Highlights
- Cardiac-specific Dsg2 deletion induces excessive cardiac fibrosis in mice.
- Fenofibrate alleviates cardiac fibrosis in CS-Dsg2−/− mice.
- Cardiac-specific activation of PPARα ameliorates cardiac fibrosis in CS-Dsg2−/− mice.
- The inhibitory effect of PPARα on cardiac fibrosis is mediated by STAT3 and TGF-β /SMAD3 signaling.
- PPARα is a promising target for the intervention of ACM by ameliorating cardiac fibrosis.
Abstract
Background: Arrhythmogenic cardiomyopathy (ACM) is a genetic heart muscle disease characterized by progressive fibro-fatty replacement of cardiac myocytes. Up to now, the existing therapeutic modalities for ACM are mostly palliative. About 50% of ACM is caused by mutations in genes encoding desmosomal proteins including Desmoglein-2 (Dsg2). In the current study, the cardiac fibrosis of ACM and its underlying mechanism were investigated by using a cardiac-specific knockout of Dsg2 mouse model. Methods: Cardiac-specific Dsg2 knockout (CS-Dsg2−/−) mice and wild-type (WT) mice were respectively used as the animal model of ACM and controls. The myocardial collagen volume fraction was determined by histological analysis. The expression levels of fibrotic markers such as α-SMA and Collagen I as well as signal transducers such as STAT3, SMAD3, and PPARα were measured by Western blot and quantitative real-time PCR. Results: Increased cardiac fibrosis was observed in CS-Dsg2−/− mice according to Masson staining. PPARα deficiency and hyperactivation of STAT3 and SMAD3 were observed in the myocardium of CS-Dsg2−/− mice. The biomarkers of fibrosis such as α-SMA and Collagen I were upregulated after gene silencing of Dsg2 in HL-1 cells. Furthermore, STAT3 gene silencing by Stat3 siRNA inhibited the expression of fibrotic markers. The activation of PPARα by fenofibrate or AAV9-Pparα improved the cardiac fibrosis and decreased the phosphorylation of STAT3, SMAD3, and AKT in CS-Dsg2−/− mice. Conclusions: Activation of PPARα alleviates the cardiac fibrosis in ACM.
1. Introduction
Arrhythmogenic cardiomyopathy (ACM) is a fatal heart disease characterized by cardiac dysfunction, heart failure, and life-threatening ventricular arrhythmias [1,2]. The population prevalence of ACM has been estimated between 1:1000 and 1:5000 [2]. Studies have shown that ACM causes 10% to 15% of sudden cardiac death (SCD) cases, especially among young people and athletes [2]. Pathological features of ACM include loss of myocytes and progressive fibro-fatty replacement. These pathological features tend to occur in the right ventricle (RV), with left ventricular (LV) or bilateral ventricular involvement [2,3]. Previous studies found that one or more mutations in genes encoding desmosomal proteins led to about 50% of ACM cases [4], including desmoglein2 (DSG2) [5], desmocollin2 [6], plakoglobin [7], desmoplakin [8], and plakophilin-2 [9]. DSG2 is a major cadherin of the cardiac desmosome; it was reported that mutations in the Dsg2 gene are associated with severe lethal heart muscle diseases such as ACM [10]. Till now, the main purpose of existing treatment for ACM is to prevent SCD [11].
Necrotic and apoptotic cardiomyocytes are replaced by fibrosis during the ACM disease’s progress [12]. Although cardiac fibrosis plays a critical role in enhancing cardiac structural stability, it results in cardiac structural remodeling and impaired cardiac function, finally increasing the risk of potentially lethal cardiac arrhythmias [13]. Thus, improvement of cardiac fibrosis might be beneficial to avoid further deterioration of the ACM. The signal transducer and activator of transcription 3 (STAT3) is hyperactivated in fibrotic diseases and STAT3 inhibitors are currently used in the treatments of fibrotic diseases, especially in cardiac fibrosis [14]. Transforming growth factor-β (TGF-β) is a central mediator in hypertrophic and fibrotic of the heart. Canonical and non-canonical pathways for TGF-β-induced fibrosis in the heart are known [15]. Furthermore, interaction of Stat3 and TGF-β/Smad3 signaling is regarded as playing a critical role in cardiac fibrotic processes [16]. Recent studies illustrated the antagonistic effects and bidirectional regulation between STATs and peroxisome proliferator-activated receptors (PPARs) and suggested a potential cross-talk between STAT and PPAR pathways [17,18,19]. PPARs are the nuclear receptor superfamily of ligand-activated transcription factors. As the predominant PPAR isoform in the heart, peroxisome proliferator-activated receptor α (PPARα) modulates cardiac metabolism substrate conversion in cardiac hypertrophy, cardiac hypoxia, and diabetic heart [20]. PPARα gene deletion contributes to cardiac hypertrophy and deterioration of cardiac function [21]. Previous studies illustrated that PPARα activation alleviated cardiac fibrosis and reversed cardiac dysfunction [22] and PPARα could inhibit the TGF-β-induced profibrotic pathway in cardiac fibrosis [23,24]. Fenofibrate alleviated myocardial inflammation and collagen deposition in Ang II-infused rats [25]. Recently, we reported that activation of PPARα reduced the cardiac lipid accumulation and restored cardiac function in ACM mice [26]. Although PPARα plays a critical role in lipid accumulation in ACM, the effects of PPARα on cardiac fibrosis in ACM is still unclear. We hypothesized that the PPARα-STAT3/SMAD pathway is critical to cardiac fibrosis in ACM mice. In our current study, we found that PPARα was downregulated in the hearts of cardiac-specific Dsg2 knockout mice; restoring the activity of PPARα by using fenofibrate (a PPARα agonist) or AAV9-Pparα improved cardiac fibrosis via the PPARα-STAT3/SMAD pathway in cardiac-specific Dsg2 deletion mice. Our findings suggest that PPARα is a potential therapeutic target of cardiac fibrosis in ACM.
2. Materials and Methods
2.1. Materials
Fenofibrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit anti-Phospho-stat3 (Tyr705), rabbit anti-Phospho-SMAD3 (Ser423/425), rabbit anti-SMAD, rabbit anti-Phospho-AKT (Ser473), rabbit anti-AKT, rabbit anti-α-SMA, rabbit anti-Collagen I antibodies, mouse anti-stat3, and mouse monoclonal anti-β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Rabbit anti-DSG2, rabbit anti-PPARα, rabbit anti-GAPDH antibodies were from Abcam Inc. (Cambridge, MA, USA).
2.2. Animals and Treatments
Cardiac-specific dsg2 gene knockout (CS-Dsg2−/−) on C57-based genetic backgrounds were successfully constructed by mating DSG2 flox with CKMM cre [26]. Mice were housed in standard plastic rodent cages and maintained in a regulated environment (24 °C, 12 h light and 12 h dark cycles with lights on at 7:00 a.m.). All mice used in this study were 8–12 weeks old.
In order to activate PPARα in vivo, PPARα agonist fenofibrate (150 mg/kg body weight) was administrated by daily oral gavage for 28 days. To overexpress PPARα in the heart, male CS-Dsg2−/− mice were tail-vein infused with adeno-associated virus carrying PPARα (AAV9-cTnT-Pparα, 5 × 1011 vg per mouse). Adeno-associated virus carrying GFP (AAV9-cTnT-GFP, 5 × 1011 vg per mouse) was used as control. The mice were sacrificed 28 days after AAV injections [26].
2.3. Histological Analysis
Hearts were harvested from mice, fixed overnight in 4% paraformaldehyde, embedded in paraffin, and then, sectioned serially. Masson’s trichrome staining was performed to evaluate collagen deposition using a kit following manufacturer’s instructions (G1006-20 ML, Servicebio, Wuhan, China). The collagen volume fraction (CVF) was determined by Image J software as an index of cardiac fibrosis. The ratio of myocardial collagen area to the total myocardial area was used to calculate the collagen volume fraction.
2.4. Cell Culture and Treatment
The murine atrial cardiac myocyte cell line HL-1 was maintained in 10% fetal bovine serum (FBS) in Dulbecco’s modified Eagle’s medium at 37 °C in an atmosphere of 5% CO2. For transient transfection, cells were plated at optimal densities and grown for 24 h. Cells were then transfected with Dsg2 siRNA (MBS828119, MyBioSource, San Diego, CA, USA) or Stat3 siRNA (6354, Cell Signaling Technology) using lipofectamine reagent according to the manufacturer’s instructions.
2.5. Western Blot Analysis
The tissues and cells were homogenized in the lysis buffer. After protein quantification, 40 μg of protein was loaded onto SDS-PAGE gels. Then, protein extracts were electrophoresed, blotted, and then, incubated with primary antibodies. The antibodies were detected using 1:10,000 horseradish peroxidase-conjugated donkey anti-rabbit IgG and donkey anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA). Western blotting luminol reagent was used to visualize bands. The band intensities were quantitated by Image J software.
2.6. RNA Extraction, Quantitative Real-Time PCR
For gene expression analysis, RNA was isolated from mouse tissues and cells by using Trizol (Takara, Kusatsu, Shiga) and reverse-transcribed into cDNAs using the first-strand synthesis system for RT-PCR kit (Takara). SYBR green-based real-time PCR was performed using the Bio-Rad IQ5 PCR system (Bio-Rad, Foster City, CA, USA). Sequences for the primer pairs used in this study are shown in Table 1.

Table 1.
List and sequences of primers used in RT-PCR experiments.
2.7. Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance was analyzed with a student’s t-test. Differences were considered statistically significant with p values < 0.05.
3. Results
3.1. Cardiac-Specific Dsg2 Gene Deletion Provokes Cardiac Fibrosis
The ACM mouse model was generated by crossing Dsg2fl-neo/+ mice with Ckmm-Cre mice which resulted in cardiac-specific Dsg2 deletion (CS-Dsg2−/−). Increased cardiac fibrosis was observed in CS-Dsg2−/− mice according to Masson staining (Figure 1A). Several studies indicated that the activation of STAT3 contributes to cardiac fibrosis [27,28,29]. In our study, increased phosphorylation levels of STAT3 at Tyr705, SMAD3 at Ser423/425, and AKT at Ser473, and decreased expression levels of PPARα were observed in the LV, interventricular septum (IVS), and RV of CS-Dsg2−/− mice (Figure 1B). We next investigated the expression of TGF-β, α-smooth muscle actin (α-SMA), and collagen type I (Collagen I) in CS-Dsg2−/− mice. Expression levels of TGF-β, α-SMA, and Collagen I in LV, IVS, and RV of CS-Dsg2−/− mice were higher than that of littermate controls (Figure 1B,C).
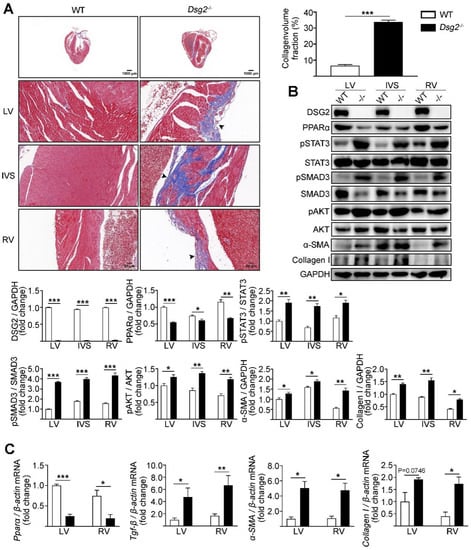
Figure 1.
Cardiac-specific Dsg2 knockout induced cardiac fibrosis. (A) Masson staining of heart sections in WT and CS-Dsg2−/− (−/−) mice. Arrow shows cardiac fibrosis. Collagen volume fraction in the hearts of WT and CS-Dsg2−/− mice was assessed. (B) Representative Western blots from mouse left ventricular (LV), interventricular septum (IVS), and right ventricle (RV). DSG2, PPARα, pSTAT3, pSMAD3, pAKT, α-SMA, and Collagen I were detected using specific antibodies. STAT3, SMAD3, AKT, and GAPDH were used as loading controls. (C) Results of quantitative PCR analysis of PPARα, TGF-β, α-SMA, and Collagen I mRNA levels in mouse LV and RV are expressed as fold change of control using β-actin as loading control. Results are expressed as mean values ± SEM. n = 6. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. WT.
The effects of Dsg2 on STAT3 activity and fibrosis were assessed in the cardiac myocyte cell line HL-1. To silence the expression of Dsg2 and Stat3, HL-1 cells were transfected with Dsg2 siRNA and Stat3 siRNA. The knockdown efficiency of Dsg2 siRNA was 66% and 51% for Stat3 siRNA. Consistent to the in vivo study, knockdown of Dsg2 in the HL-1 cells led to an increase in the phosphorylation of STAT3 (Tyr705) and the expression levels of α-SMA and Collagen I (Figure 2A,B). Furthermore, knockdown of Stat3 in the HL-1 cells decreased the expression levels of α-SMA and Collagen I (Figure 2C,D).
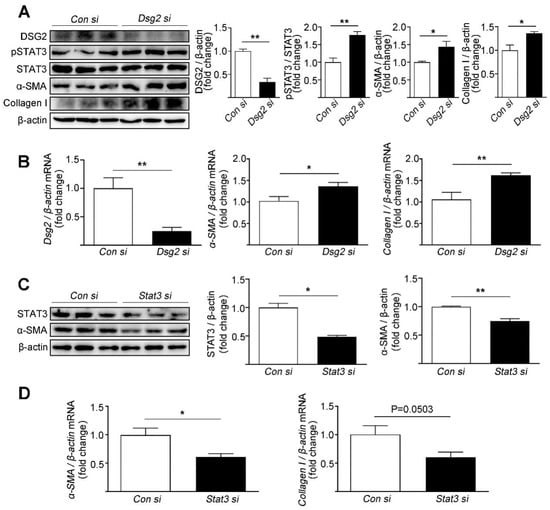
Figure 2.
Effects of Dsg2 siRNA and Stat3 siRNA on the expression levels of fibrotic markers in HL-1 cells. (A,B) HL-1 cells were transfected with control siRNA or Dsg2 siRNA. (A) Representative Western blots for DSG2, pSTAT3, α-SMA, and Collagen I were detected using specific antibodies. STAT3 and β-actin were used as loading controls. (B) Results of quantitative PCR analysis of Dsg2, α-SMA, and Collagen I mRNA levels in HL-1 cells treated with control or Dsg2 siRNA are expressed as fold change of control using β-actin as loading control. Results are expressed as mean values ± SEM. n = 3. * p < 0.05, ** p < 0.01 vs. control. (C,D) HL-1 cells were transfected with control siRNA or Stat3 siRNA. (C) Representative Western blots for STAT3 and α-SMA were detected using specific antibodies. β-actin were used as loading controls. (D) Results of quantitative PCR analysis of α-SMA and Collagen I mRNA levels in HL-1 cells treated with control or Stat3 siRNA are expressed as fold change of control using β-actin as loading control. Results are expressed as mean values ± SEM. n = 3. * p < 0.05, ** p < 0.01, vs. control.
3.2. Fenofibrate Alleviated Cardiac Fibrosis in CS-Dsg2−/− Mice
Fenofibrate, a PPARα agonist, affords myocardial protection apart from its lipid lowering effects [30]. We next assessed the effect of fenofibrate on cardiac fibrosis in CS-Dsg2−/− mice. Interestingly, a significant improvement in cardiac fibrosis was observed in CS-Dsg2−/− mice after being treated with fenofibrate (150 mg/kg/day, for 4 weeks) (Figure 3A). Fenofibrate decreased the phosphorylation levels of STAT3, SMAD3, and AKT as well as the expression levels of TGF-β, α-SMA, and Collagen I in CS-Dsg2−/− mice (Figure 3B,C).
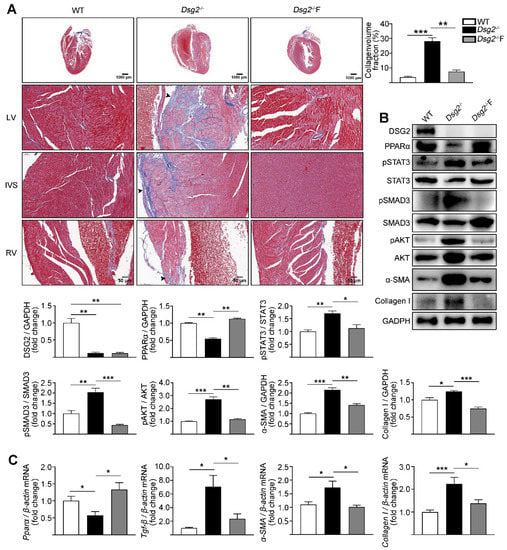
Figure 3.
Fenofibrate alleviated cardiac fibrosis in CS-Dsg2−/− mice. (A) Masson staining of heart sections in WT, CS-Dsg2−/− mice, and CS-Dsg2−/− mice treated with fenofibrate (Dsg2−/−F). Collagen volume fraction in the hearts of WT, CS-Dsg2−/−, and Dsg2−/−F mice were assessed. (B) Representative Western blots from ventricles of WT, CS-Dsg2−/−, and Dsg2−/−F mice. DSG2, PPARα, pSTAT3, pSMAD3, pAKT, α-SMA, and Collagen I were detected using specific antibodies. STAT3, SMAD3, AKT, and GAPDH were used as loading controls. (C) Results of quantitative PCR analysis of PPARα, TGF-β, α-SMA, and Collagen I mRNA levels in mouse ventricles are expressed as fold change of control using β-actin as loading control. Results are expressed as mean values ± SEM. n = 6. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control.
3.3. Cardiac-Specific Activation of PPARα Alleviated Cardiac Fibrosis in CS-Dsg2−/− Mice
To further confirm that PPARα activation in the heart could improve cardiac fibrosis in Dsg2−/− mice, cardiac-specific activation of PPARα was performed by tail-vein infusion of AAV9. AAV9-cTnT promoter-Pparα significantly reduced cardiac fibrosis in CS-Dsg2−/− mice (Figure 4A). Simultaneously, the levels of phosphorylated STAT3, phosphorylated SMAD3, phosphorylated AKT, and the expression levels of TGF-β, α-SMA, and Collagen I were decreased after cardiac-specific activation of PPARα (Figure 4B,C).
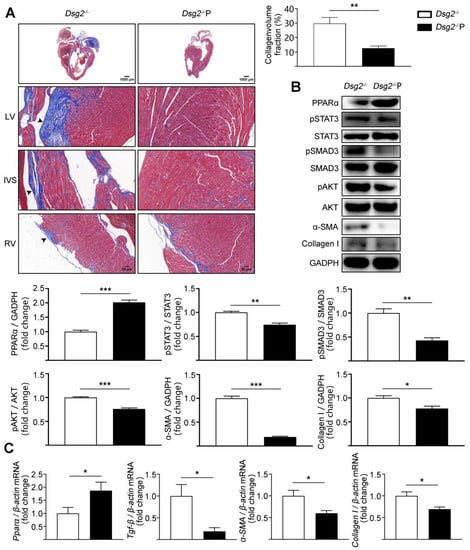
Figure 4.
AAV9-Pparα alleviated cardiac fibrosis in CS-Dsg2−/− mice. (A) Masson staining of heart sections in CS-Dsg2−/− mice and CS-Dsg2−/− mice received AAV9-Pparα (Dsg2−/−P). Collagen volume fraction in the hearts of CS-Dsg2−/− and Dsg2−/−P mice were assessed. (B) Representative Western blots from ventricles of CS-Dsg2−/− and Dsg2−/−P mice. PPARα, pSTAT3, pSMAD3, pAKT, α-SMA, and Collagen I were detected using specific antibodies. STAT3, SMAD3, AKT, and GAPDH were used as loading controls. (C) Results of quantitative PCR analysis of PPARα, TGF-β, α-SMA, and Collagen I mRNA levels in mouse ventricles are expressed as fold change of control using β-actin as loading control. Results are expressed as mean values ± SEM. n = 6. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. CS-Dsg2−/−.
4. Discussion
ACM is characterized by progressive replacement of cardiomyocytes by fibro-fatty tissue, cardiac dysfunction, ventricular arrhythmias, and heart failure. Mutation of desmoglein-2 (Dsg2) is one of the major causes of ACM and has been shown to lead to a loss of adhesive function [31]. Dsg2 mutation carriers display more severe heart muscle disease, which is associated with biventricular involvement and rapid evolution to end-stage heart failure [32]. In our previous study, we generated an ACM mouse model by cardiac-specific knockout of the DSG2 gene and discovered that downregulation of PPARα contributed to the impairment of fatty acid oxidation and, thus, to lipid accumulation in the DSG2 deletion-induced ACM [26]. However, whether downregulation of PPARα also contributes to the fibrosis in ACM was unsolved. In the present study, we uncovered a previously unrecognized role of PPARα in cardiac fibrosis in Dsg2-deficient ACM mice. Cardiac-specific Dsg2 knockout contributed to a severe cardiac fibrosis. Decreased expression of PPARα and the increased phosphorylation of STAT3 and SMAD3 were observed in this model. Moreover, activation of PPARα, either by fenofibrate or AAV9-Pparα, decreased the activity of STAT3 and SMAD3 and improved cardiac fibrosis in Dsg2 deletion-induced ACM.
Cardiac fibrosis is defined as excessive deposition of extracellular matrix (ECM) proteins by cardiac fibroblasts (CFs). CFs are transformed into myofibroblasts when they respond to stress and pathological stimuli [33]. Myocardial fibrosis reduces tissue compliance and accelerates the progression to heart failure [34]. In our study, histological analysis showed excessive deposition of collagen in the hearts of CS-Dsg2−/− mice when compared to WT mice. Furthermore, fibrotic markers such as TGF-β, α-SMA, and Collagen I were activated after cardiac-specific Dsg2 deletion. Cardiac fibrosis has been implicated in the progression of ACM. Increased cardiac fibrosis has been associated with altered cardiac conduction, resulting in conduction slowing, blockage, and re-entry [35]. Recent evidence indicates that the fibrosis state preceded the development of cardiac dysfunction in cardiomyopathies [36]. Thus, identification of druggable targets that can alleviate cardiac fibrosis might be beneficial to the treatment of ACM.
As a ligand-activated transcription factor which is highly expressed in cardiomyocytes, the role of PPARα in the heart is complex and vital. PPARα involvement in the regulation of inflammation [37], hypertrophy [38], energy metabolism [39], ischemia/reperfusion injury [40], and cardiac fibrosis [25] in hearts has been established in recent studies. Fenofibrate is a member of the fibrate family of PPARα receptor agonists and has regulating efficacy of inflammation and extracellular matrix remodeling of the heart [25,41]. As a PPARα agonist, fenofibrate has been widely used for hyperlipidemia in clinics and can also promote fatty acid oxidation in the mitochondria and improve myocardial energy metabolism [42]. Fenofibrate alleviated myocardial inflammation and fibrosis in diabetic mice via PPARα receptor [43]. Our previous study showed that PPARα was downregulated in the heart of the Dsg2 deletion ACM model and reactivation of PPARα significantly alleviated the lipid accumulation and improved cardiac function in CS-Dsg2−/− mice [26]. In the current study, we demonstrated that downregulation of PPARα also contributed to the cardiac fibrosis in the Dsg2 deletion-induced ACM model. Moreover, reactivation of PPARα either by tail-vein injection of AAV9-Pparα or oral treatment of fenofibrate improved the cardiac fibrosis in CS-Dsg2−/− mice. Our results suggested that PPARα is a promising therapeutic target for ACM intervention which not only alleviates lipid accumulation but also improves cardiac fibrosis.
Although cardiac fibrosis is one of the pathological characteristics of ACM, the mechanism of how mutations of desmosomal proteins lead to fibrosis is elusive. TGF-β is a core mediator in cardiac fibrosis. Canonical (SMAD-dependent) and non-canonical (SMAD-independent) pathways for TGF-β-induced fibrosis in the heart are documented [44]. In the canonical pathway, TGF-β activates SMAD2/3 signaling, which in turn regulates the expressions of collagen and α-SMA in myofibroblasts [45]. Non-canonical pathways involve STAT, MAPK, and PI3K pathways [46,47,48]. Our study showed that cardiac-specific Dsg2 deletion led to enhanced phosphorylation of SMAD3, STAT3, and AKT, suggesting that both canonical and non-canonical TGF-β pathways are activated in Dsg2 deletion-induced ACM. Among these pathways, STAT3 is reported to be critical to cardiac fibrosis and hypertrophy and activated in the hearts of mouse models of cardiac hypertrophy and heart failure [49]. Several studies have demonstrated that STAT3 maintains ECM homeostasis by regulating collagen synthesis and secretion in CFs [50]. Continuous STAT3 activation (tyrosine 705 residue phosphorylation) was regarded as a poor indicator in cardiac hypertrophy and heart failure [51]. Our study showed that knockdown of Dsg2 by Dsg2 siRNA induced the activation of fibrotic markers and STAT3 in HL-1 cells, while Stat3 siRNA did the reverse. These results suggested that activation of STAT3 contributes to cardiac fibrosis in cardiac-specific Dsg2 deletion mice. Furthermore, reactivation of PPARα either by AAV9-PPARα or fenofibrate decreased the phosphorylation of SMAD3, STAT3, and AKT in the Dsg2 deletion-induced ACM model, implying that PPARα modulated these pathways and deficiency in PPARα contributed to the activation of them and, thus, to cardiac fibrosis. Although the mechanistic link between PPARα and the STAT3 and TGF-β /SMAD3 pathways remains unclear, potential cross-talk between PPARα and STAT3 and TGF-β /SMAD3 pathways were reported in recent studies [17]. Chang H et al. reported that activation of PPARα ameliorates autoimmune myocarditis by suppressing Th17 cell differentiation through reducing phosphorylated STAT3 [52]. Gervois et al. demonstrated that fenofibrate treatment decreased the phosphorylation of STAT3 in livers [53]. Bansal T et al. reported that activation of PPARα improves cardiac fibrosis by inhibiting non-canonical TGF-β signaling [24]. Sekiguchi K et al. demonstrated that TGF-β signaling pathways directly inhibit PPARα activity in cardiac myocytes [54]. These studies suggest a role of PPARα in modulating STAT3 and TGF-β /SMAD3 pathways.
Current treatments for ACM lack effective treatment to improve or reverse cardiac fibrosis. In the present study, we established that activation of PPARα by fenofibrate or AAV9-Pparα improved the cardiac fibrosis in Dsg2 deletion-induced ACM. At the same time, activation of PPARα provided a cardioprotective effect through reducing the phosphorylation of STAT3 and SMAD3. These results indicated that the inhibitory effect of PPARα on cardiac fibrosis is mediated by a downregulation of STAT3 and TGF-β /SMAD3, and PPARα may be a significant target of ACM treatment. PPARα agonist fenofibrate may be a potential drug against cardiac fibrosis in ACM. In conclusion, our study generated an ACM model by cardiac-specific Dsg2 knockout and suggested that activation of PPARα ameliorates the excessive cardiac fibrosis in ACM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11203184/s1, Figure S1: The expression of PPARα in the skeletal muscle of cardiac specific DSG2 null mice received with AAV9-cTnT-GFP (DSG2−/−) or AAV9-cTnT-Pparα (DSG2−/− P).
Author Contributions
G.X. designed research; Z.Q., Y.Z., T.T., W.G., R.L. and J.H. performed research; Z.Q., Y.Z. and G.X. analyzed data; G.X., Z.Q. and Y.Z. wrote and edited the paper. All authors contributed to the discussion and revised the article, and all approved the final versions of the manuscript. G.X. was responsible for the integrity of the work as a whole. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82170818, 81770794) and the Fundamental Research Funds for the Central Universities (21620423).
Institutional Review Board Statement
Animal studies were conducted in accordance with the Guidelines on Laboratory Animals of Jinan University and were reviewed and approved by the Animal Care and Use Committee of Jinan University (No:20191128-09).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.
Acknowledgments
We thank Yubi Lin from Guangdong Medical University for providing the CS-Dsg2−/− mice.
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Abbreviations
| ACM | arrhythmogenic cardiomyopathy |
| CFs | cardiac fibroblasts |
| Collagen I | collagen type I |
| DSG2 | Desmoglein-2 |
| LV | left ventricular |
| PPARα | peroxisome proliferator-activated receptor α |
| RV | right ventricle |
| STAT3 | signal transducer and activator of transcription 3 |
| SMAD | mothers against decapentaplegic homolog 3 |
| SCD | sudden cardiac death |
| TGF-β | transforming growth factor–β |
| α-SMA | alpha-smooth muscle actin |
References
- Thiene, G.; Nava, A.; Corrado, D.; Rossi, L.; Pennelli, N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 1988, 318, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Beti, C.; Asimaki, A. Histopathological Features and Protein Markers of Arrhythmogenic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 746321. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.M.; Trembley, M.A.; Chandler, S.F.; Sanders, S.P.; Saffitz, J.E.; Abrams, D.J.; Pu, W.T. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat. Rev. Cardiol. 2019, 16, 519–537. [Google Scholar] [CrossRef]
- Awad, M.M.; Dalal, D.; Cho, E.; Amat-Alarcon, N.; James, C.; Tichnell, C.; Tucker, A.; Russell, S.D.; Bluemke, D.A.; Dietz, H.C.; et al. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am. J. Hum. Genet. 2006, 79, 136–142. [Google Scholar] [CrossRef]
- Heuser, A.; Plovie, E.R.; Ellinor, P.T.; Grossmann, K.S.; Shin, J.T.; Wichter, T.; Basson, C.T.; Lerman, B.B.; Sasse-Klaassen, S.; Thierfelder, L.; et al. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2006, 79, 1081–1088. [Google Scholar] [CrossRef]
- McKoy, G.; Protonotarios, N.; Crosby, A.; Tsatsopoulou, A.; Anastasakis, A.; Coonar, A.; Norman, M.; Baboonian, C.; Jeffery, S.; McKenna, W.J. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 2000, 355, 2119–2124. [Google Scholar] [CrossRef]
- Norgett, E.E.; Hatsell, S.J.; Carvajal-Huerta, L.; Cabezas, J.C.; Common, J.; Purkis, P.E.; Whittock, N.; Leigh, I.M.; Stevens, H.P.; Kelsell, D.P. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum. Mol. Genet. 2000, 9, 2761–2766. [Google Scholar] [CrossRef]
- Gerull, B.; Heuser, A.; Wichter, T.; Paul, M.; Basson, C.T.; McDermott, D.A.; Lerman, B.B.; Markowitz, S.M.; Ellinor, P.T.; MacRae, C.A.; et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat. Genet. 2004, 36, 1162–1164. [Google Scholar] [CrossRef]
- Pilichou, K.; Nava, A.; Basso, C.; Beffagna, G.; Bauce, B.; Lorenzon, A.; Frigo, G.; Vettori, A.; Valente, M.; Towbin, J.; et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation 2006, 113, 1171–1179. [Google Scholar] [CrossRef]
- Rigato, I.; Corrado, D.; Basso, C.; Zorzi, A.; Pilichou, K.; Bauce, B.; Thiene, G. Pharmacotherapy and other therapeutic modalities for managing Arrhythmogenic Right Ventricular Cardiomyopathy. Cardiovasc. Drugs Ther. 2015, 29, 171–177. [Google Scholar] [CrossRef]
- de Jong, S.; van Veen, T.A.; van Rijen, H.V.; de Bakker, J.M. Fibrosis and cardiac arrhythmias. J. Cardiovasc. Pharmacol. 2011, 57, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Qu, Z.; Weiss, J.N. Cardiac fibrosis and arrhythmogenesis: The road to repair is paved with perils. J. Mol. Cell. Cardiol. 2014, 70, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Šumová, B.; Mallano, T.; Chen, C.W.; Distler, A.; Bergmann, C.; Ludolph, I.; Horch, R.E.; Gelse, K.; Ramming, A.; et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat. Commun. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Su, S.A.; Yang, D.; Wu, Y.; Xie, Y.; Zhu, W.; Cai, Z.; Shen, J.; Fu, Z.; Wang, Y.; Jia, L.; et al. EphrinB2 Regulates Cardiac Fibrosis Through Modulating the Interaction of Stat3 and TGF-β/Smad3 Signaling. Circ. Res. 2017, 121, 617–627. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Waxman, D.J. Cross-talk between janus kinase-signal transducer and activator of transcription (JAK-STAT) and peroxisome proliferator-activated receptor-alpha (PPARalpha) signaling pathways. Growth hormone inhibition of pparalpha transcriptional activity mediated by stat5b. J. Biol. Chem. 1999, 274, 2672–2681. [Google Scholar]
- Shipley, J.M.; Waxman, D.J. Down-regulation of STAT5b transcriptional activity by ligand-activated peroxisome proliferator-activated receptor (PPAR) alpha and PPARgamma. Mol. Pharmacol. 2003, 64, 355–364. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Waxman, D.J. STAT5b down-regulates peroxisome proliferator-activated receptor alpha transcription by inhibition of ligand-independent activation function region-1 trans-activation domain. J. Biol. Chem. 1999, 274, 29874–29882. [Google Scholar] [CrossRef]
- Finck, B.N.; Kelly, D.P. Peroxisome proliferator-activated receptor alpha (PPARalpha) signaling in the gene regulatory control of energy metabolism in the normal and diseased heart. J. Mol. Cell. Cardiol. 2002, 34, 1249–1257. [Google Scholar] [CrossRef]
- Loichot, C.; Jesel, L.; Tesse, A.; Tabernero, A.; Schoonjans, K.; Roul, G.; Carpusca, I.; Auwerx, J.; Andriantsitohaina, R. Deletion of peroxisome proliferator-activated receptor-alpha induces an alteration of cardiac functions. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H161–H166. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Miyauchi, T.; Sakai, S.; Takanashi, M.; Irukayama-Tomobe, Y.; Yamaguchi, I. Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J. Am. Coll. Cardiol. 2004, 43, 1481–1488. [Google Scholar] [PubMed]
- Zhang, Y.; Ji, H.; Qiao, O.; Li, Z.; Pecoraro, L.; Zhang, X.; Han, X.; Wang, W.; Zhang, X.; Man, S.; et al. Nanoparticle conjugation of ginsenoside Rb3 inhibits myocardial fibrosis by regulating PPARα pathway. Biomed. Pharmacother. 2021, 139, 111630. [Google Scholar] [CrossRef]
- Bansal, T.; Chatterjee, E.; Singh, J.; Ray, A.; Kundu, B.; Thankamani, V.; Sengupta, S.; Sarkar, S. Arjunolic acid, a peroxisome proliferator-activated receptor α agonist, regresses cardiac fibrosis by inhibiting non-canonical TGF-β signaling. J. Biol. Chem. 2017, 292, 16440–16462. [Google Scholar] [CrossRef] [PubMed]
- Diep, Q.N.; Benkirane, K.; Amiri, F.; Cohn, J.S.; Endemann, D.; Schiffrin, E.L. PPAR alpha activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin II-infused rats. J. Mol. Cell. Cardiol. 2004, 36, 295–304. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, R.; Huang, Y.; Yang, Z.; Xian, J.; Huang, J.; Qiu, Z.; Lin, X.; Zhang, M.; Chen, H.; et al. Reactivation of PPARα alleviates myocardial lipid accumulation and cardiac dysfunction by improving fatty acid β-oxidation in Dsg2-deficient arrhythmogenic cardiomyopathy. In Acta Pharmaceutica Sinica B; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, B.; Suo, Y.; Liu, C.; Yang, Q.; Zhang, K.; Yuan, M.; Yuan, M.; Zhang, Y.; Li, G. Intermittent hypoxia mediated by TSP1 dependent on STAT3 induces cardiac fibroblast activation and cardiac fibrosis. eLife 2020, 9, e49923. [Google Scholar] [CrossRef]
- Cao, W.; Shi, P.; Ge, J.J. miR-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via CADM1/STAT3 pathway. BMC Cardiovasc. Disord. 2017, 17, 88. [Google Scholar] [CrossRef]
- Chen, X.; Su, J.; Feng, J.; Cheng, L.; Li, Q.; Qiu, C.; Zheng, Q. TRIM72 contributes to cardiac fibrosis via regulating STAT3/Notch-1 signaling. J. Cell. Physiol. 2019, 234, 17749–17756. [Google Scholar] [CrossRef]
- Balakumar, P.; Rohilla, A.; Mahadevan, N. Pleiotropic actions of fenofibrate on the heart. Pharmacol. Res. 2011, 63, 8–12. [Google Scholar] [CrossRef]
- Kant, S.; Holthöfer, B.; Magin, T.M.; Krusche, C.A.; Leube, R.E. Desmoglein 2-Dependent Arrhythmogenic Cardiomyopathy Is Caused by a Loss of Adhesive Function. Circ. Cardiovasc. Genet. 2015, 8, 553–563. [Google Scholar] [CrossRef]
- Hermida, A.; Fressart, V.; Hidden-Lucet, F.; Donal, E.; Probst, V.; Deharo, J.C.; Chevalier, P.; Klug, D.; Mansencal, N.; Delacretaz, E.; et al. High risk of heart failure associated with desmoglein-2 mutations compared to plakophilin-2 mutations in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur. J. Heart Fail. 2019, 21, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Souders, C.A.; Bowers, S.L.; Baudino, T.A. Cardiac fibroblast: The renaissance cell. Circ. Res. 2009, 105, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Mandel, W.J.; Kobayashi, Y.; Karagueuzian, H.S. Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J. Arrhythm. 2014, 30, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; López, B.; Coelho-Filho, O.R.; Lakdawala, N.K.; Cirino, A.L.; Jarolim, P.; Kwong, R.; González, A.; Colan, S.D.; Seidman, J.G.; et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N. Engl. J. Med. 2010, 363, 552–563. [Google Scholar] [CrossRef]
- Smeets, P.J.; Planavila, A.; van der Vusse, G.J.; van Bilsen, M. Peroxisome proliferator-activated receptors and inflammation: Take it to heart. Acta Physiol. 2007, 191, 171–188. [Google Scholar] [CrossRef]
- Smeets, P.J.; Teunissen, B.E.; Willemsen, P.H.; van Nieuwenhoven, F.A.; Brouns, A.E.; Janssen, B.J.; Cleutjens, J.P.; Staels, B.; van der Vusse, G.J.; van Bilsen, M. Cardiac hypertrophy is enhanced in PPAR alpha−/− mice in response to chronic pressure overload. Cardiovasc. Res. 2008, 78, 79–89. [Google Scholar] [CrossRef]
- Finck, B.N. The PPAR regulatory system in cardiac physiology and disease. Cardiovasc. Res. 2007, 73, 269–277. [Google Scholar] [CrossRef]
- Li, L.X.; Yin, L.H.; Gao, M.; Xu, L.N.; Qi, Y.; Peng, J.Y. MiR-23a-5p exacerbates intestinal ischemia-reperfusion injury by promoting oxidative stress via targeting PPAR alpha. Biochem. Pharmacol. 2020, 180, 114194. [Google Scholar] [CrossRef]
- Lockyer, P.; Schisler, J.C.; Patterson, C.; Willis, M.S. Minireview: Won't get fooled again: The nonmetabolic roles of peroxisome proliferator-activated receptors (PPARs) in the heart. Mol. Endocrinol. 2010, 24, 1111–1119. [Google Scholar] [CrossRef][Green Version]
- Li, P.; Luo, S.; Pan, C.; Cheng, X. Modulation of fatty acid metabolism is involved in the alleviation of isoproterenol-induced rat heart failure by fenofibrate. Mol. Med. Rep. 2015, 12, 7899–7906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Cheng, Y.; Gu, J.; Wang, S.; Zhou, S.; Wang, Y.; Tan, Y.; Feng, W.; Fu, Y.; Mellen, N.; et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin. Sci. 2016, 130, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.J.; et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Wang, B.; Zhang, H.; Qin, G.; Li, C.; Cao, L.; Gao, Q.; Ping, Y.; Zhang, K.; et al. Regulatory T cells promote glioma cell stemness through TGF-β-NF-κB-IL6-STAT3 signaling. Cancer Immunol. Immunother. 2021, 70, 2601–2616. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Flavell, R.A.; Choi, M.E. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for activation of p38alpha and p38delta MAPK isoforms by TGF-beta 1 in murine mesangial cells. J. Biol. Chem. 2002, 277, 47257–47262. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, N.; Vera, R.; Fraile, B.; Martínez-Onsurbe, P.; Paniagua, R.; Royuela, M. TGF-β/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer. Aging Male 2020, 23, 801–811. [Google Scholar] [CrossRef]
- Zhuang, L.; Jia, K.; Chen, C.; Li, Z.; Zhao, J.; Hu, J.; Zhang, H.; Fan, Q.; Huang, C.; Xie, H.; et al. DYRK1B-STAT3 Drives Cardiac Hypertrophy and Heart Failure by Impairing Mitochondrial Bioenergetics. Circulation 2022, 145, 829–846. [Google Scholar] [CrossRef]
- Patel, N.J.; Nassal, D.M.; Gratz, D.; Hund, T.J. Emerging therapeutic targets for cardiac arrhythmias: Role of STAT3 in regulating cardiac fibroblast function. Expert Opin. Ther. Targets 2021, 25, 63–73. [Google Scholar] [CrossRef]
- Boengler, K.; Hilfiker-Kleiner, D.; Drexler, H.; Heusch, G.; Schulz, R. The myocardial JAK/STAT pathway: From protection to failure. Pharmacol. Ther. 2008, 120, 172–185. [Google Scholar] [CrossRef]
- Chang, H.; Zhao, F.; Xie, X.; Liao, Y.; Song, Y.; Liu, C.; Wu, Y.; Wang, Y.; Liu, D.; Wang, Y.; et al. PPARα suppresses Th17 cell differentiation through IL-6/STAT3/RORγt pathway in experimental autoimmune myocarditis. Exp. Cell Res. 2019, 375, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Kleemann, R.; Pilon, A.; Percevault, F.; Koenig, W.; Staels, B.; Kooistra, T. Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-alpha activator fenofibrate. J. Biol. Chem. 2004, 279, 16154–16160. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Tian, Q.; Ishiyama, M.; Burchfield, J.; Gao, F.; Mann, D.L.; Barger, P.M. Inhibition of PPAR-alpha activity in mice with cardiac-restricted expression of tumor necrosis factor: Potential role of TGF-beta/Smad3. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).