The Effect of Heat Shock on Myogenic Differentiation of Human Skeletal-Muscle-Derived Mesenchymal Stem/Stromal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. The Isolation and Cultivation of Total SM-MSCs Population
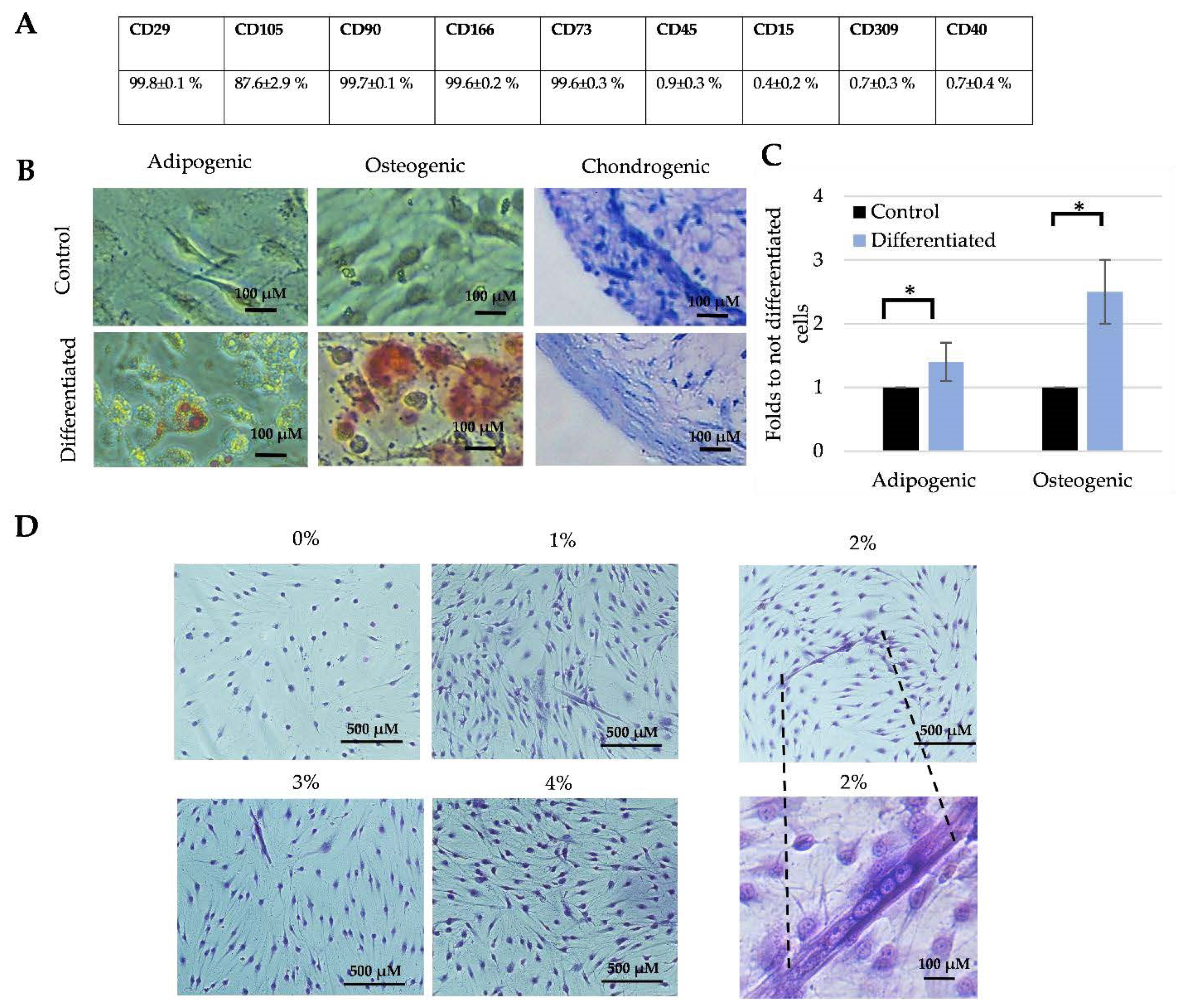
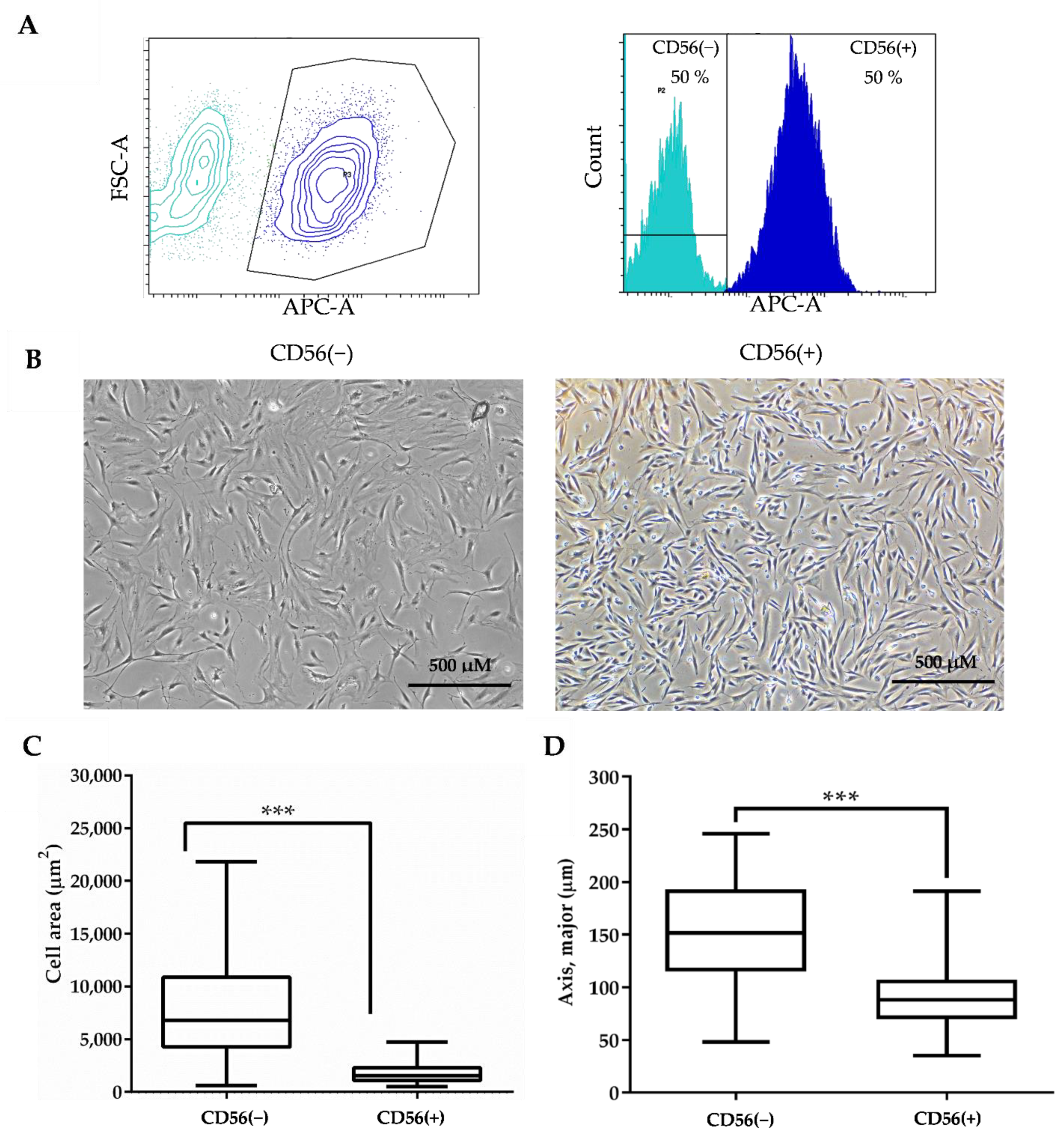
2.2. Flow Cytometry Analysis
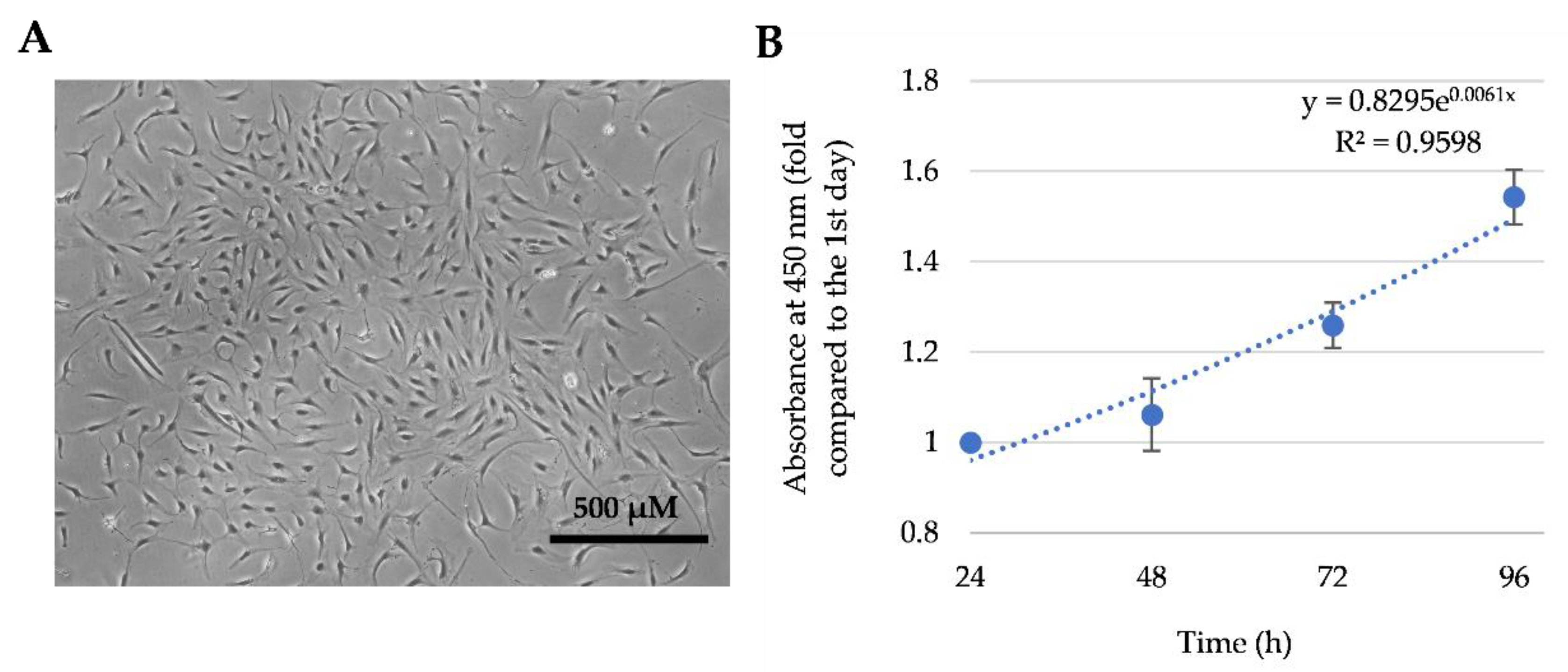
2.3. Cell Proliferation Measurement
2.4. Differentiation to the MSCs Typical Directions
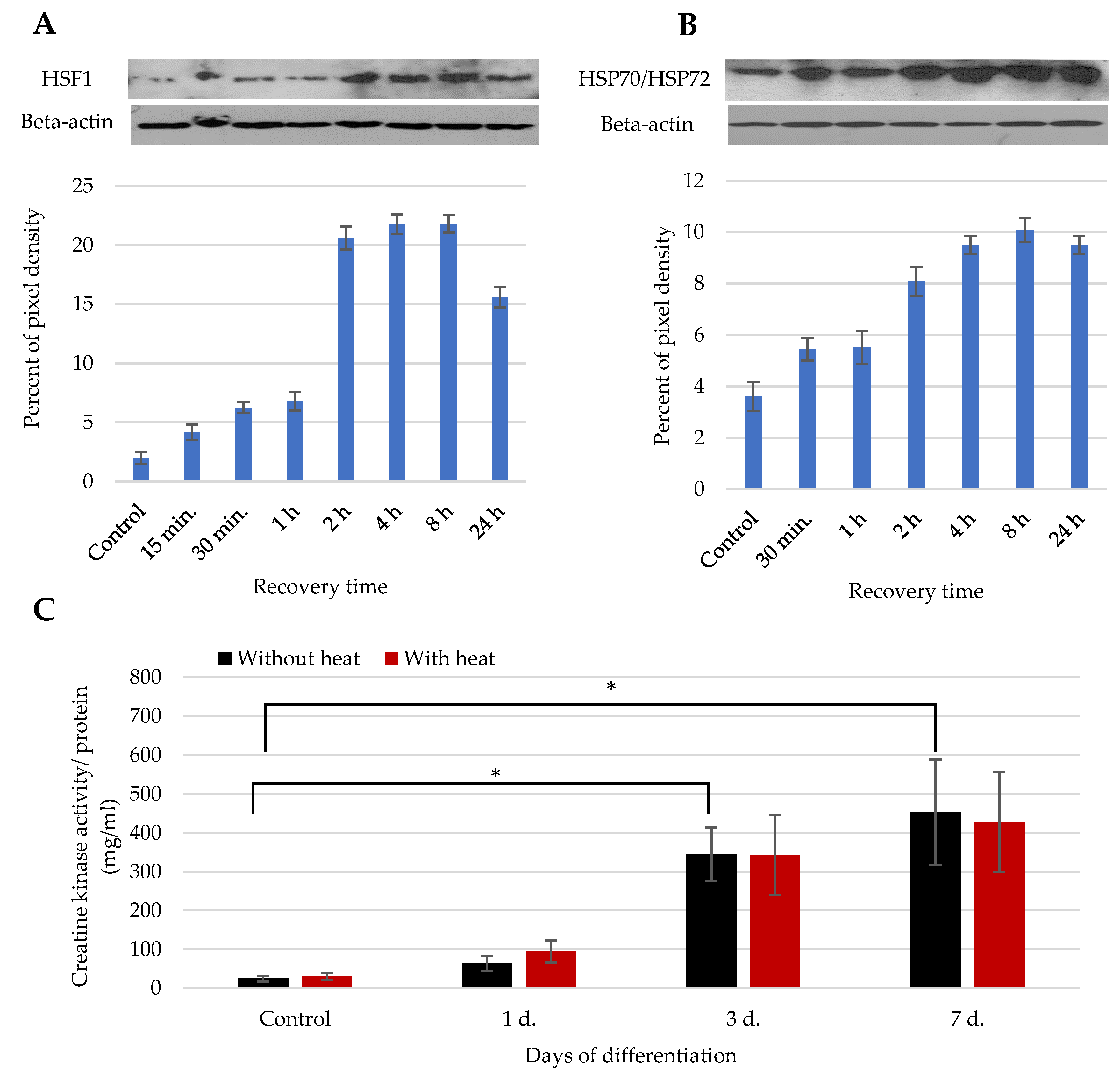
2.5. External Heat Stress/Stimulus and Myogenic Differentiation
2.6. Gene Expression Analysis
2.7. Measurement of Creatine Kinase Activity
2.8. Western Blotting
2.9. Imunocitochemistry
2.10. Statistical Analysis
3. Results
3.1. The Characterization of Total SM-MSCs Population
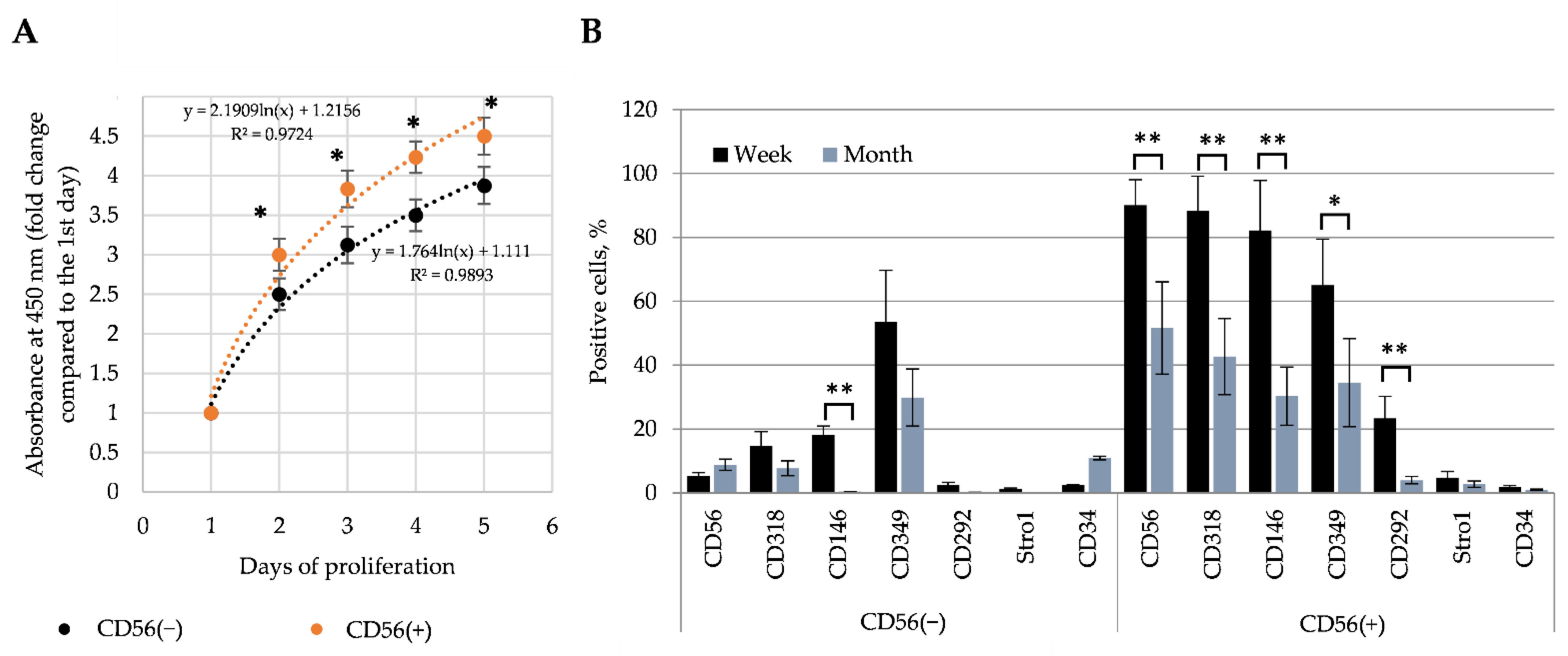
3.2. The Impact of Heat Stimulus on the Total SM-MSCs Population
3.3. Sorting and Identification of CD56(−) and CD56(+) SM-MSCs Subpopulations
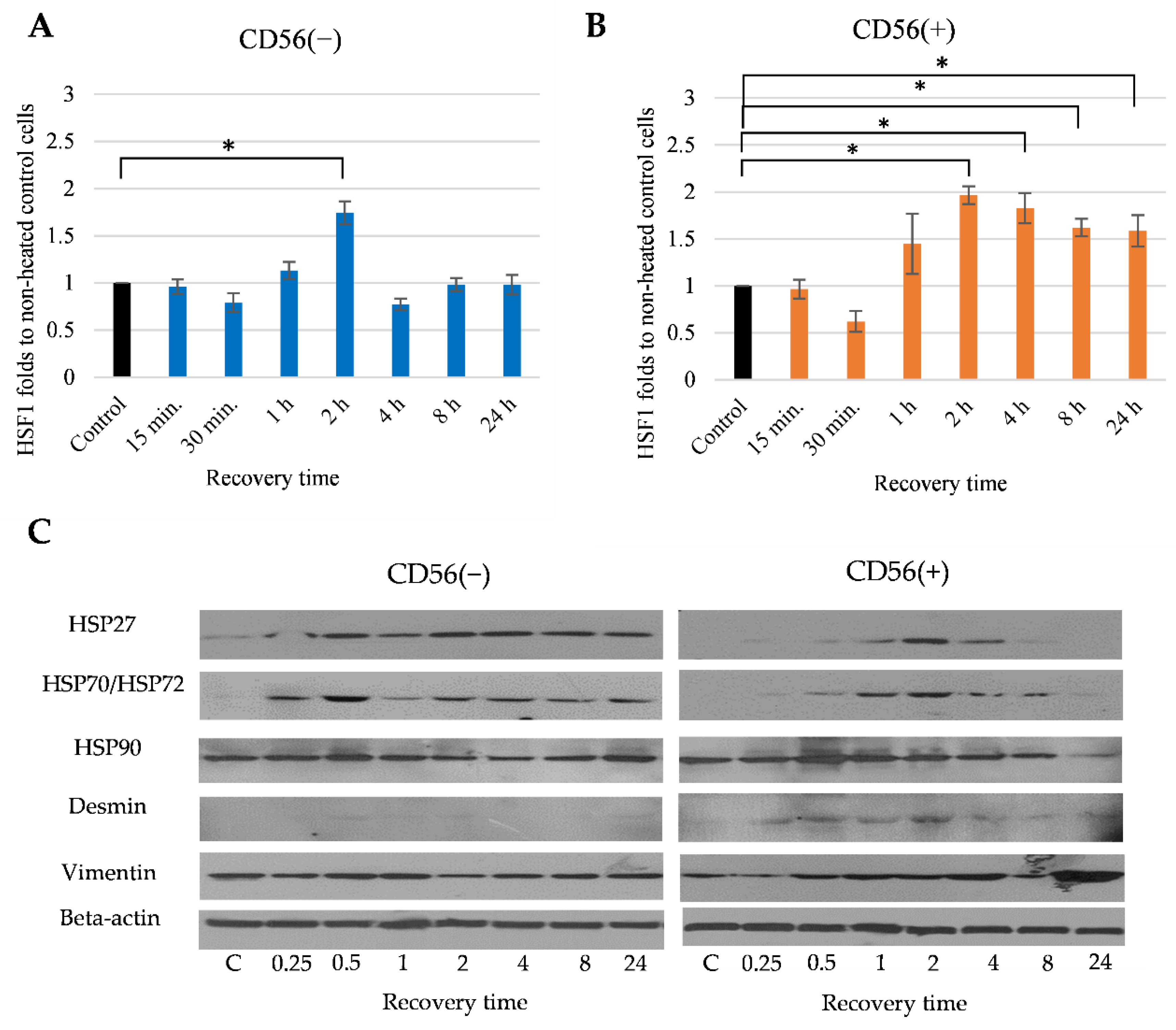
3.4. The Effect of Heat Stimulus on CD56(−) and CD56(+) Cells
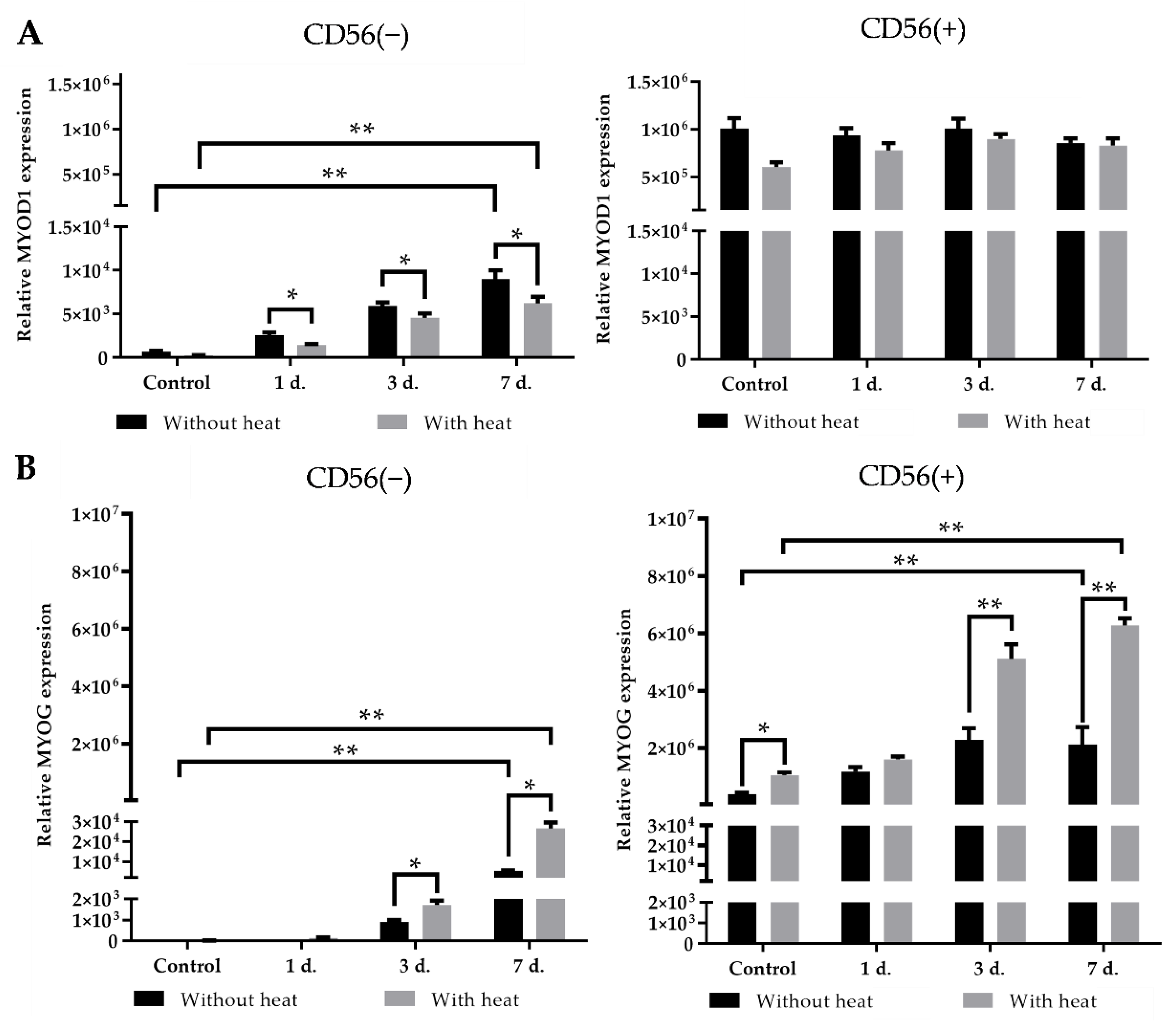
3.5. The Effect of Heat Stimulus on the Expression of Myogenic Transcription Factors in CD56(−) and CD56(+) Subpopulations
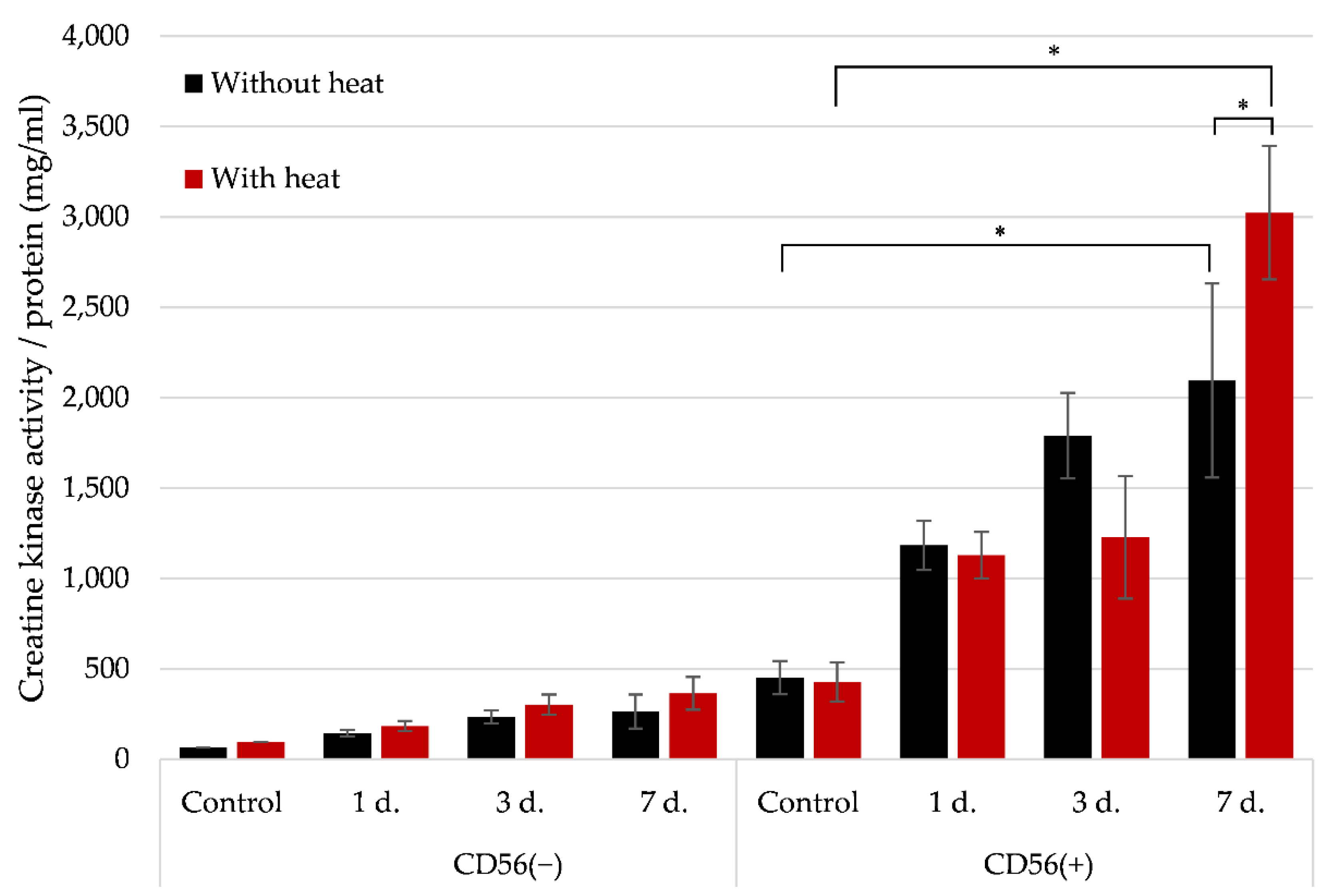
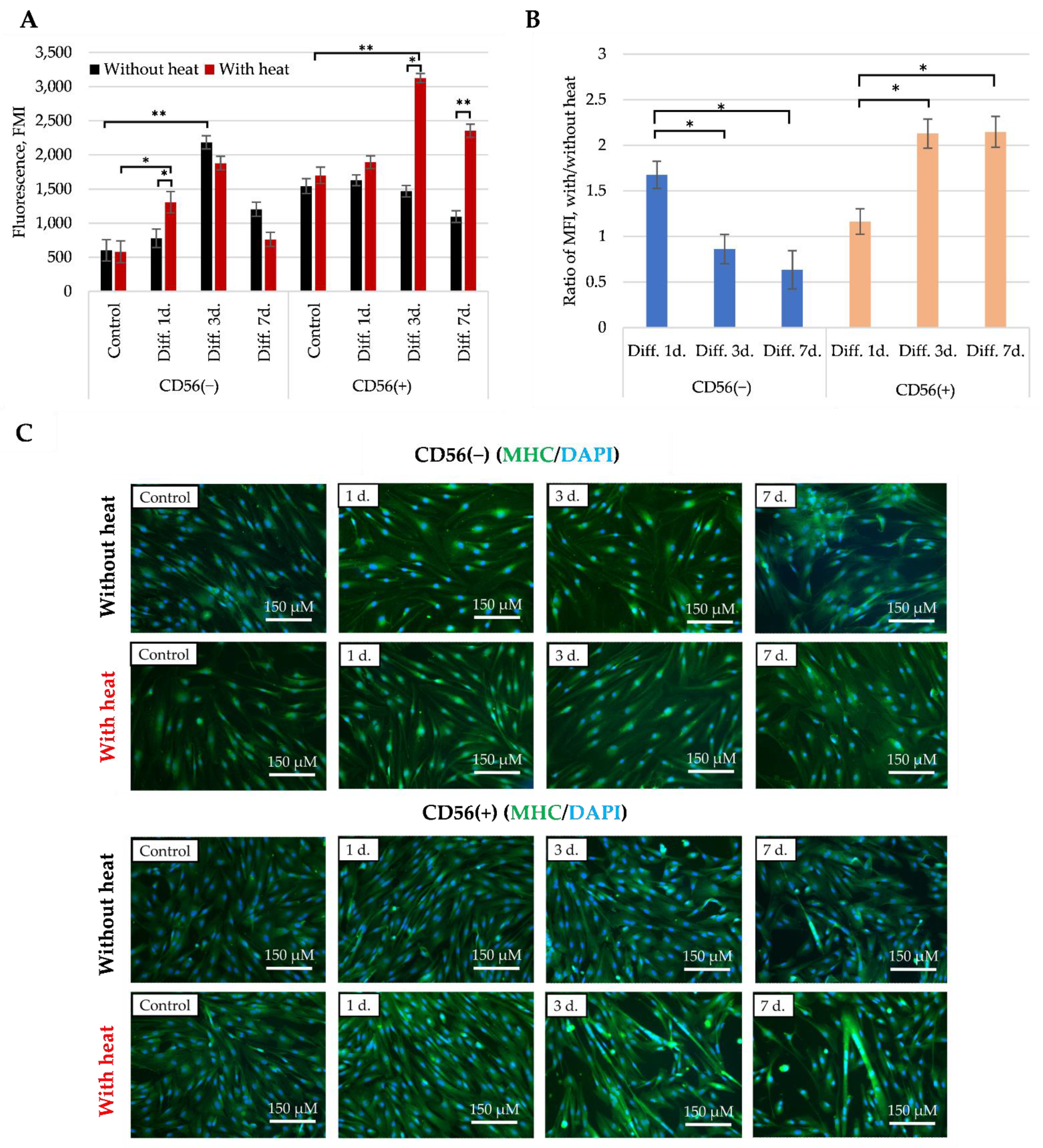
3.6. The Effect of Heat Stimulus on the Late Myogenic Differentiation Markers in CD56(−) and CD56(+) Subpopulations
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, T.J. Mechanotransduction in skeletal muscle. Front. Biosci. 2007, 12, 174–191. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.J.; Pyne, D.B.; Thompson, K.G.; Rattray, B. Warm-Up Strategies for Sport and Exercise: Mechanisms and Applications. Sport. Med. 2015, 45, 1523–1546. [Google Scholar] [CrossRef]
- McGorm, H.; Roberts, L.A.; Coombes, J.S.; Peake, J.M. Turning Up the Heat: An Evaluation of the Evidence for Heating to Promote Exercise Recovery, Muscle Rehabilitation and Adaptation. Sports Med. 2018, 48, 1311–1328. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.P.; Reardon, F.D.; Zaleski, W.; Reardon, M.L.; Haman, F.; Ducharme, M.B. Muscle temperature transients before, during, and after exercise measured using an intramuscular multisensor probe. J. Appl. Physiol. 2003, 94, 2350–2357. [Google Scholar] [CrossRef]
- Clijsen, R.; Stoop, R.; Hohenauer, E.; Aerenhouts, D.; Clarys, P.; Deflorin, C.; Taeymans, J. Local Heat Applications as a Treatment of Physical and Functional Parameters in Acute and Chronic Musculoskeletal Disorders or Pain. Arch. Phys. Med. Rehabil. 2022, 103, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Deasy, B.; Gavina, M.; Zheng, B.; Huard, J.; Lazzari, L.; Péault, B. Purification and Long-Term Culture of Multipotent Progenitor Cells Affiliated with the Walls of Human Blood Vessels: Myoendothelial Cells and Pericytes. Methods Cell Biol. 2008, 86, 295–309. [Google Scholar] [CrossRef]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The role of innate and adaptive immune cells in skeletal muscle regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef] [PubMed]
- Sancricca, C. Vessel-associated stem cells from skeletal muscle: From biology to future uses in cell therapy. World J. Stem Cells 2010, 2, 39. [Google Scholar] [CrossRef]
- Kumar, A.; D’Souza, S.S.; Moskvin, O.V.; Toh, H.; Wang, B.; Zhang, J.; Swanson, S.; Guo, L.W.; Thomson, J.A.; Slukvin, I.I. Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell Rep. 2017, 19, 1902–1916. [Google Scholar] [CrossRef] [PubMed]
- Hejbøl, E.K.; Hajjaj, M.A.; Nielsen, O.; Schrøder, H.D. Marker Expression of Interstitial Cells in Human Skeletal Muscle: An Immunohistochemical Study. J. Histochem. Cytochem. 2019, 67, 825–844. [Google Scholar] [CrossRef]
- Arrighi, N.; Moratal, C.; Clément, N.; Giorgetti-Peraldi, S.; Peraldi, P.; Loubat, A.; Kurzenne, J.Y.; Dani, C.; Chopard, A.; Dechesne, C.A. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015, 6, e1733. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J.; Pannérec, A.; Cadot, B.; Parlakian, A.; Besson, V.; Gomes, E.R.; Marazzi, G.; Sassoon, D.A. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010, 12, 257–266. [Google Scholar] [CrossRef]
- Asakura, A.; Seale, P.; Girgis-Gabardo, A.; Rudnicki, M.A. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 2002, 159, 123–134. [Google Scholar] [CrossRef]
- Dellavalle, A.; Sampaolesi, M.; Tonlorenzi, R.; Tagliafico, E.; Sacchetti, B.; Perani, L.; Innocenzi, A.; Galvez, B.G.; Messina, G.; Morosetti, R.; et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007, 9, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Testa, S.; Riera, C.S.; Fornetti, E.; Riccio, F.; Fuoco, C.; Bernardini, S.; Baldi, J.; Costantini, M.; Foddai, M.L.; Cannata, S.; et al. Skeletal Muscle-Derived Human Mesenchymal Stem Cells: Influence of Different Culture Conditions on Proliferative and Myogenic Capabilities. Front. Physiol. 2020, 11, 553198. [Google Scholar] [CrossRef]
- Cunningham, B.A.; Hemperly, J.J.; Murray, B.A.; Prediger, E.A.; Brackenbury, R.; Edelman, G.M. Neural cell adhesion molecule: Structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science 1987, 236, 799–806. [Google Scholar] [CrossRef]
- Shipley, W.R.; Hammer, R.D.; Lennington, W.J.; Macon, W.R. Paraffin immunohistochemical detection of CD56, a useful marker for neural cell adhesion molecule (NCAM), in normal and neoplastic fixed tissues. Appl. Immunohistochem. Mol. Morphol. 1997, 5, 87–93. [Google Scholar] [CrossRef]
- Krauss, R.S.; Joseph, G.A.; Goel, A.J. Keep your friends close: Cell–cell contact and skeletal myogenesis. Cold Spring Harb. Perspect. Biol. 2017, 9, a029298. [Google Scholar] [CrossRef]
- Hirayama, E.; Kim, J. Identification and characterization of a novel neural cell adhesion molecule (NCAM)-associated protein from quail myoblasts: Relationship to myotube formation and induction of neurite-like protrusions. Differentiation 2008, 76, 253–266. [Google Scholar] [CrossRef]
- Dickson, G.; Peck, D.; Moore, S.E.; Barton, C.H.; Walsh, F.S. Enhanced myogenesis in NCAM-transfected mouse myoblasts. Nature 1990, 344, 348–351. [Google Scholar] [CrossRef]
- Lindström, M.; Pedrosa-Domellöf, F.; Thornell, L.E. Satellite cell heterogeneity with respect to expression of MyoD, myogenin, Dlk1 and c-Met in human skeletal muscle: Application to a cohort of power lifters and sedentary men. Histochem. Cell Biol. 2010, 134, 371–385. [Google Scholar] [CrossRef]
- Fry, C.S.; Lee, J.D.; Mula, J.; Kirby, T.J.; Jackson, J.R.; Liu, F.; Yang, L.; Mendias, C.L.; Dupont-Versteegden, E.E.; McCarthy, J.J.; et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2015, 21, 76–80. [Google Scholar] [CrossRef]
- Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 2012, 490, 355–360. [Google Scholar] [CrossRef]
- Bironaite, D.; Brunk, U.; Venalis, A. Protective induction of Hsp70 in heat-stressed primary myoblasts: Involvement of MAPKs. J. Cell. Biochem. 2013, 114, 2024–2031. [Google Scholar] [CrossRef]
- Bironaite, D.; Pivoriunas, A.; Venalis, A. Upregulation of iHsp70 by mild heat shock protects rabbit myogenic stem cells: Involvement of JNK signalling and c-Jun. Cell Biol. Int. 2012, 36, 1089–1096. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Murgia, M.; Nagaraj, N.; Treebak, J.T.; Cox, J.; Mann, M. Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol. Cell. Proteom. 2015, 14, 841–853. [Google Scholar] [CrossRef]
- Tidball, J.G. Mechanisms of muscle injury, repair, and regeneration. Compr. Physiol. 2011, 1, 2029–2062. [Google Scholar] [CrossRef]
- Biressi, S.; Rando, T.A. Heterogeneity in the muscle satellite cell population. Semin. Cell Dev. Biol. 2010, 21, 845–854. [Google Scholar] [CrossRef]
- Ambrosio, F.; Kadi, F.; Lexell, J.; Kelley Fitzgerald, G.; Boninger, M.L.; Huard, J. The effect of muscle loading on skeletal muscle regenerative potential: An update of current research findings relating to aging and neuromuscular pathology. Am. J. Phys. Med. Rehabil. 2009, 88, 145–155. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No identical “mesenchymal stem cells” at different times and sites: Human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef]
- Cerletti, M.; Jurga, S.; Witczak, C.A.; Hirshman, M.F.; Shadrach, J.L.; Goodyear, L.J.; Wagers, A.J. Highly Efficient, Functional Engraftment of Skeletal Muscle Stem Cells in Dystrophic Muscles. Cell 2008, 134, 37–47. [Google Scholar] [CrossRef]
- Poltavtseva, R.A.; Marey, M.V.; Aleksandrova, M.A.; Revishchin, A.V.; Korochkin, L.I.; Sukhikh, G.T. Evaluation of progenitor cell cultures from human embryos for neurotransplantation. Dev. Brain Res. 2002, 134, 149–154. [Google Scholar] [CrossRef]
- Lindström, M.; Thornell, L.E. New multiple labelling method for improved satellite cell identification in human muscle: Application to a cohort of power-lifters and sedentary men. Histochem. Cell Biol. 2009, 132, 141–157. [Google Scholar] [CrossRef]
- Trapecar, M.; Kelc, R.; Gradisnik, L.; Vogrin, M.; Rupnik, M.S. Myogenic progenitors and imaging single-cell flow analysis: A model to study commitment of adult muscle stem cells. J. Muscle Res. Cell Motil. 2014, 35, 249–257. [Google Scholar] [CrossRef]
- Snijders, T.; Nederveen, J.P.; McKay, B.R.; Joanisse, S.; Verdijk, L.B.; van Loon, L.J.C.; Parise, G. Satellite cells in human skeletal muscle plasticity. Front. Physiol. 2015, 6, 283. [Google Scholar] [CrossRef]
- Agley, C.C.; Rowlerson, A.M.; Velloso, C.P.; Lazarus, N.R.; Harridge, S.D.R. Human skeletal muscle fibroblasts, but not myogenic cells, readily undergo adipogenic differentiation. J. Cell Sci. 2013, 126, 5610–5625. [Google Scholar] [CrossRef]
- Sinanan, A.C.M.; Hunt, N.P.; Lewis, M.P. Human adult craniofacial muscle-derived cells: Neural-cell adhesion-molecule (NCAM; CD56)-expressing cells appear to contain multipotential stem cells. Biotechnol. Appl. Biochem. 2004, 40, 25. [Google Scholar] [CrossRef]
- Sivasubramaniyan, K.; Ilas, D.C.; Harichandan, A.; Bos, P.K.; Santos, D.L.; de Zwart, P.; Koevoet, W.J.L.M.; Owston, H.; Bühring, H.J.; Jones, E.; et al. Bone Marrow–Harvesting Technique Influences Functional Heterogeneity of Mesenchymal Stem/Stromal Cells and Cartilage Regeneration. Am. J. Sports Med. 2018, 46, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Nakatani, M.; Ikemoto-Uezumi, M.; Yamamoto, N.; Morita, M.; Yamaguchi, A.; Yamada, H.; Kasai, T.; Masuda, S.; Narita, A.; et al. Cell-Surface Protein Profiling Identifies Distinctive Markers of Progenitor Cells in Human Skeletal Muscle. Stem Cell Rep. 2016, 7, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Lapan, A.D.; Rozkalne, A.; Gussoni, E. Human fetal skeletal muscle contains a myogenic side population that expresses the melanoma cell-adhesion molecule. Hum. Mol. Genet. 2012, 21, 3668–3680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darabi, R.; Arpke, R.W.; Irion, S.; Dimos, J.T.; Grskovic, M.; Kyba, M.; Perlingeiro, R.C.R. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 2012, 10, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Covas, D.T.; Panepucci, R.A.; Fontes, A.M.; Silva, W.A.; Orellana, M.D.; Freitas, M.C.C.; Neder, L.; Santos, A.R.D.; Peres, L.C.; Jamur, M.C.; et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp. Hematol. 2008, 36, 642–654. [Google Scholar] [CrossRef]
- Russell, K.C.; Phinney, D.G.; Lacey, M.R.; Barrilleaux, B.L.; Meyertholen, K.E.; O’Connor, K.C. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 2010, 28, 788–798. [Google Scholar] [CrossRef]
- Joshkon, A.; Heim, X.; Dubrou, C.; Bachelier, R.; Traboulsi, W.; Stalin, J.; Fayyad-Kazan, H.; Badran, B.; Foucault-Bertaud, A.; Leroyer, A.S.; et al. Role of CD146 (MCAM) in physiological and pathological angiogenesis—Contribution of new antibodies for therapy. Biomedicines 2020, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Persichini, T.; Funari, A.; Colasanti, M.; Sacchetti, B. Clonogenic, myogenic progenitors expressing MCAM/CD146 are incorporated as adventitial reticular cells in the microvascular compartment of human post-natal skeletal muscle. PLoS ONE 2017, 12, e0188844. [Google Scholar] [CrossRef]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef]
- Wang, Y.X.; Rudnicki, M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2012, 13, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M. Skeletal muscle progenitor cells and the role of Pax genes. Comptes Rendus Biol. 2007, 330, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Kuroda, K.; le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, M.; Badodi, S.; Ortuste Quiroga, H.P.; Zammit, P.S.; Hinits, Y.; Hughes, S.M. Myogenin promotes myocyte fusion to balance fibre number and size. Nat. Commun. 2018, 9, 4232. [Google Scholar] [CrossRef] [PubMed]
- Kassar-Duchossoy, L.; Gayraud-Morel, B.; Gomès, D.; Rocancourt, D.; Buckingham, M.; Shinin, V.; Tajbakhsh, S. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 2004, 431, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S. Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis. Elife 2020, 9, e60445. [Google Scholar] [CrossRef]
- Caldow, M.K.; Thomas, E.E.; Dale, M.J.; Tomkinson, G.R.; Buckley, J.D.; Cameron-Smith, D. Early myogenic responses to acute exercise before and after resistance training in young men. Physiol. Rep. 2015, 3, e125. [Google Scholar] [CrossRef] [PubMed]
- Mackey, A.L.; Magnan, M.; Chazaud, B.; Kjaer, M. Human skeletal muscle fibroblasts stimulate in vitro myogenesis and in vivo muscle regeneration. J. Physiol. 2017, 595, 5115–5127. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Meza, R.; Lieber, R.L. Skeletal muscle fibroblasts in health and disease. Differentiation 2016, 92, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Mackey, A.L.; Brandstetter, S.; Schjerling, P.; Bojsen-Moller, J.; Qvortrup, K.; Pedersen, M.M.; Doessing, S.; Kjaer, M.; Magnusson, S.P.; Langberg, H. Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle. FASEB J. 2011, 25, 1943–1959. [Google Scholar] [CrossRef] [PubMed]
- Fortini, P.; Ferretti, C.; Iorio, E.; Cagnin, M.; Garribba, L.; Pietraforte, D.; Falchi, M.; Pascucci, B.; Baccarini, S.; Morani, F.; et al. The fine tuning of metabolism, autophagy and differentiation during in vitro myogenesis. Cell Death Dis. 2016, 7, e2168. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A.; Kot, M.; Skrzypek, K.; Szewczyk, B.; Majka, M. Myogenic differentiation of ips cells shows different efficiency in simultaneous comparison of protocols. Cells 2021, 10, 1671. [Google Scholar] [CrossRef]
- Knight, J.D.R.; Kothary, R. The myogenic kinome: Protein kinases critical to mammalian skeletal myogenesis. Skelet. Muscle 2011, 1, 29. [Google Scholar] [CrossRef]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The “phosphocreatine circuit” for cellular energy homeostasis. Biochem. J. 1992, 281, 21–40. [Google Scholar] [CrossRef]
- Wallimann, T.; Hemmer, W. Creatine kinase in non-muscle tissues and cells. Mol. Cell. Biochem. 1994, 133, 193–220. [Google Scholar] [CrossRef]
- Toivola, D.M.; Strnad, P.; Habtezion, A.; Omary, M.B. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010, 20, 79–91. [Google Scholar] [CrossRef]
- Li, Z.; Mericskay, M.; Agbulut, O.; Butler-Browne, G.; Carlsson, L.; Thornell, L.E.; Babinet, C.; Paulin, D. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J. Cell Biol. 1997, 139, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors. Nat. Rev. Mol. Cell Biol. 2010, 11, 537. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Miyabara, E.H.; Nascimento, T.L.; Rodrigues, D.C.; Moriscot, A.S.; Davila, W.F.; AitMou, Y.; DeTombe, P.P.; Mestril, R. Overexpression of inducible 70-kDa heat shock protein in mouse improves structural and functional recovery of skeletal muscles from atrophy. Pflug. Arch. Eur. J. Physiol. 2012, 463, 733–741. [Google Scholar] [CrossRef] [PubMed]
- McArdle, A.; Broome, C.S.; Kayani, A.C.; Tully, M.D.; Close, G.L.; Vasilaki, A.; Jackson, M.J. HSF expression in skeletal muscle during myogenesis: Implications for failed regeneration in old mice. Exp. Gerontol. 2006, 41, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Locke, M.; Celotti, C. The effect of heat stress on skeletal muscle contractile properties. Cell Stress Chaperones 2014, 19, 519–527. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikšiūnas, R.; Labeit, S.; Bironaitė, D. The Effect of Heat Shock on Myogenic Differentiation of Human Skeletal-Muscle-Derived Mesenchymal Stem/Stromal Cells. Cells 2022, 11, 3209. https://doi.org/10.3390/cells11203209
Mikšiūnas R, Labeit S, Bironaitė D. The Effect of Heat Shock on Myogenic Differentiation of Human Skeletal-Muscle-Derived Mesenchymal Stem/Stromal Cells. Cells. 2022; 11(20):3209. https://doi.org/10.3390/cells11203209
Chicago/Turabian StyleMikšiūnas, Rokas, Siegfried Labeit, and Daiva Bironaitė. 2022. "The Effect of Heat Shock on Myogenic Differentiation of Human Skeletal-Muscle-Derived Mesenchymal Stem/Stromal Cells" Cells 11, no. 20: 3209. https://doi.org/10.3390/cells11203209
APA StyleMikšiūnas, R., Labeit, S., & Bironaitė, D. (2022). The Effect of Heat Shock on Myogenic Differentiation of Human Skeletal-Muscle-Derived Mesenchymal Stem/Stromal Cells. Cells, 11(20), 3209. https://doi.org/10.3390/cells11203209





