DLX Genes in the Development and Maintenance of the Vertebrate Skeleton: Implications for Human Pathologies
Abstract
1. Skeletal Development and Maintenance
2. The Distal-Less Gene Family in Vertebrates
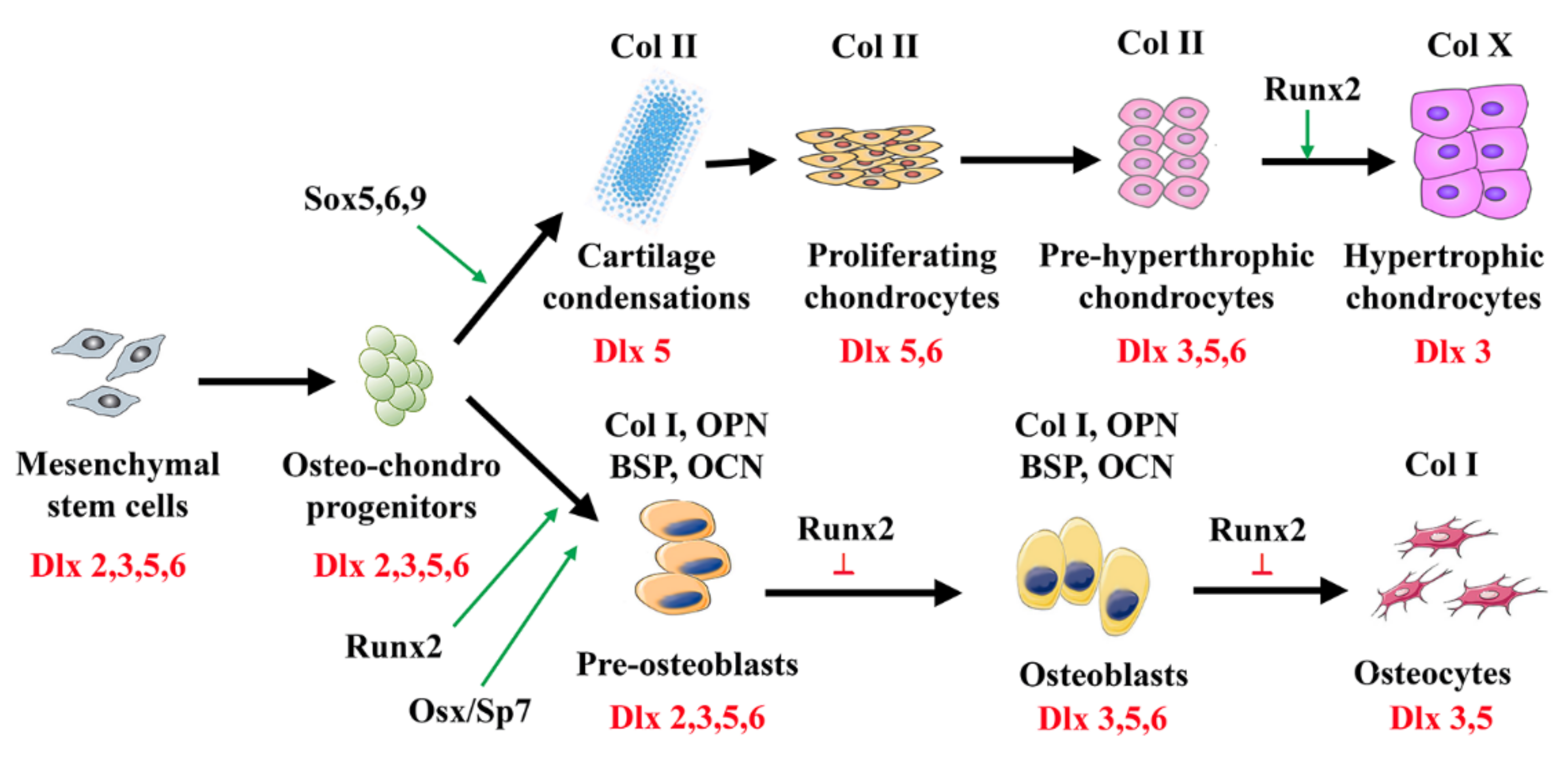
3. Dlx Genes in Skeletal Morphogenesis, Differentiation, and Remodeling
4. Dlx Genes in Cartilage Development and Maintenance
4.1. Dlx1 and Dlx2
4.2. Dlx3
4.3. Dlx5 and Dlx6
4.4. Implication of Dlx5 in Osteoarthritis
5. Roles of Dlx Genes in Osteoblast Differentiation
5.1. Dlx1 and Dlx2
5.2. Dlx3 and Dlx4
5.3. Dlx5 and Dlx6
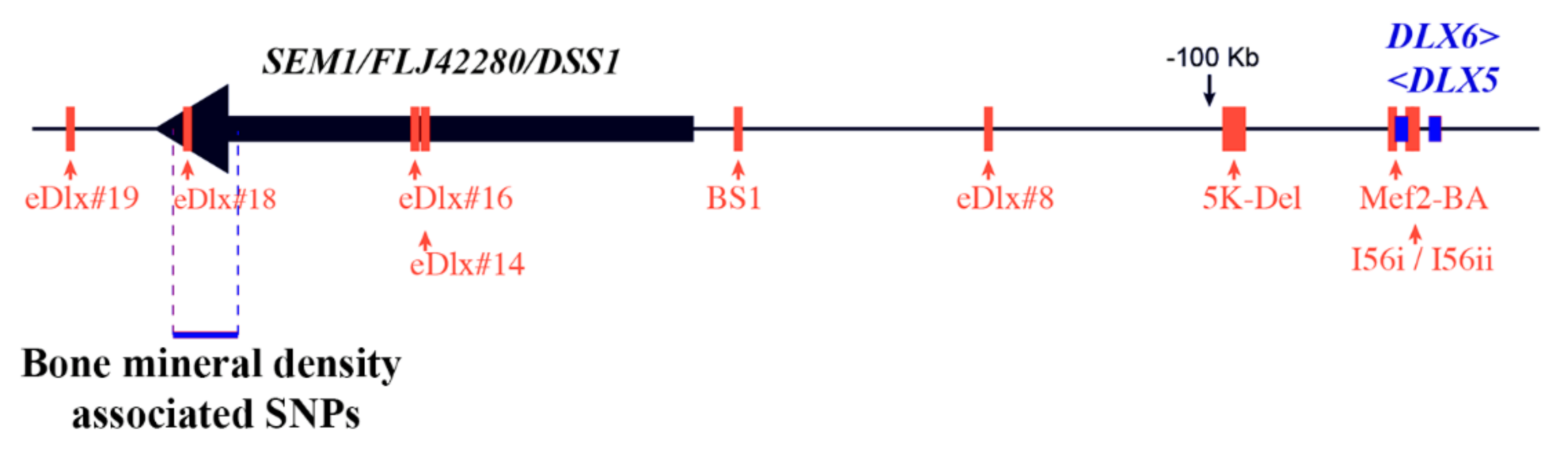
5.4. Association between DLX5/6 and Human Bone Mineral Density
6. Dlx Genes as Modulators of Osteoclast Activity
7. Regulatory Cascades Involving Dlx Genes during Skeletal Formation
7.1. Interaction between BMPs and Dlx
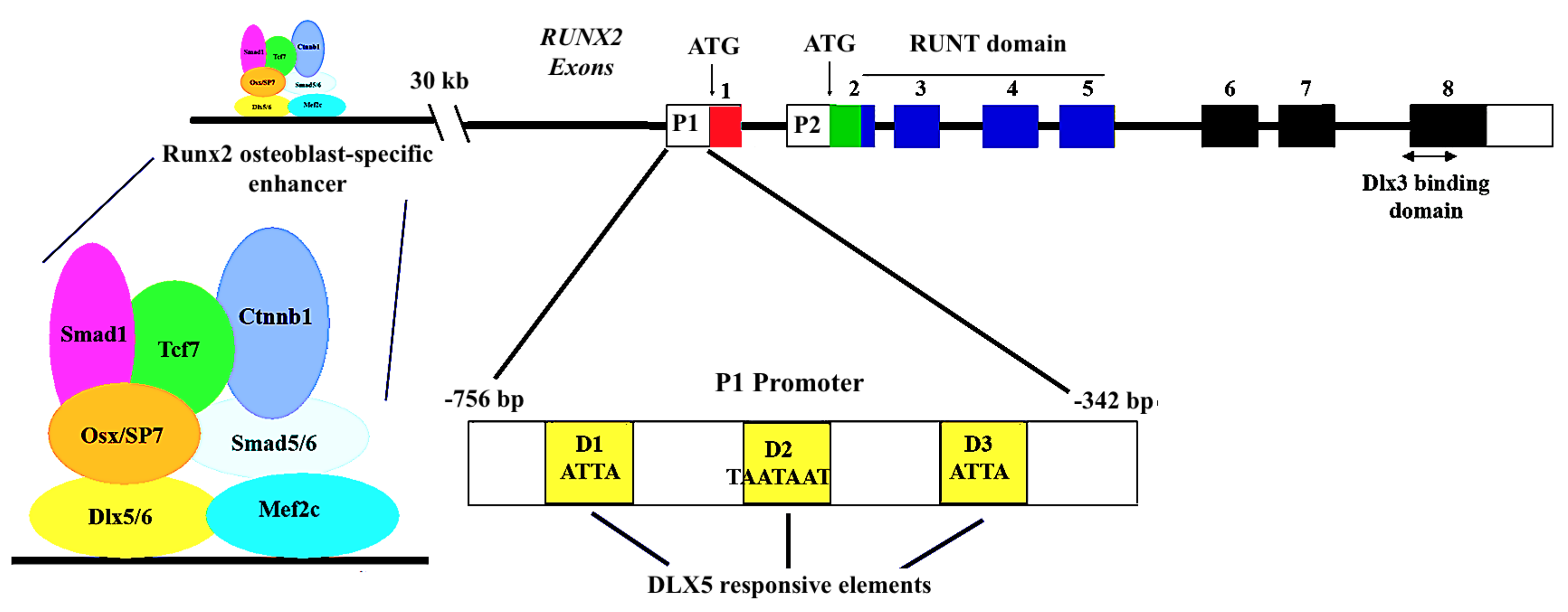
7.2. Contribution of Dlx5 in Regulating the Expression of Runx2
7.3. A Direct sp7/dlx5 Interaction in the Regulation of Bone Gene Expression
7.4. Dlx5/Runx2 Interplay in the Regulation of Osteoblast Differentiation
7.5. Antagonistic Action of Dlx5 and Msx2 in the Control of Bone Specific Gene Expression
8. Possible Involvement of Dlx5 in Osteosarcoma
9. Concluding Remark
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Hojo, H.; Ohba, S. Sp7 Action in the Skeleton: Its Mode of Action, Functions, and Relevance to Skeletal Diseases. Int. J. Mol. Sci. 2022, 23, 5647. [Google Scholar] [CrossRef]
- Komori, T. Molecular Mechanism of Runx2-Dependent Bone Development. Mol. Cells 2020, 43, 168–175. [Google Scholar] [CrossRef]
- Karsenty, G.; Ducy, P.; Starbuck, M.; Priemel, M.; Shen, J.; Geoffroy, V.; Amling, M. Cbfa1 as a regulator of osteoblast differentiation and function. Bone 1999, 25, 107–108. [Google Scholar] [CrossRef]
- Hall, B.K.; Miyake, T. All for one and one for all: Condensations and the initiation of skeletal development. Bioessays 2000, 22, 138–147. [Google Scholar] [CrossRef]
- Ikeda, T.; Kawaguchi, H.; Kamekura, S.; Ogata, N.; Mori, Y.; Nakamura, K.; Ikegawa, S.; Chung, U.I. Distinct roles of Sox5, Sox6, and Sox9 in different stages of chondrogenic differentiation. J. Bone Miner. Metab. 2005, 23, 337–340. [Google Scholar] [CrossRef]
- Hartmann, C.; Tabin, C.J. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 2000, 127, 3141–3159. [Google Scholar] [CrossRef]
- Kronenberg, H.M. PTHrP and skeletal development. Ann. N. Y. Acad. Sci. 2006, 1068, 1–13. [Google Scholar] [CrossRef]
- Merino, R.; Rodriguez-Leon, J.; Macias, D.; Ganan, Y.; Economides, A.N.; Hurle, J.M. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development 1999, 126, 5515–5522. [Google Scholar] [CrossRef]
- Enomoto, H.; Enomoto-Iwamoto, M.; Iwamoto, M.; Nomura, S.; Himeno, M.; Kitamura, Y.; Kishimoto, T.; Komori, T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J. Biol. Chem. 2000, 275, 8695–8702. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 73. [Google Scholar] [CrossRef]
- Blair, H.C.; Robinson, L.J.; Zaidi, M. Osteoclast signalling pathways. Biochem. Biophys. Res. Commun. 2005, 328, 728–738. [Google Scholar] [CrossRef]
- Yang, X.; Matsuda, K.; Bialek, P.; Jacquot, S.; Masuoka, H.C.; Schinke, T.; Li, L.; Brancorsini, S.; Sassone-Corsi, P.; Townes, T.M.; et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 2004, 117, 387–398. [Google Scholar] [CrossRef]
- Orestes-Cardoso, S.; Nefussi, J.R.; Lezot, F.; Oboeuf, M.; Pereira, M.; Mesbah, M.; Robert, B.; Berdal, A. Msx1 is a regulator of bone formation during development and postnatal growth: In vivo investigations in a transgenic mouse model. Connect. Tissue Res. 2002, 43, 153–160. [Google Scholar] [CrossRef]
- Cheng, S.L.; Shao, J.S.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J. Biol. Chem. 2003, 278, 45969–45977. [Google Scholar] [CrossRef]
- Kronenberg, H.M. Twist genes regulate Runx2 and bone formation. Dev. Cell 2004, 6, 317–318. [Google Scholar] [CrossRef][Green Version]
- Ishii, M.; Merrill, A.E.; Chan, Y.S.; Gitelman, I.; Rice, D.P.; Sucov, H.M.; Maxson, R.E., Jr. Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development 2003, 130, 6131–6142. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F. Functions of AP1 (Fos/Jun) in bone development. Ann. Rheum. Dis. 2002, 61 (Suppl. 2), ii40–ii42. [Google Scholar] [CrossRef] [PubMed]
- Levi, G.; Topilko, P.; Schneider-Maunoury, S.; Lasagna, M.; Mantero, S.; Cancedda, R.; Charnay, P. Defective bone formation in Krox-20 mutant mice. Development 1996, 122, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gollner, H.; Dani, C.; Phillips, B.; Philipsen, S.; Suske, G. Impaired ossification in mice lacking the transcription factor Sp3. Mech. Dev. 2001, 106, 77–83. [Google Scholar] [CrossRef]
- Cohen, S.M.; Bronner, G.; Kuttner, F.; Jurgens, G.; Jackle, H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature 1989, 338, 432–434. [Google Scholar] [CrossRef]
- O’Hara, E.; Cohen, B.; Cohen, S.M.; McGinnis, W. Distal-less is a downstream gene of Deformed required for ventral maxillary identity. Development 1993, 117, 847–856. [Google Scholar] [CrossRef]
- Panganiban, G.; Rubenstein, J.L. Developmental functions of the Distal-less/Dlx homeobox genes. Development 2002, 129, 4371–4386. [Google Scholar] [CrossRef]
- Cohen, S.M. Specification of limb development in the Drosophila embryo by positional cues from segmentation genes. Nature 1990, 343, 173–177. [Google Scholar] [CrossRef]
- Gorfinkiel, N.; Sanchez, L.; Guerrero, I. Drosophila terminalia as an appendage-like structure. Mech. Dev. 1999, 86, 113–123. [Google Scholar] [CrossRef]
- Stock, D.W.; Ellies, D.L.; Zhao, Z.; Ekker, M.; Ruddle, F.H.; Weiss, K.M. The evolution of the vertebrate Dlx gene family. Proc. Natl. Acad. Sci. USA 1996, 93, 10858–10863. [Google Scholar] [CrossRef]
- Ellies, D.L.; Stock, D.W.; Hatch, G.; Giroux, G.; Weiss, K.M.; Ekker, M. Relationship between the genomic organization and the overlapping embryonic expression patterns of the zebrafish dlx genes. Genomics 1997, 45, 580–590. [Google Scholar] [CrossRef]
- Nakamura, S.; Stock, D.W.; Wydner, K.L.; Bollekens, J.A.; Takeshita, K.; Nagai, B.M.; Chiba, S.; Kitamura, T.; Freeland, T.M.; Zhao, Z.; et al. Genomic analysis of a new mammalian distal-less gene: Dlx7. Genomics 1996, 38, 314–324. [Google Scholar] [CrossRef]
- Scherer, S.W.; Poorkaj, P.; Massa, H.; Soder, S.; Allen, T.; Nunes, M.; Geshuri, D.; Wong, E.; Belloni, E.; Little, S.; et al. Physical mapping of the split hand/split foot locus on chromosome 7 and implication in syndromic ectrodactyly. Hum. Mol. Genet. 1994, 3, 1345–1354. [Google Scholar] [CrossRef]
- Quinn, L.M.; Johnson, B.V.; Nicholl, J.; Sutherland, G.R.; Kalionis, B. Isolation and identification of homeobox genes from the human placenta including a novel member of the Distal-less family, DLX4. Gene 1997, 187, 55–61. [Google Scholar] [CrossRef]
- McGuinness, T.; Porteus, M.H.; Smiga, S.; Bulfone, A.; Kingsley, C.; Qiu, M.; Liu, J.K.; Long, J.E.; Xu, D.; Rubenstein, J.L. Sequence, organization, and transcription of the Dlx-1 and Dlx-2 locus. Genomics 1996, 35, 473–485. [Google Scholar] [CrossRef]
- Simeone, A.; Acampora, D.; Pannese, M.; D’Esposito, M.; Stornaiuolo, A.; Gulisano, M.; Mallamaci, A.; Kastury, K.; Druck, T.; Huebner, K.; et al. Cloning and characterization of two members of the vertebrate Dlx gene family. Proc. Natl. Acad. Sci. USA 1994, 91, 2250–2254. [Google Scholar] [CrossRef]
- Pfeffer, U.; Ferro, P.; Pavia, V.; Trombino, S.; Dell’Eva, R.; Merlo, G.; Levi, G. The coding region of the human DLX6 gene contains a polymorphic CAG/CCG repeat. Int. J. Oncol. 2001, 18, 1293–1297. [Google Scholar] [CrossRef]
- Price, M.; Lemaistre, M.; Pischetola, M.; Di Lauro, R.; Duboule, D. A mouse gene related to Distal-less shows a restricted expression in the developing forebrain. Nature 1991, 351, 748–751. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Wang, W.; Lufkin, T. Dlx5 and Dlx6: An evolutionary conserved pair of murine homeobox genes expressed in the embryonic skeleton. Ann. N. Y. Acad. Sci. 1996, 785, 38–47. [Google Scholar] [CrossRef]
- Sumiyama, K.; Irvine, S.Q.; Stock, D.W.; Weiss, K.M.; Kawasaki, K.; Shimizu, N.; Shashikant, C.S.; Miller, W.; Ruddle, F.H. Genomic structure and functional control of the Dlx3-7 bigene cluster. Proc. Natl. Acad. Sci. USA 2002, 99, 780–785. [Google Scholar] [CrossRef]
- Eisenstat, D.D.; Liu, J.K.; Mione, M.; Zhong, W.; Yu, G.; Anderson, S.A.; Ghattas, I.; Puelles, L.; Rubenstein, J.L. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J. Comp. Neurol. 1999, 414, 217–237. [Google Scholar] [CrossRef]
- Depew, M.J.; Simpson, C.A.; Morasso, M.; Rubenstein, J.L. Reassessing the Dlx code: The genetic regulation of branchial arch skeletal pattern and development. J. Anat. 2005, 207, 501–561. [Google Scholar] [CrossRef]
- Vieux-Rochas, M.; Mantero, S.; Heude, E.; Barbieri, O.; Astigiano, S.; Couly, G.; Kurihara, H.; Levi, G.; Merlo, G.R. Spatio-temporal dynamics of gene expression of the Edn1-Dlx5/6 pathway during development of the lower jaw. Genesis 2010, 48, 262–373. [Google Scholar] [CrossRef]
- Zerucha, T.; Stuhmer, T.; Hatch, G.; Park, B.K.; Long, Q.; Yu, G.; Gambarotta, A.; Schultz, J.R.; Rubenstein, J.L.; Ekker, M. A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J. Neurosci. 2000, 20, 709–721. [Google Scholar] [CrossRef]
- Ghanem, N.; Jarinova, O.; Amores, A.; Long, Q.; Hatch, G.; Park, B.K.; Rubenstein, J.L.; Ekker, M. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res. 2003, 13, 533–543. [Google Scholar]
- Zhou, Q.P.; Le, T.N.; Qiu, X.; Spencer, V.; de Melo, J.; Du, G.; Plews, M.; Fonseca, M.; Sun, J.M.; Davie, J.R.; et al. Identification of a direct Dlx homeodomain target in the developing mouse forebrain and retina by optimization of chromatin immunoprecipitation. Nucleic Acids Res. 2004, 32, 884–892. [Google Scholar] [CrossRef]
- Okita, C.; Meguro, M.; Hoshiya, H.; Haruta, M.; Sakamoto, Y.K.; Oshimura, M. A new imprinted cluster on the human chromosome 7q21-q31, identified by human-mouse monochromosomal hybrids. Genomics 2003, 81, 556–559. [Google Scholar]
- Kimura, M.I.; Kazuki, Y.; Kashiwagi, A.; Kai, Y.; Abe, S.; Barbieri, O.; Levi, G.; Oshimura, M. Dlx5, the mouse homologue of the human-imprinted DLX5 gene, is biallelically expressed in the mouse brain. J. Hum. Genet. 2004, 49, 273–277. [Google Scholar] [CrossRef][Green Version]
- Horike, S.; Cai, S.; Miyano, M.; Cheng, J.F.; Kohwi-Shigematsu, T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat. Genet. 2005, 37, 31–40. [Google Scholar] [CrossRef]
- Feng, J.; Bi, C.; Clark, B.S.; Mady, R.; Shah, P.; Kohtz, J.D. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006, 20, 1470–1484. [Google Scholar] [CrossRef]
- Masuda, Y.; Sasaki, A.; Shibuya, H.; Ueno, N.; Ikeda, K.; Watanabe, K. Dlxin-1, a novel protein that binds Dlx5 and regulates its transcriptional function. J. Biol. Chem. 2001, 276, 5331–5338. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Lee, S.; Lee, J.; Ghil, S. Necdin modulates osteogenic cell differentiation by regulating Dlx5 and MAGE-D1. Biochem. Biophys. Res. Commun. 2017, 489, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Robledo, R.F.; Rajan, L.; Li, X.; Lufkin, T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002, 16, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Bulfone, A.; Ghattas, I.; Meneses, J.J.; Christensen, L.; Sharpe, P.T.; Presley, R.; Pedersen, R.A.; Rubenstein, J.L. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: Mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev. Biol. 1997, 185, 165–184. [Google Scholar] [CrossRef]
- Beverdam, A.; Merlo, G.R.; Paleari, L.; Mantero, S.; Genova, F.; Barbieri, O.; Janvier, P.; Levi, G. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: Mirror of the past? Genesis 2002, 34, 221–227. [Google Scholar] [CrossRef]
- Merlo, G.R.; Paleari, L.; Mantero, S.; Genova, F.; Beverdam, A.; Palmisano, G.L.; Barbieri, O.; Levi, G. Mouse model of split hand/foot malformation type I. Genesis 2002, 33, 97–101. [Google Scholar] [CrossRef]
- Conte, D.; Garaffo, G.; Lo Iacono, N.; Mantero, S.; Piccolo, S.; Cordenonsi, M.; Perez-Morga, D.; Orecchia, V.; Poli, V.; Merlo, G.R. The apical ectodermal ridge of the mouse model of ectrodactyly Dlx5;Dlx6-/- shows altered stratification and cell polarity, which are restored by exogenous Wnt5a ligand. Hum. Mol. Genet. 2016, 25, 740–754. [Google Scholar] [CrossRef]
- Scherer, S.W.; Poorkaj, P.; Allen, T.; Kim, J.; Geshuri, D.; Nunes, M.; Soder, S.; Stephens, K.; Pagon, R.A.; Patton, M.A.; et al. Fine mapping of the autosomal dominant split hand/split foot locus on chromosome 7, band q21.3-q22.1. Am. J. Hum. Genet. 1994, 55, 12–20. [Google Scholar]
- Birnbaum, R.Y.; Everman, D.B.; Murphy, K.K.; Gurrieri, F.; Schwartz, C.E.; Ahituv, N. Functional characterization of tissue-specific enhancers in the DLX5/6 locus. Hum. Mol. Genet. 2012, 21, 4930–4938. [Google Scholar] [CrossRef]
- Levi, G.; Gitton, Y. Dlx genes and the maintenance of bone homeostasis and skeletal integrity. Cell Death Differ. 2014, 21, 1345–1346. [Google Scholar] [CrossRef]
- Li, H.; Marijanovic, I.; Kronenberg, M.S.; Erceg, I.; Stover, M.L.; Velonis, D.; Mina, M.; Heinrich, J.G.; Harris, S.E.; Upholt, W.B.; et al. Expression and function of Dlx genes in the osteoblast lineage. Dev. Biol. 2008, 316, 458–470. [Google Scholar] [CrossRef]
- Acampora, D.; Merlo, G.R.; Paleari, L.; Zerega, B.; Postiglione, M.P.; Mantero, S.; Bober, E.; Barbieri, O.; Simeone, A.; Levi, G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development 1999, 126, 3795–3809. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone quality--the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef]
- Noden, D.M. Interactions and fates of avian craniofacial mesenchyme. Development 1988, 103 (Suppl. 121), 121–140. [Google Scholar] [CrossRef]
- Couly, G.F.; Coltey, P.M.; Le Douarin, N.M. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development 1993, 117, 409–429. [Google Scholar] [CrossRef]
- Leucht, P.; Kim, J.B.; Amasha, R.; James, A.W.; Girod, S.; Helms, J.A. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development 2008, 135, 2845–2854. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Shi, J.; Dai, J.; Shen, S.G. Dlx2 overexpression enhanced accumulation of type II collagen and aggrecan by inhibiting MMP13 expression in mice chondrocytes. Biochem. Biophys. Res. Commun. 2018, 503, 528–535. [Google Scholar] [CrossRef]
- Xu, S.C.; Harris, M.A.; Rubenstein, J.L.; Mundy, G.R.; Harris, S.E. Bone morphogenetic protein-2 (BMP-2) signaling to the Col2alpha1 gene in chondroblasts requires the homeobox gene Dlx-2. DNA Cell Biol. 2001, 20, 359–365. [Google Scholar] [CrossRef]
- Takaishi, H.; Kimura, T.; Dalal, S.; Okada, Y.; D’Armiento, J. Joint diseases and matrix metalloproteinases: A role for MMP-13. Curr. Pharm. Biotechnol. 2008, 9, 47–54. [Google Scholar] [CrossRef]
- Thomas, B.L.; Tucker, A.S.; Qui, M.; Ferguson, C.A.; Hardcastle, Z.; Rubenstein, J.L.; Sharpe, P.T. Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development 1997, 124, 4811–4818. [Google Scholar] [CrossRef]
- Dai, J.; Si, J.; Zhu, X.; Zhang, L.; Wu, D.; Lu, J.; Ouyang, N.; Wang, X.; Shen, G. Overexpression of Dlx2 leads to postnatal condyle degradation. Mol. Med. Rep. 2016, 14, 1624–1630. [Google Scholar] [CrossRef][Green Version]
- Ghoul-Mazgar, S.; Hotton, D.; Lezot, F.; Blin-Wakkach, C.; Asselin, A.; Sautier, J.M.; Berdal, A. Expression pattern of Dlx3 during cell differentiation in mineralized tissues. Bone 2005, 37, 799–809. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Javed, A.; Morasso, M.I.; Karlin, J.; Montecino, M.; van Wijnen, A.J.; Stein, G.S.; Stein, J.L.; Lian, J.B. Dlx3 transcriptional regulation of osteoblast differentiation: Temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol. Cell. Biol. 2004, 24, 9248–9261. [Google Scholar] [CrossRef]
- Isaac, J.; Erthal, J.; Gordon, J.; Duverger, O.; Sun, H.W.; Lichtler, A.C.; Stein, G.S.; Lian, J.B.; Morasso, M.I. DLX3 regulates bone mass by targeting genes supporting osteoblast differentiation and mineral homeostasis in vivo. Cell Death Differ. 2014, 21, 1365–1376. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhao, S.; Zhou, X.; Eberspaecher, H.; Solursh, M.; de Crombrugghe, B. rDlx, a novel distal-less-like homeoprotein is expressed in developing cartilages and discrete neuronal tissues. Dev. Biol. 1994, 164, 37–51. [Google Scholar] [CrossRef]
- Ferrari, D.; Sumoy, L.; Gannon, J.; Sun, H.; Brown, A.M.; Upholt, W.B.; Kosher, R.A. The expression pattern of the Distal-less homeobox-containing gene Dlx-5 in the developing chick limb bud suggests its involvement in apical ectodermal ridge activity, pattern formation, and cartilage differentiation. Mech. Dev. 1995, 52, 257–264. [Google Scholar] [CrossRef]
- Hsu, S.H.; Noamani, B.; Abernethy, D.E.; Zhu, H.; Levi, G.; Bendall, A.J. Dlx5- and Dlx6-mediated chondrogenesis: Differential domain requirements for a conserved function. Mech. Dev. 2006, 123, 819–830. [Google Scholar] [CrossRef]
- Bendall, A.J.; Hu, G.; Levi, G.; Abate-Shen, C. Dlx5 regulates chondrocyte differentiation at multiple stages. Int. J. Dev. Biol. 2003, 47, 335–344. [Google Scholar]
- Ferrari, D.; Kosher, R.A. Dlx5 is a positive regulator of chondrocyte differentiation during endochondral ossification. Dev. Biol. 2002, 252, 257–270. [Google Scholar] [CrossRef]
- Naski, M.C.; Ornitz, D.M. FGF signaling in skeletal development. Front. Biosci. 1998, 3, d781–d794. [Google Scholar] [PubMed]
- Chung, U.I.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J. Clin. Investig. 2001, 107, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Mandl, L.A. Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K.P. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Lo Monaco, M.; Merckx, G.; Ratajczak, J.; Gervois, P.; Hilkens, P.; Clegg, P.; Bronckaers, A.; Vandeweerd, J.M.; Lambrichts, I. Stem Cells for Cartilage Repair: Preclinical Studies and Insights in Translational Animal Models and Outcome Measures. Stem Cells Int. 2018, 2018, 9079538. [Google Scholar] [CrossRef]
- Deng, Y.; Lei, G.; Lin, Z.; Yang, Y.; Lin, H.; Tuan, R.S. Engineering hyaline cartilage from mesenchymal stem cells with low hypertrophy potential via modulation of culture conditions and Wnt/β-catenin pathway. Biomaterials 2019, 192, 569–578. [Google Scholar] [CrossRef]
- Twomey-Kozak, J.; Desai, S.; Liu, W.; Li, N.Y.; Lemme, N.; Chen, Q.; Owens, B.D.; Jayasuriya, C.T. Distal-Less Homeobox 5 Is a Therapeutic Target for Attenuating Hypertrophy and Apoptosis of Mesenchymal Progenitor Cells. Int. J. Mol. Sci. 2020, 21, 4823. [Google Scholar] [CrossRef]
- Yang, H.; Cao, Y.; Zhang, J.; Liang, Y.; Su, X.; Zhang, C.; Liu, H.; Han, X.; Ge, L.; Fan, Z. DLX5 and HOXC8 enhance the chondrogenic differentiation potential of stem cells from apical papilla via LINC01013. Stem Cell Res. Ther. 2020, 11, 271. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, C.; Lu, Y.; Yuan, F. The molecular mechanism research of cartilage calcification induced by osteoarthritis. Bioengineered 2022, 13, 13082–13088. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, C.; Jiang, S.; Yuan, F. Anti-Dlx5 Retards the Progression of Osteoarthritis through Inhibiting Chondrocyte Hypertrophy and Apoptosis. Evid. Based Complement. Altern. Med. 2022, 2022, 5019920. [Google Scholar] [CrossRef]
- Priam, F.; Ronco, V.; Locker, M.; Bourd, K.; Bonnefoix, M.; Duchene, T.; Bitard, J.; Wurtz, T.; Kellermann, O.; Goldberg, M.; et al. New cellular models for tracking the odontoblast phenotype. Arch. Oral Biol. 2005, 50, 271–277. [Google Scholar] [CrossRef]
- Lichtenstein, J.; Warson, R.; Jorgenson, R.; Dorst, J.P.; McKusick, V.A. The tricho-dento-osseous (TDO) syndrome. Am. J. Hum. Genet. 1972, 24, 569–582. [Google Scholar]
- Wright, J.T.; Kula, K.; Hall, K.; Simmons, J.H.; Hart, T.C. Analysis of the tricho-dento-osseous syndrome genotype and phenotype. Am. J. Med. Genet. 1997, 72, 197–204. [Google Scholar] [CrossRef]
- Price, J.A.; Bowden, D.W.; Wright, J.T.; Pettenati, M.J.; Hart, T.C. Identification of a mutation in DLX3 associated with tricho-dento-osseous (TDO) syndrome. Hum. Mol. Genet. 1998, 7, 563–569. [Google Scholar] [CrossRef]
- Price, J.A.; Wright, J.T.; Kula, K.; Bowden, D.W.; Hart, T.C. A common DLX3 gene mutation is responsible for tricho-dento-osseous syndrome in Virginia and North Carolina families. J. Med. Genet. 1998, 35, 825–828. [Google Scholar] [CrossRef]
- Price, J.A.; Wright, J.T.; Walker, S.J.; Crawford, P.J.; Aldred, M.J.; Hart, T.C. Tricho-dento-osseous syndrome and amelogenesis imperfecta with taurodontism are genetically distinct conditions. Clin. Genet. 1999, 56, 35–40. [Google Scholar] [CrossRef]
- Haldeman, R.J.; Cooper, L.F.; Hart, T.C.; Phillips, C.; Boyd, C.; Lester, G.E.; Wright, J.T. Increased bone density associated with DLX3 mutation in the tricho-dento-osseous syndrome. Bone 2004, 35, 988–997. [Google Scholar] [CrossRef]
- Bryan, J.T.; Morasso, M.I. The Dlx3 protein harbors basic residues required for nuclear localization, transcriptional activity and binding to Msx1. J. Cell Sci. 2000, 113 Pt 22, 4013–4023. [Google Scholar] [CrossRef]
- Choi, S.J.; Roodman, G.D.; Feng, J.Q.; Song, I.S.; Amin, K.; Hart, P.S.; Wright, J.T.; Haruyama, N.; Hart, T.C. In vivo impact of a 4 bp deletion mutation in the DLX3 gene on bone development. Dev. Biol. 2009, 325, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Tare, R.S.; Lee, S.H.; Mandeville, M.; Morasso, M.I.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network. J. Biol. Chem. 2006, 281, 40515–40526. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Testa, J.R. DLX Genes: Roles in Development and Cancer. Cancers 2021, 13, 3005. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.G.; Fu, S.W.; Schwartz, A.; Pinzone, J.J.; Simmens, S.J.; Berg, P.E. Expression of BP1, a novel homeobox gene, correlates with breast cancer progression and invasion. Breast Cancer Res. Treat. 2005, 90, 241–247. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Man, Y.G.; Rezaei, M.K.; Simmens, S.J.; Berg, P.E. BP1, a homeoprotein, is significantly expressed in prostate adenocarcinoma and is concordant with prostatic intraepithelial neoplasia. Mod. Pathol. 2009, 22, 1–6. [Google Scholar] [CrossRef][Green Version]
- Tomida, S.; Yanagisawa, K.; Koshikawa, K.; Yatabe, Y.; Mitsudomi, T.; Osada, H.; Takahashi, T. Identification of a metastasis signature and the DLX4 homeobox protein as a regulator of metastasis by combined transcriptome approach. Oncogene 2007, 26, 4600–4608. [Google Scholar] [CrossRef][Green Version]
- Haga, S.B.; Fu, S.; Karp, J.E.; Ross, D.D.; Williams, D.M.; Hankins, W.D.; Behm, F.; Ruscetti, F.W.; Chang, M.; Smith, B.D.; et al. BP1, a new homeobox gene, is frequently expressed in acute leukemias. Leukemia 2000, 14, 1867–1875. [Google Scholar] [CrossRef][Green Version]
- Hara, F.; Samuel, S.; Liu, J.; Rosen, D.; Langley, R.R.; Naora, H. A homeobox gene related to Drosophila distal-less promotes ovarian tumorigenicity by inducing expression of vascular endothelial growth factor and fibroblast growth factor-2. Am. J. Pathol. 2007, 170, 1594–1606. [Google Scholar] [CrossRef][Green Version]
- Wu, D.; Mandal, S.; Choi, A.; Anderson, A.; Prochazkova, M.; Perry, H.; Gil-Da-Silva-Lopes, V.L.; Lao, R.; Wan, E.; Tang, P.L.; et al. DLX4 is associated with orofacial clefting and abnormal jaw development. Hum. Mol. Genet. 2015, 24, 4340–4352. [Google Scholar] [CrossRef]
- Ryoo, H.M.; Hoffmann, H.M.; Beumer, T.; Frenkel, B.; Towler, D.A.; Stein, G.S.; Stein, J.L.; van Wijnen, A.J.; Lian, J.B. Stage-specific expression of Dlx-5 during osteoblast differentiation: Involvement in regulation of osteocalcin gene expression. Mol. Endocrinol. 1997, 11, 1681–1694. [Google Scholar] [CrossRef]
- Samee, N.; Geoffroy, V.; Marty, C.; Schiltz, C.; Vieux-Rochas, M.; Clement-Lacroix, P.; Belleville, C.; Levi, G.; de Vernejoul, M.C. Increased bone resorption and osteopenia in Dlx5 heterozygous mice. J. Cell. Biochem. 2009, 107, 865–872. [Google Scholar] [CrossRef]
- Samee, N.; Geoffroy, V.; Marty, C.; Schiltz, C.; Vieux-Rochas, M.; Levi, G.; de Vernejoul, M.C. Dlx5, a positive regulator of osteoblastogenesis, is essential for osteoblast-osteoclast coupling. Am. J. Pathol. 2008, 173, 773–780. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, Y.J.; Yoon, W.J.; Kim, J.I.; Kim, B.G.; Hwang, Y.S.; Wozney, J.M.; Chi, X.Z.; Bae, S.C.; Choi, K.Y.; et al. Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J. Biol. Chem. 2005, 280, 35579–35587. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, Y.J.; Kim, H.J.; Park, H.D.; Kang, A.R.; Kyung, H.M.; Sung, J.H.; Wozney, J.M.; Kim, H.J.; Ryoo, H.M. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J. Biol. Chem. 2003, 278, 34387–34394. [Google Scholar] [CrossRef]
- Kiyoshima, T.; Yamauchi, M.; Wong, C.; Jheon, A.; Ganss, B.; Sodek, J. An L1 element disrupts human bone sialoprotein promoter: Lack of tissue-specific regulation by distalless5 (Dlx5) and runt homeodomain protein2 (Runx2)/core binding factor a1 (Cbfa1) elements. Gene 2002, 299, 205–217. [Google Scholar] [CrossRef]
- Crackower, M.A.; Scherer, S.W.; Rommens, J.M.; Hui, C.C.; Poorkaj, P.; Soder, S.; Cobben, J.M.; Hudgins, L.; Evans, J.P.; Tsui, L.C. Characterization of the split hand/split foot malformation locus SHFM1 at 7q21.3-q22.1 and analysis of a candidate gene for its expression during limb development. Hum. Mol. Genet. 1996, 5, 571–579. [Google Scholar] [CrossRef]
- Zhang, L.; Choi, H.J.; Estrada, K.; Leo, P.J.; Li, J.; Pei, Y.F.; Zhang, Y.; Lin, Y.; Shen, H.; Liu, Y.Z.; et al. Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum. Mol. Genet. 2014, 23, 1923–1933. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.T.; Liu, H.; Li, M.; Yang, T.L.; Zhang, X.W.; Liu, Y.Z.; Tian, Q.; Deng, H.W. Are bone mineral density loci associated with hip osteoporotic fractures? A validation study on previously reported genome-wide association loci in a Chinese population. Genet. Mol. Res. 2012, 11, 202–210. [Google Scholar] [CrossRef]
- Styrkarsdottir, U.; Halldorsson, B.V.; Gudbjartsson, D.F.; Tang, N.L.; Koh, J.M.; Xiao, S.M.; Kwok, T.C.; Kim, G.S.; Chan, J.C.; Cherny, S.; et al. European bone mineral density loci are also associated with BMD in East-Asian populations. PLoS ONE 2010, 5, e13217. [Google Scholar] [CrossRef]
- Rivadeneira, F.; Styrkársdottir, U.; Estrada, K.; Halldórsson, B.V.; Hsu, Y.H.; Richards, J.B.; Zillikens, M.C.; Kavvoura, F.K.; Amin, N.; Aulchenko, Y.S.; et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 2009, 41, 1199–1206. [Google Scholar] [CrossRef]
- Estrada, K.; Styrkarsdottir, U.; Evangelou, E.; Hsu, Y.H.; Duncan, E.L.; Ntzani, E.E.; Oei, L.; Albagha, O.M.; Amin, N.; Kemp, J.P.; et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012, 44, 491–501. [Google Scholar] [CrossRef]
- Roca-Ayats, N.; Cozar Morillo, M.; Gerousi, M.; Czwan, E.; Urreizti, R.; Martínez-Gil, N.; García-Giralt, N.; Mellibovsky, L.; Nogués, X.; Díez-Pérez, A.; et al. Identification of genetic variants associated with bone mineral density (BMD) in the FLJ42280 gene. Rev. Osteoporos. Metab. Miner. 2017, 9, 28–34. [Google Scholar] [CrossRef]
- Felson, D.T. Clinical practice. Osteoarthritis of the knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef]
- Roodman, G.D. Cell biology of the osteoclast. Exp. Hematol. 1999, 27, 1229–1241. [Google Scholar] [CrossRef]
- Vaananen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113 Pt 3, 377–381. [Google Scholar] [CrossRef]
- Lézot, F.; Thomas, B.L.; Blin-Wakkach, C.; Castaneda, B.; Bolanos, A.; Hotton, D.; Sharpe, P.T.; Heymann, D.; Carles, G.F.; Grigoriadis, A.E.; et al. Dlx homeobox gene family expression in osteoclasts. J. Cell. Physiol. 2010, 223, 779–787. [Google Scholar] [CrossRef]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004, 15, 457–475. [Google Scholar] [CrossRef]
- Atkins, G.J.; Kostakis, P.; Pan, B.; Farrugia, A.; Gronthos, S.; Evdokiou, A.; Harrison, K.; Findlay, D.M.; Zannettino, A.C. RANKL expression is related to the differentiation state of human osteoblasts. J. Bone Miner. Res. 2003, 18, 1088–1098. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Hjelmeland, A.B.; Quarles, L.D. Selective deficiency of the “bone-related” Runx2-II unexpectedly preserves osteoblast-mediated skeletogenesis. J. Biol. Chem. 2004, 279, 20307–20313. [Google Scholar] [CrossRef]
- Jee, W.S.; Ma, Y. Animal models of immobilization osteopenia. Morphologie 1999, 83, 25–34. [Google Scholar]
- Yanagisawa, M.; Suzuki, N.; Mitsui, N.; Koyama, Y.; Otsuka, K.; Shimizu, N. Effects of compressive force on the differentiation of pluripotent mesenchymal cells. Life Sci. 2007, 81, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Depew, M.J.; Lufkin, T.; Rubenstein, J.L. Specification of jaw subdivisions by Dlx genes. Science 2002, 298, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Celeste, A.J.; Iannazzi, J.A.; Taylor, R.C.; Hewick, R.M.; Rosen, V.; Wang, E.A.; Wozney, J.M. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc. Natl. Acad. Sci. USA 1990, 87, 9843–9847. [Google Scholar] [CrossRef] [PubMed]
- Hay, E.; Lemonnier, J.; Fromigue, O.; Guenou, H.; Marie, P.J. Bone morphogenetic protein receptor IB signaling mediates apoptosis independently of differentiation in osteoblastic cells. J. Biol. Chem. 2004, 279, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X. Shaping limbs by apoptosis. J. Exp. Zool. 1998, 282, 691–702. [Google Scholar] [CrossRef]
- Miyama, K.; Yamada, G.; Yamamoto, T.S.; Takagi, C.; Miyado, K.; Sakai, M.; Ueno, N.; Shibuya, H. A BMP-inducible gene, dlx5, regulates osteoblast differentiation and mesoderm induction. Dev. Biol. 1999, 208, 123–133. [Google Scholar] [CrossRef]
- Holleville, N.; Quilhac, A.; Bontoux, M.; Monsoro-Burq, A.H. BMP signals regulate Dlx5 during early avian skull development. Dev. Biol. 2003, 257, 177–189. [Google Scholar] [CrossRef]
- Singh, M.; Del Carpio-Cano, F.E.; Monroy, M.A.; Popoff, S.N.; Safadi, F.F. Homeodomain transcription factors regulate BMP-2-induced osteoactivin transcription in osteoblasts. J. Cell. Physiol. 2012, 227, 390–399. [Google Scholar] [CrossRef]
- Balint, E.; Lapointe, D.; Drissi, H.; van der Meijden, C.; Young, D.W.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Phenotype discovery by gene expression profiling: Mapping of biological processes linked to BMP-2-mediated osteoblast differentiation. J. Cell. Biochem. 2003, 89, 401–426. [Google Scholar] [CrossRef]
- Merlo, G.R.; Paleari, L.; Mantero, S.; Zerega, B.; Adamska, M.; Rinkwitz, S.; Bober, E.; Levi, G. The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Dev. Biol. 2002, 248, 157–169. [Google Scholar] [CrossRef]
- Lee, M.H.; Javed, A.; Kim, H.J.; Shin, H.I.; Gutierrez, S.; Choi, J.Y.; Rosen, V.; Stein, J.L.; van Wijnen, A.J.; Stein, G.S.; et al. Transient upregulation of CBFA1 in response to bone morphogenetic protein-2 and transforming growth factor beta1 in C2C12 myogenic cells coincides with suppression of the myogenic phenotype but is not sufficient for osteoblast differentiation. J. Cell. Biochem. 1999, 73, 114–125. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, H.J.; Li, Q.L.; Chi, X.Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.G.; Choi, J.Y.; Ryoo, H.M.; et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000, 20, 8783–8792. [Google Scholar] [CrossRef]
- Lee, M.H.; Kwon, T.G.; Park, H.S.; Wozney, J.M.; Ryoo, H.M. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem. Biophys. Res. Commun. 2003, 309, 689–694. [Google Scholar] [CrossRef]
- Ryoo, H.M.; Lee, M.H.; Kim, Y.J. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene 2006, 366, 51–57. [Google Scholar] [CrossRef]
- Park, M.H.; Shin, H.I.; Choi, J.Y.; Nam, S.H.; Kim, Y.J.; Kim, H.J.; Ryoo, H.M. Differential expression patterns of Runx2 isoforms in cranial suture morphogenesis. J. Bone Miner. Res. 2001, 16, 885–892. [Google Scholar] [CrossRef]
- Choi, K.Y.; Lee, S.W.; Park, M.H.; Bae, Y.C.; Shin, H.I.; Nam, S.; Kim, Y.J.; Kim, H.J.; Ryoo, H.M. Spatio-temporal expression patterns of Runx2 isoforms in early skeletogenesis. Exp. Mol. Med. 2002, 34, 426–433. [Google Scholar] [CrossRef]
- Banerjee, C.; Javed, A.; Choi, J.Y.; Green, J.; Rosen, V.; van Wijnen, A.J.; Stein, J.L.; Lian, J.B.; Stein, G.S. Differential regulation of the two principal Runx2/Cbfa1 n-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype. Endocrinology 2001, 142, 4026–4039. [Google Scholar] [CrossRef][Green Version]
- Drissi, H.; Luc, Q.; Shakoori, R.; Chuva De Sousa Lopes, S.; Choi, J.Y.; Terry, A.; Hu, M.; Jones, S.; Neil, J.C.; Lian, J.B.; et al. Transcriptional autoregulation of the bone related CBFA1/RUNX2 gene. J. Cell. Physiol. 2000, 184, 341–350. [Google Scholar] [CrossRef]
- Kawane, T.; Komori, H.; Liu, W.; Moriishi, T.; Miyazaki, T.; Mori, M.; Matsuo, Y.; Takada, Y.; Izumi, S.; Jiang, Q.; et al. Dlx5 and mef2 regulate a novel runx2 enhancer for osteoblast-specific expression. J. Bone Miner. Res. 2014, 29, 1960–1969. [Google Scholar] [CrossRef]
- Hojo, H.; Ohba, S.; He, X.; Lai, L.P.; McMahon, A.P. Sp7/Osterix Is Restricted to Bone-Forming Vertebrates where It Acts as a Dlx Co-factor in Osteoblast Specification. Dev. Cell 2016, 37, 238–253. [Google Scholar] [CrossRef]
- Suske, G. The Sp-family of transcription factors. Gene 1999, 238, 291–300. [Google Scholar] [CrossRef]
- Roca, H.; Phimphilai, M.; Gopalakrishnan, R.; Xiao, G.; Franceschi, R.T. Cooperative interactions between RUNX2 and homeodomain protein-binding sites are critical for the osteoblast-specific expression of the bone sialoprotein gene. J. Biol. Chem. 2005, 280, 30845–30855. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, M.H.; Wozney, J.M.; Cho, J.Y.; Ryoo, H.M. Bone morphogenetic protein-2-induced alkaline phosphatase expression is stimulated by Dlx5 and repressed by Msx2. J. Biol. Chem. 2004, 279, 50773–50780. [Google Scholar] [CrossRef]
- Shirakabe, K.; Terasawa, K.; Miyama, K.; Shibuya, H.; Nishida, E. Regulation of the activity of the transcription factor Runx2 by two homeobox proteins, Msx2 and Dlx5. Genes Cells 2001, 6, 851–856. [Google Scholar] [CrossRef]
- Benson, M.D.; Bargeon, J.L.; Xiao, G.; Thomas, P.E.; Kim, A.; Cui, Y.; Franceschi, R.T. Identification of a homeodomain binding element in the bone sialoprotein gene promoter that is required for its osteoblast-selective expression. J. Biol. Chem. 2000, 275, 13907–13917. [Google Scholar] [CrossRef]
- Li, J.J.; Sodek, J. Cloning and characterization of the rat bone sialoprotein gene promoter. Biochem. J. 1993, 289 Pt 3, 625–629. [Google Scholar] [CrossRef][Green Version]
- Kim, R.H.; Shapiro, H.S.; Li, J.J.; Wrana, J.L.; Sodek, J. Characterization of the human bone sialoprotein (BSP) gene and its promoter sequence. Matrix Biol. 1994, 14, 31–40. [Google Scholar] [CrossRef]
- Barnes, G.L.; Javed, A.; Waller, S.M.; Kamal, M.H.; Hebert, K.E.; Hassan, M.Q.; Bellahcene, A.; Van Wijnen, A.J.; Young, M.F.; Lian, J.B.; et al. Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003, 63, 2631–2637. [Google Scholar]
- Newberry, E.P.; Latifi, T.; Towler, D.A. Reciprocal regulation of osteocalcin transcription by the homeodomain proteins Msx2 and Dlx5. Biochemistry 1998, 37, 16360–16368. [Google Scholar] [CrossRef]
- Hu, G.; Lee, H.; Price, S.M.; Shen, M.M.; Abate-Shen, C. Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development 2001, 128, 2373–2384. [Google Scholar] [CrossRef]
- Longhi, A.; Errani, C.; De Paolis, M.; Mercuri, M.; Bacci, G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat. Rev. 2006, 32, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Testa, J.R. DLX5 (distal-less homeobox 5) promotes tumor cell proliferation by transcriptionally regulating MYC. J. Biol. Chem. 2009, 284, 20593–20601. [Google Scholar] [CrossRef] [PubMed]
- Kresse, S.H.; Rydbeck, H.; Skårn, M.; Namløs, H.M.; Barragan-Polania, A.H.; Cleton-Jansen, A.M.; Serra, M.; Liestøl, K.; Hogendoorn, P.C.; Hovig, E.; et al. Integrative analysis reveals relationships of genetic and epigenetic alterations in osteosarcoma. PLoS ONE 2012, 7, e48262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, H.; Wei, W.; Wang, Q.; Chen, J.; Hei, R.; Chen, C.; Wu, X.; Yuan, H.; Gu, J.; et al. DLX5 promotes osteosarcoma progression via activation of the NOTCH signaling pathway. Am. J. Cancer Res. 2021, 11, 3354–3374. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levi, G.; Narboux-Nême, N.; Cohen-Solal, M. DLX Genes in the Development and Maintenance of the Vertebrate Skeleton: Implications for Human Pathologies. Cells 2022, 11, 3277. https://doi.org/10.3390/cells11203277
Levi G, Narboux-Nême N, Cohen-Solal M. DLX Genes in the Development and Maintenance of the Vertebrate Skeleton: Implications for Human Pathologies. Cells. 2022; 11(20):3277. https://doi.org/10.3390/cells11203277
Chicago/Turabian StyleLevi, Giovanni, Nicolas Narboux-Nême, and Martine Cohen-Solal. 2022. "DLX Genes in the Development and Maintenance of the Vertebrate Skeleton: Implications for Human Pathologies" Cells 11, no. 20: 3277. https://doi.org/10.3390/cells11203277
APA StyleLevi, G., Narboux-Nême, N., & Cohen-Solal, M. (2022). DLX Genes in the Development and Maintenance of the Vertebrate Skeleton: Implications for Human Pathologies. Cells, 11(20), 3277. https://doi.org/10.3390/cells11203277






