Transposable Elements as a Source of Novel Repetitive DNA in the Eukaryote Genome
Abstract
1. Introduction
2. Transposons as a Source of Repetitive Units for the Emergence of Tandem Repeats
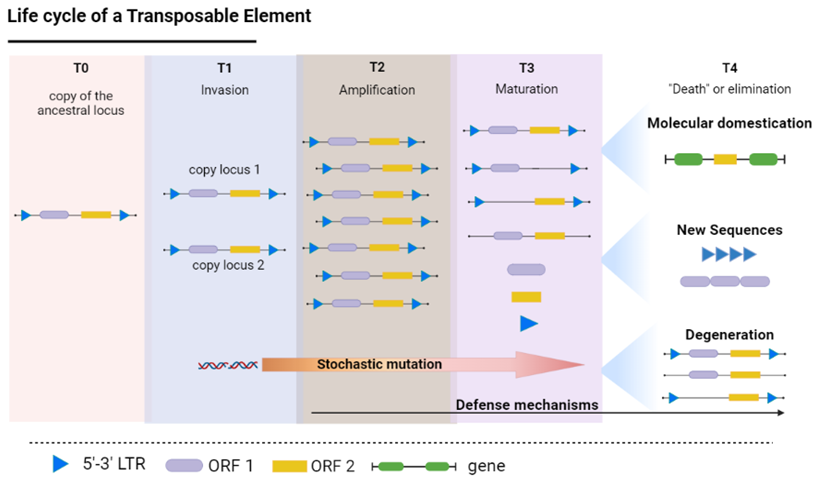
2.1. Classes and Mobility of the TEs
2.2. Are Certain Portions of a TE More Prone to the Generation of Tandem Repeat Sequences?
3. Is the Centromeric Region a Hotspot of the Emergence of de novo satDNA Derived from TEs?
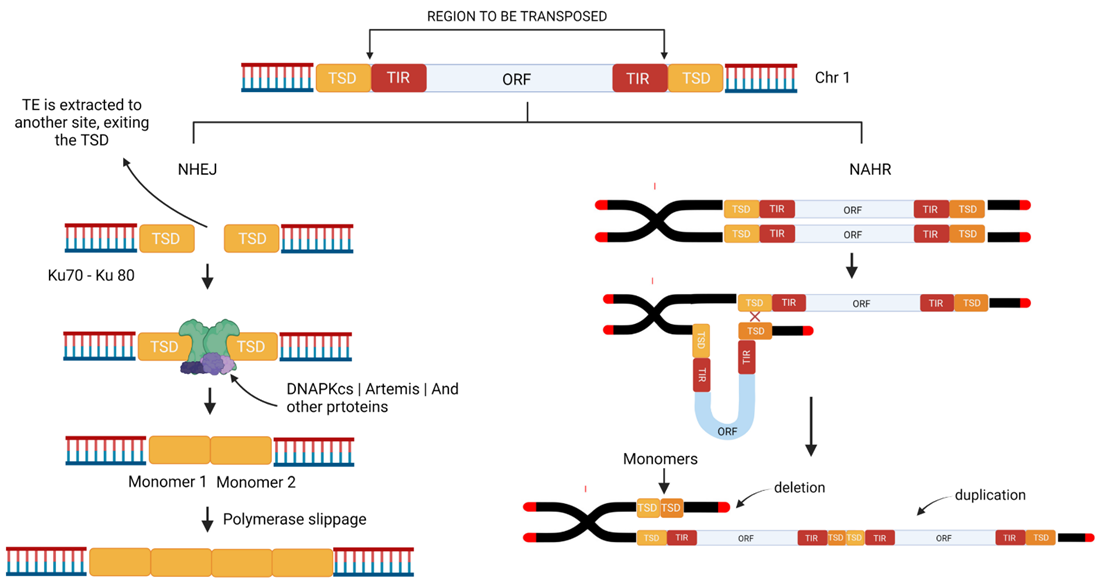
4. Mechanisms of the Production of Repeats from TEs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGurk, M.P.; Barbash, D.A. Double Insertion of Transposable Elements Provides a Substrate for the Evolution of Satellite DNA. Genome Res. 2018, 28, 714–725. [Google Scholar] [CrossRef]
- Zeljko, V.T.; Pavlek, M.; Meštrović, N.; Plohl, M. Satellite DNA-like Repeats Are Dispersed throughout the Genome of the Pacific Oyster Crassostrea Gigas Carried by Helentron Non-Autonomous Mobile Elements. Sci. Rep. 2020, 10, 15107. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, N.; Mravinac, B.; Pavlek, M.; Vojvoda-Zeljko, T.; Šatović, E.; Plohl, M. Structural and Functional Liaisons between Transposable Elements and Satellite DNAs. Chromosome Res. 2015, 23, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Belyayev, A.; Josefiová, J.; Jandová, M.; Mahelka, V.; Krak, K.; Mandák, B. Transposons and Satellite DNA: On the Origin of the Major Satellite DNA Family in the Chenopodium Genome. Mob. DNA 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Paço, A.; Freitas, R.; Vieira-da-Silva, A. Conversion of DNA Sequences: From a Transposable Element to a Tandem Repeat or to a Gene. Genes 2019, 10, 1014. [Google Scholar] [CrossRef]
- Tek, A.L.; Song, J.; Macas, J.; Jiang, J. Sobo, a Recently Amplified Satellite Repeat of Potato, and Its Implications for the Origin of Tandemly Repeated Sequences. Genetics 2005, 170, 1231–1238. [Google Scholar] [CrossRef]
- Li, J.; Leung, F.C. A CR1 Element Is Embedded in a Novel Tandem Repeat (Hin FI Repeat) within the Chicken Genome. Genome 2006, 49, 97–103. [Google Scholar] [CrossRef]
- Dias, G.B.; Heringer, P.; Svartman, M.; Kuhn, G.C.S. Helitrons Shaping the Genomic Architecture of Drosophila: Enrichment of DINE-TR1 in α- and β-Heterochromatin, Satellite DNA Emergence, and PiRNA Expression. Chromosome Res. 2015, 23, 597–613. [Google Scholar] [CrossRef]
- Wicker, T.; Sabot, F.; Hua-Van, A.; Bennetzen, J.L.; Capy, P.; Chalhoub, B.; Flavell, A.; Leroy, P.; Morgante, M.; Panaud, O.; et al. A Unified Classification System for Eukaryotic Transposable Elements. Nat. Rev. Genet. 2007, 8, 973–982. [Google Scholar] [CrossRef]
- Kidwell, M.G.; Lisch, D.R. Perspective: Transposable elements, parasitic dna, and genome evolution. Evolution 2001, 55, 1–24. [Google Scholar] [CrossRef]
- Fernández-Medina, R.D.; Ribeiro, J.M.C.; Carareto, C.M.A.; Velasque, L.; Struchiner, C.J. Losing Identity: Structural Diversity of Transposable Elements Belonging to Different Classes in the Genome of Anopheles Gambiae. BMC Genom. 2012, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.L.; Lozovskaya, E.R.; Nurminsky, D.I.; Lohe, A.R. What Restricts the Activity of Mariner-like Transposable Elements. Trends Genet. 1997, 13, 197–201. [Google Scholar] [CrossRef]
- Le Rouzic, A.; Capy, P. The First Steps of Transposable Elements Invasion. Genetics 2005, 169, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.G.; Bao, W.; Martins, C.; Jurka, J. Horizontal Transfers of Mariner Transposons between Mammals and Insects. Mob. DNA 2012, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.K.; Feschotte, C. The Evolutionary History of Human DNA Transposons: Evidence for Intense Activity in the Primate Lineage. Genome Res. 2007, 17, 422–432. [Google Scholar] [CrossRef]
- Pritham, E.J.; Feschotte, C. Massive Amplification of Rolling-Circle Transposons in the Lineage of the Bat Myotis Lucifugus. Proc. Natl. Acad. Sci. USA 2007, 104, 1895–1900. [Google Scholar] [CrossRef]
- Chen, J.; Lu, L.; Robb, S.M.C.; Collin, M.; Okumoto, Y.; Stajich, J.E.; Wessler, S.R. Genomic Diversity Generated by a Transposable Element Burst in a Rice Recombinant Inbred Population. Proc. Natl. Acad. Sci. USA 2020, 117, 26288–26297. [Google Scholar] [CrossRef]
- Nitta, N.; Farman, M.L.; Leong, S.A. Genome Organization of Magnaporthe Grisea: Integration of Genetic Maps, Clustering of Transposable Elements and Identification of Genome Duplications and Rearrangements. Theor. Appl. Genet. 1997, 95, 20–32. [Google Scholar] [CrossRef]
- Dasilva, C.; Hadji, H.; Ozouf-Costaz, C.; Nicaud, S.; Jaillon, O.; Weissenbach, J.; Crollius, H.R. Remarkable Compartmentalization of Transposable Elements and Pseudogenes in the Heterochromatin of the Tetraodon Nigroviridis Genome. Proc. Natl. Acad. Sci. USA 2002, 99, 13636–13641. [Google Scholar] [CrossRef]
- Thon, M.R.; Martin, S.L.; Goff, S.; Wing, R.A.; Dean, R.A. BAC End Sequences and a Physical Map Reveal Transposable Element Content and Clustering Patterns in the Genome of Magnaporthe Grisea. Fungal Genet. Biol. 2004, 41, 657–666. [Google Scholar] [CrossRef]
- Thon, M.; Pan, H.; Diener, S.; Papalas, J.; Taro, A.; Mitchell, T.; Dean, R. BAC End Sequences and a Physical Map Reveal Transposable Element Content and Clustering Patterns in the Genome of Magnaporthe Grisea. Genome Biol. 2006, 7, R16. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, N.; Bourc’his, D. Transposable Elements in the Mammalian Germline: A Comfortable Niche or a Deadly Trap? Heredity 2010, 105, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yan, W. Male Germline Control of Transposable Elements. Biol. Reprod. 2012, 86, 162-1. [Google Scholar] [CrossRef]
- Shi, X.; Seluanov, A.; Gorbunova, V. Cell Divisions Are Required for L1 Retrotransposition. Mol. Cell. Biol. 2007, 27, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.; Odom, D.T.; Kutter, C. The Emergence of PiRNAs against Transposon Invasion to Preserve Mammalian Genome Integrity. Nat. Commun. 2017, 8, 1411. [Google Scholar] [CrossRef]
- Feschotte, C.; Pritham, E.J. DNA Transposons and the Evolution of Eukaryotic Genomes. Annu. Rev. Genet. 2007, 41, 331–368. [Google Scholar] [CrossRef]
- Engels, W.R.; Johnson-Schlitz, D.M.; Eggleston, W.B.; Sved, J. High-Frequency P Element Loss in Drosophila Is Homolog Dependent. Cell 1990, 62, 515–525. [Google Scholar] [CrossRef]
- Maupetit-Mehouas, S.; Vaury, C. Transposon Reactivation in the Germline May Be Useful for Both Transposons and Their Host Genomes. Cells 2020, 9, 1172. [Google Scholar] [CrossRef]
- Sigman, M.J.; Slotkin, R.K. The First Rule of Plant Transposable Element Silencing: Location, Location, Location. Plant Cell 2016, 28, 304–313. [Google Scholar] [CrossRef]
- Ward, M.C.; Zhao, S.; Luo, K.; Pavlovic, B.J.; Karimi, M.M.; Stephens, M.; Gilad, Y. Silencing of Transposable Elements May Not Be a Major Driver of Regulatory Evolution in Primate IPSCs. eLife 2018, 7, e33084. [Google Scholar] [CrossRef]
- Rigal, M.; Mathieu, O. A “Mille-Feuille” of Silencing: Epigenetic Control of Transposable Elements. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2011, 1809, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xi, R. Silencing Transposable Elements in the Drosophila Germline. Cell. Mol. Life Sci. 2017, 74, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Chandler, V.; Rivin, C.; Walbot, V. Stable non-mutator stocks of maize have sequences homologous to the Mu1 transposable element. Genetics 1986, 114, 1007–1021. [Google Scholar] [CrossRef]
- Chomet, P.S.; Wessler, S.; Dellaporta, S.L. Inactivation of the Maize Transposable Element Activator (Ac) Is Associated with Its DNA Modification. EMBO J. 1987, 6, 295–302. [Google Scholar] [CrossRef]
- Banks, J.A.; Masson, P.; Fedoroff, N. Molecular Mechanisms in the Developmental Regulation of the Maize Suppressor-Mutator Transposable Element. Genes Dev. 1988, 2, 1364–1380. [Google Scholar] [CrossRef]
- Collings, C.K.; Anderson, J.N. Links between DNA Methylation and Nucleosome Occupancy in the Human Genome. Epigenetics Chromatin 2017, 10, 18. [Google Scholar] [CrossRef]
- Rose, N.R.; Klose, R.J. Understanding the Relationship between DNA Methylation and Histone Lysine Methylation. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 1362–1372. [Google Scholar] [CrossRef]
- Walter, M.; Teissandier, A.; Pérez-Palacios, R.; Bourc’his, D. An Epigenetic Switch Ensures Transposon Repression upon Dynamic Loss of DNA Methylation in Embryonic Stem Cells. eLife 2016, 5, e11418. [Google Scholar] [CrossRef]
- Ebbs, M.L.; Bender, J. Locus-Specific Control of DNA Methylation by the Arabidopsis SUVH5 Histone Methyltransferase. Plant Cell 2006, 18, 1166–1176. [Google Scholar] [CrossRef]
- Lippman, Z.; May, B.; Yordan, C.; Singer, T.; Martienssen, R. Distinct Mechanisms Determine Transposon Inheritance and Methylation via Small Interfering RNA and Histone Modification. PLoS Biol. 2003, 1, E67. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Colmenares, S.U.; Karpen, G.H. Heterochromatin: Guardian of the Genome. Annu. Rev. Cell Dev. Biol. 2018, 34, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ali, M.; Zhou, Q. Establishment and Evolution of Heterochromatin. Ann. N. Y. Acad. Sci. 2020, 1476, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, M.G. Transposable Elements and the Evolution of Genome Size in Eukaryotes. Genetica 2002, 115, 49–63. [Google Scholar] [CrossRef]
- Sirijovski, N.; Woolnough, C.; Rock, J.; Joss, J.M.P. NfCR1, the First Non-LTR Retrotransposon Characterized in the Australian Lungfish Genome, Neoceratodus Forsteri, Shows Similarities to CR1-like Elements. J. Exp. Zool. 2005, 304, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, J.; Zhu, C.; Yang, L.; Ren, Y.; Ruan, J.; Fan, G.; Hu, J.; Xu, W.; Bi, X.; et al. African Lungfish Genome Sheds Light on the Vertebrate Water-to-Land Transition. Cell 2021, 184, 1362–1376.e18. [Google Scholar] [CrossRef] [PubMed]
- Marsano, R.M.; Dimitri, P. Constitutive Heterochromatin in Eukaryotic Genomes: A Mine of Transposable Elements. Cells 2022, 11, 761. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Jurka, J. RAG1 Core and V(D)J Recombination Signal Sequences Were Derived from Transib Transposons. PLoS Biol. 2005, 3, e181. [Google Scholar] [CrossRef]
- Feschotte, C. Transposable Elements and the Evolution of Regulatory Networks. Nat. Rev. Genet. 2008, 9, 397–405. [Google Scholar] [CrossRef]
- Alzohairy, A.M.; Gyulai, G.; Jansen, R.K.; Bahieldin, A. Transposable Elements Domesticated and Neofunctionalized by Eukaryotic Genomes. Plasmid 2013, 69, 1–15. [Google Scholar] [CrossRef]
- Qiu, Y.; Köhler, C. Mobility Connects: Transposable Elements Wire New Transcriptional Networks by Transferring Transcription Factor Binding Motifs. Biochem. Soc. Trans. 2020, 48, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Han, K.; Liang, P. Role of Transposable Elements in Gene Regulation in the Human Genome. Life 2021, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, R.; Judd, J.; Feschotte, C.; Wysocka, J. Roles of Transposable Elements in the Regulation of Mammalian Transcription. Nat. Rev. Mol. Cell Biol. 2022, 23, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, Y.D.; Eckert, K.A.; Chiaromonte, F.; Makova, K.D. A Matter of Life or Death: How Microsatellites Emerge in and Vanish from the Human Genome. Genome Res. 2011, 21, 2038–2048. [Google Scholar] [CrossRef]
- Etchegaray, E.; Naville, M.; Volff, J.-N.; Haftek-Terreau, Z. Transposable Element-Derived Sequences in Vertebrate Development. Mob. DNA 2021, 12, 1. [Google Scholar] [CrossRef]
- Slotkin, R.K.; Martienssen, R. Transposable Elements and the Epigenetic Regulation of the Genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef]
- Elbarbary, R.A.; Lucas, B.A.; Maquat, L.E. Retrotransposons as Regulators of Gene Expression. Science 2016, 351, aac7247. [Google Scholar] [CrossRef]
- Buschiazzo, E.; Gemmell, N.J. The Rise, Fall and Renaissance of Microsatellites in Eukaryotic Genomes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16998838/ (accessed on 15 May 2022).
- Macas, J.; Koblízková, A.; Navrátilová, A.; Neumann, P. Hypervariable 3’ UTR Region of Plant LTR-Retrotransposons as a Source of Novel Satellite Repeats. Gene 2009, 448, 198–206. [Google Scholar] [CrossRef]
- Kazazian, H.H. Mobile Elements: Drivers of Genome Evolution. Science 2004, 303, 1626–1632. [Google Scholar] [CrossRef]
- Ahmed, M.; Liang, P. Transposable Elements Are a Significant Contributor to Tandem Repeats in the Human Genome. Comp. Funct. Genom. 2012, 2012, 947089. [Google Scholar] [CrossRef]
- Kanhayuwa, L.; Coutts, R.H.A. Short Interspersed Nuclear Element (SINE) Sequences in the Genome of the Human Pathogenic Fungus Aspergillus Fumigatus Af293. PLoS ONE 2016, 11, e0163215. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. International Human Genome Sequencing Consortium. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Dewannieux, M.; Esnault, C.; Heidmann, T. LINE-Mediated Retrotransposition of Marked Alu Sequences. Nat. Genet. 2003, 35, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Deininger, P. Alu Elements: Know the SINEs. Genome Biol. 2011, 12, 236. [Google Scholar] [CrossRef]
- Armour, J.A.L.; Wong, Z.; Wilson, V.; Royle, N.J.; Jeffreys, A.J. Sequences Flanking the Repeat Arrays of Human Minlsatellites: Association with Tandem and Dispersed Repeat Elements. Nucl. Acids Res. 1989, 17, 4925–4936. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; Dalgliesh, G.L.; Endres, D.; Gomez, M.; Taylor, J.; Bidichandani, S.I. Expansion of GAA Triplet Repeats in the Human Genome: Unique Origin of the FRDA Mutation at the Center of an Alu. Genomics 2004, 83, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Jurka, J.; Gentles, A.J. Origin and Diversification of Minisatellites Derived from Human Alu Sequences. Gene 2006, 365, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Arcot, S.S.; Wang, Z.; Weber, J.L.; Deininger, P.L.; Batzer, M.A. Alu Repeats: A Source for the Genesis of Primate Microsatellites. Genomics 1995, 29, 136–144. [Google Scholar] [CrossRef]
- Bureau, T.E.; Wessler, S.R. Mobile Inverted-Repeat Elements of the Tourist Familyare Associated with the Genes of Many Cereal Grasses. Proc. Natl. Acad. Sci. USA 1994, 91, 1411–1415. [Google Scholar] [CrossRef]
- Feschotte, C.; Swamy, L.; Wessler, S.R. Genome-Wide Analysis of Mariner -Like Transposable Elements in Rice Reveals Complex Relationships with Stowaway Miniature Inverted Repeat Transposable Elements (MITEs). Genetics 2003, 163, 747–758. [Google Scholar] [CrossRef]
- Coates, B.S.; Kroemer, J.A.; Sumerford, D.V.; Hellmich, R.L. A Novel Class of Miniature Inverted Repeat Transposable Elements (MITEs) That Contain Hitchhiking (GTCY)n Microsatellites: Lepidopteran Mobile Microsatellites. Insect Mol. Biol. 2011, 20, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, A.; Kawahara, A. Lineage-Specific Tandem Repeats Riding on a Transposable Element of MITE in Xenopus Evolution: A New Mechanism for Creating Simple Sequence Repeats. J. Mol. Evol. 2004, 59, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Scalvenzi, T.; Pollet, N. Insights on Genome Size Evolution from a Miniature Inverted Repeat Transposon Driving a Satellite DNA. Mol. Phylogenet. Evol. 2014, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Langdon, T.; Seago, C.; Jones, R.N.; Ougham, H.; Thomas, H.; Forster, J.W.; Jenkins, G. De Novo Evolution of Satellite DNA on the Rye B Chromosome. Genetics 2000, 154, 869–884. [Google Scholar] [CrossRef] [PubMed]
- López-Flores, I.; Garrido-Ramos, M.A. The Repetitive DNA Content of Eukaryotic Genomes. In Genome Dynamics; Garrido-Ramos, M.A., Ed.; S. Karger AG: Basel, Switzerland, 2012; Volume 7, pp. 1–28. [Google Scholar] [CrossRef]
- López-Flores, I.; de la Herrán, R.; Garrido-Ramos, M.A.; Boudry, P.; Ruiz-Rejón, C.; Ruiz-Rejón, M. The Molecular Phylogeny of Oysters Based on a Satellite DNA Related to Transposons. Gene 2004, 339, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Bois, P.; Williamson, J.; Brown, J.; Dubrova, Y.E.; Jeffreys, A.J. A Novel Unstable Mouse VNTR Family Expanded from SINE B1 Elements. Genomics 1998, 49, 122–128. [Google Scholar] [CrossRef]
- Kelly, R.G. Similar Origins of Two Mouse Minisatellites within Transposon-like LTRs. Genomics 1994, 24, 509–515. [Google Scholar] [CrossRef]
- Rossi, M.S.; Pesce, C.G.; Reig, O.A.; Kornblihtt, A.R.; Zorzópulos, J. Retroviral-like Features in the Monomer of the Major Satellite DNA from the South American Rodents of the Genus Ctenomys. DNA Sequence 1993, 3, 379–381. [Google Scholar] [CrossRef]
- Paço, A.; Adega, F.; Meštrović, N.; Plohl, M.; Chaves, R. The Puzzling Character of Repetitive DNA in Phodopus Genomes (Cricetidae, Rodentia). Chromosome Res. 2015, 23, 427–440. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Holmquist, G.P.; Jurka, J. L1 Repeat Is a Basic Unit of Heterochromatin Satellites in Cetaceans. Mol. Biol. Evol. 1998, 15, 611–612. [Google Scholar] [CrossRef]
- Duffy, A.J.; Coltman, D.W.; Wright, J.M. Microsatellites at a Common Site in the Second ORF of L1 Elements in Mammalian Genomes. Mamm. Genome 1996, 7, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Palomeque, T.; Antonio Carrillo, J.; Muñoz-López, M.; Lorite, P. Detection of a Mariner-like Element and a Miniature Inverted-Repeat Transposable Element (MITE) Associated with the Heterochromatin from Ants of the Genus Messor and Their Possible Involvement for Satellite DNA Evolution. Gene 2006, 371, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.J.; Nagel, A.; Bachmann, J.; Bachmann, L. Evolutionary Dynamics of the SGM Transposon Family in the Drosophila Obscura Species Group. Mol. Biol. Evol. 2000, 17, 1597–1609. [Google Scholar] [CrossRef][Green Version]
- Heikkinen, E.; Launonen, V.; Müller, E.; Bachmann, L. The PvB370 BamHI Satellite DNA Family of the Drosophila Virilis Group and Its Evolutionary Relation to Mobile Dispersed Genetic PDv Elements. J. Mol. Evol. 1995, 41, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Agudo, M.; Losada, A.; Abad, J.P.; Pimpinelli, S.; Ripoll, P.; Villasante, A. Centromeres from Telomeres? The Centromeric Region of the Y Chromosome of Drosophila Melanogaster Contains a Tandem Array of Telomeric HeT-A- and TART-Related Sequences. Nucleic Acids Res. 1999, 27, 3318–3324. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.B.; Svartman, M.; Delprat, A.; Ruiz, A.; Kuhn, G.C.S. Tetris Is a Foldback Transposon That Provided the Building Blocks for an Emerging Satellite DNA of Drosophila Virilis. Genome Biol. Evol. 2014, 6, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Batistoni, R.; Pesole, G.; Marracci, S.; Nardi, I. A Tandemly Repeated DNA Family Originated from SINE-Related Elements in the European Plethodontid Salamanders (Amphibia, Urodela). J. Mol. Evol. 1995, 40, 608–615. [Google Scholar] [CrossRef]
- Suntronpong, A.; Singchat, W.; Kruasuwan, W.; Prakhongcheep, O.; Sillapaprayoon, S.; Muangmai, N.; Somyong, S.; Indananda, C.; Kraichak, E.; Peyachoknagul, S.; et al. Characterization of Centromeric Satellite DNAs (MALREP) in the Asian Swamp Eel (Monopterus Albus) Suggests the Possible Origin of Repeats from Transposable Elements. Genomics 2020, 112, 3097–3107. [Google Scholar] [CrossRef]
- Pasero, P.; Sjakste, N.; Blettry, C.; Got, C.; Marilley, M. Long-Range Organization and Sequence-Directed Curvature of Xenopus Laevis Satellite 1 DNA. Nucleic Acids Res. 1993, 21, 4703–4710. [Google Scholar] [CrossRef]
- Plohl, M.; Petrović, V.; Luchetti, A.; Ricci, A.; Šatović, E.; Passamonti, M.; Mantovani, B. Long-Term Conservation vs High Sequence Divergence: The Case of an Extraordinarily Old Satellite DNA in Bivalve Mollusks. Heredity 2010, 104, 543–551. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Jurka, J. Molecular Paleontology of Transposable Elements from Arabidopsis Thaliana. In Transposable Elements and Genome Evolution; McDonald, J.F., Ed.; Springer: Dordrecht, The Netherlands, 2000; pp. 27–37. [Google Scholar] [CrossRef]
- Mogil, L.S.; Slowikowski, K.; Laten, H.M. Computational and Experimental Analyses of Retrotransposon-Associated Minisatellite DNAs in the Soybean Genome. BMC Bioinform. 2012, 13, S13. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wu, Y.; Koblížková, A.; Torres, G.A.; Wang, K.; Iovene, M.; Neumann, P.; Zhang, W.; Novák, P.; Buell, C.R.; et al. Repeatless and Repeat-Based Centromeres in Potato: Implications for Centromere Evolution. Plant Cell 2012, 24, 3559–3574. [Google Scholar] [CrossRef]
- Sharma, A.; Wolfgruber, T.K.; Presting, G.G. Tandem Repeats Derived from Centromeric Retrotransposons. BMC Genom. 2013, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, L.; Macaulay, M.; Cardle, L.; Morgante, M.; degli Ivanissevich, S.; Maestri, E.; Powell, W.; Waugh, R. Intimate Association of Microsatellite Repeats with Retrotransposons and Other Dispersed Repetitive Elements in Barley. Plant J. 1999, 17, 415–425. [Google Scholar] [CrossRef]
- Cheng, Z.-J.; Murata, M. A Centromeric Tandem Repeat Family Originating From a Part of Ty3/ Gypsy -Retroelement in Wheat and Its Relatives. Genetics 2003, 164, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Vondrak, T.; Ávila Robledillo, L.; Novák, P.; Koblížková, A.; Neumann, P.; Macas, J. Characterization of Repeat Arrays in Ultra-Long Nanopore Reads Reveals Frequent Origin of Satellite DNA from Retrotransposon-Derived Tandem Repeats. Plant J. 2020, 101, 484–500. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-J.; Shen, Y.-H.; Gao, Y.-H.; Chen, L.-Y.; Xiang, Z.-H.; Zhang, Z. Burst Expansion, Distribution and Diversification of MITEs in the Silkworm Genome. BMC Genom. 2010, 11, 520. [Google Scholar] [CrossRef]
- Deprá, M.; Ludwig, A.; Valente, V.L.; Loreto, E.L. Mar, a MITE Family of HAT Transposons in Drosophila. Mob. DNA 2012, 3, 13. [Google Scholar] [CrossRef]
- Lu, C.; Chen, J.; Zhang, Y.; Hu, Q.; Su, W.; Kuang, H. Miniature Inverted-Repeat Transposable Elements (MITEs) Have Been Accumulated through Amplification Bursts and Play Important Roles in Gene Expression and Species Diversity in Oryza Sativa. Mol. Biol. Evol. 2012, 29, 1005–1017. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Jurka, J. Helitrons on a Roll: Eukaryotic Rolling-Circle Transposons. Trends Genet. 2007, 23, 521–529. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Wang, H. The Contributions of Transposable Elements to the Structure, Function, and Evolution of Plant Genomes. Annu. Rev. Plant Biol. 2014, 65, 505–530. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, P.; Płucienniczak, G.; Bartosik, D. The Different Faces of Rolling-Circle Replication and Its Multifunctional Initiator Proteins. Front. Microbiol. 2017, 8, 2353. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.H.; Choo, K.H.A. Evolutionary Dynamics of Transposable Elements at the Centromere. Trends Genet. 2004, 20, 611–616. [Google Scholar] [CrossRef]
- Garrido-Ramos, M. Satellite DNA: An Evolving Topic. Genes 2017, 8, 230. [Google Scholar] [CrossRef]
- Thakur, J.; Packiaraj, J.; Henikoff, S. Sequence, Chromatin and Evolution of Satellite DNA. Int. J. Mol. Sci. 2021, 22, 4309. [Google Scholar] [CrossRef] [PubMed]
- Perea-Resa, C.; Blower, M.D. Centromere Biology: Transcription Goes on Stage. Mol. Cell Biol. 2018, 38, e00263-18. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, M.; Chuang, Y.-C.; Smith, G.R. Distributing Meiotic Crossovers for Optimal Fertility and Evolution. DNA Repair 2019, 81, 102648. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Henikoff, S. What Makes a Centromere? Exp. Cell Res. 2020, 389, 111895. [Google Scholar] [CrossRef]
- Gržan, T.; Despot-Slade, E.; Meštrović, N.; Plohl, M.; Mravinac, B. CenH3 Distribution Reveals Extended Centromeres in the Model Beetle Tribolium Castaneum. PLoS Genet. 2020, 16, e1009115. [Google Scholar] [CrossRef]
- Amorim, I.C.; Sotero-Caio, C.G.; Costa, R.G.C.; Xavier, C.; de Moura, R.d.C. Comprehensive Mapping of Transposable Elements Reveals Distinct Patterns of Element Accumulation on Chromosomes of Wild Beetles. Chromosome Res. 2021, 29, 203–218. [Google Scholar] [CrossRef]
- Jiang, J.; Birchler, J.A.; Parrott, W.A.; Kelly Dawe, R. A Molecular View of Plant Centromeres. Trends Plant Sci. 2003, 8, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, R.J.; Dawe, R.K. Distribution of Retroelements in Centromeres and Neocentromeres of Maize. Genetics 2003, 165, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Su, H.; Shi, Q.; Fu, S.; Wang, J.; Zhang, X.; Hu, Z.; Han, F. De Novo Centromere Formation and Centromeric Sequence Expansion in Wheat and Its Wide Hybrids. PLoS Genet. 2016, 12, e1005997. [Google Scholar] [CrossRef] [PubMed]
- Presting, G.G. Centromeric Retrotransposons and Centromere Function. Curr. Opin. Genet. Dev. 2018, 49, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Leo, L.; Marchetti, M.; Giunta, S.; Fanti, L. Epigenetics as an Evolutionary Tool for Centromere Flexibility. Genes 2020, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.-H.; Hori, T.; Martins, N.M.C.; Toyoda, A.; Misu, S.; Monma, N.; Hiratani, I.; Maeshima, K.; Ikeo, K.; Fujiyama, A.; et al. Chromosome Engineering Allows the Efficient Isolation of Vertebrate Neocentromeres. Dev. Cell 2013, 24, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Jiang, N.; Wing, R.A.; Jiang, J.; Jackson, S.A. Transposons Play an Important Role in the Evolution and Diversification of Centromeres among Closely Related Species. Front. Plant Sci. 2015, 6, 216. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chavan, A.; Palladino, J.; Wei, X.; Martins, N.M.C.; Santinello, B.; Chen, C.-C.; Erceg, J.; Beliveau, B.J.; Wu, C.-T.; et al. Islands of Retroelements Are Major Components of Drosophila Centromeres. PLoS Biol. 2019, 17, e3000241. [Google Scholar] [CrossRef]
- Wolfgruber, T.K.; Sharma, A.; Schneider, K.L.; Albert, P.S.; Koo, D.-H.; Shi, J.; Gao, Z.; Han, F.; Lee, H.; Xu, R.; et al. Maize Centromere Structure and Evolution: Sequence Analysis of Centromeres 2 and 5 Reveals Dynamic Loci Shaped Primarily by Retrotransposons. PLoS Genet. 2009, 5, e1000743. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Shen, Y.-H.; Xu, H.-E.; Liang, H.-Y.; Han, M.-J.; Zhang, Z. A Novel HAT Element in Bombyx Mori and Rhodnius Prolixus: Its Relationship with Miniature Inverted Repeat Transposable Elements (MITEs) and Horizontal Transfer. Insect Mol. Biol. 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Sharma, A.; Presting, G.G. Evolution of Centromeric Retrotransposons in Grasses. Genome Biol. Evol. 2014, 6, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, S.; Ishii, T.; Brown, C.T.; Houben, A.; Comai, L. Centromere Location in Arabidopsis Is Unaltered by Extreme Divergence in CENH3 Protein Sequence. Genome Res. 2017, 27, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Satović, E.; Vojvoda Zeljko, T.; Luchetti, A.; Mantovani, B.; Plohl, M. Adjacent Sequences Disclose Potential for Intra-Genomic Dispersal of Satellite DNA Repeats and Suggest a Complex Network with Transposable Elements. BMC Genom. 2016, 17, 997. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.-H.; Hori, T.; Toyoda, A.; Kato, J.; Popendorf, K.; Sakakibara, Y.; Fujiyama, A.; Fukagawa, T. Chickens Possess Centromeres with Both Extended Tandem Repeats and Short Non-Tandem-Repetitive Sequences. Genome Res. 2010, 20, 1219–1228. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhang, J.; Feng, Y.; Chen, Q.; Liu, Z.-S.; Liu, C.-L.; He, W.; Wang, H.; Yang, S.-F.; et al. Comparative Analysis of Transposable Elements and the Identification of Candidate Centromeric Elements in the Prunus Subgenus Cerasus and Its Relatives. Genes 2022, 13, 641. [Google Scholar] [CrossRef]
- Saint-Leandre, B.; Nguyen, S.C.; Levine, M.T. Diversification and Collapse of a Telomere Elongation Mechanism. Genome Res. 2019, 29, 920–931. [Google Scholar] [CrossRef]
- George, J.A.; DeBaryshe, P.G.; Traverse, K.L.; Celniker, S.E.; Pardue, M.-L. Genomic Organization of the Drosophila Telomere Retrotransposable Elements. Genome Res. 2006, 16, 1231–1240. [Google Scholar] [CrossRef]
- Dover, G. Molecular Drive: A Cohesive Mode of Species Evolution. Nature 1982, 299, 111–117. [Google Scholar] [CrossRef]
- Elliott, B.; Richardson, C.; Jasin, M. Chromosomal Translocation Mechanisms at Intronic Alu Elements in Mammalian Cells. Mol. Cell 2005, 17, 885–894. [Google Scholar] [CrossRef]
- Hedges, D.J.; Deininger, P.L. Inviting Instability: Transposable Elements, Double-Strand Breaks, and the Maintenance of Genome Integrity. Mutat. Res. 2007, 616, 46–59. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; Gao, D.; Wang, J.; Lang, Y.; Liu, T.; Li, B.; Bai, Z.; Luis Goicoechea, J.; Liang, C.; et al. Whole-Genome Sequencing of Oryza Brachyantha Reveals Mechanisms Underlying Oryza Genome Evolution. Nat. Commun. 2013, 4, 1595. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.M.; Bridges, M.C.; Strother, A.E.; Burckhalter, C.E.; Burnette, J.M.; Hancock, C.N. Precise Repair of MPing Excision Sites Is Facilitated by Target Site Duplication Derived Microhomology. Mob. DNA 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.; McVey, M. Error-Prone Repair of DNA Double-Strand Breaks. J. Cell Physiol. 2016, 231, 15–24. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-Homologous DNA End Joining and Alternative Pathways to Double-Strand Break Repair. Nat. Rev. Mol. Cell. Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Liu, P.; Lacaria, M.; Zhang, F.; Withers, M.; Hastings, P.J.; Lupski, J.R. Frequency of Nonallelic Homologous Recombination Is Correlated with Length of Homology: Evidence That Ectopic Synapsis Precedes Ectopic Crossing-Over. Am. J. Hum. Genet. 2011, 89, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Parks, M.M.; Lawrence, C.E.; Raphael, B.J. Detecting Non-Allelic Homologous Recombination from High-Throughput Sequencing Data. Genome Biol. 2015, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Al-Zain, A.M.; Symington, L.S. The Dark Side of Homology-Directed Repair. DNA Repair 2021, 106, 103181. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.T.; Drouin, G. Ectopic Gene Conversions in the Genome of Ten Hemiascomycete Yeast Species. Int. J. Evol. Biol. 2010, 2011, 970768. [Google Scholar] [CrossRef]
- Mazón, G.; Lam, A.F.; Ho, C.K.; Kupiec, M.; Symington, L.S. The Rad1–Rad10 Nuclease Promotes Chromosome Translocations between Dispersed Repeats. Nat. Struct. Mol. Biol. 2012, 19, 964–971. [Google Scholar] [CrossRef]
- Marsano, R.M.; Milano, R.; Minervini, C.; Moschetti, R.; Caggese, C.; Barsanti, P.; Caizzi, R. Organization and Possible Origin of the Bari-1 Cluster in the Heterochromatic H39 Region of Drosophila Melanogaster. Genetica 2003, 117, 281–289. [Google Scholar] [CrossRef]

| Species | Type of Transposable Element (TE) | Superfamily | Class | Mobility | Region of the TE | New Sequence | Reference |
|---|---|---|---|---|---|---|---|
| Pan paniscus and Hylobates lar | Alu elements | SINE Alu Family | Class I | non-autonomous | 3′ oligo(dA) tail and A-rich middle region | A-rich primates’ microsatellites | [69] |
| Homo sapiens | Alu | LTR –retrotransposons | SINE Alu Family | Class I | non-autonomous | TR may occur in any region of the TE | 7276 Minisatellites | [61] |
| Homo sapiens | Alu | SINE Alu Family | Class I | non-autonomous | - | pλg3, pMSl, pMS43, and pMS228 | [66] |
| Homo sapiens | Alu | SINE Alu Family | Class I | non-autonomous | Near to 3′ -UTR. | Three minisatellites | [68] |
| Homo sapiens | Alu | SINE Alu Family | Class I | non-autonomous | 3′ oligo(dA) tail. | (GAA)n | [67] |
| Mouse genome | SINE B1 | SINE Superfamily | Class I | non-autonomous | GAGGCA dimmer within the SINE | (GGCAGA)n | [78] |
| Mouse genome | MaLR | Retrotransposon-like superfamily | Class I | non-autonomous | LTR | Ms6-hm e Hm-2 | [79] |
| Ctenomys sp. | retroviral genome | - | Class I | - | LTR | RPCS satDNA | [80] |
| Phodopus roborovskii | LINE-1 elements | LINE | Class I | autonomous | ORF2 | PROsat | [81] |
| Dolphin | LINE-1 | LINE | Class I | autonomous | -3’ UTR | Common Cetacean Satellite | [82] |
| Phoca vitulina concolour | LINE-1 | LINE | Class I | autonomous | ORF2 | Pvc 20 | [83] |
| Genus Messor | Mariner-like (Mboumar) | Tc1/mariner | Class II | autonomous | Mariner is found inside the satDNA | satDNA | [84] |
| Gallus gallus | CR1 | CR1 family of LINEs | Class I | autonomous | -3′ UTR and a partial coding region of ORF 2 | HinfI (SCR1) | [7] |
| Helicoverpa zea | HzSINE1 MITE-like | SINE Superfamily | Class II | non-autonomous | 5′-IR | (GTCY)n | [72] |
| Drosophila virilis, Drosophila americana, and Drosophila biarmipes. | DINEs | Helitron | Class II | non-autonomous | Central tandem repeats (CTRs) | satDNA arrays | [8] |
| Drosophila guanche | SGM-IS | SGM Transposon Family | Class II | non-autonomous | - | SGM satDNA | [85] |
| Drosophila virilis group | pDv element | pDv transposable element family | Class II | - | Terminal repeat | pvB370 BamHI sat DNA | [86] |
| Drosophila melanogaster | TART | HeT-A | TART subfamilies of the HeT DNA family | Class I Class I | Autonomous non-autonomous | -3’ UTR | 18HT satDNA | [87] |
| Drosophila virilis | Tetris | Foldback elements | Class II | non-autonomous | TIR | satDNA-arrays (TIR-220) | [88] |
| Hydromantes imperialis and H. ambrosii | SINE-like elements | SINE Superfamily | Class I | non-autonomous | tRNA-related region | Hy/Pol III | [89] |
| Monopterus albus | LTR- RetrotransposonGypsy | LTR-Retrotransposon-like | Class I | autonomous | LTR | satDNA MALREP | [90] |
| Xenopus leavis | Xmix MITE | - | Class II | non-autonomous | TIR | Xstir satDNA | [73] |
| Xenopus leavis | SINE-like | SINE Superfamily | Class I | non-autonomous | tRNA-related region | Satellite 1 | [91] |
| Xenopus tropicalis | MITE of TC1-mariner | Tc1–Mariner | Class II | non-autonomous | stDNA located within the MITE element | miDNA4 | [74] |
| Ostrea edulis | CvA | Pearl | Class II | non-autonomous | - | HindIII | [77] |
| Venerupis decussata | MITE (Pearl) | Pearl | Class II | non-autonomous | - | BIV160 | [92] |
| Arabidopsis thaliana | Atenspm | En/Spm-like | Class II | autonomous | -5’ UTR | ENSAT1 | [93] |
| Pisum sativum | Ty3/gypsy-like ogre | Gypsy | Class I | autonomous | -3′ UTR | PisTR-A satDNA | [59] |
| Glycine max | Gmr9/GmOgre | Ty3-gypsy | Class I | autonomous | Between the 3’UTR and Repetitive LTR | Gmr9-associate minisatellite | [94] |
| Solanum bulbocastanum | Sore 1 | SORE-1 family | Class I | autonomous | LTR | Sobo satDNa | [6] |
| Solanum tuberosus | Ty3/gypsy-like | LTR-Retrotransposon-like | Class I | autonomous | LTR | St3-58; St3-238; St18; St3-294 | [95] |
| Zea mays | CRM1 and CRM4 | Centromeric Retrotransposons of Maize (CRM) | Class I | autonomous | UTR regions and LTR | CRM1TR e CRM4TR satDNA | [96] |
| Hordeum vulgare | BARE-1 | BARE-1 retrotransposon family | Class I | non-autonomous | LTR | SSR | [97] |
| Aegilops speltoides | Cereba | Ty3-gypsy | Class I | autonomous | ORF to gag | CAA microsatellite | [98] |
| Secale cereale | Crwydryn Tnr1 MITE | Crwydryn Tnr1/Stowaway family | Class I Class II | non-autonomous non-autonomous | ORF to gag - | E3900 satDNA D1100 satDNA | [75] |
| Lathyrus sativus | Ogre LTR-retrotransposons | LTR-Retrotransposon-like | Class I | autonomous | Close to the ORF to gag | nine satDNAs. | [99] |
| Chenopodium album aggregate | CACTA-Like Jozin | CACTA superfamily | Class II | non-autonomous | Tnp2 TPase | CficCl-61-40 satDNA | [4] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zattera, M.L.; Bruschi, D.P. Transposable Elements as a Source of Novel Repetitive DNA in the Eukaryote Genome. Cells 2022, 11, 3373. https://doi.org/10.3390/cells11213373
Zattera ML, Bruschi DP. Transposable Elements as a Source of Novel Repetitive DNA in the Eukaryote Genome. Cells. 2022; 11(21):3373. https://doi.org/10.3390/cells11213373
Chicago/Turabian StyleZattera, Michelle Louise, and Daniel Pacheco Bruschi. 2022. "Transposable Elements as a Source of Novel Repetitive DNA in the Eukaryote Genome" Cells 11, no. 21: 3373. https://doi.org/10.3390/cells11213373
APA StyleZattera, M. L., & Bruschi, D. P. (2022). Transposable Elements as a Source of Novel Repetitive DNA in the Eukaryote Genome. Cells, 11(21), 3373. https://doi.org/10.3390/cells11213373







