The Biological Relevance of Papaverine in Cancer Cells
Abstract
1. Introduction
2. Papaverine
3. Possible Pathways of Mechanisms of Action
3.1. Phosphodiesterases and cAMP
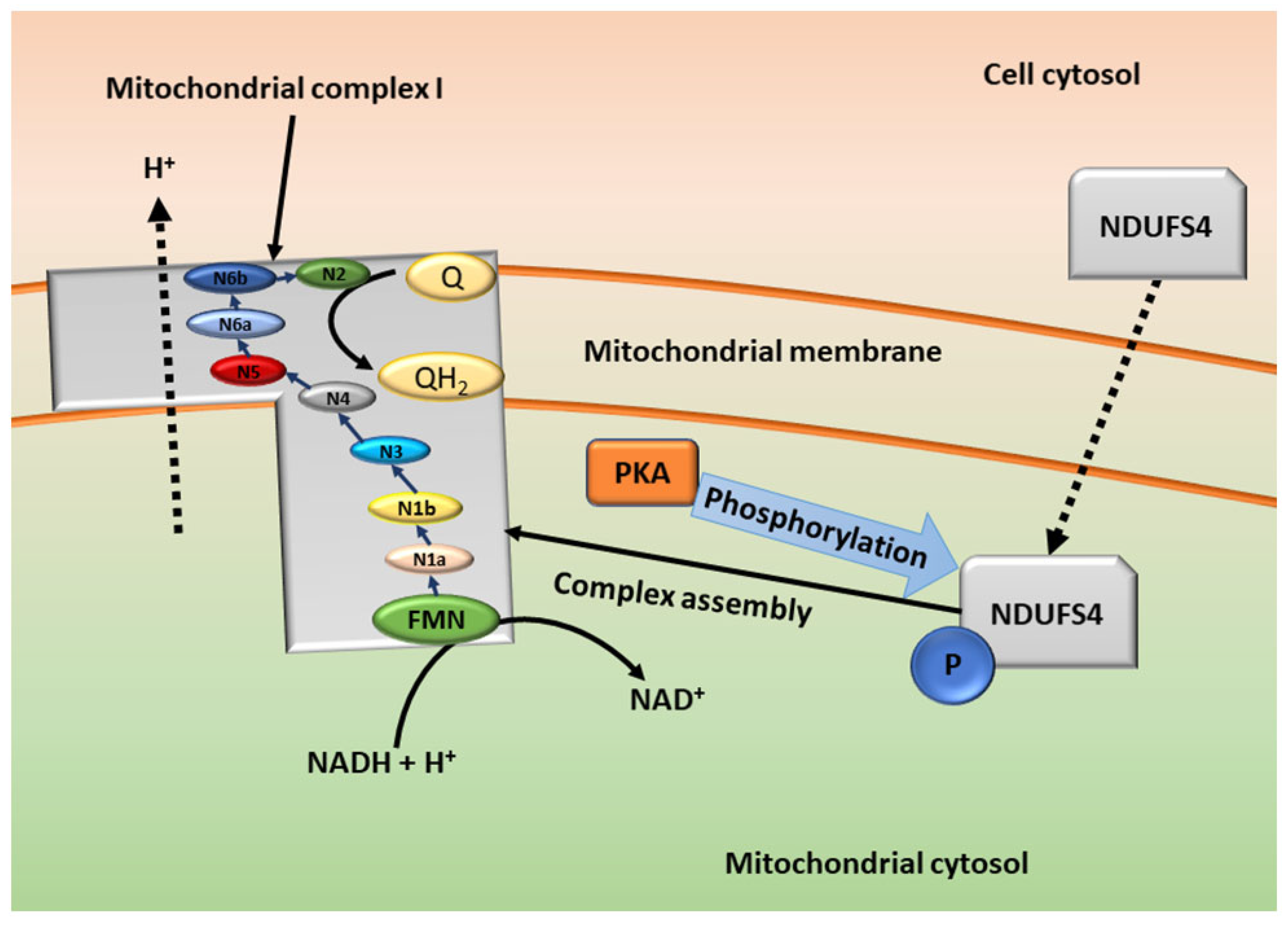
3.2. cAMP in the Mitochondria—Mitochondrial Respiration
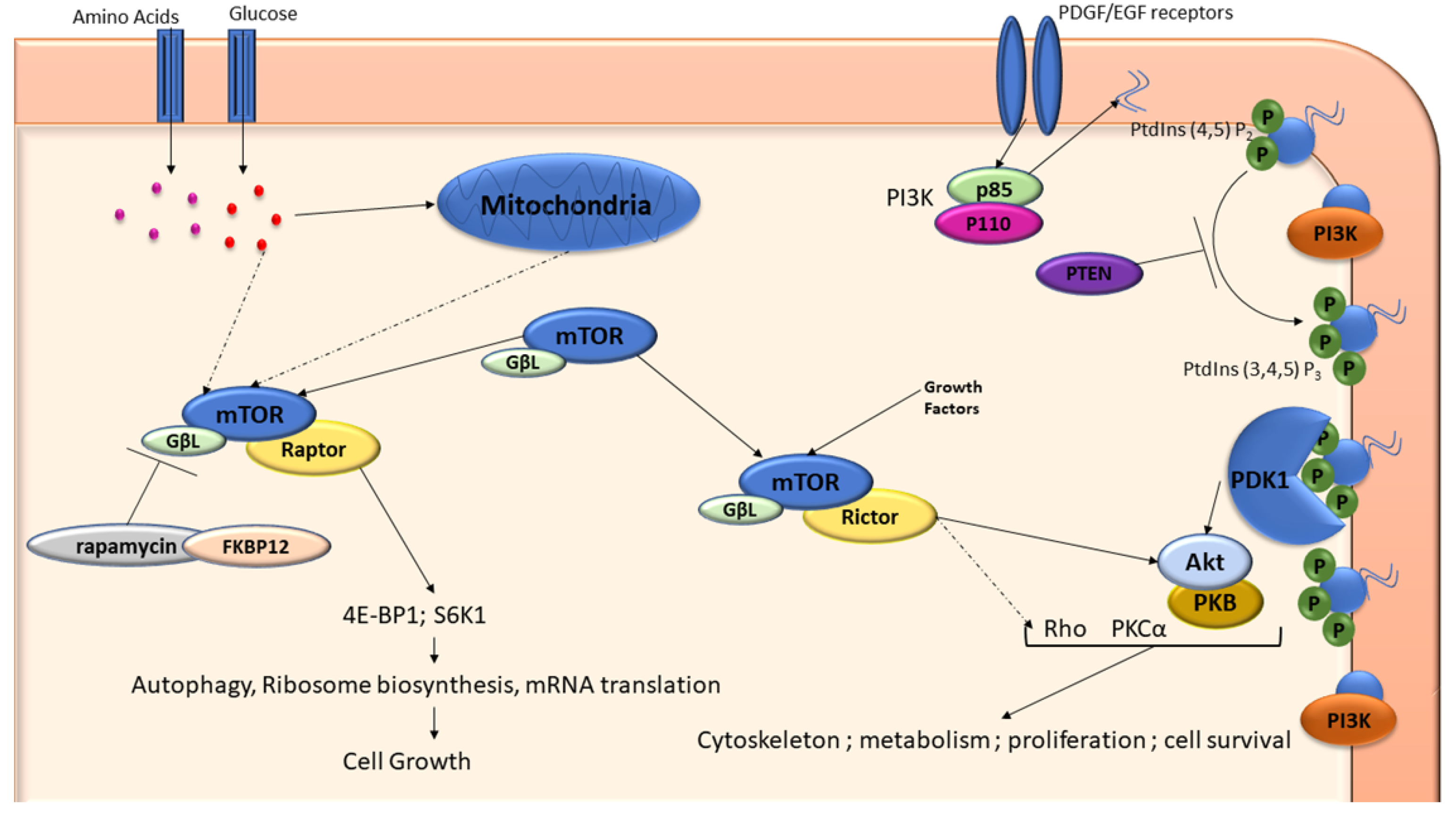
3.3. mTOR and PI3K
3.4. HMGB1 and RAGE
3.5. Vascular Endothelial Growth Factor
3.6. Apoptotic Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Medicinal plants: A review. J. Plant Sci. 2015, 3, 50–55. [Google Scholar]
- Rehman, J.u.; Zahra; Ahmad, N.; Khalid, M.; Noor ul Huda Khan Asghar, H.M.; Gilani, Z.A.; Ullah, I.; Nasar, G.; Akhtar, M.M.; Usmani, M.N. Intensity modulated radiation therapy: A review of current practice and future outlooks. J. Radiat. Res. Appl. Sci. 2018, 11, 361–367. [Google Scholar] [CrossRef]
- Benej, M.; Hong, X.; Vibhute, S.; Scott, S.; Wu, J.; Graves, E.; Le, Q.-T.; Koong, A.C.; Giaccia, A.J.; Yu, B. Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 10756–10761. [Google Scholar] [CrossRef]
- Afzali, M.; Ghaeli, P.; Khanavi, M.; Parsa, M.; Montazeri, H.; Ghahremani, M.H.; Ostad, S.N. Non-addictive opium alkaloids selectively induce apoptosis in cancer cells compared to normal cells. DARU J. Pharm. Sci. 2015, 23, 16. [Google Scholar] [CrossRef]
- Gümüşçü, A.; Arslan, N.; Sarıhan, E.O. Evaluation of selected poppy (Papaver somniferum L.) lines by their morphine and other alkaloids contents. Eur. Food Res. Technol. 2008, 226, 1213–1220. [Google Scholar] [CrossRef]
- Patel, T.R.; Schoenwald, R.D.; Lach, J.L. Comparative Bioavailability of Papaverine Hydrochloride, Papaverine Hexametaphosphate and Papaverine Polymetaphosphate. Drug Dev. Ind. Pharm. 1981, 7, 329–345. [Google Scholar] [CrossRef]
- Dittbrenner, A.; Mock, H.-P.; Börner, A.; Lohwasser, U. Variability of alkaloid content in Papaver somniferum L. J. Appl. Bot. Food Qual. 2012, 82, 103–107. [Google Scholar]
- Kassell, N.F.; Helm, G.; Simmons, N.; Phillips, C.D.; Cail, W.S. Treatment of cerebral vasospasm with intra-arterial papaverine. J. Neurosurg. 1992, 77, 848–852. [Google Scholar] [CrossRef]
- Wilson, R.F.; White, C.W. Intracoronary papaverine: An ideal coronary vasodilator for studies of the coronary circulation in conscious humans. Circulation 1986, 73, 444–451. [Google Scholar] [CrossRef]
- Virag, R.; Frydman, D.; Legman, M.; Virag, H. Intracavernous Injection of Papaverine as a Diagnostic and Therapeutic Method in Erectile Failure. Angiology 1984, 35, 79–87. [Google Scholar] [CrossRef]
- Clouston, J.E.; Numaguchi, Y.; Zoarski, G.H.; Aldrich, E.F.; Simard, J.M.; Zitnay, K.M. Intraarterial papaverine infusion for cerebral vasospasm after subarachnoid hemorrhage. Am. J. Neuroradiol. 1995, 16, 27–38. [Google Scholar]
- Sajadian, S.; Vatankhah, M.; Majdzadeh, M.; Kouhsari, S.M.; Ghahremani, M.H. Cell cycle arrest and apoptogenic properties of opium alkaloids noscapine and papaverine on breast cancer stem cells. Toxicol. Mech. Methods 2015, 25, 388–395. [Google Scholar] [CrossRef]
- Lawrence, P.F. Chapter 78—Pharmacologic Adjuncts to Endovascular Procedures. In Endovascular Surgery, 4th ed.; Moore, W.S., Ahn, S.S., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 807–813. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. 19-Antianginal Drugs. In Synthesis of Essential Drugs; Vardanyan, R.S., Hruby, V.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 257–267. [Google Scholar] [CrossRef]
- Meyer, M.C.; Gollamudi, R.; Straughn, A.B. The influence of dosage form on papaverine bioavailability. J. Clin. Pharmacol. 1979, 19, 435–444. [Google Scholar] [CrossRef]
- Hebb, A.L.O.; Robertson, H.A.; Denovan-Wright, E.M. Phosphodiesterase 10A inhibition is associated with locomotor and cognitive deficits and increased anxiety in mice. Eur. Neuropsychopharmacol. 2008, 18, 339–363. [Google Scholar] [CrossRef]
- Gomes, D.A.; Joubert, A.M.; Visagie, M.H. In Vitro Effects of Papaverine on Cell Proliferation, Reactive Oxygen Species, and Cell Cycle Progression in Cancer Cells. Molecules 2021, 26, 6388. [Google Scholar] [CrossRef]
- Hodgson, E. Chapter Fourteen—Toxins and Venoms. In Progress in Molecular Biology and Translational Science; Hodgson, E., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 112, pp. 373–415. [Google Scholar]
- Vodušek, D.B.; Aminoff, M.J. Chapter 30—Sexual Dysfunction in Patients with Neurologic Disorders. In Aminoff’s Neurology and General Medicine, 5th ed.; Aminoff, M.J., Josephson, S.A., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 633–656. [Google Scholar] [CrossRef]
- Berg, G.; Jonsson, K.A.; Hammar, M.; Norlander, B. Variable Bioavailability of Papaverine. Pharmacol. Toxicol. 1988, 62, 308–310. [Google Scholar] [CrossRef]
- Keegan, K.A.; Penson, D.F. Chapter 28—Vasculogenic Erectile Dysfunction. In Vascular Medicine: A Companion to Braunwald’s Heart Disease, 2nd ed.; Creager, M.A., Beckman, J.A., Loscalzo, J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 341–348. [Google Scholar] [CrossRef]
- Goto, T.; Matsushima, H.; Kasuya, Y.; Hosaka, Y.; Kitamura, T.; Kawabe, K.; Hida, A.; Ohta, Y.; Simizu, T.; Takeda, K. The effect of papaverine on morphologic differentiation, proliferation and invasive potential of human prostatic cancer LNCaP cells. Int. J. Urol. 1999, 6, 314–319. [Google Scholar] [CrossRef]
- Marciano, R.; Prasad, M.; Ievy, T.; Tzadok, S.; Leprivier, G.; Elkabets, M.; Rotblat, B. High-Throughput Screening Identified Compounds Sensitizing Tumor Cells to Glucose Starvation in Culture and VEGF Inhibitors In Vivo. Cancers 2019, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Tamada, K.; Nakajima, S.; Ogawa, N.; Inada, M.; Shibasaki, H.; Sato, A.; Takasawa, R.; Yoshimori, A.; Suzuki, Y.; Watanabe, N.; et al. Papaverine identified as an inhibitor of high mobility group box 1/receptor for advanced glycation end-products interaction suppresses high mobility group box 1-mediated inflammatory responses. Biochem. Biophys. Res. Commun. 2019, 511, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.; Wink, M. Antiproliferative effect of the isoquinoline alkaloid papaverine in hepatocarcinoma HepG-2 cells—Inhibition of telomerase and induction of senescence. Molecules 2014, 19, 11846–11859. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.-J.; Zhang, H.-B.; Wei, A.-Y. Papaverine selectively inhibits human prostate cancer cell (PC-3) growth by inducing mitochondrial mediated apoptosis, cell cycle arrest and downregulation of NF-κB/PI3K/Akt signalling pathway. J. B.U.ON. Off. J. Balk. Union Oncol. 2017, 22, 112–118. [Google Scholar]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Schafer, K. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Puig, P.E.; Guilly, M.N.; Bouchot, A.; Droin, N.; Cathelin, D.; Bouyer, F.; Favier, L.; Ghiringhelli, F.; Kroemer, G.; Solary, E. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol. Int. 2008, 32, 1031–1043. [Google Scholar] [CrossRef]
- Chen, J.; Niu, N.; Zhang, J.; Qi, L.; Shen, W.; Donkena, K.V.; Feng, Z.; Liu, J. Polyploid giant cancer cells (PGCCs): The evil roots of cancer. Curr. Cancer Drug Targets 2019, 19, 360–367. [Google Scholar] [CrossRef]
- Inada, M.; Shindo, M.; Kobayashi, K.; Sato, A.; Yamamoto, Y.; Akasaki, Y.; Ichimura, K.; Tanuma, S.-i. Anticancer effects of a non-narcotic opium alkaloid medicine, papaverine, in human glioblastoma cells. PLoS ONE 2019, 14, e0216358. [Google Scholar] [CrossRef]
- Pöch, G.; Kukovetz, W. Papaverine-induced inhibition of phosphodiesterase activity in various mammalian tissues. Life Sci. 1971, 10, 133–144. [Google Scholar] [CrossRef]
- Triner, L.; Vulliemoz, Y.; Schwartz, I.; Nahas, G.G. Cyclic phosphodiesterase activity and the action of papaverine. Biochem. Biophys. Res. Commun. 1970, 40, 64–69. [Google Scholar] [CrossRef]
- Fujishige, K.; Kotera, J.; Michibata, H.; Yuasa, K.; Takebayashi, S.-i.; Okumura, K.; Omori, K. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J. Biol. Chem. 1999, 274, 18438–18445. [Google Scholar] [CrossRef]
- Beavo, J.A. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol. Rev. 1995, 75, 725–748. [Google Scholar] [CrossRef]
- Handa, N.; Mizohata, E.; Kishishita, S.; Toyama, M.; Morita, S.; Uchikubo-Kamo, T.; Akasaka, R.; Omori, K.; Kotera, J.; Terada, T. Crystal structure of the GAF-B domain from human phosphodiesterase 10A complexed with its ligand, cAMP. J. Biol. Chem. 2008, 283, 19657–19664. [Google Scholar] [CrossRef]
- Fajardo, A.M.; Piazza, G.A.; Tinsley, H.N. The role of cyclic nucleotide signaling pathways in cancer: Targets for prevention and treatment. Cancers 2014, 6, 436–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lindsey, A.; Li, N.; Gary, B.; Andrews, J.; Keeton, A.; Piazza, G. β-catenin nuclear translocation in colorectal cancer cells is suppressed by PDE10A inhibition, cGMP elevation, and activation of PKG. Oncotarget 2015, 7, 5353–5365. [Google Scholar] [CrossRef]
- Coskran, T.M.; Morton, D.; Menniti, F.S.; Adamowicz, W.O.; Kleiman, R.J.; Ryan, A.M.; Strick, C.A.; Schmidt, C.J.; Stephenson, D.T. Immunohistochemical localization of phosphodiesterase 10A in multiple mammalian species. J. Histochem. Cytochem. 2006, 54, 1205–1213. [Google Scholar] [CrossRef]
- Gross-Langenhoff, M.; Hofbauer, K.; Weber, J.; Schultz, A.; Schultz, J.E. cAMP is a ligand for the tandem GAF domain of human phosphodiesterase 10 and cGMP for the tandem GAF domain of phosphodiesterase 11. J. Biol. Chem. 2006, 281, 2841–2846. [Google Scholar] [CrossRef]
- Friedman, D.L. Role of cyclic nucleotides in cell growth and differentiation. Physiol. Rev. 1976, 56, 652–708. [Google Scholar] [CrossRef]
- New, D.; Wong, Y. Molecular mechanisms mediating the G protein-coupled regulation of cell cycle progression. J. Mol. Signal. 2007, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Vroom, C.; Ghofrani, H.A.; Weissmann, N.; Bieniek, E.; Grimminger, F.; Seeger, W.; Schermuly, R.T.; Pullamsetti, S.S. Phosphodiesterase 10A upregulation contributes to pulmonary vascular remodeling. PLoS ONE 2011, 6, e18136. [Google Scholar] [CrossRef]
- Itoh, K.; Ishima, T.; Kehler, J.; Hashimoto, K. Potentiation of NGF-induced neurite outgrowth in PC12 cells by papaverine: Role played by PLC-γ, IP3 receptors. Brain Res. 2011, 1377, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Heikaus, C.C.; Pandit, J.; Klevit, R.E. Cyclic nucleotide binding GAF domains from phosphodiesterases: Structural and mechanistic insights. Structure 2009, 17, 1551–1557. [Google Scholar] [CrossRef]
- Valsecchi, F.; Ramos-Espiritu, L.S.; Buck, J.; Levin, L.R.; Manfredi, G. cAMP and Mitochondria. Physiology 2013, 28, 199–209. [Google Scholar] [CrossRef]
- Johnson, A.R.; Moran, N.C.; Mayer, S.E. Cyclic AMP content and histamine release in rat mast cells. J. Immunol. 1974, 112, 511–519. [Google Scholar]
- Bang, Y.J.; Pirnia, F.; Fang, W.; Kang, W.; Sartor, O.; Whitesell, L.; Ha, M.; Tsokos, M.; Sheahan, M.; Nguyen, P. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc. Natl. Acad. Sci. USA 1994, 91, 5330–5334. [Google Scholar] [CrossRef]
- Shimizu, T.; Ohta, Y.; Ozawa, H.; Matsushima, H.; Takeda, K. Papaverine combined with prostaglandin E2 synergistically induces neuron-like morphological changes and decrease of malignancy in human prostatic cancer LNCaP cells. Anticancer. Res. 2000, 20, 761–767. [Google Scholar]
- Eischen, C.M.; Packham, G.; Nip, J.; Fee, B.E.; Hiebert, S.W.; Zambetti, G.P.; Cleveland, J.L. Bcl-2 is an apoptotic target suppressed by both c-Myc and E2F-1. Oncogene 2001, 20, 6983–6993. [Google Scholar] [CrossRef]
- Lazarou, M.; Thorburn, D.R.; Ryan, M.T.; McKenzie, M. Assembly of mitochondrial complex I and defects in disease. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 78–88. [Google Scholar] [CrossRef]
- Lenaz, G.; Fato, R.; Genova, M.L.; Bergamini, C.; Bianchi, C.; Biondi, A. Mitochondrial Complex I: Structural and functional aspects. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 1406–1420. [Google Scholar] [CrossRef]
- Horgan, D.J.; Singer, T.P.; Casida, J. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase XIII. Binding sites of rotenone, piericidin A, and Amytal in the respiratory chain. J. Biol. Chem. 1968, 243, 834–843. [Google Scholar] [CrossRef]
- Morikawa, N.; Nakagawa-Hattori, Y.; Mizuno, Y. Effect of dopamine, dimethoxyphenylethylamine, papaverine, and related compounds on mitochondrial respiration and complex I activity. J. Neurochem. 1996, 66, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Plitzko, B.; Loesgen, S. Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism. Bio-Protoc. 2018, 8, e2850. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ponuwei, G.A.; Moore, C.E.; Willars, G.B.; Tee, A.R.; Herbert, T.P. cAMP inhibits mammalian target of rapamycin complex-1 and -2 (mTORC1 and 2) by promoting complex dissociation and inhibiting mTOR kinase activity. Cell. Signal. 2011, 23, 1927–1935. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Sabatini, D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005, 17, 596–603. [Google Scholar] [CrossRef]
- Gomes, D.A.; Joubert, A.M.; Visagie, M.H. In Vitro Effects of Papaverine on Cell Migration and Vascular Endothelial Growth Factor in Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 4654. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Tonini, T.; Rossi, F.; Claudio, P.P. Molecular basis of angiogenesis and cancer. Oncogene 2003, 22, 6549–6556. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G. VEGF receptor signaling in tumor angiogenesis. Oncologist 2000, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, C.; Tang, Z.; Zhang, F.; Arjunan, P.; Li, Y.; Hou, X.; Kumar, A.; Dong, L. VEGF-B: A survival, or an angiogenic factor? Cell Adh. Migr. 2009, 3, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kumar, A.; Zhang, F.; Lee, C.; Tang, Z. Complicated life, complicated VEGF-B. Trends Mol. Med. 2012, 18, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Perona, R. Cell signalling: Growth factors and tyrosine kinase receptors. Clin. Transl. Oncol. 2006, 8, 77–82. [Google Scholar] [CrossRef]
- Li, X. VEGF-B: A thing of beauty. Cell Res. 2010, 20, 741–744. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Papazoglou, D.; Giatromanolaki, A.; Bougioukas, G.; Maltezos, E.; Siviridis, E. VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer 2004, 46, 293–298. [Google Scholar] [CrossRef]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef]
- Ferrer, F.A.; Miller, L.J.; Andrawis, R.I.; Kurtzman, S.H.; Albertsen, P.C.; Laudone, V.P.; Kreutzer, D.L. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: In situ and in vitro expression of VEGF by human prostate cancer cells. J. Urol. 1997, 157, 2329–2333. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; Latek, R.R.; Guntur, K.V.P.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. GβL, a Positive Regulator of the Rapamycin-Sensitive Pathway Required for the Nutrient-Sensitive Interaction between Raptor and mTOR. Mol. Cell 2003, 11, 895–904. [Google Scholar] [CrossRef]
- Yuan, T.; Cantley, L. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Wu, L.-Q.; Zhang, T.; Han, Y.-F.; Lin, X. Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol. Rep. 2015, 33, 1630–1638. [Google Scholar] [CrossRef]
- Li, R.; Zou, X.; Huang, H.; Yu, Y.; Zhang, H.; Liu, P.; Pan, S.; Ouyang, Y.; Shang, Y. HMGB1/PI3K/Akt/mTOR Signaling Participates in the Pathological Process of Acute Lung Injury by Regulating the Maturation and Function of Dendritic Cells. Front. Immunol. 2020, 11, 1104. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, E.; Span, P.; Schuuring, J.; Peters, J.; Sweep, F.; Van Der Kogel, A.; Bussink, J. Angiogenesis, hypoxia and VEGF expression during tumour growth in a human xenograft tumour model. Microvasc. Res. 2009, 77, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. New Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Wang, X.; Klein, R.D. Prostaglandin E2 induces vascular endothelial growth factor secretion in prostate cancer cells through EP2 receptor-mediated cAMP pathway. Mol. Carcinog. 2007, 46, 912–923. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 5353–5365. [Google Scholar] [CrossRef]


| Alkaloid Name | Alkaloid Content Range within Total Alkaloids (%) | Average Alkaloid Content within Total Alkaloids (%) | Pharmacological Use |
|---|---|---|---|
| Papaverine | 0.3–29.7 | 3.70 | Smooth muscle relaxant |
| Codeine | 0.5–25.5 | 7.69 | Used as a cough suppressant and pain killer |
| Morphine | 42.6–87.6 | 67.9 | Dominant alkaloid used as a naturally occurring pain killer |
| Thebaine | 1.9–18.1 | 5.66 | Used industrially to synthesize other pain killers |
| Noscapine | 1.1–41.2 | 16.05 | Cough suppressant |
| Cell Line | Highest PPV Concentration Tested (µM) | Cell Viability (%) | Period of Exposure to PPV (h) | Reference |
|---|---|---|---|---|
| MDA-MB-231 | 150 | 56 | 48 | [20] |
| A549 | 150 | 53 | 48 | [20] |
| DU145 | 150 | 64 | 48 | [20] |
| PC-3 | 200 | 15 | 24 | [29] |
| HT 29 | 100 | 35 | 48 | [7] |
| HT1080 | >100 | 15 | 48 | [7] |
| T47D | >100 | 25 | 48 | [7] |
| LNCaP | 100 | 30 | 144 | [25] |
| NIH-3 T3 | 1000 | 85 | 48 | [7] |
| NHF | 200 | 98 | 24 | [29] |
| Cell Line | PPV Concentration (µM) | Cells Occupying the Sub-G1 Phase (%) | Period of Exposure to PPV (h) | Reference |
|---|---|---|---|---|
| PC-3 | 120 | 52.4 | 48 | [29] |
| MDA-MB-231 | 150 | 47 | 72 | [20] |
| A549 | 150 | 9.6 | 72 | [20] |
| DU145 | 150 | 24 | 72 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, D.A.; Joubert, A.M.; Visagie, M.H. The Biological Relevance of Papaverine in Cancer Cells. Cells 2022, 11, 3385. https://doi.org/10.3390/cells11213385
Gomes DA, Joubert AM, Visagie MH. The Biological Relevance of Papaverine in Cancer Cells. Cells. 2022; 11(21):3385. https://doi.org/10.3390/cells11213385
Chicago/Turabian StyleGomes, Daniella Anthea, Anna Margaretha Joubert, and Michelle Helen Visagie. 2022. "The Biological Relevance of Papaverine in Cancer Cells" Cells 11, no. 21: 3385. https://doi.org/10.3390/cells11213385
APA StyleGomes, D. A., Joubert, A. M., & Visagie, M. H. (2022). The Biological Relevance of Papaverine in Cancer Cells. Cells, 11(21), 3385. https://doi.org/10.3390/cells11213385







