The Longevity-Associated Variant of BPIFB4 Reduces Senescence in Glioma Cells and in Patients’ Lymphocytes Favoring Chemotherapy Efficacy
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture and Treatment
2.2. Senescence Induction and Detection
2.3. Cytofluorimetric Analysis
2.4. Cytokine Detection
2.5. Proliferation Assay
2.6. Cytotoxicity Assay
2.7. Apoptosis Assay
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
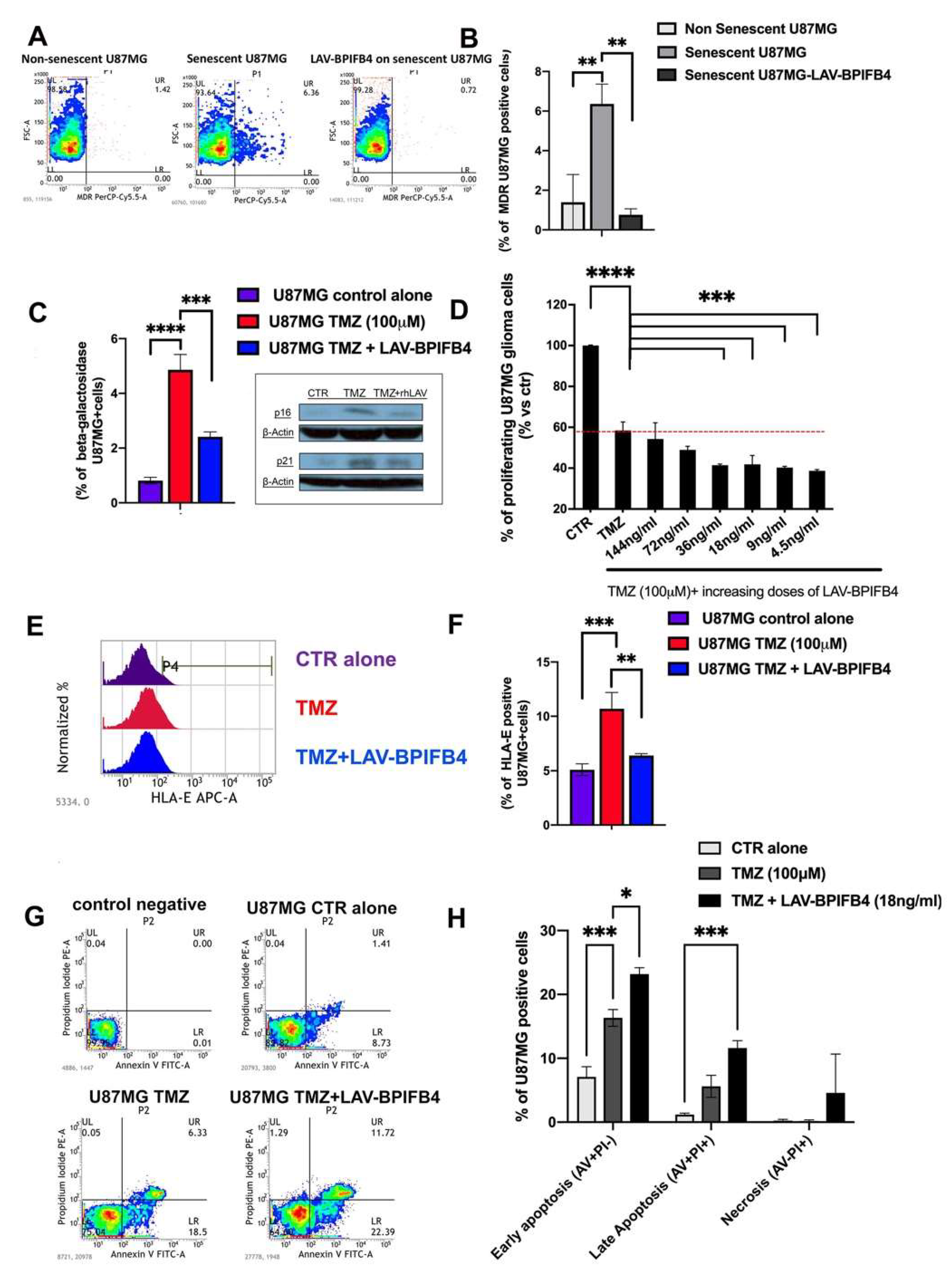
3.1. Recombinant Human LAV-BPIFB4 Influenced GBM Cell Senescence Rate
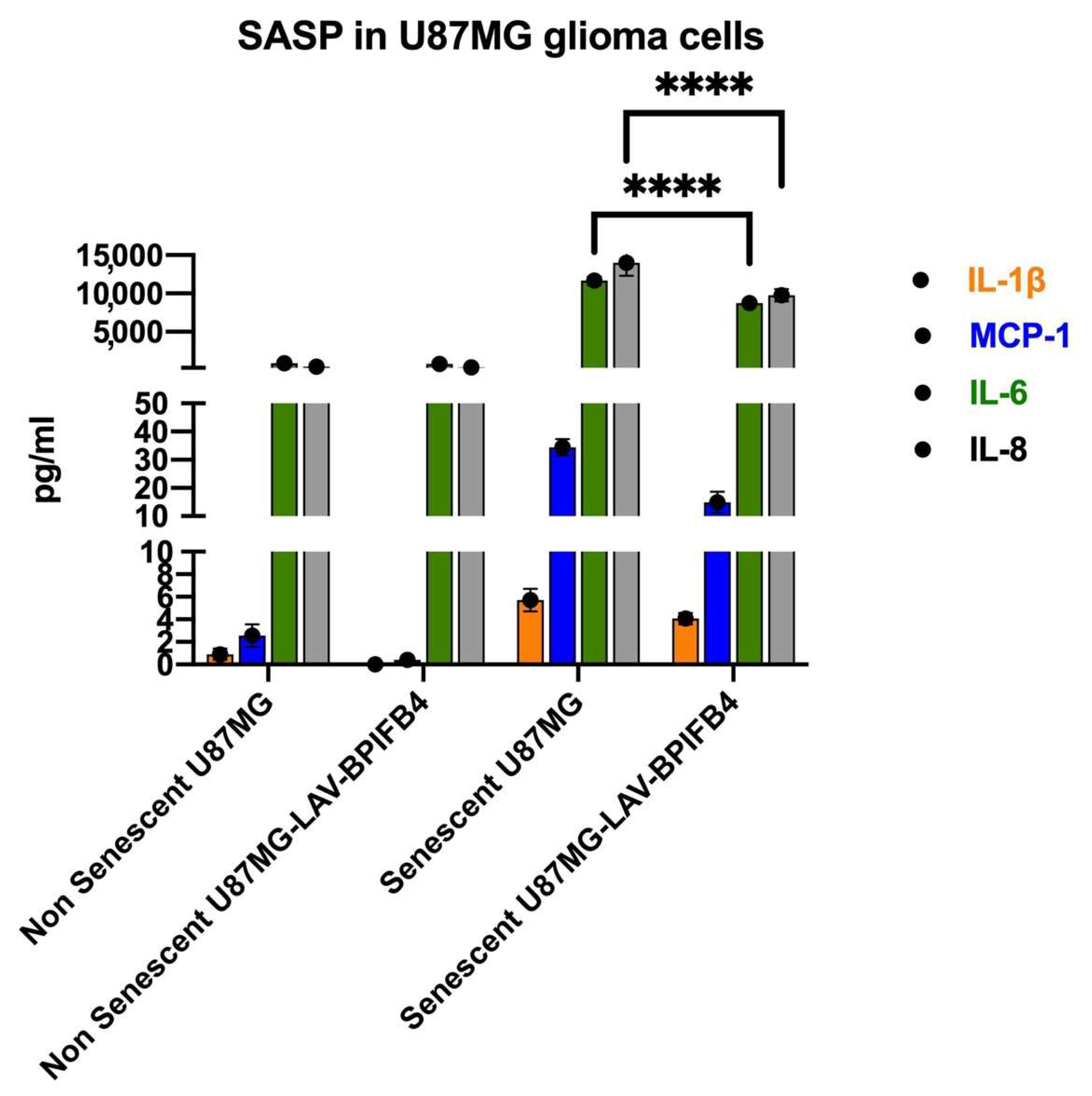
3.2. Recombinant Human LAV-BPIFB4 Decreased SASP in ETP-Treated Senescent GBM Cells
3.3. Recombinant Human LAV-BPIFB4 Sensitized U87-MG Cells to TMZ Treatment
3.4. LAV-BPIFB4 Reversed the Senescent Phenotype of T cells from GBM Patients
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quail, D.F.; Joyce, J.A. The microenvironmental landscape of brain tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv119–iv142. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Carminucci, A.S.; Amendolara, B.; Bansal, M.; Leung, R.; Lei, L.; Realubit, R.; Li, H.; Karan, C.; Yun, J.; et al. Convection-enhanced delivery of etoposide is effective against murine proneural glioblastoma. Neuro Oncol. 2014, 16, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.J.; Tyler, B.M.; Gould, T.; Veal, G.J.; Gorelick, N.; Rowlinson, J.; Serra, R.; Ritchie, A.; Berry, P.; Otto, A.; et al. Overall Survival in Malignant Glioma Is Significantly Prolonged by Neurosurgical Delivery of Etoposide and Temozolomide from a Thermo-Responsive Biodegradable Paste. Clin. Cancer Res. 2019, 25, 5094–5106. [Google Scholar] [CrossRef] [Green Version]
- Brenner, E.; Schörg, B.F.; Ahmetlić, F.; Wieder, T.; Hilke, F.J.; Simon, N.; Schroeder, C.; Demidov, G.; Riedel, T.; Fehrenbacher, B.; et al. Cancer immune control needs senescence induction by interferon-dependent cell cycle regulator pathways in tumours. Nat. Commun. 2020, 11, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, W.; Zender, L.; Miething, C.; Dickins, R.A.; Hernando, E.; Krizhanovsky, V.; Cordon-Cardo, C.; Lowe, S.W. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007, 445, 656–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamayo-Orrego, L.; Wu, C.L.; Bouchard, N.; Khedher, A.; Swikert, S.M.; Remke, M.; Skowron, P.; Taylor, M.D.; Charron, F. Evasion of Cell Senescence Leads to Medulloblastoma Progression. Cell Rep. 2016, 14, 2925–2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roninson, I.B. Tumor cell senescence in cancer treatment. Cancer Res. 2003, 63, 2705–2715. [Google Scholar]
- Eggert, T.; Wolter, K.; Ji, J.; Ma, C.; Yevsa, T.; Klotz, S.; Medina-Echeverz, J.; Longerich, T.; Forgues, M.; Reisinger, F.; et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 2016, 30, 533–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkaya, H.; Kim, G.I.; Davis, A.; Malik, F.; Henry, N.L.; Ithimakin, S.; Quraishi, A.A.; Tawakkol, N.; D’Angelo, R.; Paulson, A.K.; et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell 2012, 47, 570–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Mitsutake, N.; LaPerle, K.; Akeno, N.; Zanzonico, P.; Longo, V.A.; Mitsutake, S.; Kimura, E.T.; Geiger, H.; Santos, E.; et al. Endogenous expression of Hras(G12V) induces developmental defects and neoplasms with copy number imbalances of the oncogene. Proc. Natl. Acad. Sci. USA 2009, 106, 7979–7984. [Google Scholar] [CrossRef] [Green Version]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzmann, F.; Ortells, M.C.; Morton, J.P.; Sansom, O.J.; Zender, L.; Keyes, W.M. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Chiche, A.; Le Roux, I.; von Joest, M.; Sakai, H.; Aguín, S.B.; Cazin, C.; Salam, R.; Fiette, L.; Alegria, O.; Flamant, P.; et al. Injury-Induced Senescence Enables In Vivo Reprogramming in Skeletal Muscle. Cell Stem Cell 2017, 20, 407–414.e4. [Google Scholar] [CrossRef] [Green Version]
- Aasland, D.; Götzinger, L.; Hauck, L.; Berte, N.; Meyer, J.; Effenberger, M.; Schneider, S.; Reuber, E.E.; Roos, W.P.; Tomicic, M.T.; et al. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR–CHK1, p21, and NF-kB. Cancer Res. 2019, 79, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Awah, C.U.; Sonabend, A.M. Topoisomerase II Poisons for Glioblastoma; Existing Challenges and Opportunities to Personalize Therapy. Front. Neurol. 2018, 9, 459. [Google Scholar] [CrossRef]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [CrossRef]
- Kosar, M.; Bartkova, J.; Hubackova, S.; Hodny, Z.; Lukas, J.; Bartek, J. Senescence-Associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a). Cell Cycle 2011, 10, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Tyutynuk-Massey, L.; Cudjoe Jr, E.K.; Idowu, M.O.; Landry, J.W.; Gewirtz, D.A. Non-Cell Autonomous Effects of the Senescence-Associated Secretory Phenotype in Cancer. Front. Oncol. 2018, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Johannessen, T.C.; Bjerkvig, R. Molecular mechanisms of temozolomide resistance in glioblastoma multiforme. Expert Rev. Anticancer Ther. 2012, 12, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Tivnan, A.; Zakaria, Z.; O’Leary, C.; Kögel, D.; Pokorny, J.L.; Sarkaria, J.N.; Prehn, J.H. Inhibition of multidrug resistance protein 1 (MRP1) improves chemotherapy drug response in primary and recurrent glioblastoma multiforme. Front. Neurosci. 2015, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Wu, Q.; Sathornsumetee, S.; Hao, Y.; Li, Z.; Hjelmeland, A.B.; Shi, Q.; McLendon, R.E.; Bigner, D.D.; Rich, J.N. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006, 66, 7843–7848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Barr, J.; Kong, L.Y.; Wang, Y.; Wu, A.; Sharma, A.K.; Gumin, J.; Henry, V.; Colman, H.; Priebe, W.; et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol. Cancer Ther. 2010, 9, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Woroniecka, K.I.; Rhodin, K.E.; Chongsathidkiet, P.; Keith, K.A.; Fecci, P.E. T-Cell Dysfunction in Glioblastoma: Applying a New Framework. Clin. Cancer Res. 2018, 24, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro. Oncol. 2016, 18 (Suppl. 5), v1–v75. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Walford, R.L. The Immunologic Theory of Aging. Gerontologist 1964, 4, 195–197. [Google Scholar] [CrossRef]
- Ciaglia, E.; Lopardo, V.; Montella, F.; Sellitto, C.; Manzo, V.; De Bellis, E.; Iannaccone, T.; Franci, G.; Zannella, C.; Pagliano, P.; et al. BPIFB4 Circulating Levels and Its Prognostic Relevance in COVID-19. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1775–1783. [Google Scholar] [CrossRef]
- Di Pardo, A.; Ciaglia, E.; Cattaneo, M.; Maciag, A.; Montella, F.; Lopardo, V.; Ferrario, A.; Villa, F.; Madonna, M.; Amico, E.; et al. The longevity-associated variant of BPIFB4 improves a CXCR4-mediated striatum–microglia crosstalk preventing disease progression in a mouse model of Huntington’s disease. Cell Death Dis. 2020, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Avolio, E.; Thomas, A.C.; Faulkner, A.; Beltrami, A.P.; Cervellin, C.; Carrizzo, A.; Maciag, A.; Gu, Y.; Ciaglia, E.; et al. Transfer of a human gene variant associated with exceptional longevity improves cardiac function in obese type 2 diabetic mice through induction of the SDF-1/CXCR4 signalling pathway. Eur. J. Heart Fail. 2020, 22, 1568–1581. [Google Scholar] [CrossRef] [PubMed]
- Puca, A.A.; Carrizzo, A.; Spinelli, C.; Damato, A.; Ambrosio, M.; Villa, F.; Ferrario, A.; Maciag, A.; Fornai, F.; Lenzi, P.; et al. Single systemic transfer of a human gene associated with exceptional longevity halts the progression of atherosclerosis and inflammation in ApoE knockout mice through a CXCR4-mediated mechanism. Eur. Heart J. 2020, 41, 2487–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malavolta, M.; Dato, S.; Villa, F.; De Rango, F.; Iannone, F.; Ferrario, A.; Maciag, A.; Ciaglia, E.; D’Amato, A.; Carrizzo, A.; et al. LAV-BPIFB4 associates with reduced frailty in humans and its transfer prevents frailty progression in old mice. Aging 2019, 11, 6555–6568. [Google Scholar] [CrossRef]
- Villa, F.; Malovini, A.; Carrizzo, A.; Spinelli, C.C.; Ferrario, A.; Maciąg, A.; Madonna, M.; Bellazzi, R.; Milanesi, L.; Vecchione, C.; et al. Serum BPIFB4 levels classify health status in long-living individuals. Immun. Ageing. 2015, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Dossena, M.; Ferrario, A.; Lopardo, V.; Ciaglia, E.; Puca, A.A. New Insights for BPIFB4 in Cardiovascular Therapy. Int. J. Mol. Sci. 2020, 21, 7163. [Google Scholar] [CrossRef]
- Ciaglia, E.; Montella, F.; Maciag, A.; Scala, P.; Ferrario, A.; Banco, C.; Carrizzo, A.; Spinelli, C.C.; Cattaneo, M.; De Candia, P.; et al. Longevity-Associated Variant of BPIFB4 Mitigates Monocyte-Mediated Acquired Immune Response. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74 (Suppl. 1), S38–S44. [Google Scholar] [CrossRef]
- Tamamori-Adachi, M.; Koga, A.; Susa, T.; Fujii, H.; Tsuchiya, M.; Okinaga, H.; Hisaki, H.; Iizuka, M.; Kitajima, S.; Okazaki, T. DNA damage response induced by Etoposide promotes steroidogenesis via GADD45A in cultured adrenal cells. Sci. Rep. 2018, 8, 9636. [Google Scholar] [CrossRef]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Ruth, A.C.; Roninson, I.B. Effects of the multidrug transporter P-glycoprotein on cellular responses to ionizing radiation. Cancer Res. 2000, 60, 2576–2578. [Google Scholar] [PubMed]
- Stein, G.H.; Drullinger, L.F.; Soulard, A.; Dulić. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell Biol. 1999, 19, 2109–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Rivera-Pérez, J.A. Senescence-Associated β-galactosidase activity marks the visceral endoderm of mouse embryos but is not indicative of senescence. Genesis 2014, 52, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Ciaglia, E.; Lopardo, V.; Montella, F.; Carrizzo, A.; Di Pietro, P.; Malavolta, M.; Giacconi, R.; Orlando, F.; Cattaneo, M.; Madeddu, P.; et al. Transfer of the Longevity associated variant of BPIFB4 gene rejuvenates immune system and vasculature by a reduction of CD38+macrophages and NAD+ decline. Cell Death Dis. 2021, in press.
- Putavet, D.A.; de Keizer, P.L.J. Residual Disease in Glioma Recurrence: A Dangerous Liaison with Senescence. Cancers 2021, 13, 1560. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Ito, K.; Kawana, M.; Iwata, T.; Higai, K. Transcriptional Regulation of the Natural Cytotoxicity Receptor NKp44 Gene in Human NK Cell Leukemia. J. Glycomics Lipidomics 2019, 8, 144. [Google Scholar] [CrossRef]
- Bozek, K.; Relógio, A.; Kielbasa, S.M.; Heine, M.; Dame, C.; Kramer, A.; Herzel, H. Regulation of clock-controlled genes in mammals. PLoS ONE 2009, 4, e4882. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.R.D.; Cuzzubbo, S.; McArthur, S.; Durrant, L.G.; Adhikaree, J.; Tinsley, C.J.; Pockley, A.G.; McArdle, S.E.B. Immune Escape in Glioblastoma Multiforme and the Adaptation of Immunotherapies for Treatment. Front. Immunol. 2020, 11, 582106. [Google Scholar] [CrossRef]
- Munoz, J.L.; Rodriguez-Cruz, V.; Greco, S.J.; Nagula, V.; Scotto, K.W.; Rameshwar, P. Temozolomide induces the production of epidermal growth factor to regulate MDR1 expression in glioblastoma cells. Mol. Cancer Ther. 2014, 13, 2399–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granada, A.E.; Jiménez, A.; Stewart-Ornstein, J.; Blüthgen, N.; Reber, S.; Jambhekar, A.; Lahav, G. The effects of proliferation status and cell cycle phase on the responses of single cells to chemotherapy. Mol. Biol. Cell 2020, 31, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Stover, D.G.; Coloff, J.L.; Barry, W.T.; Brugge, J.S.; Winer, E.P.; Selfors, L.M. The Role of Proliferation in Determining Response to Neoadjuvant Chemotherapy in Breast Cancer: A Gene Expression-Based Meta-Analysis. Clin. Cancer Res. 2016, 22, 6039–6050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schosserer, M.; Grillari, J.; Breitenbach, M. The Dual Role of Cellular Senescence in Developing Tumors and Their Response to Cancer Therapy. Front. Oncol. 2017, 7, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puca, A.A.; Lopardo, V.; Montella, F.; Di Pietro, P.; Cesselli, D.; Rolle, I.G.; Bulfoni, M.; Di Sarno, V.; Iaconetta, G.; Campiglia, P.; et al. The Longevity-Associated Variant of BPIFB4 Reduces Senescence in Glioma Cells and in Patients’ Lymphocytes Favoring Chemotherapy Efficacy. Cells 2022, 11, 294. https://doi.org/10.3390/cells11020294
Puca AA, Lopardo V, Montella F, Di Pietro P, Cesselli D, Rolle IG, Bulfoni M, Di Sarno V, Iaconetta G, Campiglia P, et al. The Longevity-Associated Variant of BPIFB4 Reduces Senescence in Glioma Cells and in Patients’ Lymphocytes Favoring Chemotherapy Efficacy. Cells. 2022; 11(2):294. https://doi.org/10.3390/cells11020294
Chicago/Turabian StylePuca, Annibale Alessandro, Valentina Lopardo, Francesco Montella, Paola Di Pietro, Daniela Cesselli, Irene Giulia Rolle, Michela Bulfoni, Veronica Di Sarno, Giorgio Iaconetta, Pietro Campiglia, and et al. 2022. "The Longevity-Associated Variant of BPIFB4 Reduces Senescence in Glioma Cells and in Patients’ Lymphocytes Favoring Chemotherapy Efficacy" Cells 11, no. 2: 294. https://doi.org/10.3390/cells11020294
APA StylePuca, A. A., Lopardo, V., Montella, F., Di Pietro, P., Cesselli, D., Rolle, I. G., Bulfoni, M., Di Sarno, V., Iaconetta, G., Campiglia, P., Vecchione, C., Beltrami, A. P., & Ciaglia, E. (2022). The Longevity-Associated Variant of BPIFB4 Reduces Senescence in Glioma Cells and in Patients’ Lymphocytes Favoring Chemotherapy Efficacy. Cells, 11(2), 294. https://doi.org/10.3390/cells11020294









