The Extracellular Molecular Chaperone Clusterin Inhibits Amyloid Fibril Formation and Suppresses Cytotoxicity Associated with Semen-Derived Enhancer of Virus Infection (SEVI)
Abstract
1. Introduction
2. Materials and Methods
2.1. SEVI Amyloid Fibril Formation
2.2. Clusterin
2.3. Thioflavin T Fluorescence Assay
2.4. Transmission Electron Microscopy
2.5. Cellular Toxicity
2.6. MTT Assay
3. Results
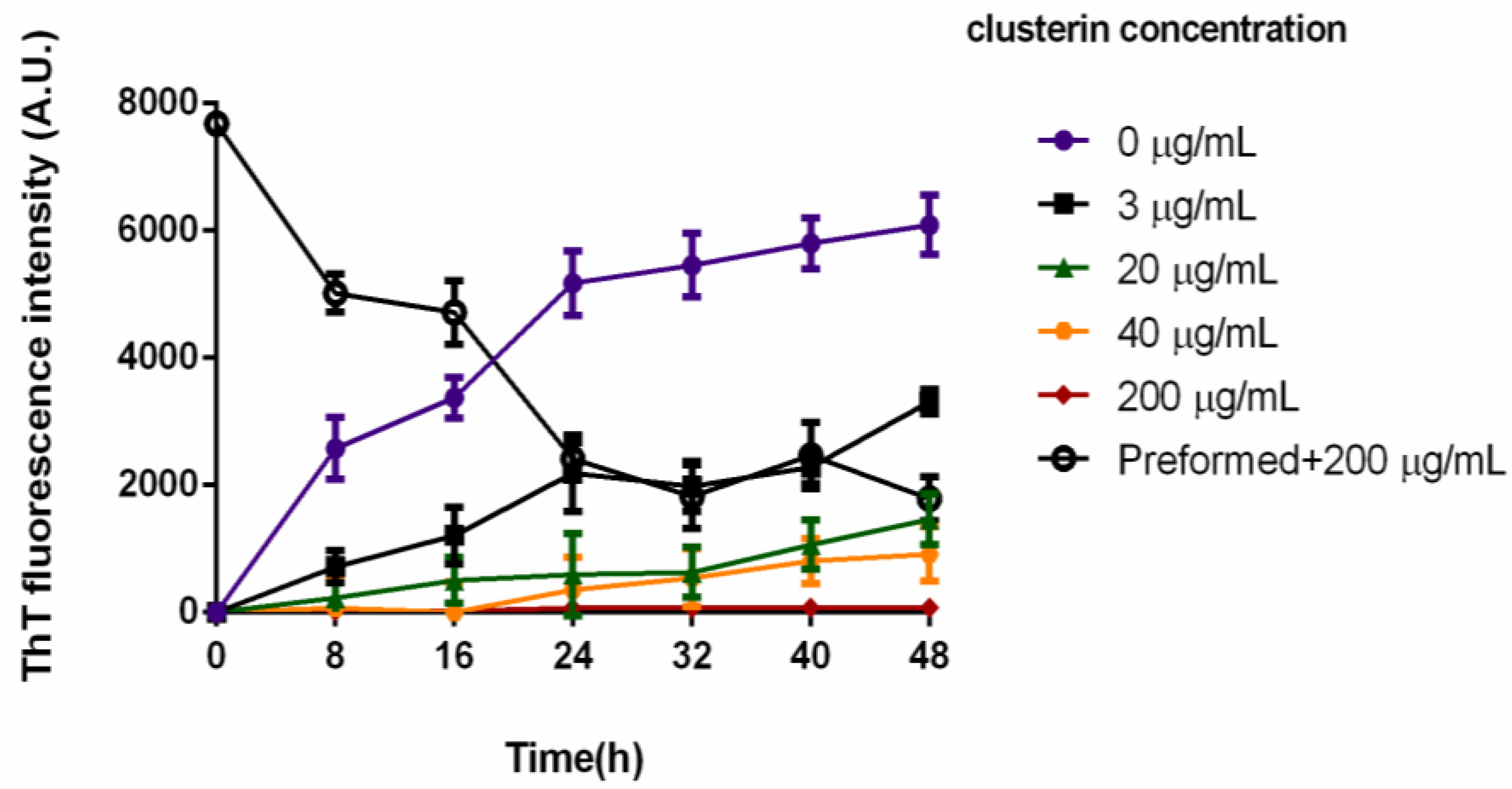
3.1. The Effect of Clusterin on the Aggregation and Amyloid Fibril Formation of PAP248–286
3.2. Transmission Electron Microscopic Analysis of the Effect of Clusterin on SEVI Amyloid Fibril Formation
3.3. Clusterin Inhibits the Cytotoxicity of SEVI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Ann. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Yerbury, J.J.; Poon, S.; Meehan, S.; Thompson, B.; Kumita, J.R.; Dobson, C.M.; Wilson, M.R. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007, 21, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.A.; Rekas, A.; Thorn, D.C.; Wilson, M.R. Small heat-shock proteins and clusterin: Intra- and extracellular molecular chaperones with a common mechanism of action and function? IUBMB Life 2003, 55, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. Protein misfolding, evolution and disease. TIBS 1999, 24, 329–332. [Google Scholar] [CrossRef]
- Kumita, J.R.; Poon, S.; Caddy, G.L.; Hagan, C.L.; Dumoulin, M.; Yerbury, J.J.; Stewart, E.M.; Robinson, C.V.; Wilson, M.R.; Dobson, C.M. The extracellular chaperone clusterin potently inhibits human lysozyme amyloid formation by interacting with prefibrillar species. J. Mol. Biol. 2007, 369, 157–167. [Google Scholar] [CrossRef][Green Version]
- Humphreys, D.T.; Carver, J.A.; Easterbrook-Smith, S.B.; Wilson, M.R. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J. Biol. Chem. 1999, 274, 6875–6881. [Google Scholar] [CrossRef] [PubMed]
- Yerbury, J.J.; Rybchyn, M.S.; Easterbrook-Smith, S.B.; Henriques, C.; Wilson, M.R. The acute phase protein haptoglobin is a mammalian extracellular chaperone with an action similar to clusterin. Biochemistry 2005, 44, 10914–10925. [Google Scholar] [CrossRef]
- French, K.; Yerbury, J.J.; Wilson, M.R. Protease activation of alpha2-macroglobulin modulates a chaperone-like action with broad specificity. Biochemistry 2008, 47, 1176–1185. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Das, K.P. Molecular chaperone-like properties of an unfolded protein, αs-casein. J. Biol. Chem. 1999, 274, 15505–15509. [Google Scholar] [CrossRef]
- Morgan, P.E.; Treweek, T.M.; Lindner, R.A.; Price, W.E.; Carver, J.A. Casein proteins as molecular chaperones. J. Agric. Food Chem. 2005, 53, 2670–2683. [Google Scholar] [CrossRef]
- Geraghty, N.J.; Satapathy, S.; Kelly, M.; Cheng, F.; Lee, A.; Wilson, M.R. Expanding the family of extracellular chaperones: Identification of human plasma proteins with chaperone activity. Prot. Sci. 2021, 30, 2272–2286. [Google Scholar] [CrossRef]
- West, J.; Satapathy, S.; Whiten, D.R.; Kelly, M.; Geraghty, N.J.; Proctor, E.J.; Sormanni, P.; Vendruscolo, M.; Buxbaum, J.N.; Ranson, M.; et al. Neuroserpin and transthyretin are extracellular chaperones that preferentially inhibit amyloid formation. Sci. Adv. 2021, 7, eabf7606. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, A.R.; Yerbury, J.J.; Ecroyd, H.; Wilson, M.R. Extracellular chaperones and proteostasis. Ann. Rev. Biochem. 2013, 82, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Jenne, D.E.; Lowin, B.; Peitsch, M.C.; Bottcher, A.; Schmitz, G.; Tschopp, J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J. Biol. Chem. 1991, 266, 11030–11036. [Google Scholar] [CrossRef]
- Sabatte, J.; Faigle, W.; Ceballos, A.; Morelle, W.; Rodrigues, C.R.; Lenicov, F.R.; Thepaut, M.; Fieschi, F.; Malchiodi, E.; Fernandez, M.; et al. Semen clusterin is a novel DC-SIGN ligand. J. Immunol. 2011, 187, 5299–5309. [Google Scholar] [CrossRef]
- Wilson, M.R.; Easterbrook-Smith, S.B. Clusterin is a secreted mammalian chaperone. TIBS 2000, 25, 95–98. [Google Scholar] [CrossRef]
- Thambisetty, M.; Simmons, A.; Velayudhan, L.; Hye, A.; Campbell, J.; Zhang, Y.; Wahlund, L.O.; Westman, E.; Kinsey, A.; Guntert, A.; et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch. Gen. Psych. 2010, 67, 739–748. [Google Scholar] [CrossRef]
- Poon, S.; Treweek, T.M.; Wilson, M.R.; Easterbrook-Smith, S.B.; Carver, J.A. Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett. 2002, 513, 259–266. [Google Scholar] [CrossRef]
- Wilson, M.R.; Yerbury, J.J.; Poon, S. Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol. BioSys. 2008, 4, 42–52. [Google Scholar] [CrossRef]
- Wyatt, A.R.; Yerbury, J.J.; Berghofer, P.; Greguric, I.; Katsifis, A.; Dobson, C.M.; Wilson, M.R. Clusterin facilitates in vivo clearance of extracellular misfolded proteins. Cell. Mol. Life Sci 2011, 68, 3919–3931. [Google Scholar] [CrossRef]
- Elias, A.K.; Scanlon, D.; Musgrave, I.F.; Carver, J.A. SEVI, the semen enhancer of HIV infection along with fragments from its central region, form amyloid fibrils that are toxic to neuronal cells. Biochim. Biophys. Acta Prot. Proteom. 2014, 1844, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Rücker, E.; Ständker, L.; Adermann, K.; Goffinet, C.; Schindler, M.; Wildum, S.; Chinnadurai, R.; Rajan, D.; Specht, A.; et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 2007, 131, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.R.; Nanga, R.P.R.; Popovych, N.; Soong, R.; Macdonald, P.M.; Ramamoorthy, A. The amyloidogenic SEVI precursor, PAP248–286, is highly unfolded in solution despite an underlying helical tendency. Biochim. Biophys. Acta Biomemb. 2011, 1808, 1161–1169. [Google Scholar] [CrossRef]
- Nanga, R.P.R.; Brender, J.R.; Vivekanandan, S.; Popovych, N.; Ramamoorthy, A. NMR structure in a membrane environment reveals putative amyloidogenic regions of the SEVI precursor peptide PAP248−286. J. Am. Chem. Soc. 2009, 131, 17972–17979. [Google Scholar] [CrossRef]
- Hughes, S.R.; Khorkova, O.; Goyal, S.; Knaeblein, J.; Heroux, J.; Riedel, N.G.; Sahasrabudhe, S. Alpha2-macroglobulin associates with beta-amyloid peptide and prevents fibril formation. Proc. Natl. Acad. Sci. USA 1998, 95, 3275–3280. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, E.; Soto, C.; Governale, S.; Frangione, B.; Ghiso, J. Apolipoprotein J and Alzheimer's amyloid beta solubility. Biochem. J. 1996, 316, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Wals, P.; Osterburg, H.H.; Johnson, S.A.; Pasinetti, G.M.; Morgan, T.E.; Rozovsky, I.; Stine, W.B.; Snyder, S.W.; Holzman, T.F.; et al. Clusterin (apoJ) alters the aggregation of amyloid beta-peptide (A beta 1-42) and forms slowly sedimenting A beta complexes that cause oxidative stress. Exp. Neurol. 1995, 136, 22–31. [Google Scholar] [CrossRef]
- McHattie, S.; Edington, N. Clusterin prevents aggregation of neuropeptide 106-126 in vitro. Biochem. Biophys. Res. Comm. 1999, 259, 336–340. [Google Scholar] [CrossRef]
- Hatters, D.M.; Wilson, M.R.; Easterbrook-Smith, S.B.; Howlett, G.J. Suppression of apolipoprotein C-II amyloid formation by the extracellular chaperone, clusterin. Eur. J. Biochem. 2002, 269, 2789–2794. [Google Scholar] [CrossRef]
- DeMattos, R.B.; O’Dell, M.A.; Parsadanian, M.; Taylor, J.W.; Harmony, J.A.; Bales, K.R.; Paul, S.M.; Aronow, B.J.; Holtzman, D.M. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10843–10848. [Google Scholar] [CrossRef]
- Narayan, P.; Orte, A.; Clarke, R.W.; Bolognesi, B.; Hook, S.; Ganzinger, K.A.; Meehan, S.; Wilson, M.R.; Dobson, C.M.; Klenerman, D. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-beta(1-40) peptide. Nat. Struct. Mol. Biol. 2012, 19, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.M.; Zirafi, O.; Müller, J.; Sandi-Monroy, N.; Yadav, J.K.; Meier, C.; Weil, T.; Roan, N.R.; Greene, W.C.; Walther, P.; et al. Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat. Commun. 2014, 5, 3508. [Google Scholar] [CrossRef] [PubMed]
- Röcker, A.; Roan, N.R.; Yadav, J.K.; Fändrich, M.; Münch, J. Structure, function and antagonism of semen amyloids. Chem. Commun. 2018, 54, 7557–7569. [Google Scholar] [CrossRef] [PubMed]
- Roan, N.R.; Liu, H.; Usmani, S.M.; Neidleman, J.; Müller, J.A.; Avila-Herrera, A.; Gawanbacht, A.; Zirafi, O.; Chu, S.; Dong, M.; et al. Liquefaction of semen generates and later degrades a conserved semenogelin peptide that enhances HIV infection. J. Virol. 2014, 88, 7221–7234. [Google Scholar] [CrossRef] [PubMed]
- Castellano, L.M.; Bart, S.M.; Holmes, V.M.; Weissman, D.; Shorter, J. Repurposing Hsp104 to antagonise seminal amyloid and counter HIV infection. Chem. Biol. 2015, 22, 1074–1086. [Google Scholar] [CrossRef]
- LoRicco, J.G.; Xu, C.S.; Neidleman, J.; Bergkvist, M.; Greene, W.C.; Roan, N.R.; Makhatadze, G.I. Gallic acid is an antagonist of semen amyloid fibrils that enhance HIV-1 infection. J. Biol. Chem. 2010, 291, 14045–14055. [Google Scholar] [CrossRef]
- Popovych, N.; Brender, J.R.; Soong, R.; Vivekanandan, S.; Hartman, K.; Basrur, V.; Macdonald, P.M.; Ramamoorthy, A. Site specific interaction of the polyphenol EGCG with the SEVI amyloid precursor peptide PAP(248–286). J. Phys. Chem. B 2012, 116, 3650–3658. [Google Scholar] [CrossRef]
- Ren, R.; Yin, S.; Lai, B.; Ma, L.; Wen, J.; Zhang, X.; Lai, F.; Liu, S.; Li, L. Myricetin antagonizes semen-derived enhancer of viral infection (SEVI) formation and influences its infection-enhancing activity. Retrovirology 2018, 15, 49. [Google Scholar] [CrossRef]
- Mohapatra, S.; Viswanathan, G.K.K.; Wettstein, L.; Arad, E.; Paul, A.; Kumar, V.; Jelinek, R.; Münch, J.; Segal, D. Dual concentration-dependent effect of ascorbic acid on PAP(248–286) amyloid formation and SEVI-mediated HIV infection. RSC Chem. Biol. 2021, 5, 1534–1545. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Liu, H.; Qiu, M.; Zhang, T.; Li, W.; Li, Z.; Qi, T.; Qiu, Y.; Li, L.; et al. ADS-J1 disaggregates semen-derived amyloid fibrils. Biochem. J. 2019, 476, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Sheik, D.A.; Chamberlain, J.M.; Brooks, L.; Clark, M.; Kim, Y.H.; Leriche, G.; Kubiak, C.P.; Dewhurst, S.; Yang, J. Hydrophobic nanoparticles reduce the β-sheet content of SEVI amyloid fibrils and inhibit SEVI-enhanced HIV infectivity. Langmuir 2017, 33, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.A.; Ecroyd, H.; Dehle, F.C.; Musgrave, I.F.; Carver, J.A. (-)-Epigallocatechin-3-gallate (EGCG) maintains κ-casein in its pre-fibrillar state without redirecting its aggregation pathway. J. Mol. Biol. 2009, 392, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, L.; Carver, J.A.; Pukala, T.L. Ion mobility mass spectrometry studies of the inhibition of α-synuclein amyloid fibril formation by (-)-epigallocatechin-3-gallate (EGCG). Aust. J. Chem. 2011, 64, 36–40. [Google Scholar] [CrossRef]
- Liu, Y.; Pukala, T.L.; Musgrave, I.F.; Williams, D.M.; Dehle, F.C.; Carver, J.A. Gallic acid is the major component of grape seed extract that inhibits amyloid fibril formation. Bioorg. Med. Chem. Lett. 2013, 23, 6336–6340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carver, J.A.; Calabrese, A.N.; Pukala, T.L. Gallic acid interacts with α-synuclein to prevent the structural collapse necessary for its aggregation. Biochim. Biophys. Acta Prot. Proteom. 2014, 1844, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.A.; Grosas, A.B.; Ecroyd, H.; Quinlan, R.A. The functional roles of the unstructured N- and C-terminal regions in αB-crystallin and other mammalian small heat-shock proteins. Cell Stress Chap. 2017, 22, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, A.N.; Liu, Y.; Wang, T.; Musgrave, I.F.; Pukala, T.L.; Tabor, R.F.; Martin, L.L.; Carver, J.A.; Bowie, J.H. The amyloid fibril-forming properties of the amphibian antimicrobial peptide uperin 3.5. ChemBioChem 2016, 17, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.L.; Kubeil, C.; Piantavigna, S.; Tikkoo, T.; Gray, N.P.; John, T.; Calabrese, A.N.; Liu, Y.; Hong, Y.; Hossain, M.A.; et al. Amyloid aggregation and membrane activity of the antimicrobial peptide uperin 3.5. Pep. Sci. 2018, 110, e24052. [Google Scholar] [CrossRef]
- John, T.; Dealey, T.J.A.; Gray, N.P.; Patil, N.A.; Hossain, M.A.; Abel, B.; Carver, J.A.; Hong, Y.; Martin, L.L. Kinetics of amyloid fibrillar aggregation of uperin 3.5 is directed by the peptide’s secondary structure. Biochemistry 2019, 58, 3656–3668. [Google Scholar] [CrossRef]
- Baltutis, V.; Calabrese, A.N.; Carver, J.A.; Martin, L.L. Uperin antimicrobial peptides: Structures, activity, and amyloid fibril formation. In Antimicrobial Peptides: Function, Mechanisms of Action and Role in Health and Disease; Ray, P.C., Ed.; Nova Sciences Publishers Inc.: Hauppauge, NY, USA, 2021; Chapter 4; pp. 95–124. [Google Scholar]
- Pham, C.L.L.; Kwan, A.H.; Sunde, M. Functional amyloid: Widespread in Nature, diverse in purpose. Essays Biochem. 2014, 56, 207–219. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elias, A.K.; Wilson, M.R.; Carver, J.A.; Musgrave, I.F. The Extracellular Molecular Chaperone Clusterin Inhibits Amyloid Fibril Formation and Suppresses Cytotoxicity Associated with Semen-Derived Enhancer of Virus Infection (SEVI). Cells 2022, 11, 3259. https://doi.org/10.3390/cells11203259
Elias AK, Wilson MR, Carver JA, Musgrave IF. The Extracellular Molecular Chaperone Clusterin Inhibits Amyloid Fibril Formation and Suppresses Cytotoxicity Associated with Semen-Derived Enhancer of Virus Infection (SEVI). Cells. 2022; 11(20):3259. https://doi.org/10.3390/cells11203259
Chicago/Turabian StyleElias, Abigail K., Mark R. Wilson, John A. Carver, and Ian F. Musgrave. 2022. "The Extracellular Molecular Chaperone Clusterin Inhibits Amyloid Fibril Formation and Suppresses Cytotoxicity Associated with Semen-Derived Enhancer of Virus Infection (SEVI)" Cells 11, no. 20: 3259. https://doi.org/10.3390/cells11203259
APA StyleElias, A. K., Wilson, M. R., Carver, J. A., & Musgrave, I. F. (2022). The Extracellular Molecular Chaperone Clusterin Inhibits Amyloid Fibril Formation and Suppresses Cytotoxicity Associated with Semen-Derived Enhancer of Virus Infection (SEVI). Cells, 11(20), 3259. https://doi.org/10.3390/cells11203259






