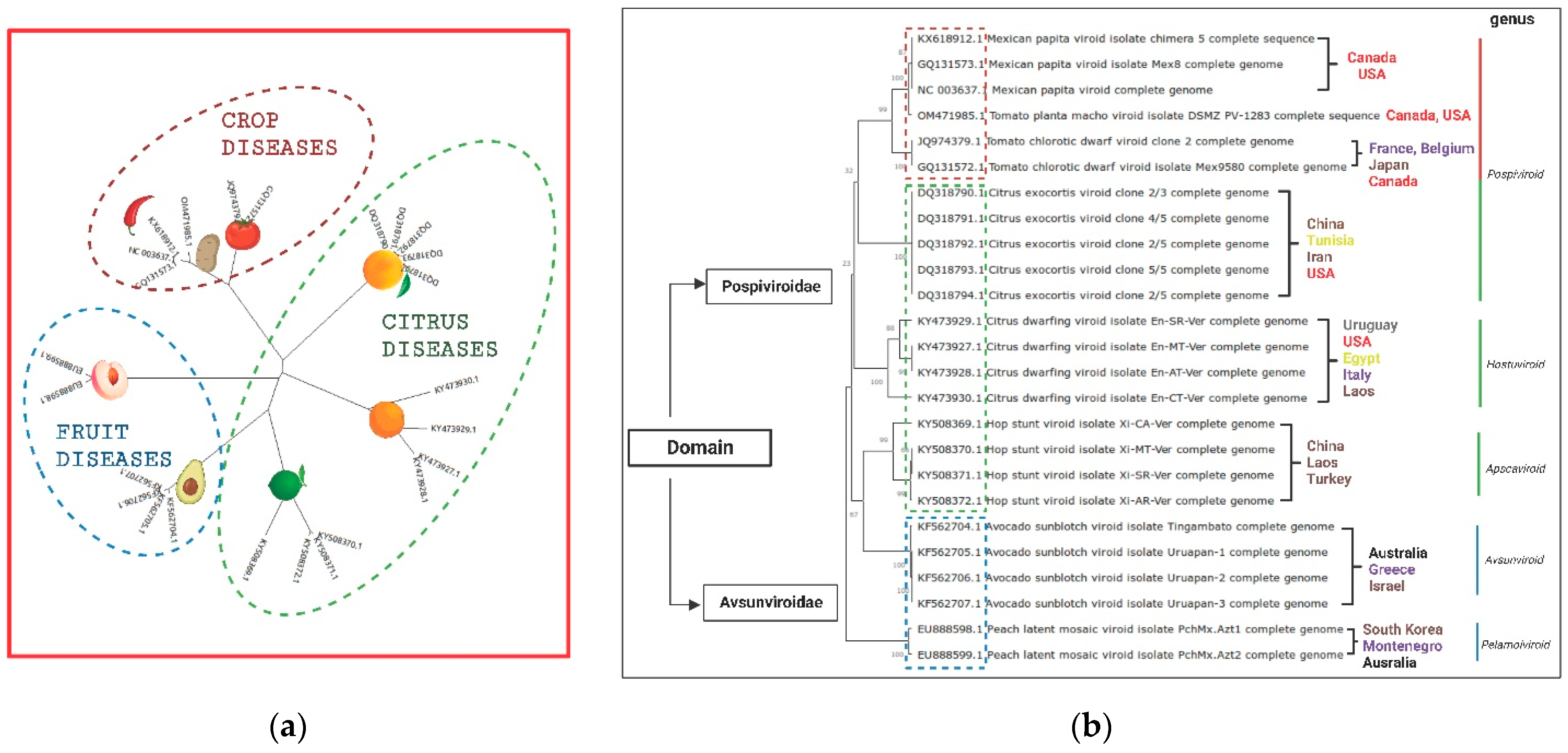
3. Genetic Variants of Pospiviroid Species Endemic to Mexico
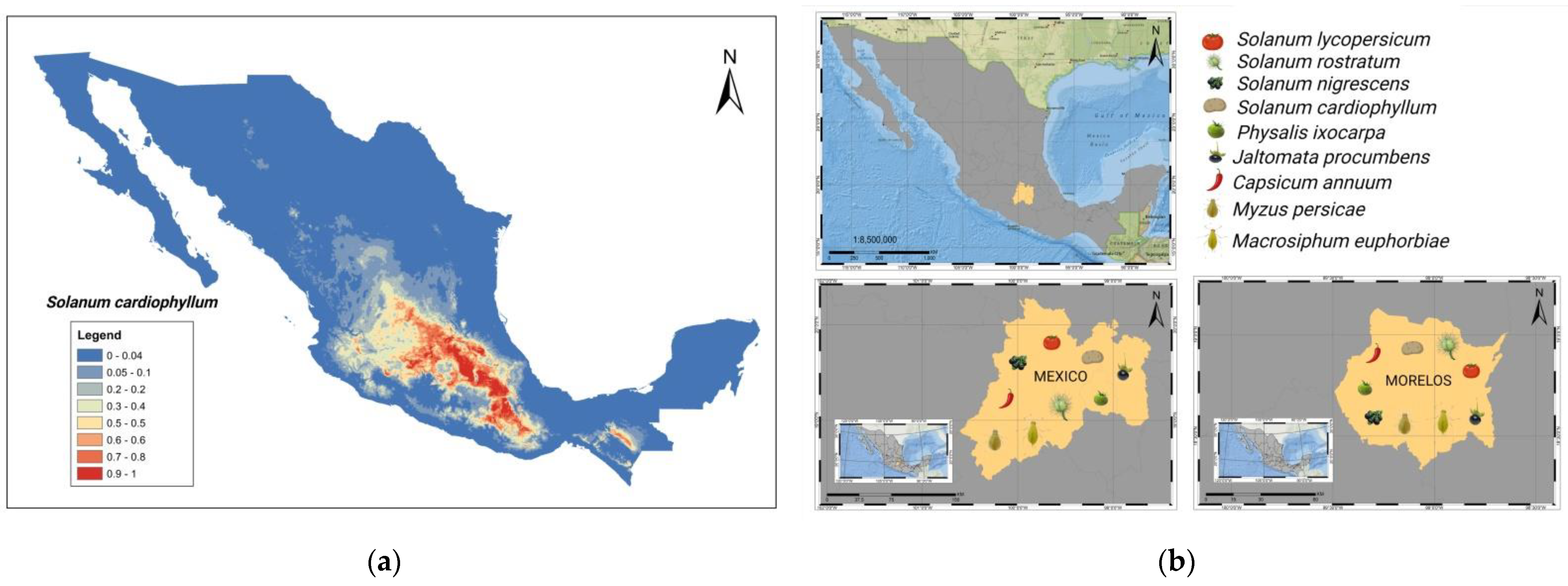
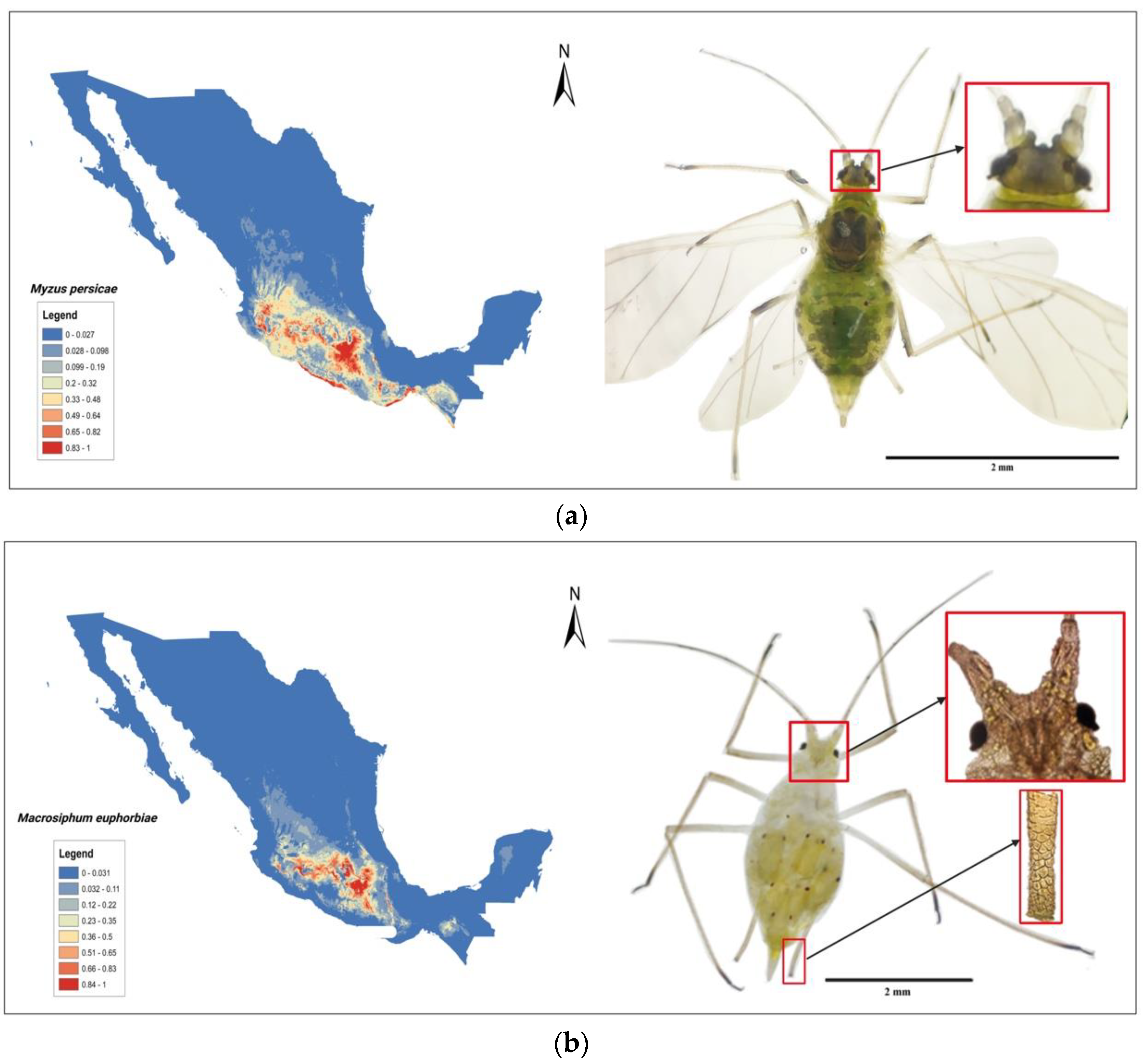
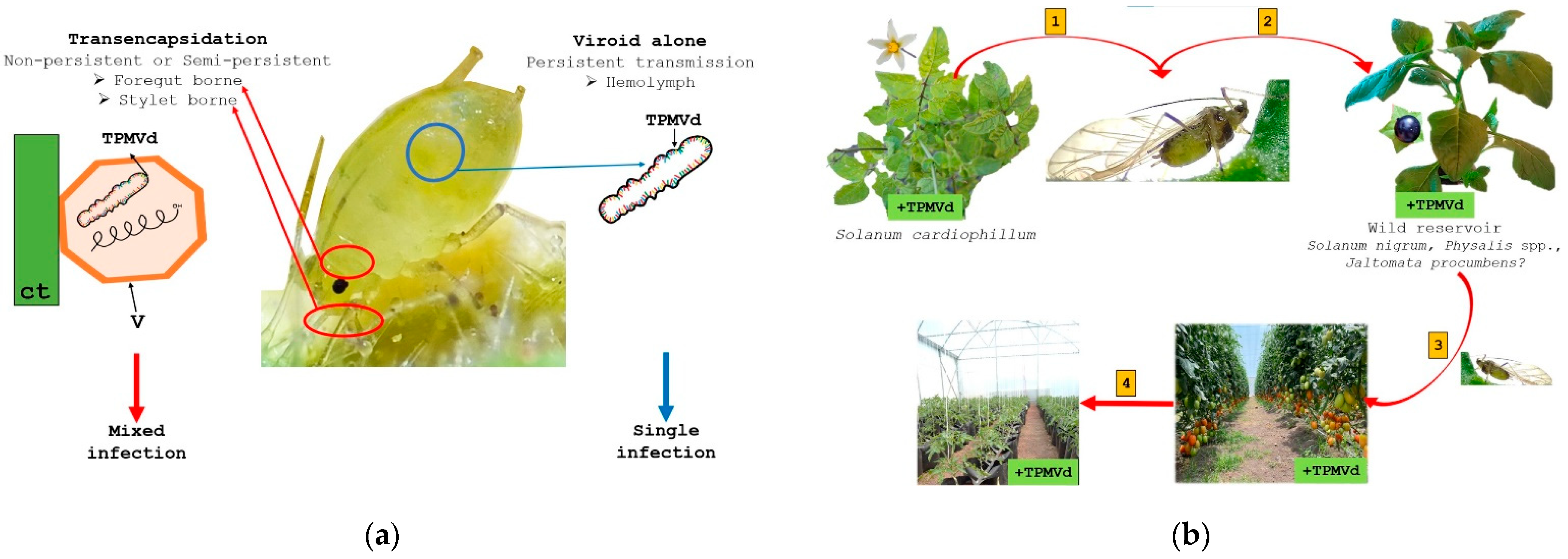
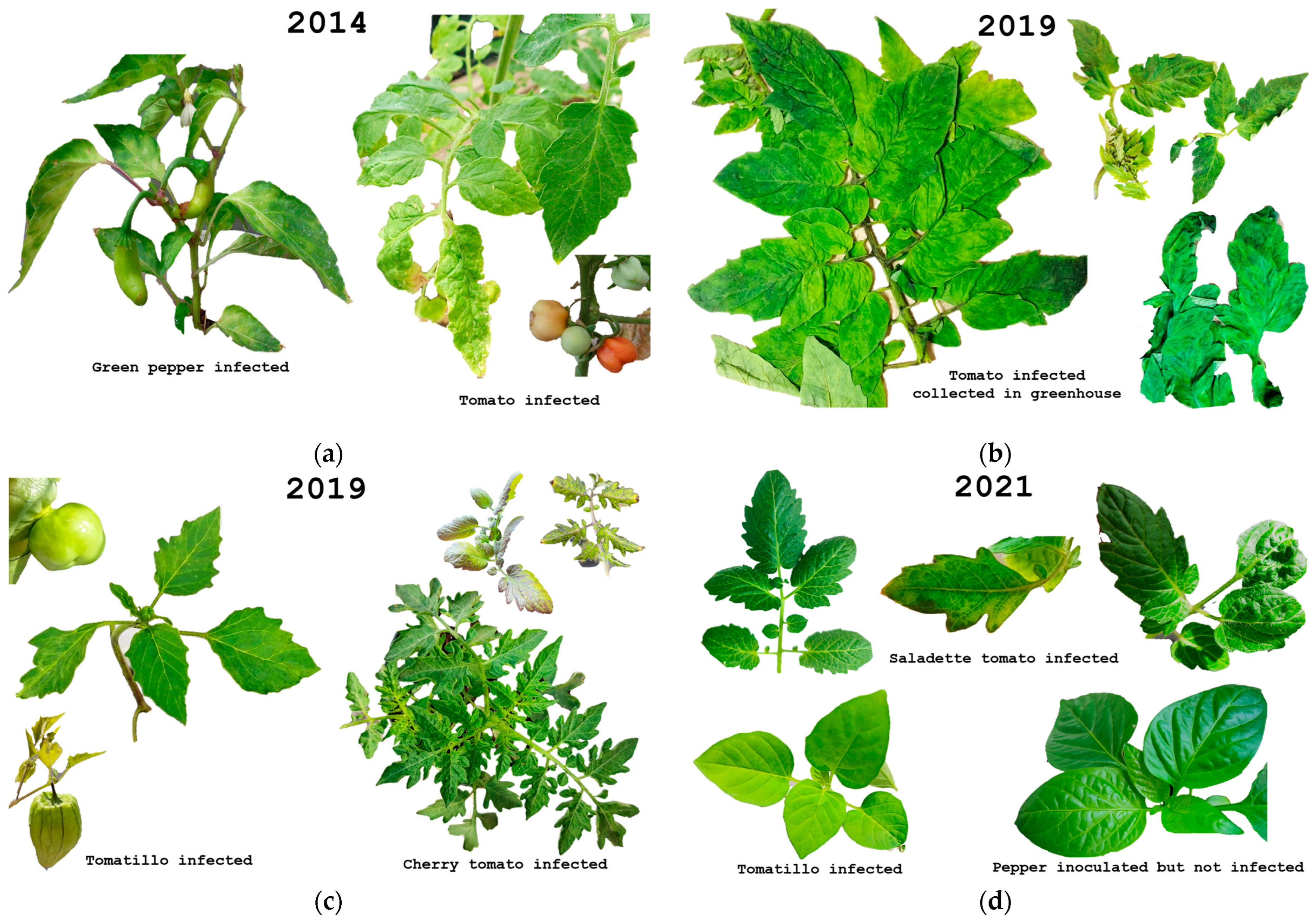
4. Tomato Crop Diseases: Role of Solanaceae Host Endemic Species and Putative Vectors
5. ASBVd and the Origin of Avocado Plants
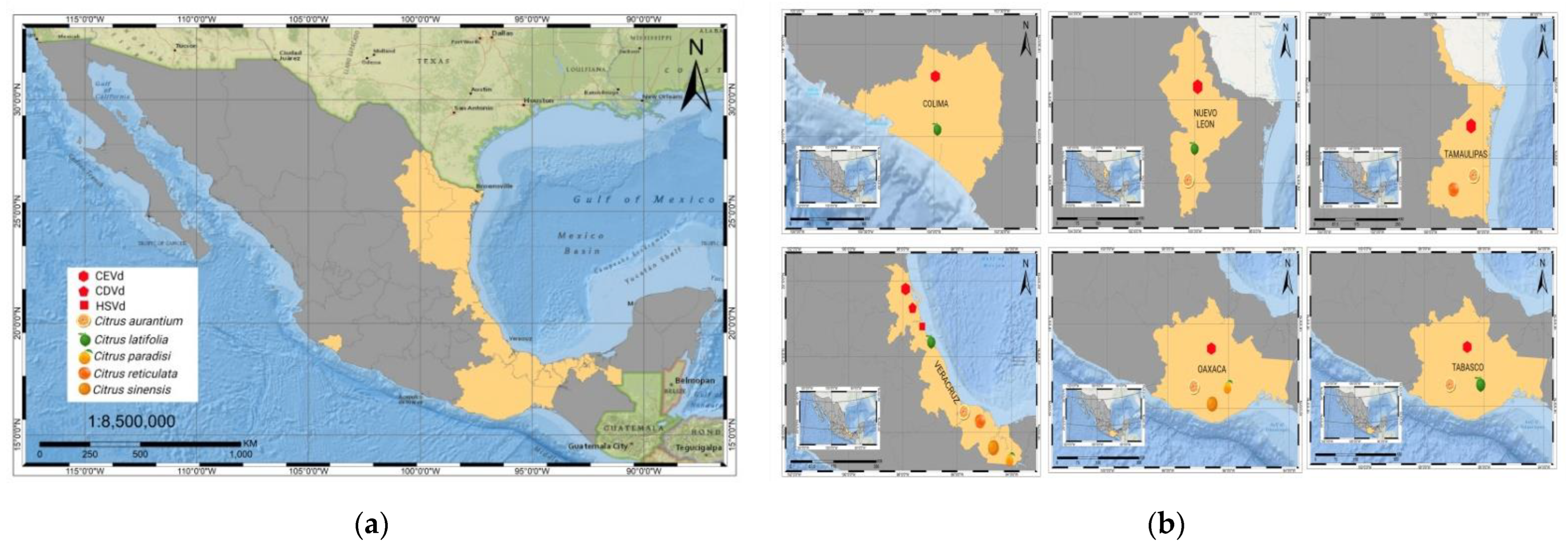
6. Citrus Exocortis and Other Citrus Viroid Species
7. Emerging Diseases
8. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diener, T. Potato spindle tuber “virus”: IV. A replicating, low molecular weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef]
- Stollar, B.D.; Diener, T. Potato spindle tuber viroid: V. Failure of immunological tests to disclose double-stranded RNA or RNA-DNA hybrids. Virology 1971, 46, 168–170. [Google Scholar] [CrossRef]
- Diener, T.; Schneider, I.; Smith, D. Potato spindle tuber viroid: XI. A comparison of the ultraviolet light sensitivities of PSTV, tobacco ringspot virus, and its satellite. Virology 1974, 57, 577–581. [Google Scholar] [CrossRef]
- Diener, T. Viroids: The smallest known agents of infectious disease. Annu. Rev. Microbiol. 1974, 28, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Semancik, J.; Weathers, L. Exocortis virus of citrus: Association of infectivity with nucleic acid preparations. Virology 1968, 36, 326–328. [Google Scholar] [CrossRef]
- Semancik, J.; Magnuson, D.; Weathers, L. Potato spindle tuber disease produced by pathogenic RNA from citrus exocortis disease: Evidence for the identity of the causal agent. Virology 1973, 52, 292–294. [Google Scholar] [CrossRef]
- Galindo, A.J. Tomato planta macho. In The Viroids; Diener, T.O., Ed.; Springer: Boston, MA, USA, 1987; pp. 315–320. [Google Scholar]
- Galindo, A.; Rodriguez, M. Rectificacion del agente causal de la planta macho del jitomate. Resumenes VIII Congr. Soc. Mex. Fitopatol. 1978. [Google Scholar]
- Galindo, J.; Smith, D.R.; Diener, T.O. Etiology of Planta Macho, a viroid disease of tomato. Phytopathology 1982, 72, 49–54. [Google Scholar] [CrossRef]
- Orozco Vargas, G.; Galindo Alonso, J. Ecology of Tomato planta macho viroid: I. natural host plants; agroecosystem effect on viroid incidence and influence of temperature on viroid distribution. Rev. Mex. Fitopatol. 1986, 4, 19–28. [Google Scholar]
- Martínez, M.; Vargas-Ponce, O.; Rodríguez, A.; Chiang, F.; Ocegueda, S. Solanaceae family in Mexico. Bot. Sci. 2017, 95, 131–145. [Google Scholar] [CrossRef]
- Martínez-Soriano, J.P.; Galindo-Alonso, J.; Maroon, C.; Yucel, I.; Smith, D.R.; Diener, T.O. Mexican papita viroid: Putative ancestor of crop viroids. Proc. Natl. Acad. Sci. USA 1996, 93, 9397–9401. [Google Scholar] [CrossRef] [PubMed]
- Almeyda-León, I.H.; Iracheta-Cárdenas, M.M.; Jasso-Argumedo, J.; Curti-Díaz, S.A.; Ruiz-Beltrán, P.; Rocha-Peña, M. Reexamination of citrus viroids of Tahiti lime in Mexico. Rev. Mex. Fitopatol. 2002, 20, 152–160. [Google Scholar]
- Alvarado-Gómez, O.; Rocha-Peña, M.; Silva-Vara, S.; Martínez-Soriano, J.; Lee, R.; Rivera-Bustamante, R.; Beltrán, P.R.; Citrus exocortis and citrus cachexia viroids in commercial groves of Tahiti lime in México. In Fourteenth IOCV Conference; 2000; pp. 289–293. Available online: https://escholarship.org/uc/item/4g26v7td (accessed on 10 June 2022).
- Rocha-Peña, M.; Martínez-Soriano, J.; Byerly-Murphy, K. Viroides de cítricos: Amenaza oculta para la citricultura de México. Rev. Mex. Fitopatol. 1995, 13, 146–149. [Google Scholar]
- Uc-Várguez, A.; Ochoa-Martínez, D.L.; Cárdenas-Soriano, E.; Mora-Aguilera, G. Sintomatología e histopatología del amarillamiento letal de la lima persa Citrus latifolia Tanaka. Rev. Mex. Fitopatol. 2005, 23, 169–175. [Google Scholar]
- Ramírez-Pool, J.A.; Xoconostle-Cázares, B.; Calderón-Pérez, B.; Ibarra-Laclette, E.; Villafán, E.; Lira-Carmona, R.; Ruiz-Medrano, R. Transcriptomic analysis of the host response to mild and severe CTV Strains in naturally infected Citrus sinensis orchards. Int. J. Mol. Sci. 2022, 23, 2435. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-A, R.; Téliz-Ortiz, D.; Pallás, V.; Sánchez-Navarro, J. First report of Avocado sunblotch viroid in avocado from Michoacán, México. Plant Dis. 2009, 93, 202. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Almaraz, R.; Pallas, V.; Sanchez-Navarro, J. First report of Peach latent mosaic viroid in peach trees from Mexico. Plant Dis. 2015, 99, 899. [Google Scholar] [CrossRef]
- Saucedo Carabez, J.R.; Téliz Ortiz, D.; Vallejo Pérez, M.R.; Beltrán Peña, H. The Avocado sunblotch viroid: An invisible foe of avocado. Viruses 2019, 11, 491. [Google Scholar] [CrossRef]
- Contreras, R. Detección de tristeza, psorosis, exocortis, cachexia y eneanismo en lima persa (Citrus latifolia) en Veracruz, México. Master’s Thesis, Colegio de Postgraduados, Institución de Enseñanza e Investigación en Ciencias Agrígolas, Montecillo, Mexico, December 2016. [Google Scholar]
- Pagliano, G.; Peyrou, M.; Del Campo, R.; Orlando, L.; Gravina, A.; Wettstein, R.; Francis, M. Detection and characterization of citrus viroids in Uruguay. In Proceedings of the Fourteenth IOCV Conference, 2000; pp. 282–288. Available online: https://escholarship.org/uc/item/8t2199rt (accessed on 10 June 2022).
- Gámez, C.E.G.; Gómez, O.G.A.; Mauleón, H.G.; Garza, R.G.; Ojeda, M.G.Á.; Rodríguez, M.L. Detección por RT-PCR punto final y tiempo real de tres especies de viroides en cítricos de Nuevo León y Tamaulipas, México. Rev. Mex. Fitopatol. 2013, 31, 20–28. [Google Scholar]
- Hanani, A.; Khater, M.; Drais, I.; Djelouah, K. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MN379512.1 (accessed on 10 June 2022).
- Kubaa, R.A.; Saponari, M.; El-Khateeb, A.; Djelouah, K. First identification of Citrus exocortis viroid (CEVd) and Citrus dwarf viroid (CVd-III) in citrus orchards in Syria. J. Plant Pathol. 2016, 98, 171–185. [Google Scholar]
- Tessitori, M.; Rizza, S.; Reina, A.; Causarano, G.; Di Serio, F. The genetic diversity of Citrus dwarfing viroid populations is mainly dependent on the infected host species. J. Gen. Virol. 2013, 94, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Donovan, N.; Chambers, G.; Englezou, A.; Phanthavong, S.; Daly, A.; Wildman, O.; Holford, P.; Burgess, L. First report of citrus exocortis viroid, citrus bent leaf viroid, hop stunt viroid and citrus dwarfing viroid in Lao PDR. Australas. Plant Pathol. 2020, 49, 661–663. [Google Scholar] [CrossRef]
- Sánchez-Salas, J.; Robles-Serna, R.; González-Garza, R. Ocurrencia de exocortis de los cítricos en el Estado de Nuevo León y su efecto en una combinación patrón-injerto susceptible. Proc. Am. Soc. Hortic. Sci. 1979, 75–78. [Google Scholar]
- Xu, W.; Shu, J.; Hong, N.; Perreault, J.-P. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/HQ284028.1/ (accessed on 10 June 2022).
- Najar, A.; Hamdi, I.; Varsani, A.; Durán-Vila, N. Citrus viroids in Tunisia: Prevalence and molecular characterization. J. Plant Pathol. 2017, 99, 787–792. [Google Scholar]
- Ovando, E. Detección de tristeza, huanglongbing, xiloporosis y exocortis en huertos citrícolas de la zona norte de Veracruz. Master’s Thesis, Colegio de Postgraduados, Postgrado de Recursos Genéticos y Productividad Montecillo, Texcoco, Estado de Mexico, November 2018. [Google Scholar]
- Ebrahimi-Moghadam, L.; Zakiaghl, M.; Jafarpour, B.; Mehrvar, M. Construction of infectious clones and demonstration of pathogenicity of citrus viroids. Iran. J. Plant Pathol. 2017, 53, 399–415. [Google Scholar]
- Rocha-Peña, M.; López Arroyo, J.; Peña del Río, M.; Almeyda-León, I. Current situation on citrus virus and virus-like diseases and their vectors in Mexico. In Proceedings of the International Organization of Citrus Virologists Conference Proceedings (1957–2010), 2005; pp. 421–422. Available online: https://escholarship.org/uc/item/0sr3q361 (accessed on 10 June 2022).
- Massawe, D.P. High-Throughput Sequencing and Identification of Viruses and Non-Viral Vasculature-Limited Pathogens in maize and Blueberry. Ph.D. Thesis, The Ohio State University, Plant Pathology, Columbus, OH, USA, 2020. [Google Scholar]
- Lin, C.-Y.; Wu, M.-L.; Shen, T.-L.; Yeh, H.-H.; Hung, T.-H. Multiplex detection, distribution, and genetic diversity of Hop stunt viroid and Citrus exocortis viroid infecting citrus in Taiwan. Virol. J. 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Ferraro, R.; Scuderi, G.; Russo, M.; Catara, A.; Licciardello, G. Metagenomic detection of viruses and viroids associated with isolates of citrus tristeza virus. Paper presented at the XXIV Congress of the Italian Phytopathological Society, Department of Agricultural, Food and Environmental Sciences, Marche Polytechnic University, Ancona, Italy, 5–7 September 2018; Volume 100, pp. 613–653. [Google Scholar]
- Loconsole, G.; Önelge, N.; Yokomi, R.K.; Abou Kubaa, R.; Savino, V.; Saponari, M. Rapid differentiation of citrus Hop stunt viroid variants by real-time RT-PCR and high resolution melting analysis. Mol. Cell. Probes 2013, 27, 221–229. [Google Scholar] [CrossRef]
- Ling, K.-S.; Bledsoe, M. First report of Mexican papita viroid infecting greenhouse tomato in Canada. Plant Dis. 2009, 93, 839. [Google Scholar] [CrossRef]
- Ling, K.-S.; Zhang, W. First report of a natural infection by Mexican papita viroid and Tomato chlorotic dwarf viroid on greenhouse tomatoes in Mexico. Plant Dis. 2009, 93, 1216. [Google Scholar] [CrossRef]
- Candresse, T.; Marais, A.; Tassus, X.; Suhard, P.; Renaudin, I.; Leguay, A.; Poliakoff, F.; Blancard, D. First report of Tomato chlorotic dwarf viroid in tomato in France. Plant Dis. 2010, 94, 633. [Google Scholar] [CrossRef]
- Avina-Padilla, K.; de la Vega, O.M.; Rivera-Bustamante, R.; Martinez-Soriano, J.P.; Owens, R.A.; Hammond, R.W.; Vielle-Calzada, J.-P. In silico prediction and validation of potential gene targets for pospiviroid-derived small RNAs during tomato infection. Gene 2015, 564, 197–205. [Google Scholar] [CrossRef]
- Van Bogaert, N. Viroid-Insect-Plant Interactions in View of Transmission Routes. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2016. [Google Scholar]
- Matsushita, Y.; Kanda, A.; Usugi, T.; Tsuda, S. First report of a tomato chlorotic dwarf viroid disease on tomato plants in Japan. J. Gen. Plant Pathol. 2008, 74, 182–184. [Google Scholar] [CrossRef]
- Belalcazar, C.; Galindo, A. Estudio sobre el virus de la “planta macho” del jitomate. Agrociencia 1974, 18, 79. [Google Scholar]
- Geering, A.; Steele, V.; Kopittke, R. Final Report for Horticulture Australia Limited Project AV03009: Development of Avocado Sunblotch Viroid Indexing Protocols for the Avocado Nursery Industry; University of Queensland: St Lucia, Australia, 2006. [Google Scholar]
- Lotos, L.; Kavroulakis, N.; Navarro, B.; Di Serio, F.; Olmos, A.; Ruiz-García, A.B.; Katis, N.; Maliogka, V. First report of Avocado sunblotch viroid (ASBVd) naturally infecting Avocado (Persea americana) in Greece. Plant Dis. 2018, 102, 1470. [Google Scholar] [CrossRef]
- Blickle, W.; Saenger, H.L. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/X52045.1 (accessed on 10 June 2022).
- Jo, Y.; Yoo, S.-H.; Chu, H.; Cho, J.K.; Choi, H.; Yoon, J.-Y.; Choi, S.-K.; Cho, W.K. Complete genome sequences of peach latent mosaic viroid from a single peach cultivar. Genome Announc. 2015, 3, e01098-15. [Google Scholar] [CrossRef]
- Pleško, I.M.; Marn, M.V.; Miladinovic, Z.; Zindovic, J. First report of peach latent mosaic viroid in peach Trees in Montenegro. Plant Dis. 2012, 96, 150. [Google Scholar] [CrossRef] [PubMed]
- Yazarlou, A.; Jafarpour, B.; Tarighi, S.; Habili, N.; Randles, J.W. New Iranian and Australian peach latent mosaic viroid variants and evidence for rapid sequence evolution. Arch. Virol. 2012, 157, 343–347. [Google Scholar] [CrossRef]
- Sánchez-Del-Castillo, F.; Moreno-Pérez, E.d.C.; Contreras-Magaña, E.; Sahagún-Castellanos, J. Rendimiento de jitomate con diferentes métodos de cultivo hidropónico basados en doseles escaleriformes. Rev. Chapingo Ser. Hortic. 2014, 20, 239–251. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization. Database FAOSTAT. Available online: http://faostat.fao.org (accessed on 10 June 2022).
- Hernández-Martínez, J.; García-Mata, R.; Valdivia-Alcalá, R.; Omaña-Silvestre, J.M. Evolución de la competitividad y rentabilidad del cultivo del tomate rojo (Lycopersicon esculentum L.) en Sinaloa, México. Agrociencia 2004, 38, 431–436. [Google Scholar]
- Ling, K.-S.; Verhoeven, J.; Singh, R.; Brown, J. First report of Tomato chlorotic dwarf viroid in greenhouse tomatoes in Arizona. Plant Dis. 2009, 93, 1075. [Google Scholar] [CrossRef]
- Ling, K.-S.; Sfetcu, D. First report of natural infection of greenhouse tomatoes by Potato spindle tuber viroid in the United States. Plant Dis. 2010, 94, 1376. [Google Scholar] [CrossRef] [PubMed]
- Avina-Padilla, K.; Ochoa-Sanchez, J.C.; Rivera-Bustamante, R. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/JQ974379.1 (accessed on 10 June 2022).
- Guerrero Gámez, C.E.; Alvarado Gómez, O.G.; Gutiérrez Mauleón, H.; González Garza, R.; Álvarez Ojeda, M.G.; Luna Rodríguez, M. Detection of three citrus viroids species from Nuevo Leon and Tamaulipas, Mexico by conventional and real time RT-PCR. Rev. Mex. Fitopatol. 2013, 31, 20–28. [Google Scholar]
- Bernad, L.; Duran-Vila, N.; Elena, S.F. Effect of citrus hosts on the generation, maintenance and evolutionary fate of genetic variability of citrus exocortis viroid. J. Gen. Virol. 2009, 90, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ieki, H.; Ozaki, K. Simultaneous detection of six citrus viroids and Apple stem grooving virus from citrus plants by multiplex reverse transcription polymerase chain reaction. J. Virol. Methods. 2002, 106, 235–239. [Google Scholar] [CrossRef]
- SIAVI. Sistema de Información Arancelaria Vía Internet. 08044001 Aguacates (paltas). México. Secretaría de Economía. Available online: http://www.economia-snci.gob.mx/ (accessed on 20 June 2022).
- REHASA. REHASA Mexican products since 1958. Available online: http://rehasa.com.mx/index.html (accessed on 20 June 2022).
- Peiró, A.; Pallás, V.; Sánchez-Navarro, J.Á. Simultaneous detection of eight viruses and two viroids affecting stone fruit trees by using a unique polyprobe. Eur. J. Plant Pathol. 2012, 132, 469–475. [Google Scholar] [CrossRef]
- Singh, R.P.; Nie, X.; Singh, M. Tomato chlorotic dwarf viroid: An evolutionary link in the origin of pospiviroids. J. Gen. Virol. 1999, 80, 2823–2828. [Google Scholar] [CrossRef]
- Li, R.; Padmanabhan, C.; Ling, K.-S. A single base pair in the right terminal domain of tomato planta macho viroid is a virulence determinant factor on tomato. Virology 2017, 500, 238–246. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Jansen, C.; Werkman, A.; Roenhorst, J. First report of Tomato chlorotic dwarf viroid in Petunia hybrida from the United States of America. Plant Dis. 2007, 91, 324. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Sano, T.; Hase, S.; Matsushita, Y. Influence of the terminal left domain on horizontal and vertical transmissions of tomato planta macho viroid and potato spindle tuber viroid through pollen. Virology 2019, 526, 22–31. [Google Scholar] [CrossRef]
- Tsushima, T.; Sano, T. A point-mutation of Coleus blumei viroid 1 switches the potential to transmit through seed. J. Gen. Virol. 2018, 99, 393–401. [Google Scholar] [CrossRef]
- Sano, T.; Ishiguro, A. Viability and pathogenicity of intersubgroup viroid chimeras suggest possible involvement of the terminal right region in replication. Virology 1998, 240, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Keese, P.; Symons, R.H. Domains in viroids: Evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc. Natl. Acad. Sci. USA 1985, 82, 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- Diener, T.; Smith, D. Potato spindle tuber viroid. VI. Monodisperse distribution after electrophoresis in 20 per cent polyacrylamide gels. Virology 1971, 46, 498–499. [Google Scholar] [CrossRef]
- Di Serio, F.; Flores, R.; Verhoeven, J.T.J.; Li, S.-F.; Pallás, V.; Randles, J.; Sano, T.; Vidalakis, G.; Owens, R. Current status of viroid taxonomy. Arch. Virol. 2014, 159, 3467–3478. [Google Scholar] [CrossRef]
- Kiefer, M.C.; Owens, R.A.; Diener, T. Structural similarities between viroids and transposable genetic elements. Proc. Natl. Acad. Sci. USA 1983, 80, 6234–6238. [Google Scholar] [CrossRef]
- Sastry, K.S. Plant Virus and Viroid Diseases in the Tropics: Volume 1: Introduction of Plant Viruses and Sub-Viral Agents, Classification, Assessment of Loss, Transmission and Diagnosis; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Hammond, R.W. Economic significance of viroids in vegetable and field crops. In Viroids and Satellites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 5–13. [Google Scholar]
- Hadidi, A.; Sun, L.; Randles, J.W. Modes of viroid transmission. Cells 2022, 11, 719. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Jansen, C.; Willemen, T.; Kox, L.; Owens, R.; Roenhorst, J. Natural infections of tomato by Citrus exocortis viroid, Columnea latent viroid, Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur. J. Plant Pathol. 2004, 110, 823–831. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Matsushita, Y. Host ranges and seed transmission of Tomato planta macho viroid and Pepper chat fruit viroid. Eur. J. Plant Pathol. 2017, 149, 211–217. [Google Scholar] [CrossRef]
- Database, E.G. Tomato Planta Macho Viroid. Available online: https://gd.eppo.int/taxon/TPMVD0/categorization (accessed on 10 June 2022).
- Peña, R.; Muñoz, A.; Bujanos, R.; Luévano, J.; Tamayo, F.; Cortez, E. Formas sexuales del complejo pulgón amarillo del sorgo, Melanaphis sacchari/sorghi en México. Southwest. Entomol. 2016, 41, 127–131. [Google Scholar] [CrossRef]
- Gibson, W.; Carrillo, J. Lista de la Colección Entomológica de la Oficina de Estudios Especiales; Folleto Misceláneo; Secretaria de Agricultura y Ganaderia: México City, Mexico, 1959; 254p. [Google Scholar]
- Holman, J. Host Plant Catalog of Aphids: Palaearctic Region; Springer: Berlin, Germany, 2009; Volume 1216. [Google Scholar]
- López Reyna, M. Aspectos Biológicos del pulgón Myzus Persicae, Transmisor del Viroide Planta Macho del Jitomate (VPMJ); Universidad Autonoma Chapingo: Chapingo, México, 1986. [Google Scholar]
- Querci, M.; Owens, R.A.; Bartolini, I.; Lazarte, V.; Salazar, L.F. Evidence for heterologous encapsidation of potato spindle tuber viroid in particles of potato leafroll virus. J. Gen. Virol. 1997, 78, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Syller, J.; Marczewski, W. Potato leafroll virus-assisted aphid transmission of potato spindle tuber viroid to potato leafroll virus-resistant potato. J. Phytopathol. 2001, 149, 195–201. [Google Scholar] [CrossRef]
- Võ, T.T.; Dehne, H.-W.; Hamacher, J. Transmission of Tomato chlorotic dwarf viroid by Myzus persicae assisted by Potato leafroll virus. J. Plant Dis. Prot. 2018, 125, 259–266. [Google Scholar] [CrossRef]
- Leichtfried, T.; Reisenzein, H.; Steinkellner, S.; Gottsberger, R.A. Transmission studies of the newly described apple chlorotic fruit spot viroid using a combined RT-qPCR and droplet digital PCR approach. Arch. Virol. 2020, 165, 2665–2671. [Google Scholar] [CrossRef]
- Falk, B.; Passmore, B.; Watson, M.; Chin, L. The specificity and significance of heterologous encapsidation of virus and virus-like RNA’s. In Biotechnology and Plant Protection: Viral Pathogenesis and Disease Resistance-Proceedings of the Fifth International Symposium; World Scientific: London, UK, 1995; p. 432. [Google Scholar]
- Wallace, J. The sun-blotch disease of avocado. J. Rio Grande Valley Hort. 1958, 12, 69–74. [Google Scholar]
- Coit, J.E. Sunblotch of the avocado. In California Avocado Society Yearbook, 1928; CAB Direct: San Diego, CA, USA, 1928; p. 27. Available online: https://www.cabdirect.org/cabdirect/abstract/20057003110 (accessed on 10 June 2022).
- Geering, A.D. A review of the status of Avocado sunblotch viroid in Australia. Australas. Plant Dis. 2018, 47, 555–559. [Google Scholar] [CrossRef]
- Allen, R.; Palukaitis, P.; Symons, R. Purified avocado sunblotch viroid causes disease in avocado seedlings. Australas. Plant Pathol. 1981, 10, 31–32. [Google Scholar] [CrossRef]
- Yee-Rendon, A.; Torres-Pacheco, I.; Trujillo-Lopez, A.S.; Romero-Bringas, K.P.; Millan-Almaraz, J.R. Analysis of New RGB Vegetation Indices for PHYVV and TMV Identification in Jalapeño Pepper (Capsicum annuum) leaves using CNNs-based model. Plants 2021, 10, 1977. [Google Scholar] [CrossRef]
- Saucedo-Carabez, J.; Téliz-Ortiz, D.; Ochoa-Ascencio, S.; Ochoa-Martínez, D.; Vallejo-Pérez, M.; Beltrán-Peña, H. Effect of Avocado sunblotch viroid (ASBVd) on avocado yield in Michoacan, Mexico. Eur. J. Plant Pathol. 2014, 138, 799–805. [Google Scholar] [CrossRef]
- Téliz, D.; Mora, A. El Aguacate y su Manejo Integrado; Colegio de Postgraduados: Texcoco, Mexico, 2015. [Google Scholar]
- Vallejo-Pérez, M.R.; Téliz-Ortiz, D.; Colinas-León, M.T.; La Torre-Almaraz, D.; Valdovinos-Ponce, G.; Nieto-Ángel, D.; Ochoa-Martínez, D.L. Alterations induced by Avocado sunblotch viroid in the postharvest physiology and quality of avocado ‘Hass’ fruit. Phytoparasitica 2015, 43, 355–364. [Google Scholar] [CrossRef]
- Agricultura, B. Origen del Aguacate. Available online: https://blogagricultura.com/ (accessed on 10 July 2022).
- Smith, C.E. Archeological evidence for selection in avocado. Econ. Bot. 1966, 20, 169–175. [Google Scholar] [CrossRef]
- Reanwarakorn, K.; Semancik, J. Regulation of pathogenicity in hop stunt viroid-related group II citrus viroids. J. Gen. Virol. 1998, 79, 3163–3171. [Google Scholar] [CrossRef] [PubMed]
- Reanwarakorn, K.; Semancik, J. Correlation of hop stunt viroid variants to cachexia and xyloporosis diseases of citrus. Phytopathology 1999, 89, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Maya, R.; Nava-Diaz, C.; Villegas-Monter, A.; Mora-Aguilera, J.A.; Ochoa-Martinez, D.L. First report of citrus psorosis virus (CPsV) in persian lime in Veracruz, Mexico. J. Plant Pathol. 2018, 100, 115. [Google Scholar] [CrossRef]
- Zuñiga Romano, M.d.C.; Ochoa Martínez, D.L.; Ortega Acosta, C.; Zamora Macorra, E.J. Natural Coinfection of Mexican Papita Viroid (MPVd) and Tomato Severe Leaf Curl Virus (ToSLCV) in Tomato (Solanum lycopersicum); Universidad Autonoma Chapingo: Chapingo, Mexico, 2022; Revista Mexicana de Fitopatologia (Submitted). [Google Scholar]
- Matoušek, J.; Kozlova, P.; Orctova, L.; Schmitz, A.; Pešina, K.; Bannach, O.; Diermann, N.; Steger, G.; Riesner, D. Accumulation of viroid-specific small RNAs and increase in nucleolytic activities linked to viroid-caused pathogenesis. Biol. Chem. 2007, 388, 1–13. [Google Scholar] [CrossRef]
- Murcia, N.; Bernad, L.; DURAN-VILA, N.; Serra, P. Two nucleotide positions in the Citrus exocortis viroid RNA associated with symptom expression in Etrog citron but not in experimental herbaceous hosts. Mol. Plant Pathol. 2011, 12, 203–208. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Roenhorst, J.; Hooftman, M.; Meekes, E.; Flores, R.; Serra, P. A pospiviroid from symptomless portulaca plants closely related to iresine viroid 1. Virus Res. 2015, 205, 22–26. [Google Scholar] [CrossRef]


| Family | Species a | Economic Host/Disease | Damage | Cost | Mode of Spread b | Control Strategy |
|---|---|---|---|---|---|---|
| Pospiviroidae | CDVd | Citrus groves | Variable | Reduced yield | Vegetative: + | Eradication viroid-free |
| Seed/pollen: − | ||||||
| Insect: − | ||||||
| Mechanical: + | ||||||
| CEVd | Dwarfing | Variable | Reduced yield | Vegetative: + | Eradication viroid-free | |
| Seed/pollen: +/− | ||||||
| Insect: − | ||||||
| Mechanical: + | ||||||
| HSVd | Exocortis | Variable | Reduced yield | Vegetative: + | Eradication viroid-free | |
| Cachexia | Seed/pollen: +/− | Propagation material | ||||
| Insect: − | ||||||
| Mechanical: + | ||||||
| MPVd | Tomato | Severe | Yield | Vegetative: NA | Eradication | |
| Greenhouses | Seed/pollen: NK | |||||
| Dwarfing | Insect: NK | |||||
| Chlorosis | Mechanical: + | |||||
| TCDVd | Tomato | Severe | Yield | Vegetative: NA | Eradication | |
| Greenhouse | Seed/pollen: +/− | |||||
| Dwarfing | Insect: + | |||||
| Chlorosis | Mechanical: + | |||||
| TPMVd | Tomate field | Severe | Yield | Vegetative:NA | Eradication | |
| Planta macho | Seed/pollen: + | |||||
| Insect: + | ||||||
| Mechanical: + | ||||||
| Avsunviroidae | ASBVd | Avocado groves | Severe | Reduced yield | Vegetative: + | Eradication viroid-free |
| Sunblotch | Discarded fruit | Seed/pollen: + | Propagation material | |||
| Insect: − | ||||||
| Mechanical: + | ||||||
| PLMVd | Peach orchards | Variable | Vegetative: + | Propagation material | ||
| Seed/pollen: + | ||||||
| Insect: + | ||||||
| Mechanical: + |
| Family | Species a | Distribution in Mexico | Other Countries | Detection b | Reports |
|---|---|---|---|---|---|
| Pospiviroidae | CDVd | Veracruz | Uruguay | 2016 1, 2002 2,3 | [21,22] |
| Nuevo León | USA | 2013 1, 2020 2,3 | [23,24] | ||
| Tamaulipas | Egypt | 2013 1, 2017 2,3 | [23,25] | ||
| Mexico | Italy | 2013 1, 2013 2,3 | [23,26] | ||
| Laos | 2020 2,3 | [27] | |||
| CEVd | Nuevo León | China | 1979 1, 2013 1, 2012 2,3 | [23,28,29] | |
| Tamaulipas | Tunisia | 1995 1, 2013 1, 2014 2,3 | [15,23,30] | ||
| Veracruz | Iran | 19991, 20181,2017 2,3 | [13,31,32] | ||
| Tabasco | USA | 1999 1,2000 1, 2021 2,3 | [13,33,34] | ||
| Mexico | 2013 1 | [23] | |||
| HSVd | Nuevo León | China | 2013 1, 2015 2,3 | [23,35] | |
| Tamaulipas | Italy | 2013 1, 2021 2,3 | [23,36] | ||
| Mexico | Laos | 2013 1, 2020 2,3 | [23,27] | ||
| Veracruz | Turkey | 19991,2018 1, 20132,3 | [13,31,37] | ||
| Tabasco | 1999 1 | [38] | |||
| MPVd | Aguascalientes | Canada | 1996 1, 2010 2,3 | [12] | |
| Mexico City | 2009 1 | [39] | |||
| TCDVd | Mexico City | France | 2009 1, 2010 2,3 | [39,40] | |
| Jalisco | Belgium | 2011 1, 2016 2,3 | [41,42] | ||
| Japan | 2009 2,3 | [43] | |||
| TPMVd | Mexico | Canada | 1974 1, 2010 2 | [38,44] | |
| Morelos | 1978 1 | [8] | |||
| Avsunviroidae | ASBVd | Michoacán | Australia | 2009 1, 2011 2,3 | [18,45] |
| Greece | 2018 2,3 | [46] | |||
| Israel | 2013 2,3 | [47] | |||
| PLMVd | Mexico | South Korea | 2013 1, 2018 2,3 | [19,48] | |
| Puebla | Montenegro | 2014 1, 2011 2,3 | [19,49] | ||
| Morelos | Australia | 1978 1,2012 2,3 | [19,50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aviña-Padilla, K.; Zamora-Macorra, E.J.; Ochoa-Martínez, D.L.; Alcántar-Aguirre, F.C.; Hernández-Rosales, M.; Calderón-Zamora, L.; Hammond, R.W. Mexico: A Landscape of Viroid Origin and Epidemiological Relevance of Endemic Species. Cells 2022, 11, 3487. https://doi.org/10.3390/cells11213487
Aviña-Padilla K, Zamora-Macorra EJ, Ochoa-Martínez DL, Alcántar-Aguirre FC, Hernández-Rosales M, Calderón-Zamora L, Hammond RW. Mexico: A Landscape of Viroid Origin and Epidemiological Relevance of Endemic Species. Cells. 2022; 11(21):3487. https://doi.org/10.3390/cells11213487
Chicago/Turabian StyleAviña-Padilla, Katia, Erika Janet Zamora-Macorra, Daniel Leobardo Ochoa-Martínez, Flor Citlally Alcántar-Aguirre, Maribel Hernández-Rosales, Loranda Calderón-Zamora, and Rosemarie W. Hammond. 2022. "Mexico: A Landscape of Viroid Origin and Epidemiological Relevance of Endemic Species" Cells 11, no. 21: 3487. https://doi.org/10.3390/cells11213487
APA StyleAviña-Padilla, K., Zamora-Macorra, E. J., Ochoa-Martínez, D. L., Alcántar-Aguirre, F. C., Hernández-Rosales, M., Calderón-Zamora, L., & Hammond, R. W. (2022). Mexico: A Landscape of Viroid Origin and Epidemiological Relevance of Endemic Species. Cells, 11(21), 3487. https://doi.org/10.3390/cells11213487









