Multifaceted Roles of Retromer in EGFR Trafficking and Signaling Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Culture and Treatments
2.3. Cell Lysis, SDS-PAGE, and Immunoblotting
2.4. Cell Proliferation Assay
2.5. Determination of Cell Surface EGFR Levels Using Flow Cytometry
2.6. Immunofluorescence Microscopy and Image Analysis
2.7. EGFR Transcript Analysis by RNA-Seq
2.8. Statistical Analysis
3. Results
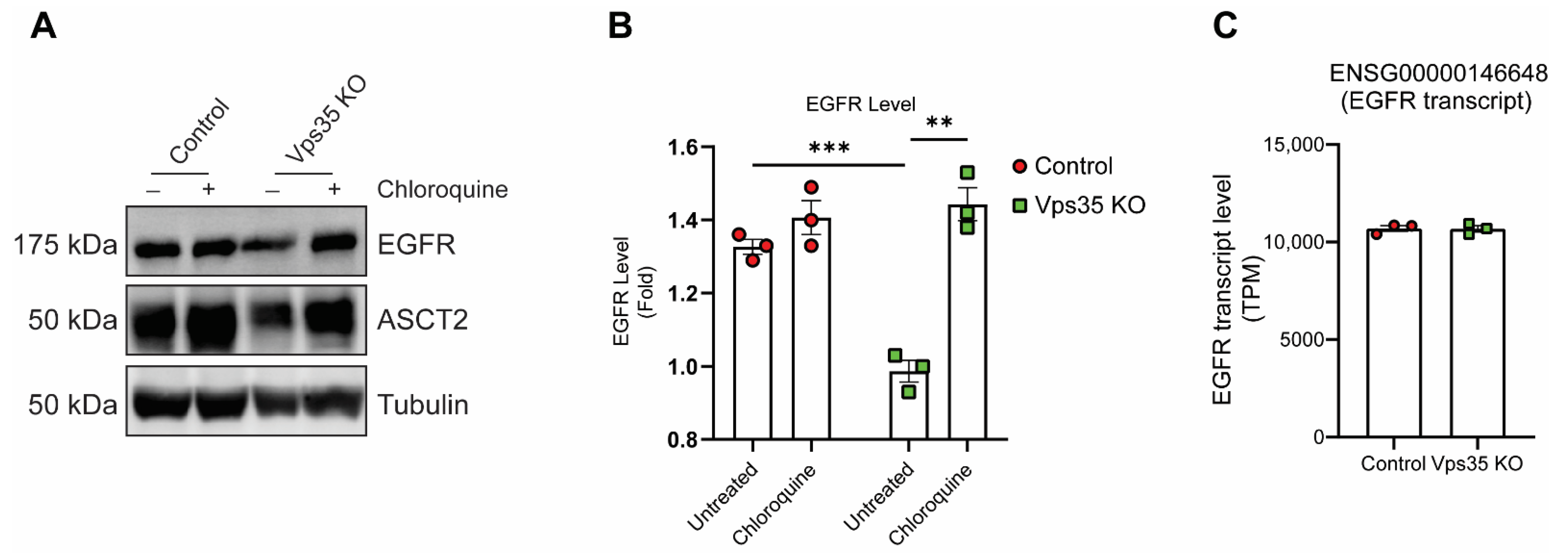
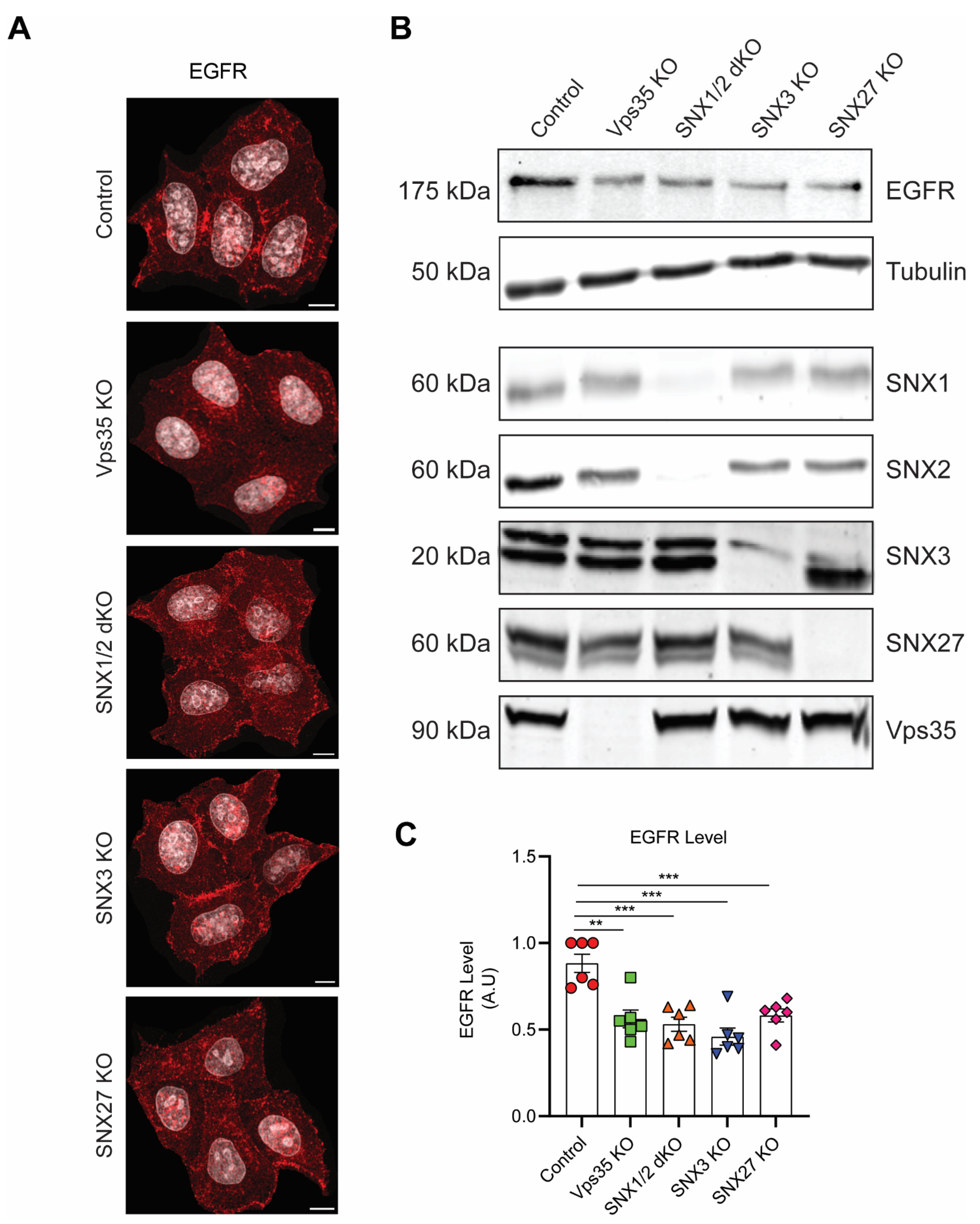
3.1. Retromer Depletion Reduces EGFR Levels but Decreases EGF-Induced EGFR Degradation
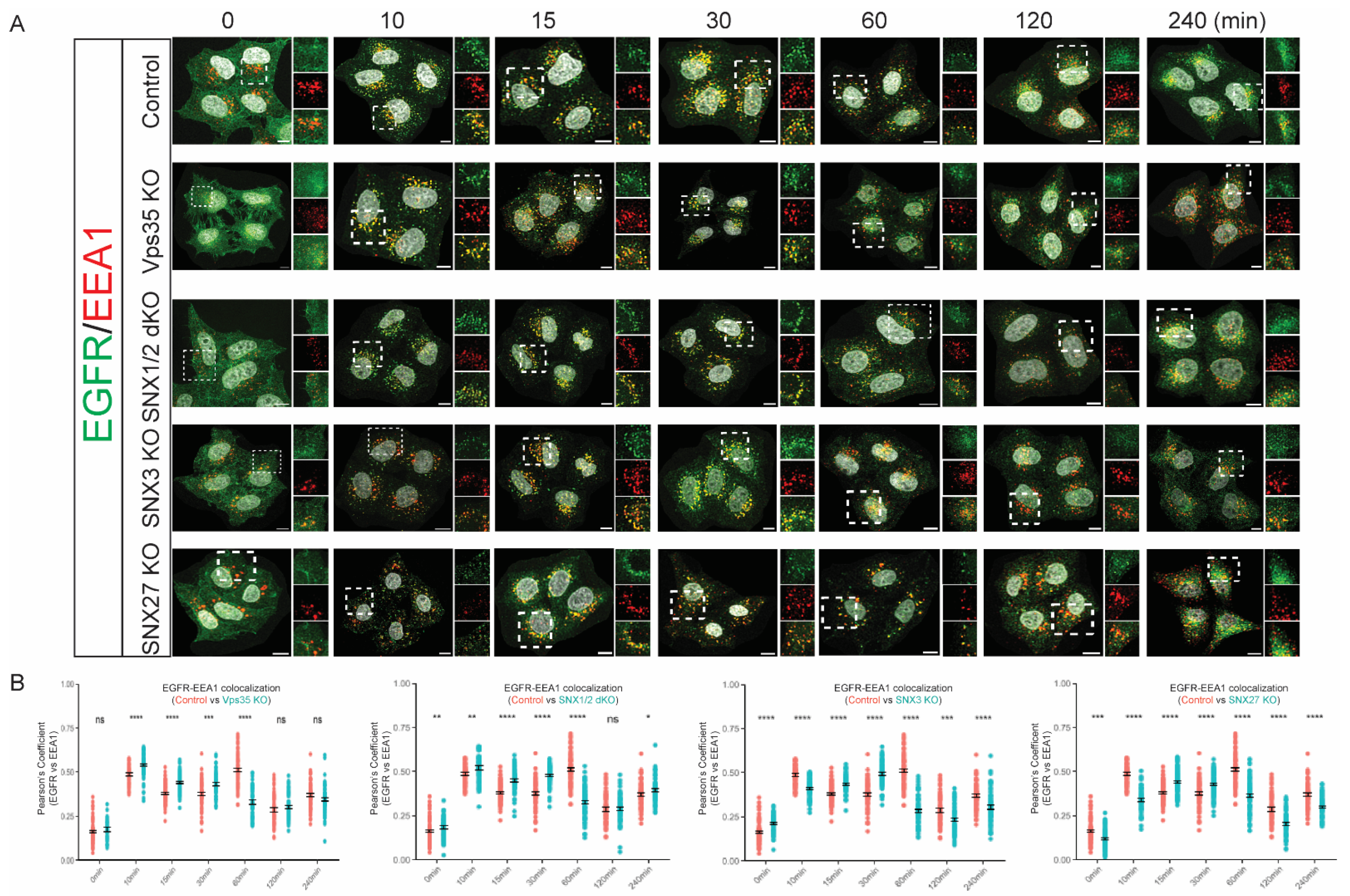
3.2. Retromer Depletion Alters the Kinetics of EGFR Endosomal Trafficking
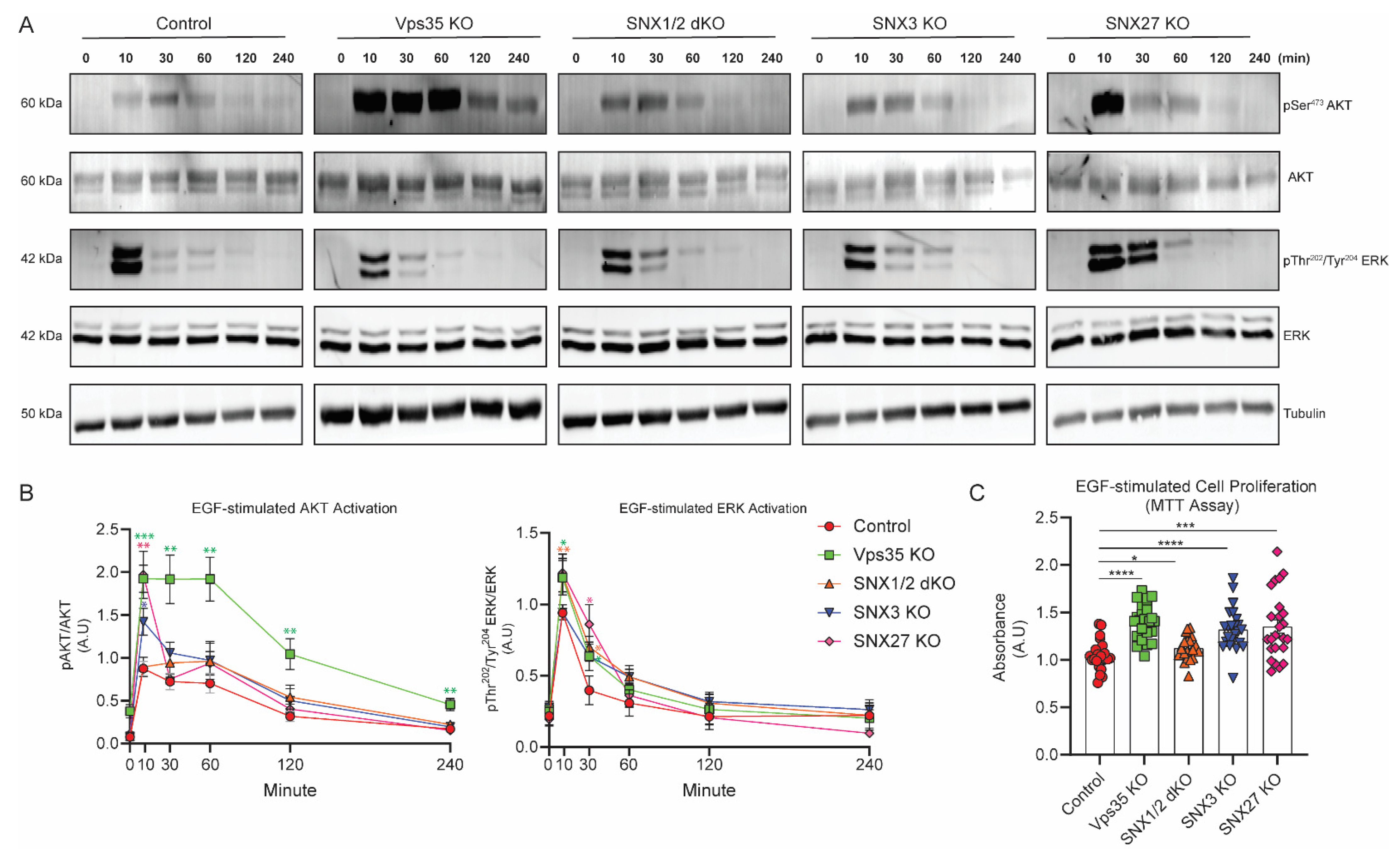
3.3. Retromer Depletion Increases EGF-Stimulated EGFR Signaling and Its Associated Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caldieri, G.; Malabarba, M.G.; Di Fiore, P.P.; Sigismund, S. EGFR Trafficking in Physiology and Cancer. Endocytosis Signal. 2018, 57, 235–272. [Google Scholar] [CrossRef]
- Bakker, J.; Spits, M.; Neefjes, J.; Berlin, I. The EGFR odyssey—From activation to destruction in space and time. J. Cell Sci. 2017, 130, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Al-Akhrass, H.; Naves, T.; Vincent, F.; Magnaudeix, A.; Durand, K.; Bertin, F.; Melloni, B.; Jauberteau, M.O.; Lalloue, F. Sortilin limits EGFR signaling by promoting its internalization in lung cancer. Nat. Commun. 2017, 8, 1182. [Google Scholar] [CrossRef] [PubMed]
- Teis, D.; Taub, N.; Kurzbauer, R.; Hilber, D.; de Araujo, M.E.; Erlacher, M.; Offterdinger, M.; Villunger, A.; Geley, S.; Bohn, G.; et al. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J. Cell Biol. 2006, 175, 861–868. [Google Scholar] [CrossRef]
- Wang, Y.; Pennock, S.; Chen, X.; Wang, Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol. Cell. Biol. 2002, 22, 7279–7290. [Google Scholar] [CrossRef]
- Taub, N.; Teis, D.; Ebner, H.L.; Hess, M.W.; Huber, L.A. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol. Biol. Cell 2007, 18, 4698–4710. [Google Scholar] [CrossRef]
- Murphy, J.E.; Padilla, B.E.; Hasdemir, B.; Cottrell, G.S.; Bunnett, N.W. Endosomes: A legitimate platform for the signaling train. Proc. Natl. Acad. Sci. USA 2009, 106, 17615–17622. [Google Scholar] [CrossRef]
- Chen, K.E.; Healy, M.D.; Collins, B.M. Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 2019, 20, 465–478. [Google Scholar] [CrossRef]
- Gallon, M.; Cullen, P.J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 2015, 43, 33–47. [Google Scholar] [CrossRef]
- Liu, J.J. Retromer-Mediated Protein Sorting and Vesicular Trafficking. J. Genet. Genom. 2016, 43, 165–177. [Google Scholar] [CrossRef]
- Lucas, M.; Gershlick, D.C.; Vidaurrazaga, A.; Rojas, A.L.; Bonifacino, J.S.; Hierro, A. Structural Mechanism for Cargo Recognition by the Retromer Complex. Cell 2016, 167, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.S.; Hung, C.S.; Liu, T.T.; Christiano, R.; Walther, T.C.; Burd, C.G. A mechanism for retromer endosomal coat complex assembly with cargo. Proc. Natl. Acad. Sci. USA 2014, 111, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Garcia-Santos, D.; Ishikawa, Y.; Seguin, A.; Li, L.; Fegan, K.H.; Hildick-Smith, G.J.; Shah, D.I.; Cooney, J.D.; Chen, W.; et al. Snx3 regulates recycling of the transferrin receptor and iron assimilation. Cell Metab. 2013, 17, 343–352. [Google Scholar] [CrossRef]
- Cui, Y.; Carosi, J.M.; Yang, Z.; Ariotti, N.; Kerr, M.C.; Parton, R.G.; Sargeant, T.J.; Teasdale, R.D. Retromer has a selective function in cargo sorting via endosome transport carriers. J. Cell Biol. 2019, 218, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.; Zhao, L.; Deng, W.; Sun, H.; Zhou, X.; Mao, L.; Hu, W.; Shen, X.; Sun, Q.; Billadeau, D.D.; et al. Mechanism of cargo recognition by retromer-linked SNX-BAR proteins. PLoS Biol. 2020, 18, e3000631. [Google Scholar] [CrossRef] [PubMed]
- Kvainickas, A.; Jimenez-Orgaz, A.; Nagele, H.; Hu, Z.; Dengjel, J.; Steinberg, F. Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J. Cell Biol. 2017, 216, 3677–3693. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, B.; Danson, C.M.; Heesom, K.J.; Cullen, P.J. Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR. J. Cell Biol. 2017, 216, 3695–3712. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, B.; Guo, Q.; Gimenez-Andres, M.; Chen, K.E.; Moody, E.R.R.; Evans, A.J.; Chandra, M.; Danson, C.M.; Williams, T.A.; Collins, B.M.; et al. SNX27-Retromer directly binds ESCPE-1 to transfer cargo proteins during endosomal recycling. PLoS Biol. 2022, 20, e3001601. [Google Scholar] [CrossRef]
- Yong, X.; Zhao, L.; Hu, W.; Sun, Q.; Ham, H.; Liu, Z.; Ren, J.; Zhang, Z.; Zhou, Y.; Yang, Q.; et al. SNX27-FERM-SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27. Proc. Natl. Acad. Sci. USA 2021, 118, e2105510118. [Google Scholar] [CrossRef]
- Clairfeuille, T.; Mas, C.; Chan, A.S.; Yang, Z.; Tello-Lafoz, M.; Chandra, M.; Widagdo, J.; Kerr, M.C.; Paul, B.; Merida, I.; et al. A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat. Struct. Mol. Biol. 2016, 23, 921–932. [Google Scholar] [CrossRef]
- Steinberg, F.; Gallon, M.; Winfield, M.; Thomas, E.C.; Bell, A.J.; Heesom, K.J.; Tavare, J.M.; Cullen, P.J. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 2013, 15, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Ogi, S.; Fujita, H.; Kashihara, M.; Yamamoto, C.; Sonoda, K.; Okamoto, I.; Nakagawa, K.; Ohdo, S.; Tanaka, Y.; Kuwano, M.; et al. Sorting nexin 2-mediated membrane trafficking of c-Met contributes to sensitivity of molecular-targeted drugs. Cancer Sci. 2013, 104, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Cicek, E.; Circir, A.; Oyken, M.; Akbulut Caliskan, O.; Dioken, D.N.; Guntekin Ergun, S.; Cetin-Atalay, R.; Sapmaz, A.; Ovaa, H.; Sahin, O.; et al. EGF-SNX3-EGFR axis drives tumor progression and metastasis in triple-negative breast cancers. Oncogene 2022, 41, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Kurten, R.C.; Cadena, D.L.; Gill, G.N. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 1996, 272, 1008–1010. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, T.; Jiang, Z.; Ge, C.; Chen, X.; Zhang, L.; Zhao, F.; Zhu, M.; Chen, T.; Cui, Y.; et al. Upregulation of SNX5 predicts poor prognosis and promotes hepatocellular carcinoma progression by modulating the EGFR-ERK1/2 signaling pathway. Oncogene 2020, 39, 2140–2155. [Google Scholar] [CrossRef]
- Nishimura, Y.; Takiguchi, S.; Yoshioka, K.; Nakabeppu, Y.; Itoh, K. Silencing of SNX1 by siRNA stimulates the ligand-induced endocytosis of EGFR and increases EGFR phosphorylation in gefitinib-resistant human lung cancer cell lines. Int. J. Oncol. 2012, 41, 1520–1530. [Google Scholar] [CrossRef]
- Gullapalli, A.; Garrett, T.A.; Paing, M.M.; Griffin, C.T.; Yang, Y.; Trejo, J. A role for sorting nexin 2 in epidermal growth factor receptor down-regulation: Evidence for distinct functions of sorting nexin 1 and 2 in protein trafficking. Mol. Biol. Cell 2004, 15, 2143–2155. [Google Scholar] [CrossRef]
- Sun, Y.; Hedman, A.C.; Tan, X.; Schill, N.J.; Anderson, R.A. Endosomal type Igamma PIP 5-kinase controls EGF receptor lysosomal sorting. Dev. Cell 2013, 25, 144–155. [Google Scholar] [CrossRef]
- Yang, Z.; Follett, J.; Kerr, M.C.; Clairfeuille, T.; Chandra, M.; Collins, B.M.; Teasdale, R.D. Sorting nexin 27 (SNX27) regulates the trafficking and activity of the glutamine transporter ASCT2. J. Biol. Chem. 2018, 293, 6802–6811. [Google Scholar] [CrossRef]
- Yang, Z.; Hong, L.K.; Follett, J.; Wabitsch, M.; Hamilton, N.A.; Collins, B.M.; Bugarcic, A.; Teasdale, R.D. Functional characterization of retromer in GLUT4 storage vesicle formation and adipocyte differentiation. FASEB J. 2016, 30, 1037–1050. [Google Scholar] [CrossRef]
- Batut, B.; van den Beek, M.; Doyle, M.A.; Soranzo, N. RNA-Seq Data Analysis in Galaxy. Methods Mol. Biol. 2021, 2284, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Curnock, R.; Calcagni, A.; Ballabio, A.; Cullen, P.J. TFEB controls retromer expression in response to nutrient availability. J. Cell Biol. 2019, 218, 3954–3966. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Duan, B.; Sun, S.; Cui, J.; Du, J.; Zhang, Y. Folliculin Interacts with Rab35 to Regulate EGF-Induced EGFR Degradation. Front. Pharmacol. 2017, 8, 688. [Google Scholar] [CrossRef] [PubMed]
- Schenck, A.; Goto-Silva, L.; Collinet, C.; Rhinn, M.; Giner, A.; Habermann, B.; Brand, M.; Zerial, M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 2008, 133, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Bugarcic, A.; Liu, H.; Norwood, S.J.; Skeldal, S.; Coulson, E.J.; Li, S.S.; Teasdale, R.D.; Collins, B.M. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E643–E652. [Google Scholar] [CrossRef] [PubMed]
- Alwan, H.A.; van Leeuwen, J.E. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J. Biol. Chem. 2007, 282, 1658–1669. [Google Scholar] [CrossRef]
- Mizuno, E.; Iura, T.; Mukai, A.; Yoshimori, T.; Kitamura, N.; Komada, M. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol. Biol. Cell 2005, 16, 5163–5174. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.M.; Jeong, D.S.; Kim, M.H. Transcriptional activation of EGFR by HOXB5 and its role in breast cancer cell invasion. Biochem. Biophys. Res. Commun. 2018, 503, 2924–2930. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Li, P.; Ma, F.; Liu, M.; Kong, S.; Xue, H. miR-200a-3p- and miR-181-5p-Mediated HOXB5 Upregulation Promotes HCC Progression by Transcriptional Activation of EGFR. Front. Oncol. 2022, 12, 822760. [Google Scholar] [CrossRef]
- Franovic, A.; Gunaratnam, L.; Smith, K.; Robert, I.; Patten, D.; Lee, S. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 13092–13097. [Google Scholar] [CrossRef]
- Kvainickas, A.; Nagele, H.; Qi, W.; Dokladal, L.; Jimenez-Orgaz, A.; Stehl, L.; Gangurde, D.; Zhao, Q.; Hu, Z.; Dengjel, J.; et al. Retromer and TBC1D5 maintain late endosomal RAB7 domains to enable amino acid-induced mTORC1 signaling. J. Cell Biol. 2019, 218, 3019–3038. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Simpson, J.; Fontana, R.; Kishi-Itakura, C.; Ktistakis, N.T.; Gammoh, N. Targeting of early endosomes by autophagy facilitates EGFR recycling and signalling. EMBO Rep. 2019, 20, e47734. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Kalaidzidis, I.V.; Kalaidzidis, Y.; Lambright, D.; Datta, S. Molecular insights into Rab7-mediated endosomal recruitment of core retromer: Deciphering the role of Vps26 and Vps35. Traffic 2015, 16, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Orgaz, A.; Kvainickas, A.; Nagele, H.; Denner, J.; Eimer, S.; Dengjel, J.; Steinberg, F. Control of RAB7 activity and localization through the retromer-TBC1D5 complex enables RAB7-dependent mitophagy. EMBO J. 2018, 37, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, A.; Wolfe, B.L.; Griffin, C.T.; Magnuson, T.; Trejo, J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: Evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol. Biol. Cell 2006, 17, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.S.; Raynor, M.C.; Wei, X.; Chen, H.Q.; Li, L. Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem. 2001, 276, 7069–7078. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.F.; Tang, F.L.; Xiong, L.; Xiong, S.; Jung, J.U.; Lee, D.H.; Li, X.S.; Feng, X.; Mei, L.; Xiong, W.C. Vps35 loss promotes hyperresorptive osteoclastogenesis and osteoporosis via sustained RANKL signaling. J. Cell Biol. 2013, 200, 821–837. [Google Scholar] [CrossRef]
- Jia, R.; Bonifacino, J.S. Lysosome Positioning Influences mTORC2 and AKT Signaling. Mol. Cell 2019, 75, 26–38. [Google Scholar] [CrossRef]
- Surve, S.; Watkins, S.C.; Sorkin, A. EGFR-RAS-MAPK signaling is confined to the plasma membrane and associated endorecycling protrusions. J. Cell Biol. 2021, 220, e202107103. [Google Scholar] [CrossRef]
- Pennock, S.; Wang, Z. Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling. Mol. Cell. Biol. 2003, 23, 5803–5815. [Google Scholar] [CrossRef]
- Andl, C.D.; Mizushima, T.; Oyama, K.; Bowser, M.; Nakagawa, H.; Rustgi, A.K. EGFR-induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 287, G1227–G1237. [Google Scholar] [CrossRef] [PubMed]
- Chmiest, D.; Sharma, N.; Zanin, N.; Viaris de Lesegno, C.; Shafaq-Zadah, M.; Sibut, V.; Dingli, F.; Hupe, P.; Wilmes, S.; Piehler, J.; et al. Spatiotemporal control of interferon-induced JAK/STAT signalling and gene transcription by the retromer complex. Nat. Commun. 2016, 7, 13476. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Feng, Z.; Li, Z.; Teasdale, R.D. Multifaceted Roles of Retromer in EGFR Trafficking and Signaling Activation. Cells 2022, 11, 3358. https://doi.org/10.3390/cells11213358
Yang Z, Feng Z, Li Z, Teasdale RD. Multifaceted Roles of Retromer in EGFR Trafficking and Signaling Activation. Cells. 2022; 11(21):3358. https://doi.org/10.3390/cells11213358
Chicago/Turabian StyleYang, Zhe, Zhengyang Feng, Zebin Li, and Rohan D. Teasdale. 2022. "Multifaceted Roles of Retromer in EGFR Trafficking and Signaling Activation" Cells 11, no. 21: 3358. https://doi.org/10.3390/cells11213358
APA StyleYang, Z., Feng, Z., Li, Z., & Teasdale, R. D. (2022). Multifaceted Roles of Retromer in EGFR Trafficking and Signaling Activation. Cells, 11(21), 3358. https://doi.org/10.3390/cells11213358






