Is IIIG9 a New Protein with Exclusive Ciliary Function? Analysis of Its Potential Role in Cancer and Other Pathologies
Abstract
1. Introduction
2. Materials and Methods
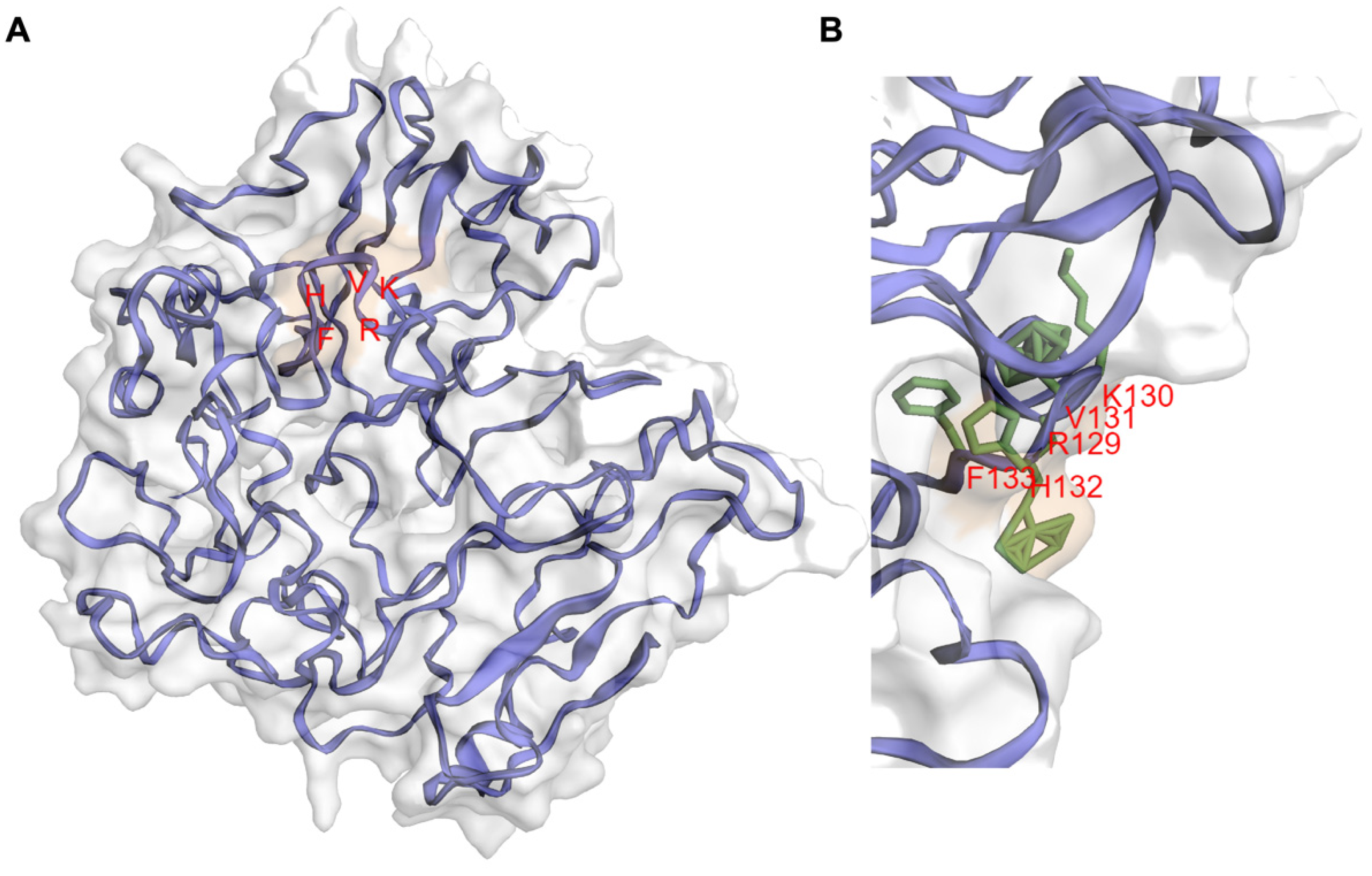
3. IIIG9 Is a Regulatory Subunit of Protein Phosphatase 1 (PP1)
4. Regulation of PP1 by IIIG9
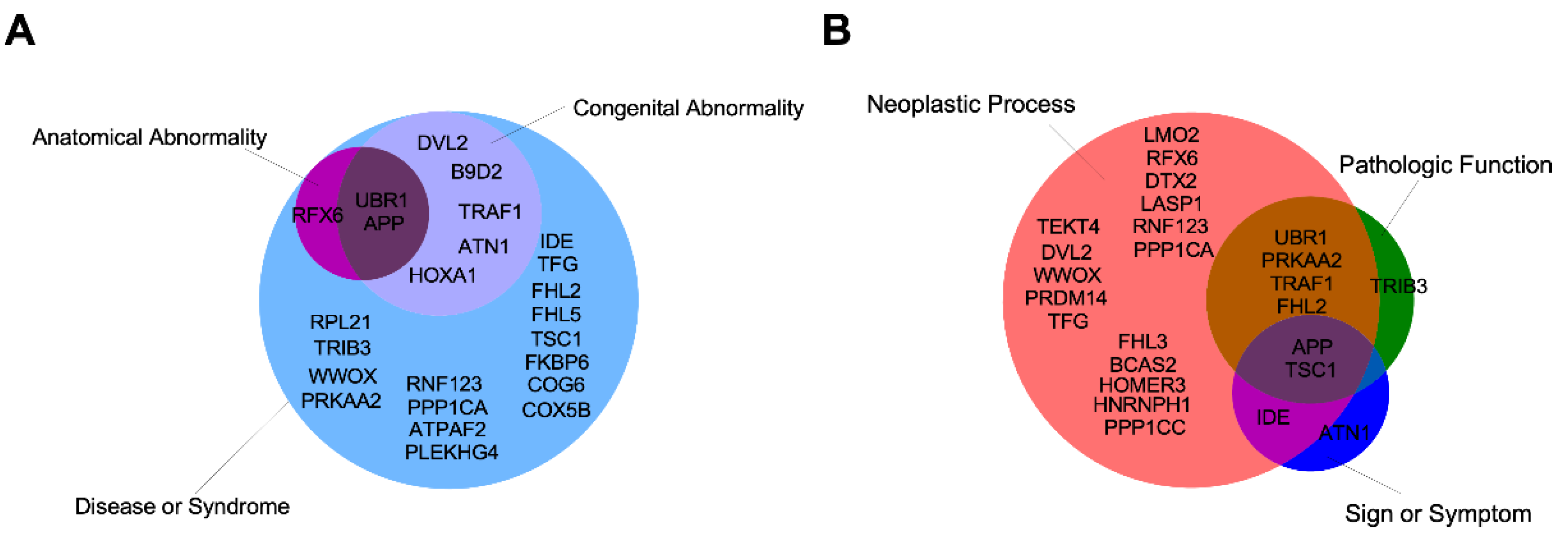
5. Other Possible IIIG9-Interacting Proteins
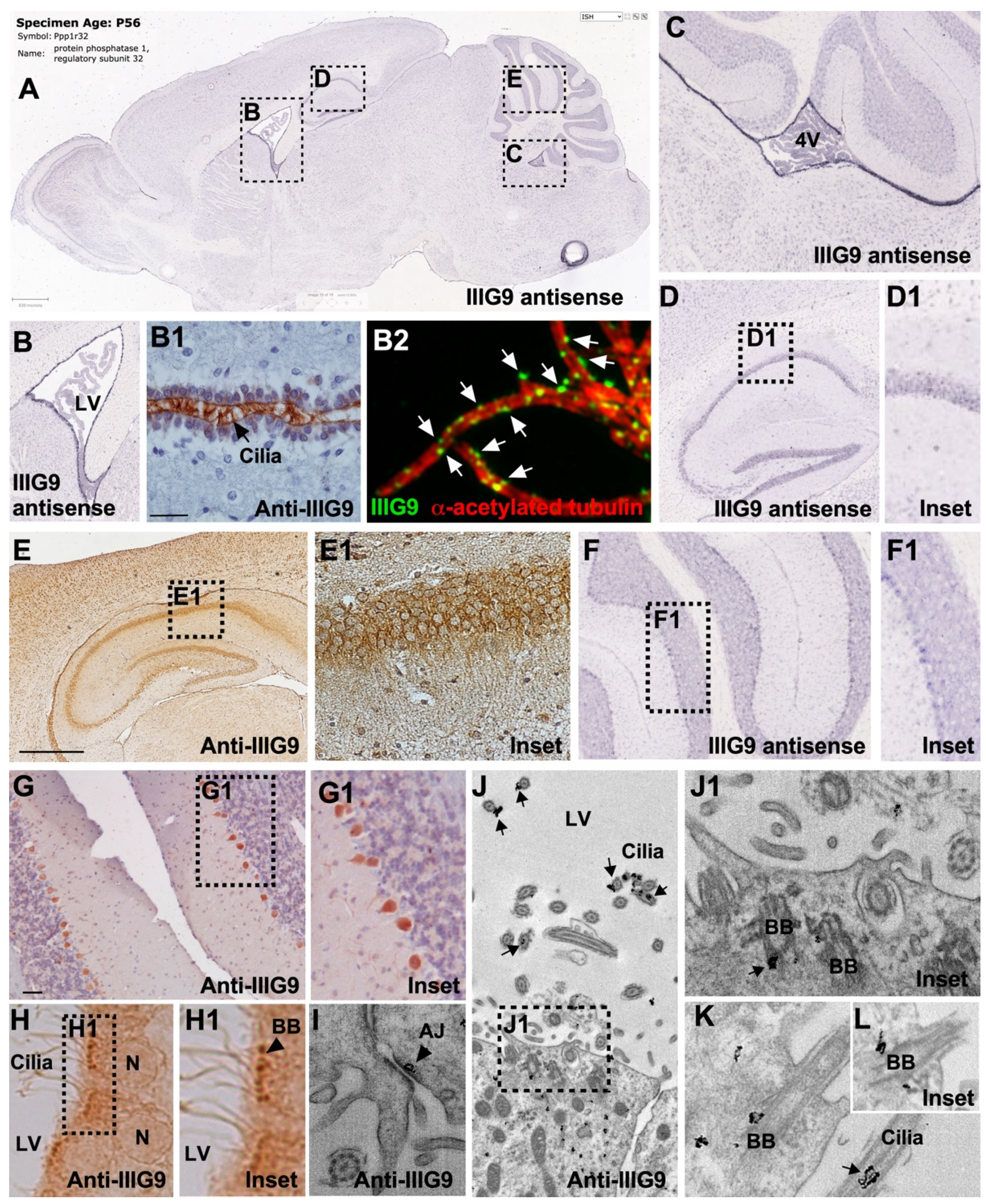
6. Expression of IIIG9 in Human Cell Lines and Adult Tissues
7. Expression of IIIG9 during CNS Development
8. Role of IIIG9 in Pathologies
8.1. Ciliopathies
8.2. Infertility
8.3. Autism Spectrum Disorder (ASD)
8.4. Drug Use Disorders
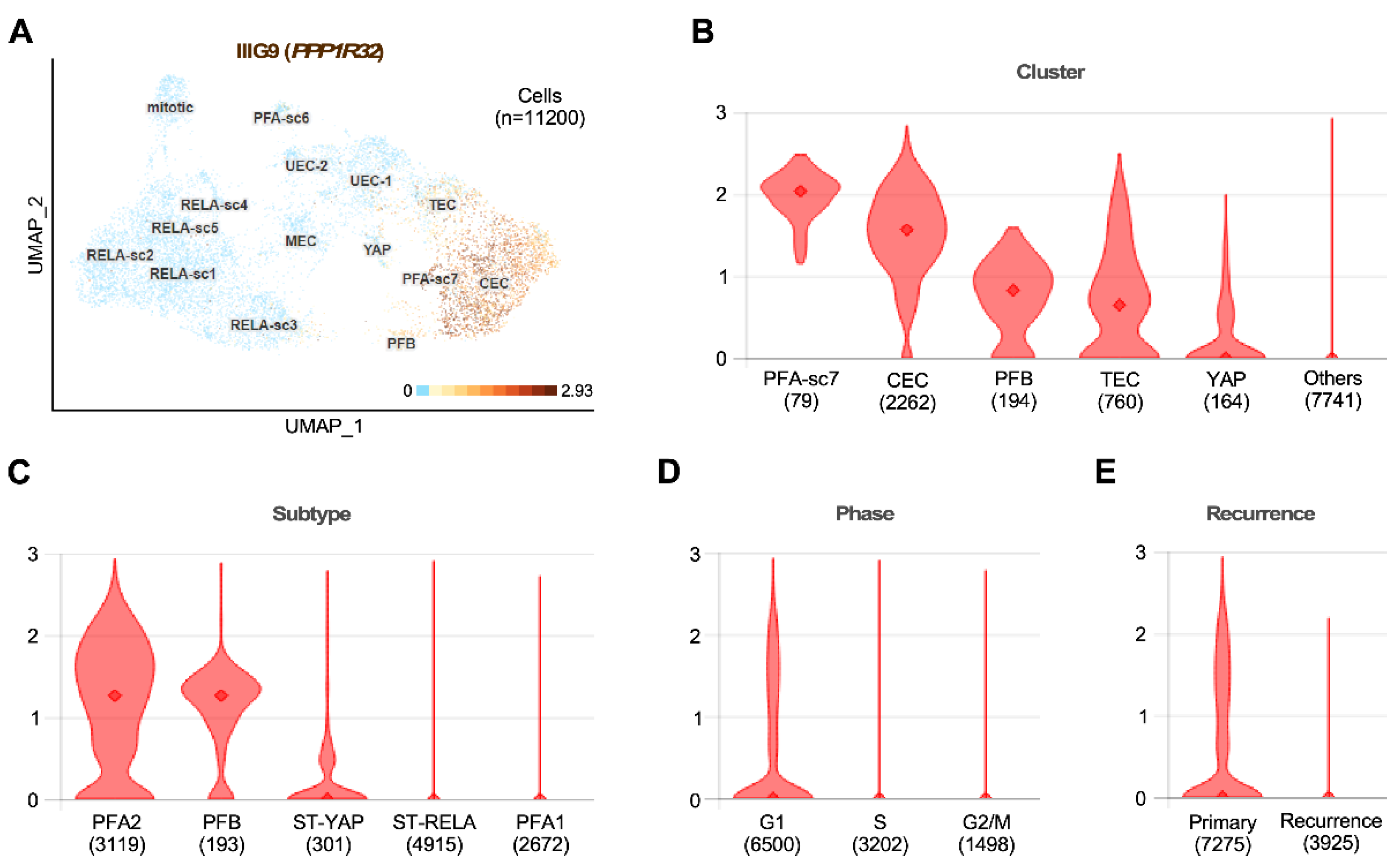
8.5. Tumors
9. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danielson, P.E.; Sautkulis, L.N.; Foye, P.E.; Hedlund, P.B.; Carson, M.J. A novel mRNA expressed along brain ventricles. Brain Res. Gene Expr. Patterns 2002, 1, 83–88. [Google Scholar] [CrossRef]
- Nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information [1988]. Accession No. NM_145017.3, NM_001170753.2, Homo Sapiens Protein Phosphatase 1 Regulatory Subunit 32 (PPP1R32), mRNA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/NM_145017.3,NM_001170753.2 (accessed on 10 June 2022).
- Nucleotide [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information [1988]. Accession No. NM_133689.2, NM_001368184.1, XM_006527302.5, XM_017318281.1, XM_017318282.1, XM_036161672.1, Mus Musculus Protein Phosphatase 1 Regulatory Subunit 32 (Ppp1r32), mRNA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/NM_133689.2,NM_001368184.1,XM_006527302.5,XM_017318281.1,XM_017318282.1,XM_036161672.1 (accessed on 10 June 2022).
- Ivliev, A.E.; t Hoen, P.A.; van Roon-Mom, W.M.; Peters, D.J.; Sergeeva, M.G. Exploring the transcriptome of ciliated cells using in silico dissection of human tissues. PLoS ONE 2012, 7, e35618. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Cifuentes, M.; Baeza, V.; Arrabal, P.M.; Visser, R.; Grondona, J.M.; Saldivia, N.; Martinez, F.; Nualart, F.; Salazar, K. Expression of a Novel Ciliary Protein, IIIG9, During the Differentiation and Maturation of Ependymal Cells. Mol. Neurobiol. 2018, 55, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- C-I-TASSER (Contact-guided Iterative Threading ASSEmbly Refinement) Server. Available online: https://zhanggroup.org/C-I-TASSER/ (accessed on 10 June 2022).
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef] [PubMed]
- Bjelkmar, P.; Larsson, P.; Cuendet, M.A.; Hess, B.; Lindahl, E. Implementation of the CHARMM Force Field in GROMACS: Analysis of Protein Stability Effects from Correction Maps, Virtual Interaction Sites, and Water Models. J. Chem. Theory Comput. 2010, 6, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ali, S.M. Molecular dynamics simulation for the test of calibrated OPLS-AA force field for binary liquid mixture of tri-iso-amyl phosphate and n-dodecane. J. Chem. Phys. 2018, 148, 74502. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- ProSA-Web (Protein Structure Analysis). Available online: https://prosa.services.came.sbg.ac.at/prosa.php (accessed on 10 June 2022).
- AlphaFold Protein Structure Database, Developed by DeepMind and EMBL-EBI. Available online: https://alphafold.ebi.ac.uk/ (accessed on 10 June 2022).
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Hendrickx, A.; Beullens, M.; Ceulemans, H.; Den Abt, T.; Van Eynde, A.; Nicolaescu, E.; Lesage, B.; Bollen, M. Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem. Biol. 2009, 16, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Heroes, E.; Lesage, B.; Gornemann, J.; Beullens, M.; Van Meervelt, L.; Bollen, M. The PP1 binding code: A molecular-lego strategy that governs specificity. FEBS J. 2013, 280, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.R.; Islam, S.A.; Sternberg, M.J.E. EzMol: A Web Server Wizard for the Rapid Visualization and Image Production of Protein and Nucleic Acid Structures. J. Mol. Biol. 2018, 430, 2244–2248. [Google Scholar] [CrossRef]
- EzMol Interface © Structural Bioinformatics Group, Imperial College London 2019. Available online: http://www.sbg.bio.ic.ac.uk/~ezmol/ (accessed on 10 June 2022).
- Casamayor, A.; Arino, J. Controlling Ser/Thr protein phosphatase PP1 activity and function through interaction with regulatory subunits. Adv. Protein Chem. Struct. Biol. 2020, 122, 231–288. [Google Scholar] [CrossRef]
- Verbinnen, I.; Ferreira, M.; Bollen, M. Biogenesis and activity regulation of protein phosphatase 1. Biochem. Soc. Trans. 2017, 45, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.; da Cruz, E.S.O.A.; Fardilha, M. Protein phosphatase 1 and its complexes in carcinogenesis. Curr. Cancer Drug Targets 2014, 14, 2–29. [Google Scholar] [CrossRef]
- Felgueiras, J.; Jeronimo, C.; Fardilha, M. Protein phosphatase 1 in tumorigenesis: Is it worth a closer look? Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188433. [Google Scholar] [CrossRef]
- Ferreira, M.; Beullens, M.; Bollen, M.; Van Eynde, A. Functions and therapeutic potential of protein phosphatase 1: Insights from mouse genetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 16–30. [Google Scholar] [CrossRef]
- Ceulemans, H.; Bollen, M. A tighter RVxF motif makes a finer Sift. Chem. Biol. 2006, 13, 6–8. [Google Scholar] [CrossRef][Green Version]
- Egloff, M.P.; Johnson, D.F.; Moorhead, G.; Cohen, P.T.; Cohen, P.; Barford, D. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997, 16, 1876–1887. [Google Scholar] [CrossRef]
- Fardilha, M.; Esteves, S.L.; Korrodi-Gregorio, L.; Vintem, A.P.; Domingues, S.C.; Rebelo, S.; Morrice, N.; Cohen, P.T.; da Cruz e Silva, O.A.; da Cruz e Silva, E.F. Identification of the human testis protein phosphatase 1 interactome. Biochem. Pharm. 2011, 82, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Vintém, A.P.B.; Fardilha, M.; Silva, O.d.C.e.; Silva, E.F.d. Subcellular Localization of a Novel Alternative Splicing of IIIG9 and Colocalization with PPP1gamma Isoforms. Microsc. Microanal. 2008, 14, 141–143. [Google Scholar] [CrossRef][Green Version]
- Chatterjee, J.; Beullens, M.; Sukackaite, R.; Qian, J.; Lesage, B.; Hart, D.J.; Bollen, M.; Kohn, M. Development of a peptide that selectively activates protein phosphatase-1 in living cells. Angew. Chem. Int. Ed. Engl. 2012, 51, 10054–10059. [Google Scholar] [CrossRef]
- Luck, K.; Kim, D.K.; Lambourne, L.; Spirohn, K.; Begg, B.E.; Bian, W.; Brignall, R.; Cafarelli, T.; Campos-Laborie, F.J.; Charloteaux, B.; et al. A reference map of the human binary protein interactome. Nature 2020, 580, 402–408. [Google Scholar] [CrossRef]
- HuRi, The Human Reference Protein Interactome Mapping Project. Available online: http://www.interactome-atlas.org/ (accessed on 10 June 2022).
- Pathway Commons. Interactions between PPP1R32 and 25 Other Genes. Available online: https://apps.pathwaycommons.org/interactions?source=PPP1R32 (accessed on 10 June 2022).
- Rodchenkov, I.; Babur, O.; Luna, A.; Aksoy, B.A.; Wong, J.V.; Fong, D.; Franz, M.; Siper, M.C.; Cheung, M.; Wrana, M.; et al. Pathway Commons 2019 Update: Integration, analysis and exploration of pathway data. Nucleic Acids Res. 2020, 48, D489–D497. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein. Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- BioGRID (Database of Protein, Genetic and Chemical Interactions). Homo Sapiens PPP1R32. Available online: https://thebiogrid.org/128618/summary/homo-sapiens/ppp1r32.html (accessed on 10 June 2022).
- Olah, J.; Vincze, O.; Virok, D.; Simon, D.; Bozso, Z.; Tokesi, N.; Horvath, I.; Hlavanda, E.; Kovacs, J.; Magyar, A.; et al. Interactions of pathological hallmark proteins: Tubulin polymerization promoting protein/p25, beta-amyloid, and alpha-synuclein. J. Biol. Chem. 2011, 286, 34088–34100. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Cho, K.S.; Kim, B.Y.; Lee, K.H. Cullin 1 (CUL1) Promotes Primary Ciliogenesis through the Induction of Ubiquitin-Proteasome-Dependent Dvl2 Degradation. Int. J. Mol. Sci. 2021, 22, 7572. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the human interactome defines protein communities and disease networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Navarrete-Perea, J.; Cannon, J.R.; Baltier, K.; Gebreab, F.; Gygi, M.P.; Thornock, A.; Zarraga, G.; Tam, S.; et al. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell 2021, 184, 3022–3040.e28. [Google Scholar] [CrossRef]
- Khanna, R.; Krishnamoorthy, V.; Parnaik, V.K. E3 ubiquitin ligase RNF123 targets lamin B1 and lamin-binding proteins. FEBS J. 2018, 285, 2243–2262. [Google Scholar] [CrossRef] [PubMed]
- Fasci, D.; van Ingen, H.; Scheltema, R.A.; Heck, A.J.R. Histone Interaction Landscapes Visualized by Crosslinking Mass Spectrometry in Intact Cell Nuclei. Mol. Cell Proteom. 2018, 17, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Belinky, F.; Nativ, N.; Stelzer, G.; Zimmerman, S.; Iny Stein, T.; Safran, M.; Lancet, D. PathCards: Multi-source consolidation of human biological pathways. Database 2015, 2015, bav006. [Google Scholar] [CrossRef]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Stein, T.I.; Dahary, D.; Fishilevich, S.; Lancet, D. The GeneCards Suite. In Practical Guide to Life Science Databases; Abugessaisa, I., Kasukawa, T., Eds.; Springer: Singapore, 2021; pp. 27–56. [Google Scholar]
- PathCards, Pathway Unification Database. Available online: https://pathcards.genecards.org/ (accessed on 10 June 2022).
- Westberg, C.; Yang, J.P.; Tang, H.; Reddy, T.R.; Wong-Staal, F. A novel shuttle protein binds to RNA helicase A and activates the retroviral constitutive transport element. J. Biol. Chem. 2000, 275, 21396–21401. [Google Scholar] [CrossRef]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef]
- Sano, E.; Shono, S.; Tashiro, K.; Konishi, H.; Yamauchi, E.; Taniguchi, H. Novel tyrosine phosphorylated and cardiolipin-binding protein CLPABP functions as mitochondrial RNA granule. Biochim. Biophys. Acta 2008, 1783, 1036–1047. [Google Scholar] [CrossRef]
- Trott, J.; Alpagu, Y.; Tan, E.K.; Shboul, M.; Dawood, Y.; Elsy, M.; Wollmann, H.; Tano, V.; Bonnard, C.; Eng, S.; et al. Mitchell-Riley syndrome iPSCs exhibit reduced pancreatic endoderm differentiation due to a mutation in RFX6. Development 2020, 147, dev194878. [Google Scholar] [CrossRef]
- Swoboda, P.; Adler, H.T.; Thomas, J.H. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol. Cell 2000, 5, 411–421. [Google Scholar] [CrossRef]
- Soyer, J.; Flasse, L.; Raffelsberger, W.; Beucher, A.; Orvain, C.; Peers, B.; Ravassard, P.; Vermot, J.; Voz, M.L.; Mellitzer, G.; et al. Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development 2010, 137, 203–212. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- El Zein, L.; Ait-Lounis, A.; Morle, L.; Thomas, J.; Chhin, B.; Spassky, N.; Reith, W.; Durand, B. RFX3 governs growth and beating efficiency of motile cilia in mouse and controls the expression of genes involved in human ciliopathies. J. Cell Sci. 2009, 122, 3180–3189. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.S.; Massey, T.H.; Wardle, M.; Peall, K.J. Dentatorubral-pallidoluysian Atrophy: An Update. Tremor Other Hyperkinet. Mov. 2018, 8, 577. [Google Scholar] [CrossRef]
- GeneCards (The Human Gene Database). Homo Sapiens ATN1. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=ATN1&keywords=ATN1 (accessed on 10 June 2022).
- Wolting, C.D.; Griffiths, E.K.; Sarao, R.; Prevost, B.C.; Wybenga-Groot, L.E.; McGlade, C.J. Biochemical and computational analysis of LNX1 interacting proteins. PLoS ONE 2011, 6, e26248. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Homo Sapiens PPP1R32. Available online: https://www.proteinatlas.org/ENSG00000162148-PPP1R32 (accessed on 10 June 2022).
- MacDonald, A.; Lu, B.; Caron, M.; Caporicci-Dinucci, N.; Hatrock, D.; Petrecca, K.; Bourque, G.; Stratton, J.A. Single Cell Transcriptomics of Ependymal Cells Across Age, Region and Species Reveals Cilia-Related and Metal Ion Regulatory Roles as Major Conserved Ependymal Cell Functions. Front. Cell Neurosci. 2021, 15, 703951. [Google Scholar] [CrossRef] [PubMed]
- Patir, A.; Fraser, A.M.; Barnett, M.W.; McTeir, L.; Rainger, J.; Davey, M.G.; Freeman, T.C. The transcriptional signature associated with human motile cilia. Sci. Rep. 2020, 10, 10814. [Google Scholar] [CrossRef]
- Wu, C.; Jin, X.; Tsueng, G.; Afrasiabi, C.; Su, A.I. BioGPS: Building your own mash-up of gene annotations and expression profiles. Nucleic Acids Res. 2016, 44, D313–D316. [Google Scholar] [CrossRef]
- BioGPS, a Free Extensible and Customizable Gene Annotation Portal. Gene Report: PPP1R32. Available online: http://biogps.org/#goto=genereport&id=220004 (accessed on 10 June 2022).
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. Gene: PPP1R32. Available online: https://maayanlab.cloud/Enrichr/#find!gene=PPP1R32 (accessed on 10 June 2022).
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef]
- Elam, C.A.; Sale, W.S.; Wirschell, M. The regulation of dynein-driven microtubule sliding in Chlamydomonas flagella by axonemal kinases and phosphatases. Methods Cell Biol. 2009, 92, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Wirschell, M.; Yamamoto, R.; Alford, L.; Gokhale, A.; Gaillard, A.; Sale, W.S. Regulation of ciliary motility: Conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme. Arch. Biochem. Biophys. 2011, 510, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Baeza, V.; Cifuentes, M.; Martinez, F.; Ramirez, E.; Nualart, F.; Ferrada, L.; Oviedo, M.J.; De Lima, I.; Troncoso, N.; Saldivia, N.; et al. IIIG9 inhibition in adult ependymal cells changes adherens junctions structure and induces cellular detachment. Sci. Rep. 2021, 11, 18537. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, M.; Bell, R.; Supekova, L.; Wang, Y.; Orth, A.P.; Batalov, S.; Miraglia, L.; Huesken, D.; Lange, J.; Martin, C.; et al. Genome-wide functional analysis of human cell-cycle regulators. Proc. Natl. Acad. Sci. USA 2006, 103, 14819–14824. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Neilson, L.J.; Zhong, H.; Murray, P.S.; Zanivan, S.; Zaidel-Bar, R. E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Sci. Signal. 2014, 7, rs7. [Google Scholar] [CrossRef]
- Lechtreck, K.F.; Delmotte, P.; Robinson, M.L.; Sanderson, M.J.; Witman, G.B. Mutations in Hydin impair ciliary motility in mice. J. Cell Biol. 2008, 180, 633–643. [Google Scholar] [CrossRef]
- van de Leemput, J.; Boles, N.C.; Kiehl, T.R.; Corneo, B.; Lederman, P.; Menon, V.; Lee, C.; Martinez, R.A.; Levi, B.P.; Thompson, C.L.; et al. CORTECON: A temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron 2014, 83, 51–68. [Google Scholar] [CrossRef]
- CORTECON. Repository of Gene Expression in the Developing Cortex. NSCI, Neural Stem Cell Institute. Gene Expression: PPP1R32. Available online: https://cortecon.neuralsci.org/index.php?cort_mode=genedisplay&geneid=220004 (accessed on 10 June 2022).
- Kawase-Koga, Y.; Low, R.; Otaegi, G.; Pollock, A.; Deng, H.; Eisenhaber, F.; Maurer-Stroh, S.; Sun, T. RNAase-III enzyme Dicer maintains signaling pathways for differentiation and survival in mouse cortical neural stem cells. J. Cell Sci. 2010, 123, 586–594. [Google Scholar] [CrossRef]
- Stubbs, J.L.; Vladar, E.K.; Axelrod, J.D.; Kintner, C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat. Cell Biol. 2012, 14, 140–147. [Google Scholar] [CrossRef]
- Terre, B.; Piergiovanni, G.; Segura-Bayona, S.; Gil-Gomez, G.; Youssef, S.A.; Attolini, C.S.; Wilsch-Brauninger, M.; Jung, C.; Rojas, A.M.; Marjanovic, M.; et al. GEMC1 is a critical regulator of multiciliated cell differentiation. EMBO J. 2016, 35, 942–960. [Google Scholar] [CrossRef]
- Choksi, S.P.; Lauter, G.; Swoboda, P.; Roy, S. Switching on cilia: Transcriptional networks regulating ciliogenesis. Development 2014, 141, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Choksi, S.P.; Babu, D.; Lau, D.; Yu, X.; Roy, S. Systematic discovery of novel ciliary genes through functional genomics in the zebrafish. Development 2014, 141, 3410–3419. [Google Scholar] [CrossRef] [PubMed]
- Meunier, A.; Azimzadeh, J. Multiciliated Cells in Animals. Cold Spring Harb. Perspect. Biol. 2016, 8, a028233. [Google Scholar] [CrossRef]
- Yevshin, I.; Sharipov, R.; Valeev, T.; Kel, A.; Kolpakov, F. GTRD: A database of transcription factor binding sites identified by ChIP-seq experiments. Nucleic Acids Res. 2017, 45, D61–D67. [Google Scholar] [CrossRef] [PubMed]
- Kolmykov, S.; Yevshin, I.; Kulyashov, M.; Sharipov, R.; Kondrakhin, Y.; Makeev, V.J.; Kulakovskiy, I.V.; Kel, A.; Kolpakov, F. GTRD: An integrated view of transcription regulation. Nucleic Acids Res. 2021, 49, D104–D111. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D. Object-oriented transcription factors database (ooTFD). Nucleic Acids Res. 2000, 28, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Farre, D.; Roset, R.; Huerta, M.; Adsuara, J.E.; Rosello, L.; Alba, M.M.; Messeguer, X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003, 31, 3651–3653. [Google Scholar] [CrossRef]
- Sigg, M.A.; Menchen, T.; Lee, C.; Johnson, J.; Jungnickel, M.K.; Choksi, S.P.; Garcia, G., 3rd; Busengdal, H.; Dougherty, G.W.; Pennekamp, P.; et al. Evolutionary Proteomics Uncovers Ancient Associations of Cilia with Signaling Pathways. Dev. Cell 2017, 43, 744–762. [Google Scholar] [CrossRef]
- Lachmann, A.; Torre, D.; Keenan, A.B.; Jagodnik, K.M.; Lee, H.J.; Wang, L.; Silverstein, M.C.; Ma’ayan, A. Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 2018, 9, 1366. [Google Scholar] [CrossRef]
- ARCHS4: Massive Mining of Publicly Available RNA-seq Data from Human and Mouse. Gene PPP1R32. Available online: https://maayanlab.cloud/archs4/gene/PPP1R32 (accessed on 10 June 2022).
- Sharma, S.; Tantisira, K.; Carey, V.; Murphy, A.J.; Lasky-Su, J.; Celedon, J.C.; Lazarus, R.; Klanderman, B.; Rogers, A.; Soto-Quiros, M.; et al. A role for Wnt signaling genes in the pathogenesis of impaired lung function in asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 328–336. [Google Scholar] [CrossRef]
- Pisani, C.; Voisin, S.; Arafah, K.; Durand, P.; Perrard, M.-H.; Guichaoua, M.-R.; Bulet, P.; Prat, O. Ex vivo assessment of testicular toxicity induced by carbendazim and iprodione, alone or in a mixture. ALTEX Altern. Anim. Exp. 2016, 33, 393–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varmuza, S.; Jurisicova, A.; Okano, K.; Hudson, J.; Boekelheide, K.; Shipp, E.B. Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1cgamma gene. Dev. Biol. 1999, 205, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano-Wells, L.; Varmuza, S. Protein phosphatase 1cgamma is required in germ cells in murine testis. Mol. Reprod. Dev. 2003, 65, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Kline, D.; Lu, J.; Orth, J.; Pilder, S.; Vijayaraghavan, S. Analysis of Ppp1cc-null mice suggests a role for PP1gamma2 in sperm morphogenesis. Biol. Reprod. 2007, 76, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Cheng, L.; Puri, P.; Soler, D.; Vijayaraghavan, S. Protein phosphatase PP1 gamma 2 in sperm morphogenesis and epididymal initiation of sperm motility. Asian J. 2007, 9, 445–452. [Google Scholar] [CrossRef]
- Mak, A.S.L.; Chiu, A.T.G.; Leung, G.K.C.; Mak, C.C.Y.; Chu, Y.W.Y.; Mok, G.T.K.; Tang, W.F.; Chan, K.Y.K.; Tang, M.H.Y.; Lau Yim, E.T.; et al. Use of clinical chromosomal microarray in Chinese patients with autism spectrum disorder-implications of a copy number variation involving DPP10. Mol. Autism 2017, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, J.; Jiang, H.; Wang, X.; Wu, L.; Wu, W.; Wang, Q. Inferring Alcoholism SNPs and Regulatory Chemical Compounds Based on Ensemble Bayesian Network. Comb. Chem. High. Throughput Screen 2017, 20, 107–115. [Google Scholar] [CrossRef]
- Nestler, E.J. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005, 8, 1445–1449. [Google Scholar] [CrossRef]
- Vallender, E.J.; Goswami, D.B.; Shinday, N.M.; Westmoreland, S.V.; Yao, W.D.; Rowlett, J.K. Transcriptomic profiling of the ventral tegmental area and nucleus accumbens in rhesus macaques following long-term cocaine self-administration. Drug Alcohol Depend. 2017, 175, 9–23. [Google Scholar] [CrossRef]
- Schmidt, H.D.; McGinty, J.F.; West, A.E.; Sadri-Vakili, G. Epigenetics and psychostimulant addiction. Cold Spring Harb. Perspect. Med. 2013, 3, a012047. [Google Scholar] [CrossRef]
- Yue, W.; Wang, T.; Zachariah, E.; Lin, Y.; Yang, C.S.; Xu, Q.; DiPaola, R.S.; Tan, X.L. Transcriptomic analysis of pancreatic cancer cells in response to metformin and aspirin: An implication of synergy. Sci. Rep. 2015, 5, 13390. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.H.; Samuel, N.; Juraschka, K.; Saleh, M.H.; Taylor, M.D.; Fehlings, M.G. The biology of ependymomas and emerging novel therapies. Nat. Rev. Cancer 2022, 22, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Shatara, M.; Schieffer, K.M.; Klawinski, D.; Thomas, D.L.; Pierson, C.R.; Sribnick, E.A.; Jones, J.; Rodriguez, D.P.; Deeg, C.; Hamelberg, E.; et al. Clinically aggressive pediatric spinal ependymoma with novel MYC amplification demonstrates molecular and histopathologic similarity to newly described MYCN-amplified spinal ependymomas. Acta Neuropathol. Commun. 2021, 9, 192. [Google Scholar] [CrossRef]
- Ghasemi, D.R.; Sill, M.; Okonechnikov, K.; Korshunov, A.; Yip, S.; Schutz, P.W.; Scheie, D.; Kruse, A.; Harter, P.N.; Kastelan, M.; et al. MYCN amplification drives an aggressive form of spinal ependymoma. Acta Neuropathol. 2019, 138, 1075–1089. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Malgulwar, P.B.; Nambirajan, A.; Pathak, P.; Rajeshwari, M.; Suri, V.; Sarkar, C.; Singh, M.; Sharma, M.C. Epithelial-to-mesenchymal transition-related transcription factors are up-regulated in ependymomas and correlate with a poor prognosis. Hum. Pathol. 2018, 82, 149–157. [Google Scholar] [CrossRef]
- Gillen, A.E.; Riemondy, K.A.; Amani, V.; Griesinger, A.M.; Gilani, A.; Venkataraman, S.; Madhavan, K.; Prince, E.; Sanford, B.; Hankinson, T.C.; et al. Single-Cell RNA Sequencing of Childhood Ependymoma Reveals Neoplastic Cell Subpopulations That Impact Molecular Classification and Etiology. Cell Rep. 2020, 32, 108023. [Google Scholar] [CrossRef]
- Falk, A.; Koch, P.; Kesavan, J.; Takashima, Y.; Ladewig, J.; Alexander, M.; Wiskow, O.; Tailor, J.; Trotter, M.; Pollard, S.; et al. Capture of neuroepithelial-like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PLoS ONE 2012, 7, e29597. [Google Scholar] [CrossRef]
- Pediatric Neuro-Oncology Cell Atlas. Available online: https://www.pneuroonccellatlas.org/ (accessed on 10 June 2022).
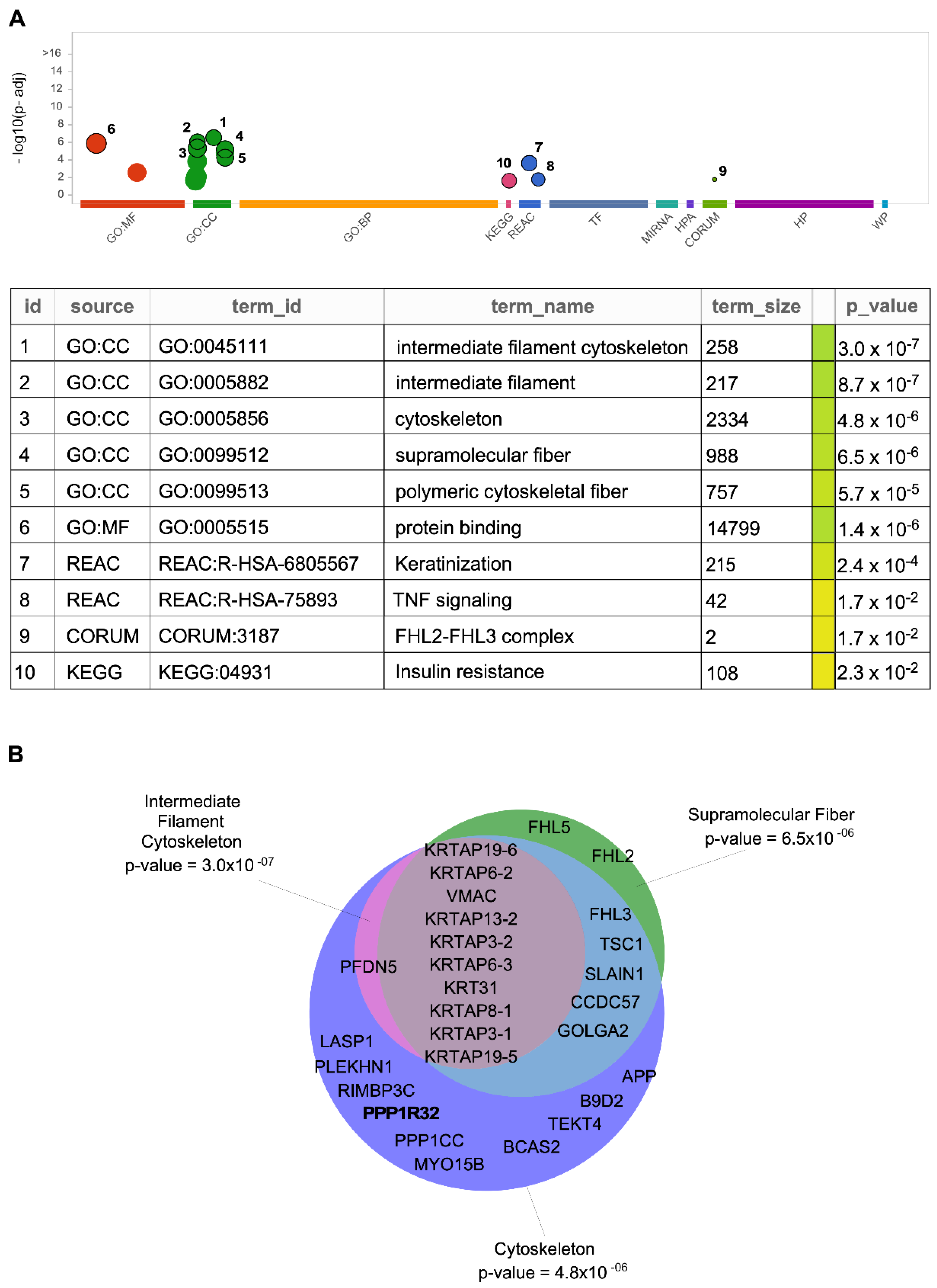
| Proteins | Experimental Approach | Ref. |
|---|---|---|
| AKAP8L, ALS2CL, ATN1, ATPAF2, B9D2, BAG4, BCAS2, C10orf55, CATSPER1, CCDC57, COG6, COX5B, CSTF2, CTDSP1, CYSRT1, DTX2, FAM168B, FHL2, FHL3, FHL5, FKBP6, FRS3, GOLGA2, HGS, HNRNPH1, HOMER3, HOXA1, HSF2BP, INCA1, KCTD9, KPRP, KRT31, KRTAP13-2, KRTAP19-5, KRTAP19-6, KRTAP3-1, KRTAP3-2, KRTAP6-2, KRTAP6-3, KRTAP8-1, LASP1, LMO2, LMO4, METTL27, MYO15B, OIP5, OTUD7B, PFDN5, PLA2G10, PLEKHG4, PLEKHN1, PPP1CC, PRDM14, PRKAA2, QARS1, RBM11, RFX6, RIMBP3C, SLAIN1, TEKT4, TFG, TGM7, TRAF1, TRAF2, TRIB3, TSC1, UNKL, VMAC, WBSCR27, WWOX, and ZMYND12 | Two-hybrid | [30] |
| APP | Reconstituted complex | [36] |
| DVL2 | Affinity Capture—MS | [37] |
| GAPDHS, GMPPB, GPX1, IDE, ISCA2, KLHL123, KLHL22, KLHL8, KLHL9, MPP7, SAMD10, UBR1, UBR2, UBR3, USP7, ZER1, and ZYG11B | Affinity Capture—MS | [38,39] |
| PPP1CA | Affinity Capture—Western | [16] |
| PPP1CA and PP1CC | Two-hybrid; reconstituted complex | [27] |
| RNF123 | Affinity Capture—MS | [40] |
| RPL21 | Proximity Label—MS | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oviedo, M.J.; Ramírez, E.; Cifuentes, M.; Farkas, C.; Mella, A.; Bertinat, R.; Gajardo, R.; Ferrada, L.; Jara, N.; De Lima, I.; et al. Is IIIG9 a New Protein with Exclusive Ciliary Function? Analysis of Its Potential Role in Cancer and Other Pathologies. Cells 2022, 11, 3327. https://doi.org/10.3390/cells11203327
Oviedo MJ, Ramírez E, Cifuentes M, Farkas C, Mella A, Bertinat R, Gajardo R, Ferrada L, Jara N, De Lima I, et al. Is IIIG9 a New Protein with Exclusive Ciliary Function? Analysis of Its Potential Role in Cancer and Other Pathologies. Cells. 2022; 11(20):3327. https://doi.org/10.3390/cells11203327
Chicago/Turabian StyleOviedo, María José, Eder Ramírez, Manuel Cifuentes, Carlos Farkas, Andy Mella, Romina Bertinat, Roberto Gajardo, Luciano Ferrada, Nery Jara, Isabelle De Lima, and et al. 2022. "Is IIIG9 a New Protein with Exclusive Ciliary Function? Analysis of Its Potential Role in Cancer and Other Pathologies" Cells 11, no. 20: 3327. https://doi.org/10.3390/cells11203327
APA StyleOviedo, M. J., Ramírez, E., Cifuentes, M., Farkas, C., Mella, A., Bertinat, R., Gajardo, R., Ferrada, L., Jara, N., De Lima, I., Martínez, F., Nualart, F., & Salazar, K. (2022). Is IIIG9 a New Protein with Exclusive Ciliary Function? Analysis of Its Potential Role in Cancer and Other Pathologies. Cells, 11(20), 3327. https://doi.org/10.3390/cells11203327






