Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions
Abstract
1. Introduction
2. Current Therapeutic Utilization of Limbal Stem Cells
2.1. LSC Transplantation
2.2. LSC Culture and Expansion
3. Future Directions: Modulation of Non-Limbal and Limbal Stem Cells to Improve Current Therapies
3.1. Molecular Identity and Modulation of LSCs
3.2. Potentially Underutilized Role of (Lymph)angiogenesis Modulation in LSCD
4. Therapeutic Hurdles of LCSD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Gonzalez, G.; Sasamoto, Y.; Ksander, B.R.; Frank, M.; Frank, N.Y. Limbal stem cells: Identity, developmental origin, and therapeutic potential. Wiley Interdiscip. Rev. Dev. Biol. 2017, 7, e303. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Hoz, L.; Tenorio, E.; Buentello, B.; Magaña, F.; Wintergerst, A.; Navas, A.; Garfias, Y.; Arzate, H. Can Human Oral Mucosa Stem Cells Differentiate to Corneal Epithelia? Int. J. Mol. Sci. 2021, 22, 5976. [Google Scholar] [CrossRef] [PubMed]
- Dilly, P.N. Contribution of the epithelium to the stability of the tear film. Trans. Ophthalmol. Soc. UK 1985, 104, 381–389. [Google Scholar] [PubMed]
- Davidson, H.J.; Kuonen, V.J. The tear film and ocular mucins. Vet. Ophthalmol. 2004, 7, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Amitai-Lange, A.; Altshuler, A.; Bubley, J.; Dbayat, N.; Tiosano, B.; Shalom-Feuerstein, R. Lineage Tracing of Stem and Progenitor Cells of the Murine Corneal Epithelium. Stem Cells 2014, 33, 230–239. [Google Scholar] [CrossRef]
- Sacchetti, M.; Rama, P.; Bruscolini, A.; Lambiase, A. Limbal Stem Cell Transplantation: Clinical Results, Limits, and Perspectives. Stem Cells Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Chauhan, T.; Yung, M.; Tseng, C.-H.; Deng, S.X. Outcomes of Limbal Stem Cell Transplant. JAMA Ophthalmol. 2020, 138, 660. [Google Scholar] [CrossRef]
- Dua, H.S.; Shanmuganathan, V.A.; Powell-Richards, A.O.; Tighe, P.J.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532. [Google Scholar] [CrossRef]
- Dorà, N.J.; Hill, R.E.; Collinson, J.M.; West, J.D. Lineage tracing in the adult mouse corneal epithelium supports the limbal epithelial stem cell hypothesis with intermittent periods of stem cell quiescence. Stem Cell Res. 2015, 15, 665–677. [Google Scholar] [CrossRef]
- Chee, K.Y.; Kicic, A.; Wiffen, S.J. Limbal stem cells: The search for a marker. Clin. Exp. Ophthalmol. 2006, 34, 64–73. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Haq, Z.; Kang, K.; Jabbehdari, S.; Rosenblatt, M.L.; Djalilian, A.R. Strategies for reconstructing the limbal stem cell niche. Ocul. Surf. 2019, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Shimmura, S.; Kawakita, T.; Miyashita, H.; Den, S.; Shimazaki, J.; Tsubota, K. Cytokeratin 15 Can Be Used to Identify the Limbal Phenotype in Normal and Diseased Ocular Surfaces. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4780–4786. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Mi, S.; Wright, B.; Connon, C.J. Investigation of K14/K5 as a Stem Cell Marker in the Limbal Region of the Bovine Cornea. PLoS ONE 2010, 5, e13192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Allinson, S.L.; Ma, A.; Bentley, A.J.; Martin, F.L.; Fullwood, N.J. Targeted Cornea Limbal Stem/Progenitor Cell Transfection in an Organ Culture Model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3395–3401. [Google Scholar] [CrossRef]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Budak, M.; Alpdogan, O.S.; Zhou, M.; Lavker, R.M.; Akinci, M.M.; Wolosin, J.M. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J. Cell Sci. 2005, 118, 1715–1724. [Google Scholar] [CrossRef]
- de Paiva, C.S.; Chen, Z.; Corrales, R.M.; Pflugfelder, S.C.; Li, D. ABCG2 Transporter Identifies a Population of Clonogenic Human Limbal Epithelial Cells. Stem Cells 2005, 23, 63–73. [Google Scholar] [CrossRef]
- Ding, X.-W.; Wu, J.-H.; Jiang, C.-P. ABCG2: A potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010, 86, 631–637. [Google Scholar] [CrossRef]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.B.; Perez, V.L.; et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.; Wang, J.; Wray, B.; Patel, P.; Yang, W.; Peng, H.; Lavker, R.M. Single-Cell RNA Transcriptome Helps Define the Limbal/Corneal Epithelial Stem/Early Transit Amplifying Cells and How Autophagy Affects This Population. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3570–3583. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Collins, C.A.; Nascimento, E.; Tan, D.W.; Frye, M.; Itami, S.; Watt, F.M. Lrig1 Expression Defines a Distinct Multipotent Stem Cell Population in Mammalian Epidermis. Cell Stem Cell 2009, 4, 427–439. [Google Scholar] [CrossRef]
- Thomas, P.B.; Liu, Y.H.; Zhuang, F.F.; Selvam, S.; Song, S.W.; Smith, R.E.; Trousdale, M.D.; Yiu, S.C. Identification of Notch-1 ex-pression in the limbal basal epithelium. Mol. Vis. 2007, 13, 337–344. [Google Scholar] [PubMed]
- Guo, Z.H.; Zhang, W.; Jia, Y.Y.S.; Liu, Q.X.; Li, Z.F.; Lin, J.S. An Insight into the Difficulties in the Discovery of Specific Biomarkers of Limbal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1982. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, V.; Testa, A.; Di Iorio, E.; Mavilio, F.; Pellegrini, G.; De Luca, M. C/EBPδ regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol. 2007, 177, 1037–1049. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Dietrich, T.; Saito, K.; Sorokin, L.; Sasaki, T.; Paulsson, M.; Kruse, F. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res. 2007, 85, 845–860. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef]
- Sangwan, V.S. Limbal Stem Cells in Health and Disease. Biosci. Rep. 2001, 21, 385–405. [Google Scholar] [CrossRef]
- Lehrer, M.; Sun, T.; Lavker, R. Strategies of epithelial repair: Modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 1998, 111, 2867–2875. [Google Scholar] [CrossRef]
- Beebe, D.C.; Masters, B.R. Cell lineage and the differentiation of corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1815–1825. [Google Scholar]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Calonge, M.; Nieto-Miguel, T.; de la Mata, A.; Galindo, S.; Herreras, J.M.; López-Paniagua, M. Goals and Challenges of Stem Cell-Based Therapy for Corneal Blindness Due to Limbal Deficiency. Pharmaceutics 2021, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Vattulainen, M.; Ilmarinen, T.; Koivusalo, L.; Viiri, K.; Hongisto, H.; Skottman, H. Modulation of Wnt/BMP pathways during corneal differentiation of hPSC maintains ABCG2-positive LSC population that demonstrates increased regenerative potential. Stem Cell Res. Ther. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Bonnet, C.; Roberts, J.S.; Deng, S.X. Limbal stem cell diseases. Exp. Eye Res. 2021, 205, 108437. [Google Scholar] [CrossRef] [PubMed]
- Gesteira, T.F.; Sun, M.; Coulson-Thomas, Y.M.; Yamaguchi, Y.; Yeh, L.-K.; Hascall, V.; Coulson-Thomas, V.J. Hyaluronan Rich Microenvironment in the Limbal Stem Cell Niche Regulates Limbal Stem Cell Differentiation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4407–4421. [Google Scholar] [CrossRef]
- Espana, E.M.; Kawakita, T.; Romano, A.; Di Pascuale, M.; Smiddy, R.; Liu, C.-Y.; Tseng, S.C.G. Stromal Niche Controls the Plasticity of Limbal and Corneal Epithelial Differentiation in a Rabbit Model of Recombined Tissue. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5130–5135. [Google Scholar] [CrossRef]
- Notara, M.; Refaian, N.; Braun, G.; Steven, P.; Bock, F.; Cursiefen, C. Short-term uvb-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell Res. 2015, 15, 643–654. [Google Scholar] [CrossRef]
- Nubile, M.; Curcio, C.; Dua, H.S.; Calienno, R.; Lanzini, M.; Iezzi, M.; Mastropasqua, R.; Agnifili, L.; Mastropasqua, L. Pathological changes of the anatomical structure and markers of the limbal stem cell niche due to inflammation. Mol. Vis. 2013, 19, 516–525. [Google Scholar]
- Puangsricharern, V.; Tseng, S.C. Cytologlogic Evidence of Corneal Diseases with Limbal Stem Cell Deficiency. Ophthalmology 1995, 102, 1476–1485. [Google Scholar] [CrossRef]
- Deng, S.X.; Sejpal, K.; Bakhtiari, P. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr. J. Ophthalmol. 2013, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Selver, Ö.B.; Yağcı, A.; Eğrilmez, S.; Gürdal, M.; Palamar, M.; Çavuşoğlu, T.; Ateş, U.; Veral, A.; Güven, Ç.; Wolosin, J.M. Limbal Stem Cell Deficiency and Treatment with Stem Cell Transplantation. Turk. J. Ophthalmol. 2017, 47, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.G. Concept and application of limbal stem cells. Eye 1989, 3, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E.; The International Limbal Stem Cell Deficiency Working G. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef]
- Rossen, J.; Amram, A.; Milani, B.; Park, D.; Harthan, J.; Joslin, C.; McMahon, T.; Djalilian, A. Contact Lens-induced Limbal Stem Cell Deficiency. Ocul. Surf. 2016, 14, 419–434. [Google Scholar] [CrossRef]
- Di Iorio, E.; Kaye, S.B.; Ponzin, D.; Barbaro, V.; Ferrari, S.; Böhm, E.; Nardiello, P.; Castaldo, G.; McGrath, J.A.; Willoughby, C.E. Limbal Stem Cell Deficiency and Ocular Phenotype in Ectrodactyly-Ectodermal Dysplasia-Clefting Syndrome Caused by p63 Mutations. Ophthalmology 2011, 119, 74–83. [Google Scholar] [CrossRef]
- Trevisan, M.; Alvisi, G.; Barbaro, V.; Barzon, L.; Raffa, P.; Migliorati, A.; Desole, G.; Ruzittu, S.; Masi, G.; Di Iorio, E.; et al. Oral Mucosa-Derived Induced Pluripotent Stem Cells from Patients with Ectrodactyly-Ectodermal Dysplasia-Clefting Syndrome. Cell. Reprogramming 2018, 20, 215–224. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Eslani, M.; Haq, Z.; Shirzadeh, E.; Huvard, M.J.; Djalilian, A.R. Current and Upcoming Therapies for Ocular Surface Chemical Injuries. Ocul. Surf. 2016, 15, 48–64. [Google Scholar] [CrossRef]
- Liotti, L.; Caimmi, S.; Bottau, P.; Bernardini, R.; Cardinale, F.; Saretta, F.; Mori, F.; Crisafulli, G.; Franceschini, F.; Caffarelli, C. Clinical features, outcomes and treatment in children with drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis. Acta Bio Med. Atenei Parm. 2019, 90, 52–60. [Google Scholar] [CrossRef]
- Stan, C.; Diaconu, E.; Hopirca, L.; Petra, N.; Rednic, A.; Stan, C. Ocular cicatricial pemphigoid. Rom. J. Ophthalmol. 2020, 64, 226–230. [Google Scholar] [CrossRef]
- Ozer, M.D.; Altinkurt, E.; Alparslan, N. The long-term surgical outcomes of conjunctival-limbal autograft procedure with or without penetrating keratoplasty in eyes with unilateral limbal stem cell deficiency. Taiwan J. Ophthalmol. 2020, 10, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shortt, A.J.; Bunce, C.; Levis, H.J.; Blows, P.; Doré, C.J.; Vernon, A.; Secker, G.A.; Tuft, S.J.; Daniels, J.T. Three-Year Outcomes of Cultured Limbal Epithelial Allografts in Aniridia and Stevens-Johnson Syndrome Evaluated Using the Clinical Outcome Assessment in Surgical Trials Assessment Tool. Stem Cells Transl. Med. 2014, 3, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Keivyon, K.R.; Tseng, S.C. Limbal Autograft Transplantation for Ocular Surface Disorders. Ophthalmology 1989, 96, 709–723. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Tsubota, K.; Toda, I.; Saito, H.; Shinozaki, N.; Shimazaki, J. Reconstruction of the Corneal Epithelium by Limbal Allograft Transplantation for Severe Ocular Surface Disorders. Ophthalmology 1995, 102, 1486–1496. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Eslani, M.; Kurji, K.H.; Wright, E.; Sarnicola, E.; Govil, A.; Holland, E.J. Long-term Outcomes of Living-Related Conjunctival Limbal Allograft Compared With Keratolimbal Allograft in Patients With Limbal Stem Cell Deficiency. Cornea 2020, 39, 980–985. [Google Scholar] [CrossRef]
- Fernandez-Buenaga, R.; Aiello, F.; Zaher, S.S.; Grixti, A.; Ahmad, S. Twenty years of limbal epithelial therapy: An update on managing limbal stem cell deficiency. BMJ Open Ophthalmol. 2018, 3, e000164. [Google Scholar] [CrossRef]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Amemiya, T.; Kanamura, N.; Kinoshita, S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280–1284. [Google Scholar] [CrossRef]
- Borderie, V.M.; Ghoubay, D.; Georgeon, C.; Borderie, M.; de Sousa, C.; Legendre, A.; Rouard, H. Long-Term Results of Cultured Limbal Stem Cell Versus Limbal Tissue Transplantation in Stage III Limbal Deficiency. Stem Cells Transl. Med. 2019, 8, 1230–1241. [Google Scholar] [CrossRef]
- Liang, L.; Sheha, H.; Li, J.; Tseng, S.C.G. Limbal stem cell transplantation: New progresses and challenges. Eye 2008, 23, 1946–1953. [Google Scholar] [CrossRef]
- Chen, J.J.; Tseng, S.C. Corneal epithelial wound healing in partial limbal deficiency. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1301–1314. [Google Scholar]
- Chen, J.J.; Tseng, S.C. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2219–2233. [Google Scholar]
- Moldovan, S.M.; Borderie, V.; Baudrimont, M.; Laroche, L. Treatment of unilateral limbal stem cell deficiency syndrome by limbal autograft. J. Fr. Ophtalmol. 1999, 22, 302–309. [Google Scholar] [PubMed]
- Rao, S.K.; Rajagopal, R.; Sitalakshmi, G.; Padmanabhan, P. Limbal Autografting: Comparison of results in the acute and chronic phases of ocular surface burns. Cornea 1999, 18, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Amescua, G.; Atallah, M.; Palioura, S.; Perez, V. Limbal stem cell transplantation: Current perspectives. Clin. Ophthalmol. 2016, 10, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, A.E.; Lako, M.; Figueiredo, F.C. Recent Advances in Stem Cell Therapy for Limbal Stem Cell Deficiency: A Narrative Review. Ophthalmol. Ther. 2020, 9, 809–831. [Google Scholar] [CrossRef]
- Shortt, A.J.; Secker, G.A.; Rajan, M.S.; Meligonis, G.; Dart, J.K.; Tuft, S.J.; Daniels, J.T. Ex Vivo Expansion and Transplantation of Limbal Epithelial Stem Cells. Ophthalmology 2008, 115, 1989–1997. [Google Scholar] [CrossRef]
- Hernáez-Moya, R.; González, S.; Urkaregi, A.; Pijoan, J.I.; Deng, S.X.; Andollo, N. Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis. Int. J. Mol. Sci. 2020, 21, 6132. [Google Scholar] [CrossRef]
- Kolli, S.; Ahmad, S.; Lako, M.; Figueiredo, F. Successful Clinical Implementation of Corneal Epithelial Stem Cell Therapy for Treatment of Unilateral Limbal Stem Cell Deficiency. Stem Cells 2009, 28, 597–610. [Google Scholar] [CrossRef]
- Brejchova, K.; Trosan, P.; Studeny, P.; Skalicka, P.; Utheim, T.P.; Bednar, J.; Jirsova, K. Characterization and comparison of human limbal explant cultures grown under defined and xeno-free conditions. Exp. Eye Res. 2018, 176, 20–28. [Google Scholar] [CrossRef]
- Bobba, S.; Chow, S.; Watson, S.; Di Girolamo, N. Clinical outcomes of xeno-free expansion and transplantation of autologous ocular surface epithelial stem cells via contact lens delivery: A prospective case series. Stem Cell Res. Ther. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Chen, L.; Deng, S.X. Comparative Study of Xenobiotic-Free Media for the Cultivation of Human Limbal Epithelial Stem/Progenitor Cells. Tissue Eng. Part C Methods 2017, 23, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.; Pasovic, L.; Raeder, S.; Sehic, A.; Roald, B.; de la Paz, M.F.; Tønseth, K.A.; Utheim, T.P. Optisol-GS Storage of Cultured Human Limbal Epithelial Cells at Ambient Temperature Is Superior to Hypothermic Storage. Curr. Eye Res. 2020, 45, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Deng, S.X. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Exp. Eye Res. 2013, 116, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; González, S.; Nakatsu, M.; Baclagon, E.R.; Chen, F.V.; Deng, S.X. Human adipose-derived stem cells support the growth of limbal stem/progenitor cells. PLoS ONE 2017, 12, e0186238. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, M.N.; González, S.; Mei, H.; Deng, S.X. Human Limbal Mesenchymal Cells Support the Growth of Human Corneal Epithelial Stem/Progenitor Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6953–6959. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González, S.; Mei, H.; Nakatsu, M.N.; Baclagon, E.R.; Deng, S.X. A 3D culture system enhances the ability of human bone marrow stromal cells to support the growth of limbal stem/progenitor cells. Stem Cell Res. 2016, 16, 358–364. [Google Scholar] [CrossRef][Green Version]
- González, S.; Oh, D.; Baclagon, E.R.; Zheng, J.J.; Deng, S.X. Wnt Signaling Is Required for the Maintenance of Human Limbal Stem/Progenitor Cells In Vitro. Investig. Ophthalmol. Vis. Sci. 2019, 60, 107–112. [Google Scholar] [CrossRef]
- Zhang, C.; Mei, H.; Robertson, S.Y.; Lee, H.-J.; Deng, S.X.; Zheng, J.J. A Small-Molecule Wnt Mimic Improves Human Limbal Stem Cell Ex Vivo Expansion. iScience 2020, 23, 101075. [Google Scholar] [CrossRef]
- González, S.; Uhm, H.; Deng, S.X. Notch Inhibition Prevents Differentiation of Human Limbal Stem/Progenitor Cells in vitro. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.-Y.; Zhao, X.-Y.; Zhang, M.-C.; Xie, H.-T. Rat Limbal Niche Cells Prevent Epithelial Stem/Progenitor Cells From Differentiation and Proliferation by Inhibiting Notch Signaling Pathway In Vitro. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Boulton, M.; Zhao, B.; Connon, C.; Cai, J.; Albon, J. A Role for Notch Signaling in Human Corneal Epithelial Cell Differentiation and Proliferation. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, A.M.; Soleimani, M.; Eleiwa, T.K.; ElSheikh, R.H.; Frank, C.R.; Naderan, M.; Yazdanpanah, G.; Rosenblatt, M.I.; Djalilian, A.R. Current and Emerging Therapies for Limbal Stem Cell Deficiency. Stem Cells Transl. Med. 2022, 11, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ilmarinen, T.; Laine, J.; Juuti-Uusitalo, K.; Numminen, J.; Seppänen-Suuronen, R.; Uusitalo, H.; Skottman, H. Towards a defined, serum- and feeder-free culture of stratified human oral mucosal epithelium for ocular surface reconstruction. Acta Ophthalmol. 2012, 91, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.; Ahmad, S.; Mudhar, H.S.; Meeny, A.; Lako, M.; Figueiredo, F.C. Successful Application of Ex Vivo Expanded Human Autologous Oral Mucosal Epithelium for the Treatment of Total Bilateral Limbal Stem Cell Deficiency. Stem Cells 2014, 32, 2135–2146. [Google Scholar] [CrossRef]
- Blazejewska, E.A.; Schlötzer-Schrehardt, U.; Zenkel, M.; Bachmann, B.; Chankiewitz, E.; Jacobi, C.; Kruse, F.E. Corneal Limbal Microenvironment Can Induce Transdifferentiation of Hair Follicle Stem Cells into Corneal Epithelial-like Cells. Stem Cells 2009, 27, 642–652. [Google Scholar] [CrossRef]
- Gomes, J.P.; Monteiro, B.G.; Melo, G.B.; Smith, R.L.; Silva, M.C.P.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Human Immature Dental Pulp Stem Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef]
- Hongisto, H.; Vattulainen, M.; Ilmarinen, T.; Mikhailova, A.; Skottman, H. Efficient and Scalable Directed Differentiation of Clinically Compatible Corneal Limbal Epithelial Stem Cells from Human Pluripotent Stem Cells. J. Vis. Exp. 2018, 140, e58279. [Google Scholar] [CrossRef]
- Monteiro, B.G.; Serafim, R.C.; Melo, G.B.; Silva, M.C.P.; Lizier, N.F.; Maranduba, C.M.C.; Smith, R.L.; Kerkis, A.; Cerruti, H.; Gomes, J.A.P.; et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009, 42, 587–594. [Google Scholar] [CrossRef]
- Reza, H.M.; Ng, B.-Y.; Gimeno, F.L.; Phan, T.T.; Ang, L.P.-K. Umbilical Cord Lining Stem Cells as a Novel and Promising Source for Ocular Surface Regeneration. Stem Cell Rev. Rep. 2011, 7, 935–947. [Google Scholar] [CrossRef]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2018, 206, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, D.; Thake, M.; MacLaren, R. Clinical applications of retinal gene therapy. Prog. Retin. Eye Res. 2013, 32, 22–47. [Google Scholar] [CrossRef]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; González, S.; Roberts, J.S.; Robertson, S.Y.; Ruiz, M.; Zheng, J.; Deng, S.X. Human limbal epithelial stem cell regulation, bioengineering and function. Prog. Retin. Eye Res. 2021, 85, 100956. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, M.N.; Vartanyan, L.; Vu, D.M.; Ng, M.Y.; Li, X.; Deng, S.X. Preferential Biological Processes in the Human Limbus by Differential Gene Profiling. PLoS ONE 2013, 8, e61833. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Oh, D.; Mei, H.; Robertson, S.; Chang, D.; Bourges, J.-L.; Behar-Cohen, F.; Zheng, J.J.; Deng, S.X. Wnt6 plays a complex role in maintaining human limbal stem/progenitor cells. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Ouyang, H.; Xue, Y.; Lin, Y.; Zhang, X.; Xi, L.; Patel, S.; Cai, H.; Luo, J.; Zhang, M.; Zhang, M.; et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 2014, 511, 358–361. [Google Scholar] [CrossRef]
- Li, M.; Huang, H.; Li, L.; He, C.; Zhu, L.; Guo, H.; Wang, L.; Liu, J.; Wu, S.; Liu, J.; et al. Core transcription regulatory circuitry orchestrates corneal epithelial homeostasis. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Mei, H.; Nakatsu, M.N.; Baclagon, E.R.; Deng, S.X. Frizzled 7 Maintains the Undifferentiated State of Human Limbal Stem/Progenitor Cells. Stem Cells 2013, 32, 938–945. [Google Scholar] [CrossRef]
- González, S.; Halabi, M.; Ju, D.; Tsai, M.; Deng, S. Role of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal Epithelium. Cells 2020, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Wang, Q.; Qi, X.; Zhang, B.; Jiang, H.; Chen, S.; Duan, H.; Lu, Y.; Dong, J.; Cao, Y.; et al. Molecular identity of human limbal heterogeneity involved in corneal homeostasis and privilege. Ocul. Surf. 2021, 21, 206–220. [Google Scholar] [CrossRef]
- Li, D.-Q.; Kim, S.; Li, J.-M.; Gao, Q.; Choi, J.; Bian, F.; Hu, J.; Zhang, Y.; Lu, R.; Li, Y.; et al. Single-cell transcriptomics identifies limbal stem cell population and cell types mapping its differentiation trajectory in limbal basal epithelium of human cornea. Ocul. Surf. 2021, 20, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Català, P.; Groen, N.; Dehnen, J.A.; Soares, E.; van Velthoven, A.J.H.; Nuijts, R.M.M.A.; Dickman, M.M.; LaPointe, V.L.S. Single cell transcriptomics reveals the heterogeneity of the human cornea to identify novel markers of the limbus and stroma. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Collin, J.; Queen, R.; Zerti, D.; Bojic, S.; Dorgau, B.; Moyse, N.; Molina, M.M.; Yang, C.; Dey, S.; Reynolds, G.; et al. A single cell atlas of human cornea that defines its development, limbal progenitor cells and their interactions with the immune cells. Ocul. Surf. 2021, 21, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Menzel-Severing, J.; Zenkel, M.; Polisetti, N.; Sock, E.; Wegner, M.; Kruse, F.E.; Schlötzer-Schrehardt, U. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Rizzetto, S.; Eltahla, A.A.; Lin, P.; Bull, R.; Lloyd, A.R.; Ho, J.W.K.; Venturi, V.; Luciani, F. Impact of sequencing depth and read length on single cell RNA sequencing data of T cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; González, S.; Dai, W.; Deng, S.; Lu, L. Effect of Hypoxia-regulated Polo-like Kinase 3 (Plk3) on Human Limbal Stem Cell Differentiation. J. Biol. Chem. 2016, 291, 16519–16529. [Google Scholar] [CrossRef]
- Nasser, W.; Amitai-Lange, A.; Soteriou, D.; Hanna, R.; Tiosano, B.; Fuchs, Y.; Shalom-Feuerstein, R. Corneal-Committed Cells Restore the Stem Cell Pool and Tissue Boundary following Injury. Cell Rep. 2018, 22, 323–331. [Google Scholar] [CrossRef]
- Notara, M.; Lentzsch, A.; Coroneo, M.; Cursiefen, C. The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Gao, X.; Guo, K.; Santosa, S.M.; Montana, M.; Yamakawa, M.; Hallak, J.A.; Han, K.-Y.; Doh, S.J.; Rosenblatt, M.I.; Chang, J.-H.; et al. Application of corneal injury models in dual fluorescent reporter transgenic mice to understand the roles of the cornea and limbus in angiogenic and lymphangiogenic privilege. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Fuchsluger, T.A.; Jurkunas, U.V. Limbal Stem Cell Deficiency and Corneal Neovascularization. Semin. Ophthalmol. 2009, 24, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Azari, A.A.; Safapour, S. Therapeutic approaches for corneal neovascularization. Eye Vis. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Deng, S.X.; Kruse, F.; Gomes, J.A.P.; Chan, C.C.; Daya, S.; Dana, R.; Figueiredo, F.C.; Kinoshita, S.; Rama, P.; Sangwan, V.; et al. Global Consensus on the Management of Limbal Stem Cell Deficiency. Cornea 2020, 39, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Kadar, T.; Amir, A.; Cohen, L.; Cohen, M.; Sahar, R.; Gutman, H.; Horwitz, V.; Dachir, S. Anti-VEGF Therapy (Bevacizumab) for Sulfur Mustard-Induced Corneal Neovascularization Associated with Delayed Limbal Stem Cell Deficiency in Rabbits. Curr. Eye Res. 2013, 39, 439–450. [Google Scholar] [CrossRef]
- Chen, H.-C.J.; Yeh, L.-K.; Tsai, Y.-J.; Lai, C.-H.; Chen, C.-C.; Lai, J.-Y.; Sun, C.-C.; Chang, G.; Hwang, T.-L.; Chen, J.-K.; et al. Expression of Angiogenesis-Related Factors in Human Corneas after Cultivated Oral Mucosal Epithelial Transplantation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5615–5623. [Google Scholar] [CrossRef]
- Guarnieri, A.; Moreno-Montañés, J.; Alfonso-Bartolozzi, B.; Sabater, A.L.; García-Guzmán, M.; Andreu, E.J.; Prosper, F. Quantification of corneal neovascularization after ex vivo limbal epithelial stem cell therapy. Int. J. Ophthalmol. 2014, 7, 988–995. [Google Scholar] [CrossRef]
- Zakaria, N.; Van Marck, V.; Koppen, C.; Berneman, Z.; Tassignon, M.-J. Lymphangiogenesis May Play a Role in Cultivated Limbal Stem Cell Transplant Rejection. Ocul. Immunol. Inflamm. 2012, 20, 381–383. [Google Scholar] [CrossRef][Green Version]
- Dekaris, I.; Gabrić, N.; Drača, N.; Pauk-Gulić, M.; Miličić, N. Three-year corneal graft survival rate in high-risk cases treated with subconjunctival and topical bevacizumab. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 253, 287–294. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, X.; Wang, S.; Han, K.; Ema, M.; Adams, S.; Adams, R.H.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Prox1-GFP/Flt1-DsRed transgenic mice: An animal model for simultaneous live imaging of angiogenesis and lymphangiogenesis. Angiogenesis 2017, 20, 581–598. [Google Scholar] [CrossRef]
- Masood, F.; Bhattaram, R.; Rosenblatt, M.I.; Kazlauskas, A.; Chang, J.-H.; Azar, D.T. Lymphatic Vessel Regression and Its Therapeutic Applications: Learning From Principles of Blood Vessel Regression. Front. Physiol. 2022, 13, 846936. [Google Scholar] [CrossRef] [PubMed]
- Shahriary, A.; Sabzevari, M.; Jadidi, K.; Yazdani, F.; Aghamollaei, H. The Role of Inflammatory Cytokines in Neovascularization of Chemical Ocular Injury. Ocul. Immunol. Inflamm. 2021, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Chauhan, T.; Cordova, D.; Tseng, C.-H.; Deng, S.X. Biomarkers of in vivo limbal stem cell function. Ocul. Surf. 2021, 23, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Angunawela, R.I.; Mehta, J.S.; Daniels, J.T. Ex-vivo ocular surface stem cell therapies: Current techniques, applications, hurdles and future directions. Expert Rev. Mol. Med. 2013, 15, e4. [Google Scholar] [CrossRef] [PubMed]
- Thokala, P.; Singh, A.; Singh, V.K.; Rathi, V.M.; Basu, S.; Singh, V.; MacNeil, S.; Sangwan, V.S. Economic, clinical and social impact of simple limbal epithelial transplantation for limbal stem cell deficiency. Br. J. Ophthalmol. 2021, 106, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Kollerup Madsen, B.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse reactions of dimethyl sulfoxide in humans: A systematic review. F1000Research 2018, 7, 1746. [Google Scholar] [CrossRef]
- Khoo, T.S.; Jamal, R.; Ghani, N.A.A.; Alauddin, H.; Hussin, N.H.; Murad, N.A.A. Retention of Somatic Memory Associated with Cell Identity, Age and Metabolism in Induced Pluripotent Stem (iPS) Cells Reprogramming. Stem Cell Rev. Rep. 2020, 16, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Mollinari, C.; Merlo, D. Direct Reprogramming of Somatic Cells to Neurons: Pros and Cons of Chemical Approach. Neurochem. Res. 2021, 46, 1330–1336. [Google Scholar] [CrossRef]
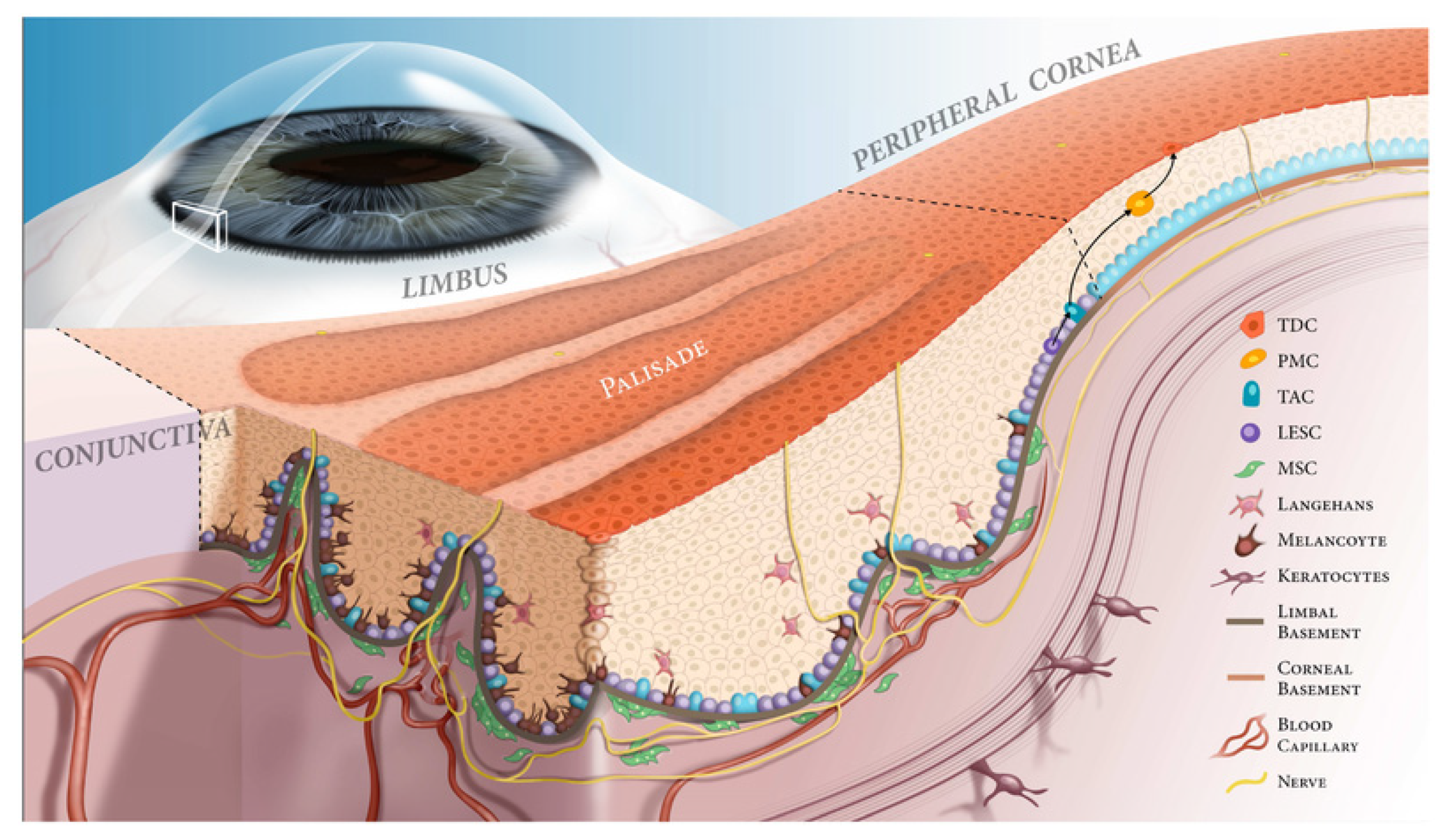
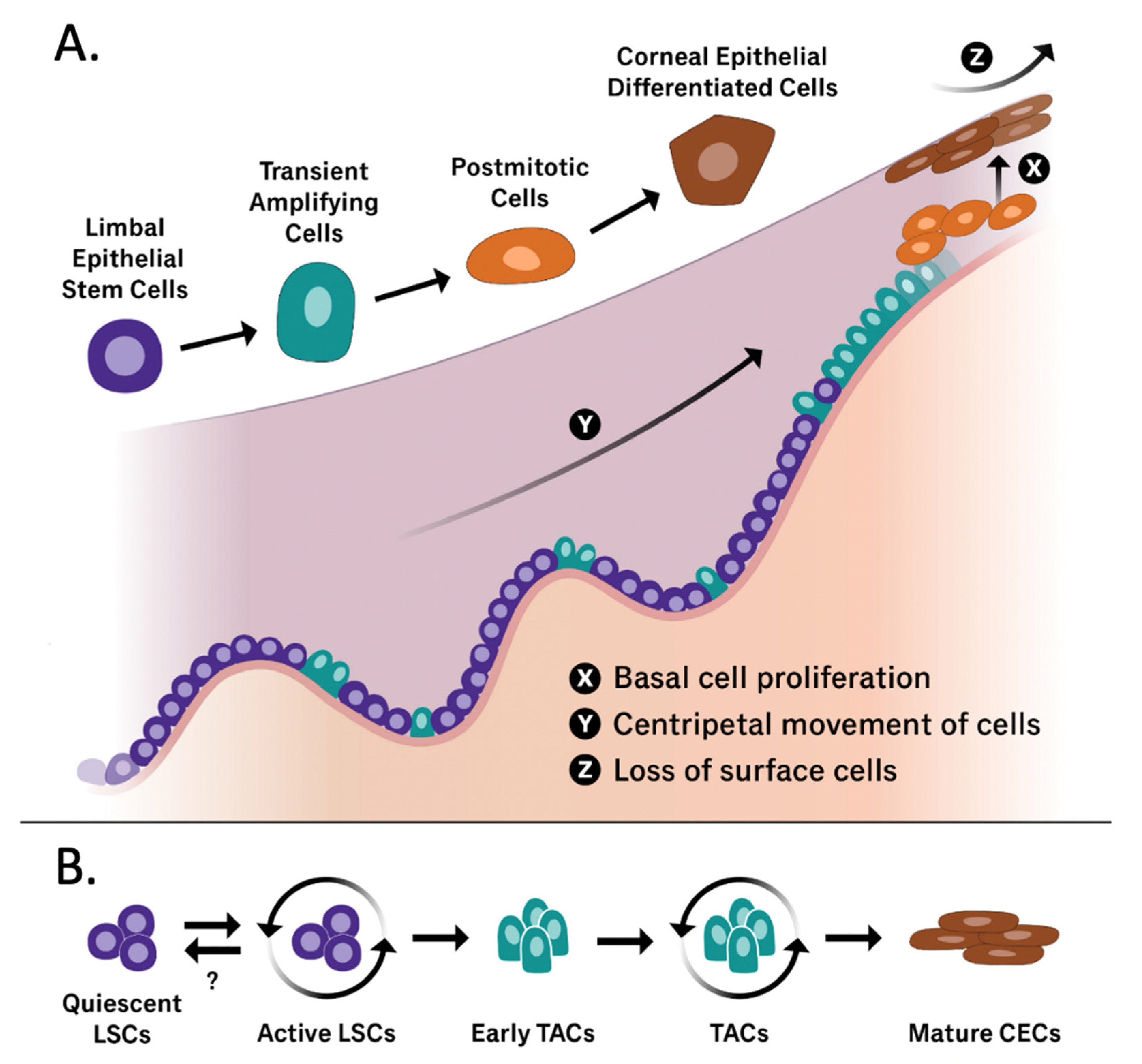
| Marker | Reference | Summary and Application |
|---|---|---|
| K15 | [13] | Cytokeratin 15 (K15) is preferentially expressed by LSCs in the basal layer of the conjunctival epithelium. In mouse models of LCSD, K15 expression is absent in the limbal epithelium. |
| K5 and K14 | [14,15] | Cytokeratin 5 (K5) and cytokeratin 14 (K14) dimerize and are a main constituent of epithelial cytoskeletons. Chen et al. and Zhao et al. independently observed K5 and K14 colocalization in the basal layer of the limbus. As a marker for LSCs, it is not an entirely specific marker, as differentiated cells derived from LSCs maintain K5/K14 expression. |
| ΔNp63α | [1,16,17] | The transcription factor p63 was proposed as a marker for LSCs by Pellegrini et al. Later Rama et al. determined that a higher proportion of p63+ cells in an autologous mixed limbal culture transplant correlates with a greater degree of transplantation success. This finding implies that p63 positivity identifies LSCs. However, nuclear p63 expression dictates that other markers must be utilized for enrichment and isolation of LSCs in culture. |
| ABCG2 | [18,19,20] | ATP-binding cassette, sub-family G, member 2 (ABCG2) is a widely expressed stem cell marker. De Paiva et al. identified ABCG2+ cells among limbal basal cells via immunofluorescence staining, and these same cells were shown to have stem cell properties, confirming their identity as LSCs. |
| ABCB5 | [21] | ATP-binding cassette, sub-family B, member 5 (ABCB5) is a known regulator of cellular differentiation. Ksander et al. identified ABCB5 as a marker for LSCs. Transplanted ABCB5+ cells were able to reconstitute the cornea in LSC-deficient mice, whereas Abcb5 knockout mice had noted depletion of quiescent LSCs. |
| LRIG1 | [22,23] | Leucine-rich repeats and immunoglobulin-like domain protein 1 (LRIG1) expression is proposed to be a marker of epithelial stem cell quiescence. Kaplan et al. utilized single-cell RNA sequencing (scRNA-seq) analysis to identify that a cluster of LSCs and early TACs expressed LRIG1. |
| TXNIP | [22] | Kaplan et al. observed thioredoxin-interacting protein (TXNIP) expression in the scRNQ-seq cluster corresponding to LSCs and early TACs. They proposed that this protein may contribute to maintaining LSC quiescence through G0/G1 cell cycle arrest. |
| Notch-1 | [7,24] | Notch-1 is a transmembrane receptor that widely regulates cell fate and is implicated in stem cell maintenance. Immunohistochemistry analysis by Thomas et al. demonstrated Notch-1 expression in the limbal basal area, mainly in the PV, as well as overlapping expression of Notch-1 and LSC marker ABCG2, suggesting that Notch-1 may be a promising LSC marker. |
| C/EBPδ | [25,26] | CCAAT/enhancer-binding protein (C/EBP) δ is a transcription factor implicated in regulating cellular proliferation and differentiation via G0/G1 cell cycle arrest. Barbaro et al. discovered colocalization of C/EBPδ, ΔNp63α, and BMI1 in quiescent LSCs and showed that forced expression of C/EBPδ could exclusively promote self-renewal of LSCs, suggesting C/EBPδ prevents asymmetric division of these stem cells. |
| Vimentin | [27,28] | Vimentin is the most abundant intermediate filament protein. Vimentin expression has been observed to colocalize with potential LSC markers such as ABCG2 and p63 in the limbal basal area. Although this marker may not provide comprehensive insight on its own, it may prove beneficial in combination with other putative LSC markers. |
| Etiology | Pathophysiology and Clinical Context | Reference |
|---|---|---|
| Contact lens (CL) wear | LSCD in CL wearers is often asymmetric and bilateral, meaning one eye is more affected than the other. Of the estimated 125 million CL wearers worldwide, roughly 2.4–5% of contact lens wearers develop signs of LSCD. LSC niche damage is hypothesized to be multifactorial, due to hypoxia, mechanical trauma, insufficient lubrication, predisposing factors, eyelid anatomy differences, etc. Presentation of LSCD secondary to CL wear is often initially asymptomatic; subsequent symptoms are generally nonspecific, e.g., pain, photophobia, visual impairment, dryness, irritation. | [45] |
| Ectrodactyly-ectodermal dysplasia-clefting (EEC) syndrome | An autosomal dominant inherited illness due to heterozygous mutation in the TP63 gene, involving progressive keratinocyte loss, culminating in LSCD and eventual blindness. Ectoderm-derived structures, such as the hair, teeth, skin, and sweat glands, are often compromised. A common defect is anomaly of the Meibomian glands and subsequent instability of the tear film. | [46,47] |
| Chemical or thermal injury | Most prevalent in males 20–40 years. Non-surgical management (e.g., irrigation, corticosteroids + ascorbic acid, bandage CL, autologous serum, treating high intraocular pressure, tetracycline) to immediately control the inflammation will influence clinical outcomes for the ocular surface and restoration of vision. Sufficient damage to LSCs or niche disarray will culminate in LSCD. After the initial healing process, reconstructive processes can be considered. | [48] |
| Stevens-Johnson syndrome | SJS is a type IV hypersensitivity adverse drug reaction with a high mortality rate. associated with SJS include anticonvulsants (phenobarbital, lamogtrigine, carbamazepine), antibiotics (sulphonamides, erythromycin, cefotaxime, cloxacillin, quinolones), and non-steroidal anti-inflammatory drugs (NSAIDs). Erythema, erosion, and pseudomembranes affect the oral, ocular, and genital mucous membranes, which may result in insults to the limbal niche. | [49] |
| Ocular cicatricial pemphigoid (OCP) | An autoimmune ocular disease and type II hypersensitivity response that requires proper management to prevent corneal conjunctivalization, opacification, and irreversible vision loss. Characteristic symptoms included progressive symblepharon (abnormal adhesions between the conjunctiva of the inner surface of the eyelid & conjunctiva of the globe), severely dry eyes, and conjunctival scarring. Twice as prevalent in females vs. males. Topical lubricants for dry eye symptom relief can be used in combination with immunosuppression. The first-line treatment is dapsone, a sulfonamide antibiotic with anti-inflammatory and immuno-modulatory properties. | [50] |
| LSC transplant donor eye | Great care is taken to preserve the donor eye, which can be a patient’s healthy eye if applicable, or the eye of a suitable donor. LSCD can be induced in the donor eye if excessive limbal tissue is removed. Dissection toward the cornea is extended through the limbal PV to obtain stem cells. | [51] |
| LSC transplant recipient eye | Measures are taken to limit inflammation and prevent LSC graft rejection. Post-operative management of inflammation involves topical steroids, preservative-free teardrops, and antibiotics. Tarsorrhaphy (stitching the eyelids closed temporarily) after LSC transplantation can decrease susceptibility to dryness. | [51] |
| Congenital aniridia | Aniridia is a disease in which the iris is partially or completely absent or abnormally developed. Implicated in aniridia-associated LSCD is an abnormality in the PAX6 gene, which has a role in the development of the anterior segment of the eye. Although the corneal epithelium is normal at birth, progressive signs of LSCD become apparent in the range of 20–40 years of age. | [52] |
| Cell Source | Summary | Reference |
|---|---|---|
| Oral mucosa epithelium | Oral mucosa epithelium transplantation into the limbal area was first reported in 2004. In this work, autologous oral mucosal biopsy samples were obtained, and the submucosal connective tissue was manually removed. The harvested mucosa was divided into smaller sections and the oral mucosa cells were enzymatically separated. Isolated oral mucosa cells were then seeded onto a prepared amniotic membrane with a supporting layer of fibroblasts. After 2–3 weeks, the cultured oral mucosa epithelium on an amniotic membrane were confluent and viable for transplantation. Three eyes afflicted by SJS-induced LSCD and three eyes afflicted by chemical burn-induced LSCD received the prepared oral mucosal epithelium transplants. All cases had improved visual acuity at a mean follow-up time of 13.9 months. Mild peripheral neovascularization was observed in all eyes. Additional clinical trials and studies with similar methodology have been published since this initial report. | [58] |
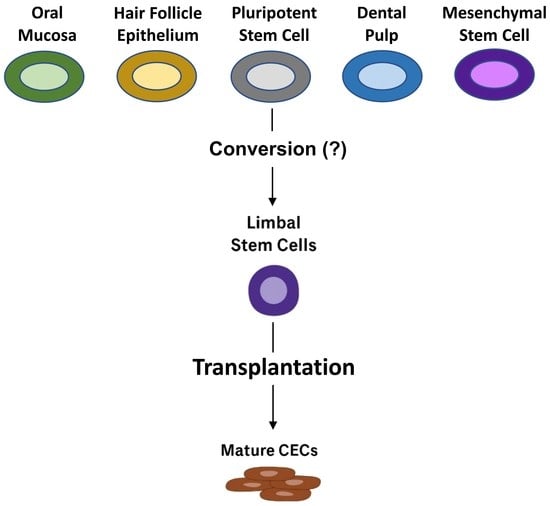
| Hair follicle epithelial stem cells | Adult murine hair follicle epithelial stem cells were harvested and cultured in an in vitro environment mimicking the limbal niche. Three- to five-week-old mouse pups were sacrificed and the upper lip pads containing vibrissae were dissected. After enzymatic digestion of the collagen capsule, hair follicles were isolated and underwent further trypsin digestion to isolate individual cells. Hair follicle epithelial stem cells were isolated via FACS. Isolated hair follicle epithelial stem cells were then expanded on a supporting 3T3 fibroblast layer and subsequently introduced to culture conditions mimicking the limbal niche. Limbal-specific extracellular matrix proteins as well as conditioned media from human limbal and corneal fibroblasts were used to emulate the limbal niche. Microscopy, RT-PCR, immunocytochemistry, and western blotting for putative LSC markers confirmed that hair follicle stem cells transdifferentiated into corneal epithelium-like cells under conditions mimicking the limbal niche. A follow-up study by the same group demonstrated an 80% transdifferentiation success rate in an ex vivo mouse model of LSCD. While these methods provide an in vitro concept of transdifferentiation of hair follicle stem cells into corneal epithelium-like cells, this work has not been extended to humans. | [86] |
| Pluripotent stem cells | A xenogeneic- and supporting feeder cell-free protocol was developed to direct differentiation of human pluripotent stem cells into human LSCs and achieved >65% LSCs in 24 days using either embryonic or induced-pluripotent stem cells. Human pluripotent stem cells were obtained from human embryos and exposed to conditions intended to emulate the limbal niche. In this protocol, Hongisto et al. describe the culture mediums required to induce and differentiate pluripotent stem cells into limbal epithelial stem cells. Furthermore, the authors outline a protocol to cryopreserve and bank the human pluripotent stem cell-derived LSCs, thereby facilitating widespread adoption and dissemination of this technology. This research laid a foundation for subsequent derivation of LSCs from pluripotent stem cells with clear therapeutic implications. | [88] |
| Dental pulp | Monteiro et al. utilized a chemical-burn rabbit model of LSCD and performed superficial keratectomy 30 days post-injury. Experimental groups then received human immature dental pulp stem cell (hIDPSC) transplants while control groups received amniotic membrane. Immunohistochemistry and RT-PCR analyses showed that hIDPSCs expressed putative LSC markers 3 months after transplantation into the limbal niche. Transplanted hIDPSCs also successfully reconstituted the corneal surface epithelium. To prepare the hIDPSC transplants, the authors first isolated and expanded the stem cells. Three days prior to surgery, the hIDPSCs were lifted and seeded directly onto a temperature-responsive cell culture dish at a density of 2 × 106 per dish. On the day of the surgery, the confluent cell sheets were harvested via a change in temperature and this layer was placed directly on the site of superficial keratectomy and covered with acellular human amniotic membrane. In a similar study, Gomes et al. transplanted a sheet of tissue-engineered hIDPSCs covered by an amniotic membrane into the same rabbit model of LSCD and again demonstrated the ability of dental pulp cells to reconstitute the corneal epithelium when transplanted into the limbal niche. This stem cell source has not been applied in human patients. | [87,89] |
| Mesenchymal stem cells | In a 2019 clinical trial, Calonge et al. transplanted allogeneic human bone marrow-derived MSCs into the limbal niche and observed MSC transplantation success rate of 76.5–85.7% at 6-month and 12-month follow-ups. They reported no significant difference in the transplantation success rate between allogeneic MSCs and cultivated limbal epithelial cells. This trial demonstrated the viability of MSC transplantation into the limbal niche to treat LSCD. | [91] |
| Umbilical cord stem cells | In this work, bone marrow was harvested from the iliac crest of allogenic donors. Mesenchymal stem cells were isolated and cultured on human amniotic membrane until 90% confluent. Transplantation involved scraping the corneal-limbal pannus and placing the stem cell amniotic membrane graft cell side down. Transplants were sutured and covered with a bandage contact lens for 4 weeks. | [90] |
| Gene | Reference | Function in LSC Niche |
|---|---|---|
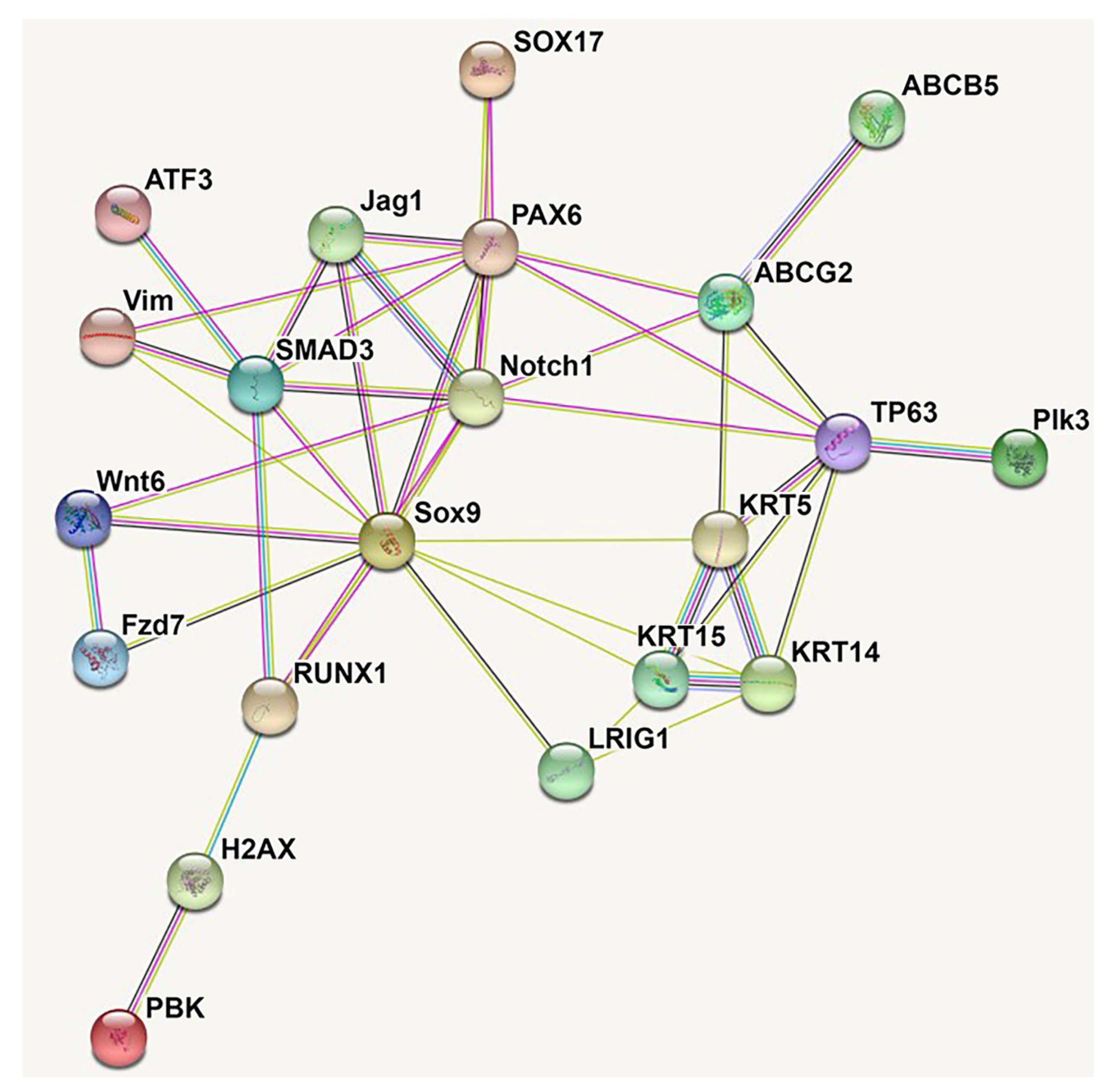
| RUNX1, PAX6, SMAD3 | [99] | Li et al. utilized chromatin accessibility assays and constructed transcription factor interaction networks to elucidate core transcription regulatory circuitries implicated in modulating LSC function. RUNX1 and SMAD3 were found to be important in maintaining the corneal epithelium, and shRNA knockdown of either RUNX1 or SMAD3 notably results in decreased Notch1 and PAX6 expression, which subsequently disrupts LSC phenotype and stemness. In summary, knockdown of either RUNX1 or SMAD3 causes LSCs to transition to keratinized epidermal-like cells. Through modulation of the epigenetic landscape, RUNX1, PAX6, and SMAD3 maintain corneal epithelium identity. RUNX1 is specifically implicated in histone acetylation that increases the transcription of LSC-specific proteins. |
| TP63, Jag1 | [82,101] | Jagged 1 (Jag1) is a protein expressed in human limbal tissue that activates Notch signaling. Gonźalez et al. demonstrated that Notch signaling activation through a recombinant Jag1 ligand decreases the LSC population and drives LSCs towards a mature corneal epithelium phenotype. By arresting mitotic division in culture limbal epithelial cells, Jag1-mediated activation of Notch signaling decreases basal limbal epithelial cell division. Overall, these findings suggest that Jag1-mediated Notch activation decreases LSC stemness, downregulates p63, diminishes the LSC population, and promotes LSC differentiation to a more mature phenotype. Although Ma et al. demonstrated contrary results in 2007, Gonźalez et al. hypothesized this could be due to differing levels of Notch activation due to different delivery systems of Jag1 ligand. |
| ABCB5 | [21] | ATP-binding cassette, sub-family B, member 5 (ABCB5) is a plasma-membrane protein found in humans. Ksander et al. demonstrated that transplanted ABCB5-positive LSCs can reconstitute the corneal epithelium in a mouse model of LSCD. Furthermore, ABCB5 knockout mice demonstrate enhanced LSC proliferation and apoptosis, ultimately resulting in loss of LSCs and perturbed corneal homeostasis characteristic of a LSCD-like phenotype. Overall, these findings suggest that ABCB5 modulates LSC quiescence and survival via anti-apoptotic signaling. |
| SOX9 | [106] | Through transcription factor gene expression profiling, Menzel-Severing et al. suggested that SOX9 is one of the major transcription factors expressed by LSCs. SOX9 is observed in the cytoplasm of basal LSCs and in the nuclei of suprabasal and corneal epithelial cells, suggesting that shunting of SOX9 to the nucleus of LSCs is associated with increased differentiation and activation. Furthermore, increased expression and nuclear localization of SOX9 is found in LSCs undergoing clonal expansion. RNAi knockdown of SOX9 in vitro results in significant upregulation of stem cell and terminal differentiation markers with simultaneously downregulation of markers of progenitor cells. Thus, a delicate signaling balance is believed to exist between SOX9 and Wnt/ß-catenin signaling that determines the fate of LSC quiescence and differentiation. Cytoplasmic SOX9 expression seems to maintain quiescent LSCs, whereas controlled nuclear translocation may promote shunting of LSCs into TACs. |
| TSPAN7, SOX17 | [103] | Li et al. utilized a scRNA-seq platform to identify subpopulations of LSCs that range from quiescent to actively proliferating and differentiating cells. Characterization of the changes in gene expression along this spectrum of quiescence identified TSPAN7 and SOX17 as novel markers of LSCs that may impact stemness and function. RNA silencing of both mRNA products inhibits cellular proliferation and perturbs corneal epithelial regeneration. Activation of these proteins is associated with increased progenitor cell marker expression. Thus, TSPAN7 and SOX17 may be markers of LSCs with the capacity to regenerate and repair corneal epithelium. |
| PBK, H2AX, ATF3 | [22] | Kaplan et al. utilized scRNA-seq in wild-type and autophagy-deficient mice to characterize molecular differences between LSCs, mature TACs, and mature differentiated corneal epithelial cells. Autophagy-deficient mice exhibit altered expression of PBK, H2AX, and ATF3. Overall, autophagy was found to be a positive regulator of LSCs, promoting differentiation and reconstitution of corneal epithelium in wound healing. Autophagy promotes expression of PBK and H2AX, two proteins that seem to promote LSC differentiation and proliferation, and downregulates expression of ATF3, a transcription factor that seems to promote LSC quiescence. Further investigation of the role of ATF3 via siATF3 treatment demonstrated that downregulation of ATF3 results in increased cell growth compared with that of control siRNA-treated cells. An important implication of this work is the potential regulatory role of ATF3 in decreasing LSC proliferation and maintaining quiescence. |
| Wnt6 | [97] | Bonnet et al. utilized 3T3 feeder cells with differential expression of Wnt6 to observe the dose-dependent effect of Wnt6 on LSC proliferation and differentiation. Co-culture of LSCs with supporting cells expressing high levels of Wnt6 results in increased proliferation of LSCs and decreased expression of differentiation markers. In addition to noncanonical Wnt/ß-catenin signaling, Wnt6 was also observed to activate noncanonical signaling in vitro. Bonnet et al. proposed that medium to high levels of Wnt6 expression are essential in promoting LSC self-renewal and stemness, thereby allowing for optimization and modulation of LSCs in culture. |
| Frizzled 7 | [100] | Mei et al. utilized qRT-PCR and immunostaining to profile the expression of various Frizzled receptors in the human limbus, identifying Frizzled 7 (Fz7) receptor as predominantly expressed. Fz7 ligand colocalizes with other LSC markers and not with mature, differentiated corneal epithelium. shRNA knockdown of Fz7 results in significantly decreased expression of LSC markers, such as ABCG2, K14, and ΔNpP63α as well as significantly decreased colony forming efficiency. These results implicate the role of Fz7 in promoting LSC stemness and function. |
| Plk3 | [108] | Wang et al. utilized a hypoxic stress culture platform to study the differential effects of the hypoxia-induced Plk3 signaling pathway on human LSCs and human corneal epithelial cells. Hypoxic conditions seem to promote LSC differentiation via downregulated Plk3 transcription, whereas hypoxic conditions have the opposite effect on mature corneal epithelial cells, resulting in upregulated Plk3 activity with subsequent apoptosis. This research suggests that downregulated Plk3 activity in LSCs as seen under hypoxic stress promotes LSC differentiation and prevents LSC apoptosis. |
| Reference/ID Number | Procedure | Summary | Trial Phase |
|---|---|---|---|
| NCT03957954 | Cultivated LSC transplantation | Cultivated LSC transplantation in uniocular cases of severe-to-total LSCD secondary to injury or ocular surgery as compared to scleral CL control | Phase 1 (Ongoing) |
| NCT03884569 | Cultivated limbal epithelial transplantation | Cultivated limbal epithelial transplantation observational study | N/A (not yet recruiting) |
| NCT03549299 | Pharmacological intervention | Study of the efficacy of investigational medicinal product LSC2 topically administered on eye affected by LSCD. LSC2 contains ABCB5-positive LSCs from cadaveric donors. | Phase 1/2a (Active) |
| NCT02592330 | Cultivated autologous limbal epithelial cell transplantation | LSCs from unaffected eye are harvested, expanded and then subsequently transplanted into the affected eye. | Phase1/2 (Active) |
| NCT01756365 | Cultured corneal epithelium graft transplantation | Autologous corneal epithelium will be cultured to produce a graft of reconstructed corneal epithelium for transplantation. | Phase1/2 (Recruiting) |
| NCT04995926 | Labial mucosal epithelium grafting for corneal limbus substitution | Transplantation of autologous labial mucosal epithelium as a substitute for LSCs, as described by Liu et al., 2011 and Choe et al., 2019. | N/A (Enrolling) |
| NCT03288844 | Cultivated autologous LSC transplantation | Multinational follow-up study of the HOLOCORE trial. Determination of long-term safety and efficacy after autologous cultivated LSC transplantation | N/A (Recruiting) |
| NCT04021134 | Allogeneic simple limbal epithelial transplantation | Observational study investigating the effects of allogeneic simple limbal epithelial transplantation | N/A (Recruiting) |
| NCT04021875 | Autologous simple limbal epithelial transplantation | Observational study investigating the effects of autologous simple limbal epithelial transplantation | N/A (Recruiting) |
| NCT03943797 | Cultivated autologous oral mucosa epithelial sheet | Evaluation of the efficacy of a protocol using collagenase instead of trypsin/EDTA to isolate and cultivate oral mucosal epithelial cells | Phase 1 (Recruiting) |
| NCT03949881 | Cultivated autologous oral mucosa epithelial sheet | Evaluation of the efficacy and patient tolerance of cultivated autologous oral mucosa epithelial sheets in bilateral cases of total LSCD with the use of collagenase as opposed to trypsin/EDTA. | Phase 2 (Recruiting) |
| NCT04932629 | LSC application for treatment of superficial corneal pathologies | Transplantation of ex-vivo cultivated allogeneic limbal stromal cells for patients with unilateral superficial corneal scars | Phase 1 (Not yet recruiting) |
| NCT02886611 | Non-therapeutic investigation | Investigation of genotype–phenotype correlation in patients with genetic etiologies of LSCD | N/A (Recruiting) |
| Completed | |||
| NCT02577861 | Holoclar: cultivated autologous LSC transplantation | Validation of the efficacy of Holoclar for patients with moderate-to-severe LSCD secondary to ocular burn at 1 year post-procedure | Phase 4 (Completed) |
| NCT03226015 | Autologous oral mucosa transplantation | Clinical and histochemical results after oral mucosa graft transplantation in eyes with LSCD | N/A (Prospective, Completed) |
| NCT02649621 | Pharmacological investigation | Prospective clinical trial comparing the improvement of LSCD in vivo after use of Amniotic Membrane Extract Eye Drop (AMEED) | Phase 1 (Completed) |
| NCT00736307 | Cultivated autologous LSC transplantation | Evaluation of the efficacy, safety, and long-term outcomes of transplantation of ex vivo cultured LSCs on amniotic membrane for corneal surface reconstruction in cases of severe LSCD | Phase 2 (Completed) |
| NCT02415218 | Cultivated autologous oral mucosa epithelial sheet | Evaluation of the efficacy and safety of cultivated oral mucosal epithelial sheet transplantation for LSCD therapy | Phase 2 (Completed) |
| NCT03217435 | Epithelial allograft transplantation from living-related donor | Comparison of efficacy of femtosecond laser-assisted corneal epithelial allograft from living-related donor vs. limbal conjunctival allograft from living-related donor for ocular surface reconstruction in patients with LSCD | N/A (Completed) |
| NCT02568527 | Simple limbal epithelial transplantation | Pilot safety and efficacy study of a synthetic biodegradable membrane as a substitute for donor human amniotic membrane [122] for LSCD treatment in combination with limbal tissue freshly excised in theatre as a one-stage procedure | N/A (Completed) |
| NCT03594370 | Non-therapeutic investigation | Study of the ability of optical coherence tomography (OCT) analysis to predict the condition of limbal epithelial stem cells as a potential patient-friendly tool to detect limbal conditions | N/A (Completed) |
| NCT04773431 | Cultivated autologous limbal epithelial cell sheet transplantation | Evaluation of the tolerability and safety of LSCD101 (cultured autologous limbal epithelial cell sheet) transplantation in patients with intractable LSCD | Phase 1 (Completed) |
| NCT01619189 | Cultivated LSC transplantation | Prospective, non-comparative monocentric study of transplantation of allogeneic or autologous LSCs cultured on human amniotic membrane [122] with no feeder cells in eyes with total limbal deficiency | Phase 2 (Completed) |
| NCT04484402 | Autologous LSC transplantation | Treatment of inflammatory-dystrophic corneal diseases using autologous LSCs (corneal epithelial stem cells) or adipose-derived MSCs | Phase 2 (Completed) |
| NCT04552730 | Pharmacological investigation | Study of the efficacy and safety of nerve growth factor in the treatment of LSCD associated with neurotrophic cornea | N/A (Completed) |
| NCT00265590 | Non-therapeutic investigation | Study of specific gene changes in patients with aniridia, a disease in which the iris is fully or partially absent, focusing particularly on corneal changes | N/A (Completed) |
| NCT01237600 | Cultivated LSC transplantation | Study to elucidate the appropriate conditions for developing cultivated corneal epithelial grafts and to evaluate transplantation outcomes | Phase 3 (Completed) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masood, F.; Chang, J.-H.; Akbar, A.; Song, A.; Hu, W.-Y.; Azar, D.T.; Rosenblatt, M.I. Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions. Cells 2022, 11, 3247. https://doi.org/10.3390/cells11203247
Masood F, Chang J-H, Akbar A, Song A, Hu W-Y, Azar DT, Rosenblatt MI. Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions. Cells. 2022; 11(20):3247. https://doi.org/10.3390/cells11203247
Chicago/Turabian StyleMasood, Faisal, Jin-Hong Chang, Anosh Akbar, Amy Song, Wen-Yang Hu, Dimitri T. Azar, and Mark I. Rosenblatt. 2022. "Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions" Cells 11, no. 20: 3247. https://doi.org/10.3390/cells11203247
APA StyleMasood, F., Chang, J.-H., Akbar, A., Song, A., Hu, W.-Y., Azar, D. T., & Rosenblatt, M. I. (2022). Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions. Cells, 11(20), 3247. https://doi.org/10.3390/cells11203247