A Combined Angelica gigas and Artemisia dracunculus Extract Prevents Dexamethasone-Induced Muscle Atrophy in Mice through the Akt/mTOR/FoxO3a Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CHDT
2.2. Animals and Treatments
2.3. Reagents
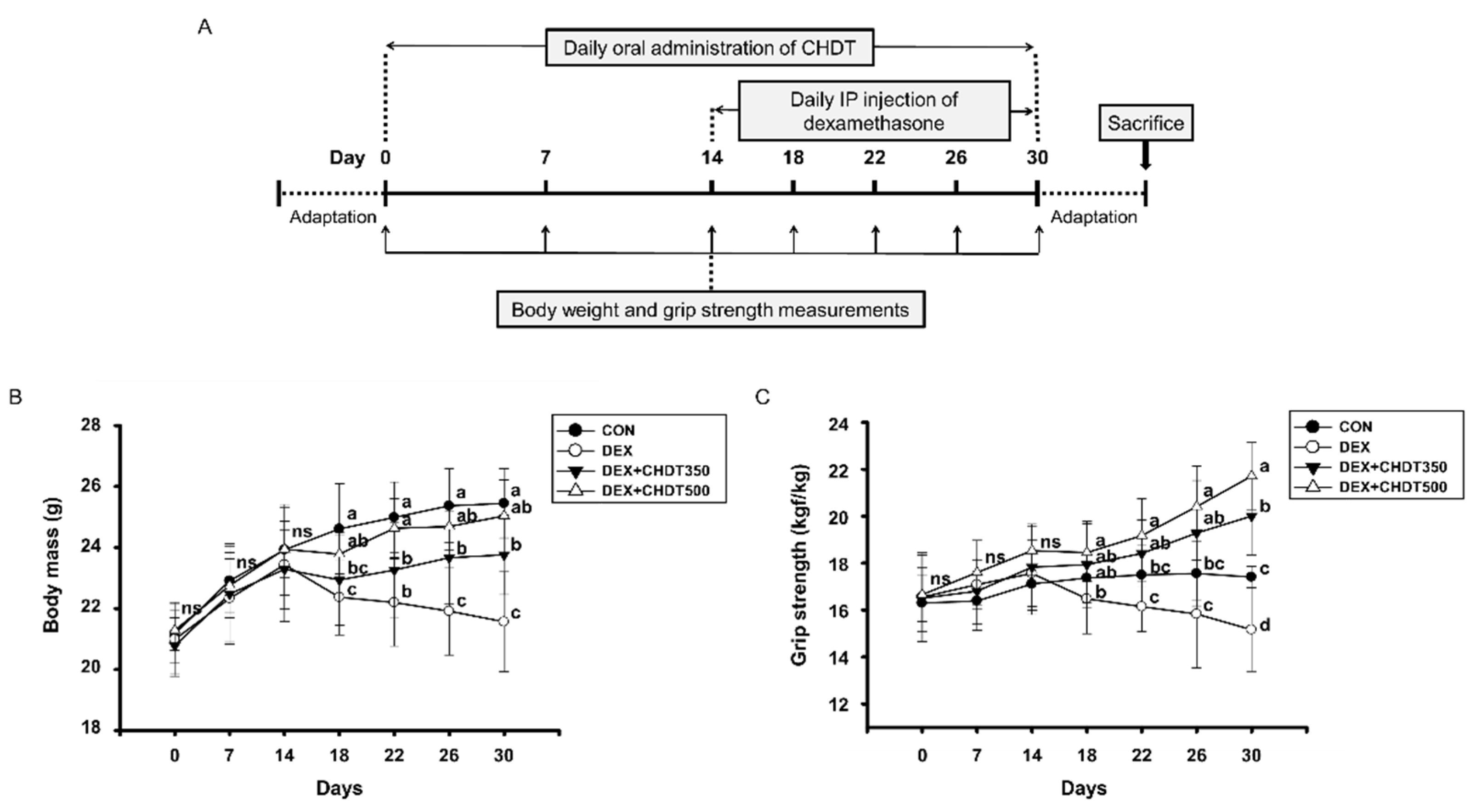
2.4. Measurement of Body Mass and Grip Strength
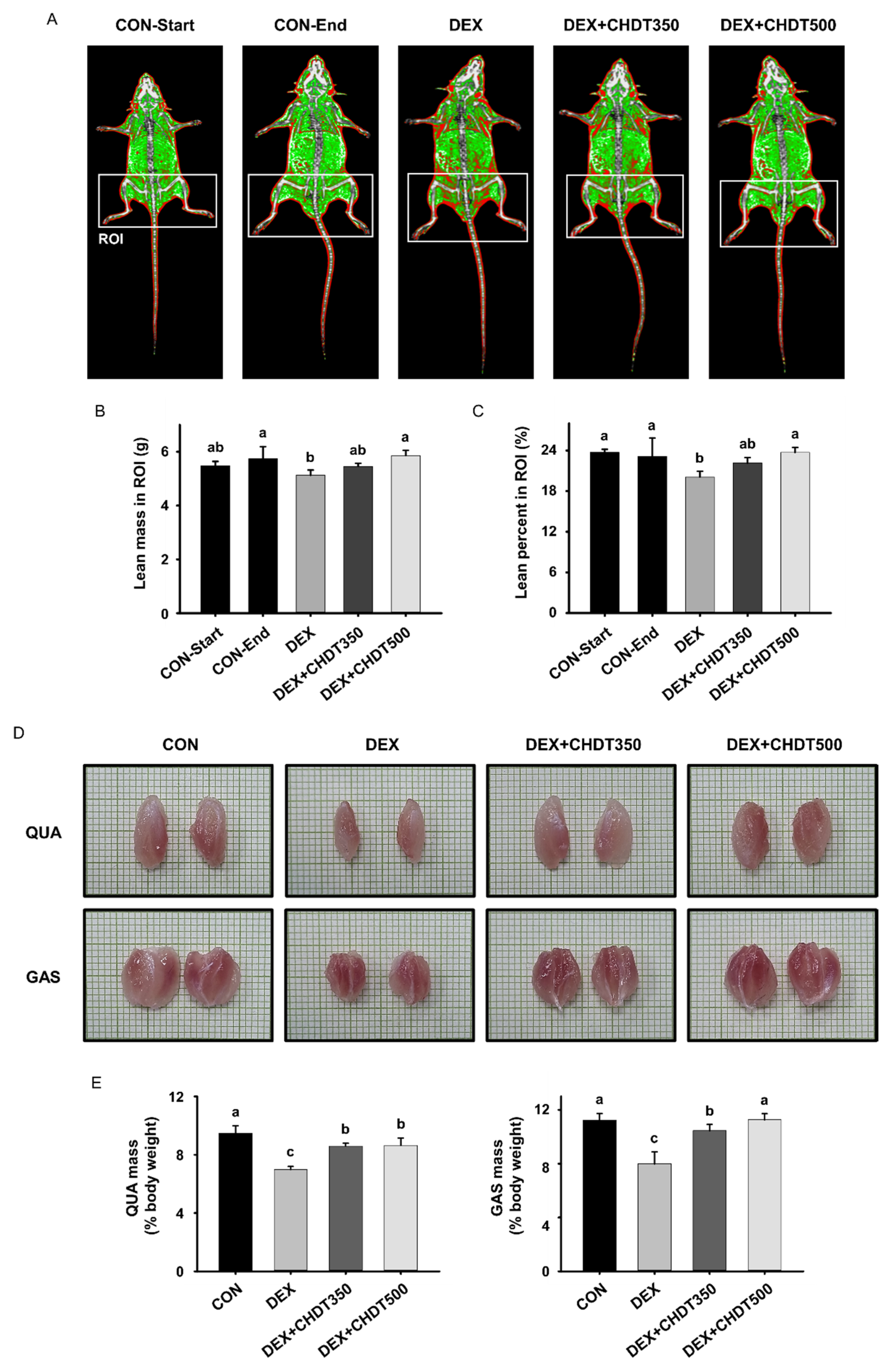
2.5. Measurement of Lean Mass
2.6. Western Blot Analysis
2.7. Histological Analysis
2.8. Serum Analysis
2.9. Statistical Analysis
3. Results
3.1. Analysis of CHDT
3.2. CHDT Ameliorates the Dex-Induced Loss of Body Mass and Muscle Strength of the Mice
3.3. CHDT Inhibits the Dex-Induced Loss of Lean Mass and Muscle Mass
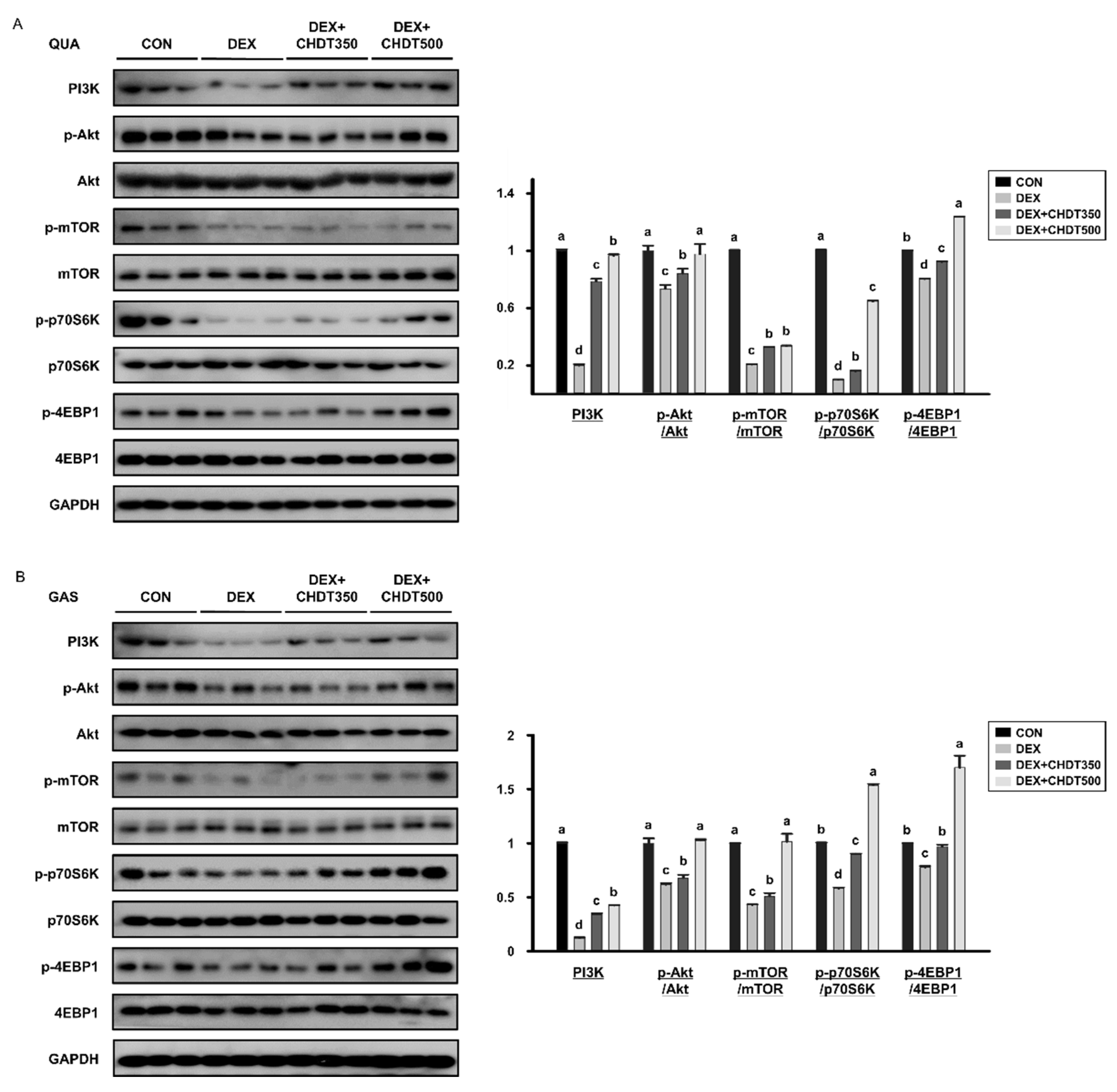
3.4. CHDT Activates the Akt/mTOR Signaling Pathway
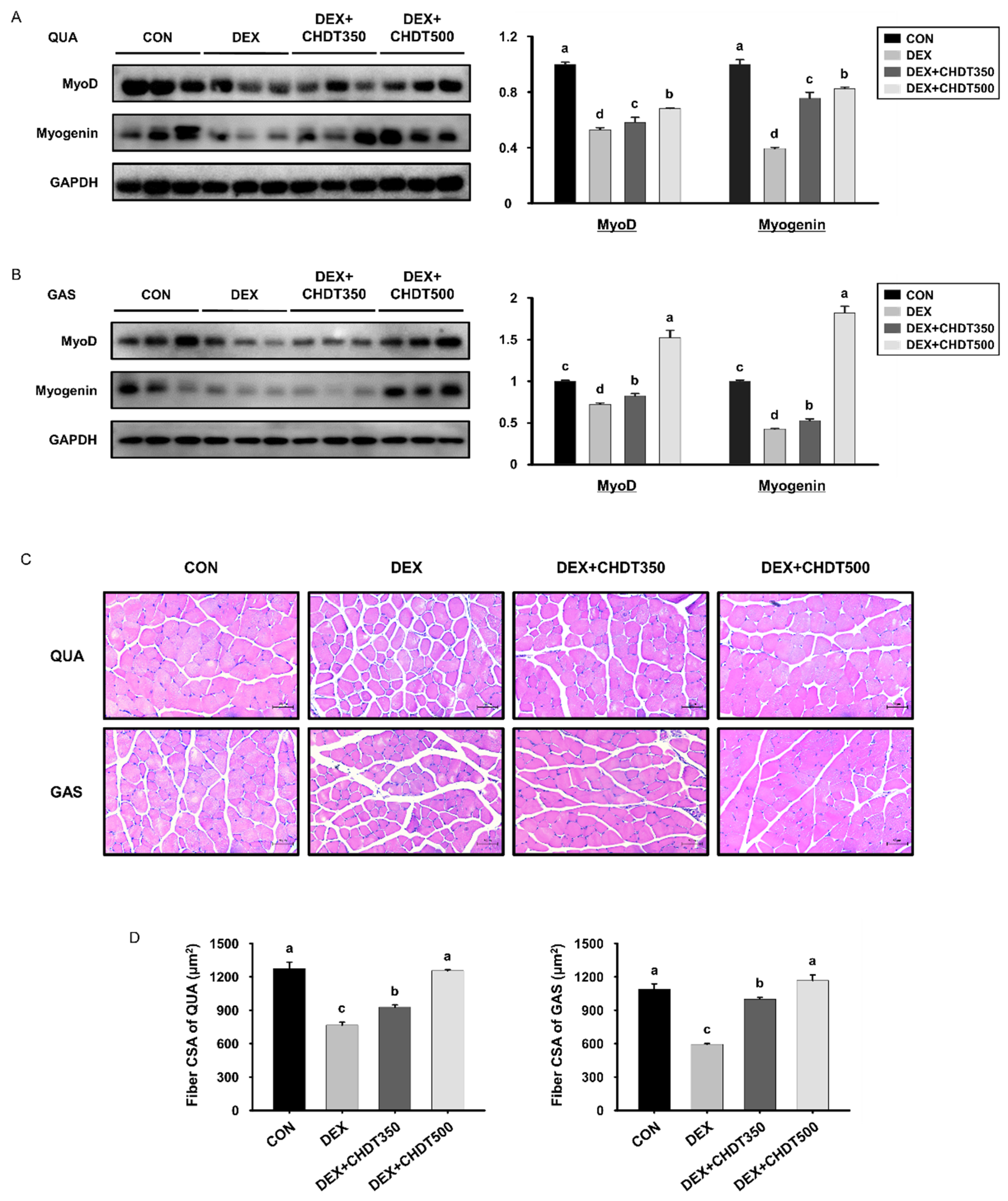
3.5. CHDT Restores the Expression of Myogenic Transcription Factors and Muscle Fiber Size in Dex-Treated Mice
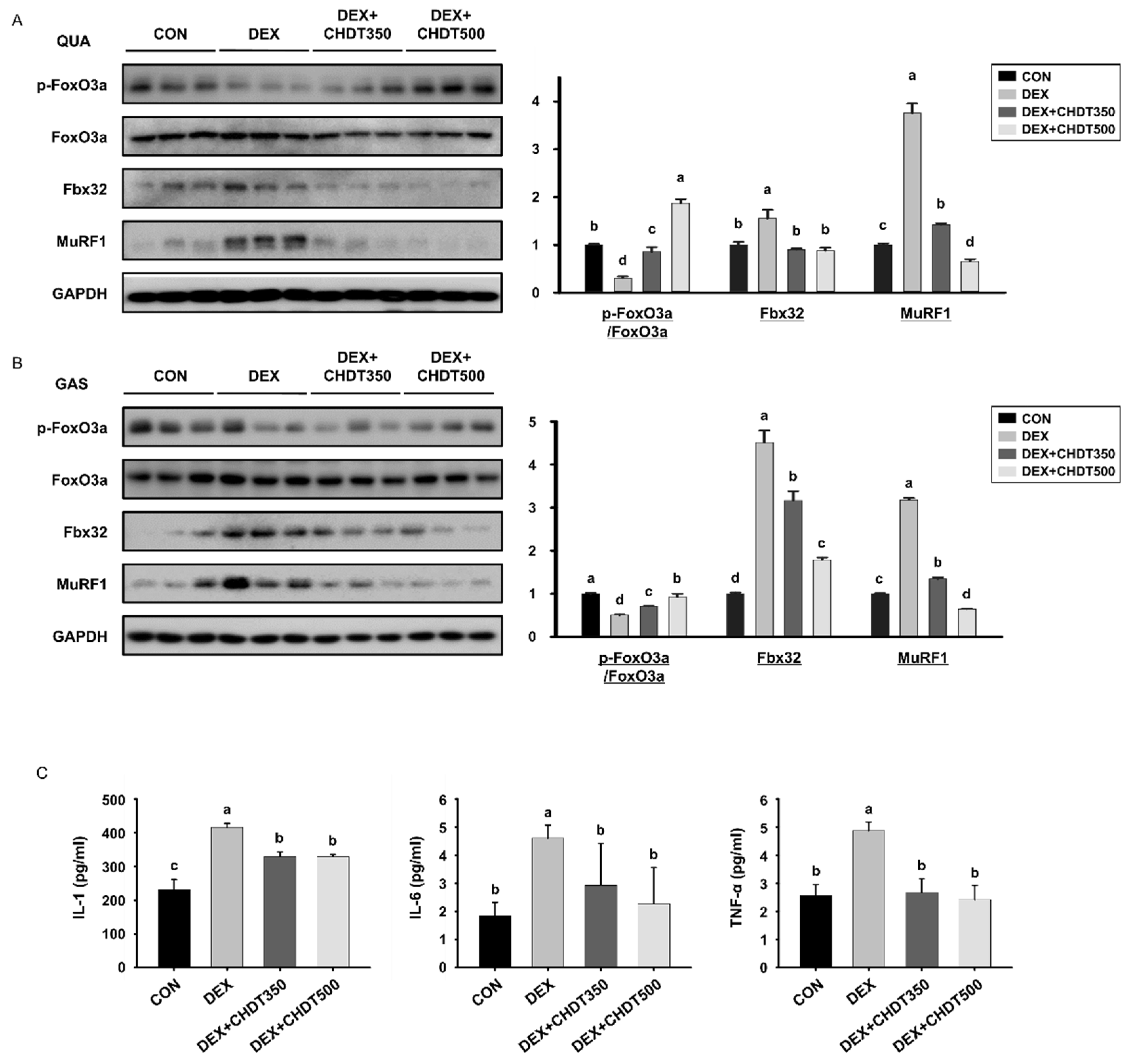
3.6. CHDT Ncreases the Hosphorylation of FoxO3a and Reduces the Circulating Concentrations of Pro-Inflammatory Cytokines in Dex-Treated Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abiri, B.; Vafa, M. Nutrition and sarcopenia: A review of the evidence of nutritional influences. Crit. Rev. Food Sci. Nutr. 2019, 59, 1456–1466. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.L.; Addison, O.; Dibble, L.E.; Foreman, K.B.; Morrell, G.; Lastayo, P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J. Aging Res. 2012, 2012, 629637. [Google Scholar] [CrossRef]
- Fry, C.S.; Rasmussen, B.B. Skeletal muscle protein balance and metabolism in the elderly. Curr. Aging Sci. 2011, 4, 260–268. [Google Scholar] [CrossRef]
- Kim, I.Y.; Park, S.; Jang, J.; Wolfe, R.R. Understanding Muscle Protein Dynamics: Technical Considerations for Advancing Sarcopenia Research. Ann. Geriatr. Med. Res. 2020, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Sitzmann, J.M.; Dastidar, S.G.; Rodriguez, A.A.; Vu, S.L.; McDonald, C.E.; Academia, E.C.; O’Leary, M.N.; Ashe, T.D.; La Spada, A.R. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J. Clin. Investig. 2015, 125, 2952–2964. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Miao, L.; Liang, F.; Huang, H.; Teng, X.; Li, S.; Nuriddinov, J.; Selzer, M.E.; Hu, Y. The mTORC1 effectors S6K1 and 4E-BP play different roles in CNS axon regeneration. Nat. Commun. 2014, 5, 5416. [Google Scholar] [CrossRef]
- Macpherson, P.C.; Wang, X.; Goldman, D. Myogenin regulates denervation-dependent muscle atrophy in mouse soleus muscle. J. Cell. Biochem. 2011, 112, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Legendre, N.P.; Biswas, A.A.; Lawton, A.; Yamamoto, S.; Tajbakhsh, S.; Kardon, G.; Goldhamer, D.J. Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration. Stem Cell Rep. 2018, 10, 956–969. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Shiozuka, M.; Takayama, Y.; Hoshino, T.; Mabuchi, K.; Matsuda, R. Effects of ageing on expression of the muscle-specific E3 ubiquitin ligases and Akt-dependent regulation of Foxo transcription factors in skeletal muscle. Mol. Cell. Biochem. 2016, 412, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Santo, E.E.; Stroeken, P.; Sluis, P.V.; Koster, J.; Versteeg, R.; Westerhout, E.M. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res. 2013, 73, 2189–2198. [Google Scholar] [CrossRef]
- Rong, Y.D.; Bian, A.L.; Hu, H.Y.; Ma, Y.; Zhou, X.Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef]
- Spate, U.; Schulze, P.C. Proinflammatory cytokines and skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 265–269. [Google Scholar] [CrossRef]
- Axelrod, L. Glucocorticoid therapy. Medicine 1976, 55, 39–65. [Google Scholar] [CrossRef]
- Shimizu, N.; Yoshikawa, N.; Ito, N.; Maruyama, T.; Suzuki, Y.; Takeda, S.; Nakae, J.; Tagata, Y.; Nishitani, S.; Takehana, K. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011, 13, 170–182. [Google Scholar] [CrossRef]
- Braun, T.P.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Long, W.; Wei, L.; Barrett, E.J. Dexamethasone inhibits the stimulation of muscle protein synthesis and PHAS-I and p70 S6-kinase phosphorylation. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E570–E575. [Google Scholar]
- Zhang, J.; Li, L.; Jiang, C.; Xing, C.; Kim, S.H.; Lu, J. Anti-cancer and other bioactivities of Korean Angelica gigas Nakai (AGN) and its major pyranocoumarin compounds. Anticancer Agents Med. Chem. 2012, 12, 1239–1254. [Google Scholar] [CrossRef]
- Cho, J.H.; Kwon, J.E.; Cho, Y.; Kim, I.; Kang, S.C. Anti-Inflammatory Effect of Angelica gigas via Heme Oxygenase (HO)-1 Expression. Nutrients 2015, 7, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.O.; Lee, I.; Paik, S.Y.; Kim, D.E.; Lim, J.D.; Kang, W.S.; Ko, S. Ultrafine Angelica gigas powder normalizes ovarian hormone levels and has antiosteoporosis properties in ovariectomized rats: Particle size effect. J. Med. Food 2012, 15, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, J.; Cai, J.; Kim, Y.; Shin, K.; Kim, T.S.; Lee, S.P.; Park, S.K.; Choi, E.K.; Kim, Y.B. An ethanolic extract of Angelica gigas improves atherosclerosis by inhibiting vascular smooth muscle cell proliferation. Lab. Anim. Res. 2014, 30, 84–89. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Yu, J.; Ji, B.; Yang, Y. Decursin inhibits the oxidation of low-density lipoprotein and protects human aortic endothelial cells against oxidative damage. Cell. Mol. Biol. 2020, 66, 155–158. [Google Scholar] [CrossRef]
- Eidi, A.; Oryan, S.; Zaringhalam, J.; Rad, M. Antinociceptive and anti-inflammatory effects of the aerial parts of Artemisia dracunculus in mice. Pharm. Biol. 2016, 54, 549–554. [Google Scholar] [CrossRef]
- Zarezade, V.; Moludi, J.; Mostafazadeh, M.; Mohammadi, M.; Veisi, A. Antioxidant and hepatoprotective effects of Artemisia dracunculus against CCl4-induced hepatotoxicity in rats. Avicenna J. Phytomed. 2018, 8, 51–62. [Google Scholar]
- Ribnicky, D.M.; Poulev, A.; Watford, M.; Cefalu, W.T.; Raskin, I. Antihyperglycemic activity of Tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine 2006, 13, 550–557. [Google Scholar] [CrossRef]
- Taylor, M.V. Muscle development: A transcriptional pathway in myogenesis. Curr. Biol. 1998, 8, R356–R358. [Google Scholar] [CrossRef][Green Version]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef]
- Ackermans, L.; Rabou, J.; Basrai, M.; Schweinlin, A.; Bischoff, S.C.; Cussenot, O.; Cancel-Tassin, G.; Renken, R.J.; Gomez, E.; Sanchez-Gonzalez, P.; et al. Screening, diagnosis and monitoring of sarcopenia: When to use which tool? Clin. Nutr. ESPEN 2022, 48, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.J.; Jeon, Y.K.; Kim, I.J. Testosterone and Sarcopenia. World J. Mens Health 2018, 36, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Desler, M.M.; Jones, S.J.; Smith, C.W.; Woods, T.L. Effects of dexamethasone and anabolic agents on proliferation and protein synthesis and degradation in C2C12 myogenic cells. J. Anim. Sci. 1996, 74, 1265–1273. [Google Scholar] [CrossRef]
- Hong, D.H.; Forsberg, N.E. Effects of dexamethasone on protein degradation and protease gene expression in rat L8 myotube cultures. Mol. Cell. Endocrinol. 1995, 108, 199–209. [Google Scholar] [CrossRef]
- Yang, H.; Wei, W.; Menconi, M.; Hasselgren, P.O. Dexamethasone-induced protein degradation in cultured myotubes is p300/HAT dependent. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R337-4. [Google Scholar] [CrossRef]
- Langendorf, E.K.; Rommens, P.M.; Drees, P.; Mattyasovszky, S.G.; Ritz, U. Detecting the Effects of the Glucocorticoid Dexamethasone on Primary Human Skeletal Muscle Cells-Differences to the Murine Cell Line. Int. J. Mol. Sci. 2020, 21, 2497. [Google Scholar] [CrossRef]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Huang, H.; Tindall, D.J. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim. Biophys. Acta 2011, 1813, 1961–1964. [Google Scholar] [CrossRef]
- Audesse, A.J.; Dhakal, S.; Hassell, L.A.; Gardell, Z.; Nemtsova, Y.; Webb, A.E. FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. PLoS Genet. 2019, 15, e1008097. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy is required to maintain muscle mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Saneyasu, T.; Ogasawara, K.; Fujiwara, Y.; Honda, K.; Kamisoyama, H. Atrogin-1 knockdown inhibits the autophagy-lysosome system in mammalian and avian myotubes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 271, 111262. [Google Scholar] [CrossRef]
- Ding, W.; Jiang, J.; Xu, J.; Cao, Y.; Xu, L. MURF contributes to skeletal muscle atrophy through suppressing autophagy. Int. J. Clin. Exp. Pathol. 2017, 10, 11075–11079. [Google Scholar] [PubMed]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef] [PubMed]
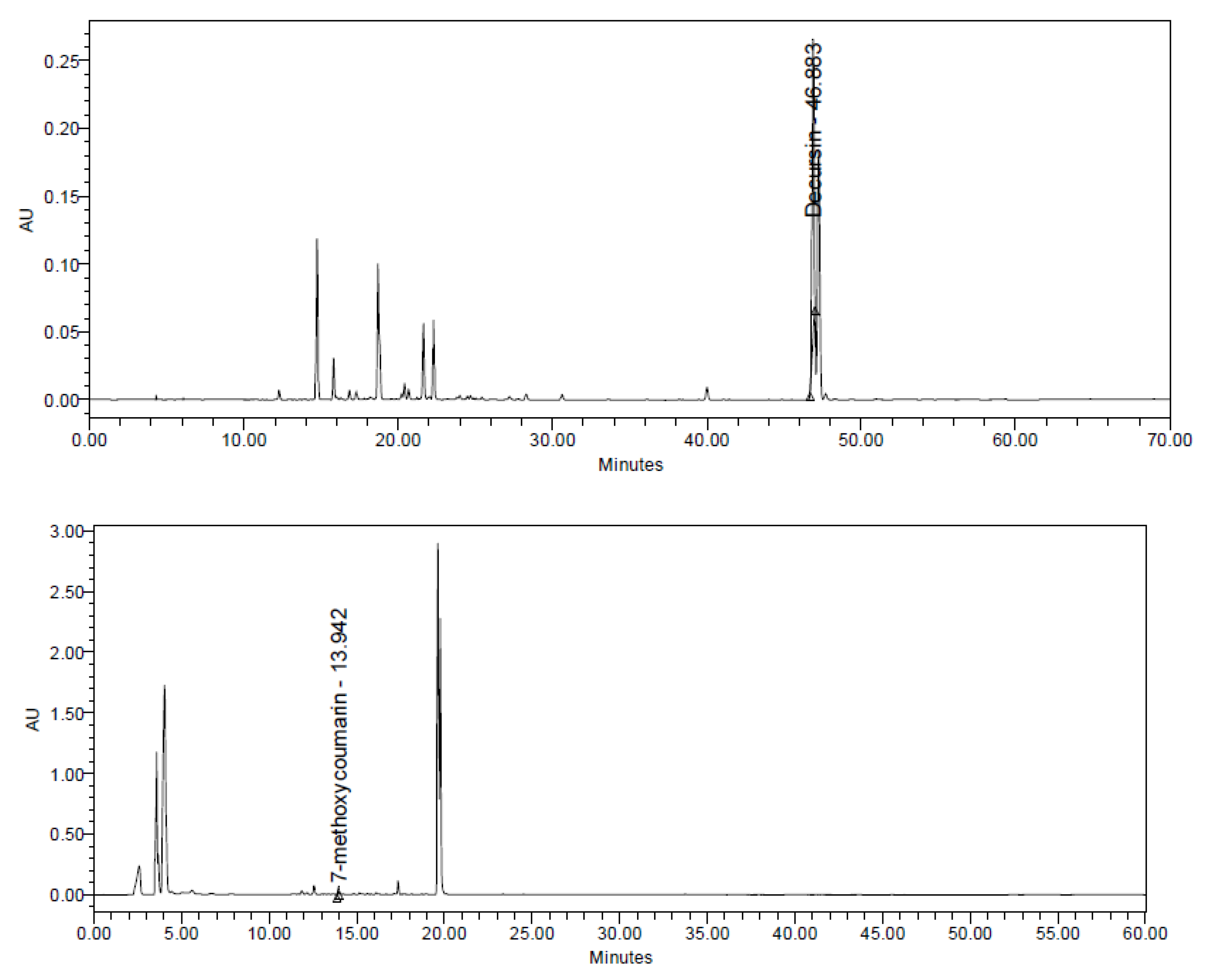
| Component | Content (%) |
|---|---|
| Crude protein | 3.22 |
| Carbohydrate | 83.70 |
| Crude fat | 5.80 |
| Ash | 4.17 |
| Moisture | 3.11 |
| Total | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.-J.; Jin, H.; Kim, B.-Y.; Lee, O.-H.; Lee, B.-Y. A Combined Angelica gigas and Artemisia dracunculus Extract Prevents Dexamethasone-Induced Muscle Atrophy in Mice through the Akt/mTOR/FoxO3a Signaling Pathway. Cells 2022, 11, 3245. https://doi.org/10.3390/cells11203245
Oh H-J, Jin H, Kim B-Y, Lee O-H, Lee B-Y. A Combined Angelica gigas and Artemisia dracunculus Extract Prevents Dexamethasone-Induced Muscle Atrophy in Mice through the Akt/mTOR/FoxO3a Signaling Pathway. Cells. 2022; 11(20):3245. https://doi.org/10.3390/cells11203245
Chicago/Turabian StyleOh, Hyun-Ji, Heegu Jin, Byung-Yong Kim, Ok-Hwan Lee, and Boo-Yong Lee. 2022. "A Combined Angelica gigas and Artemisia dracunculus Extract Prevents Dexamethasone-Induced Muscle Atrophy in Mice through the Akt/mTOR/FoxO3a Signaling Pathway" Cells 11, no. 20: 3245. https://doi.org/10.3390/cells11203245
APA StyleOh, H.-J., Jin, H., Kim, B.-Y., Lee, O.-H., & Lee, B.-Y. (2022). A Combined Angelica gigas and Artemisia dracunculus Extract Prevents Dexamethasone-Induced Muscle Atrophy in Mice through the Akt/mTOR/FoxO3a Signaling Pathway. Cells, 11(20), 3245. https://doi.org/10.3390/cells11203245








