Ion Channel Modeling beyond State of the Art: A Comparison with a System Theory-Based Model of the Shaker-Related Voltage-Gated Potassium Channel Kv1.1
Abstract
:1. Introduction
2. Methods and Results
2.1. Electrophysiological Experiments and Datasets
2.2. Data and Data Pre-Processing Considered for HMM and STB Model Parameterization
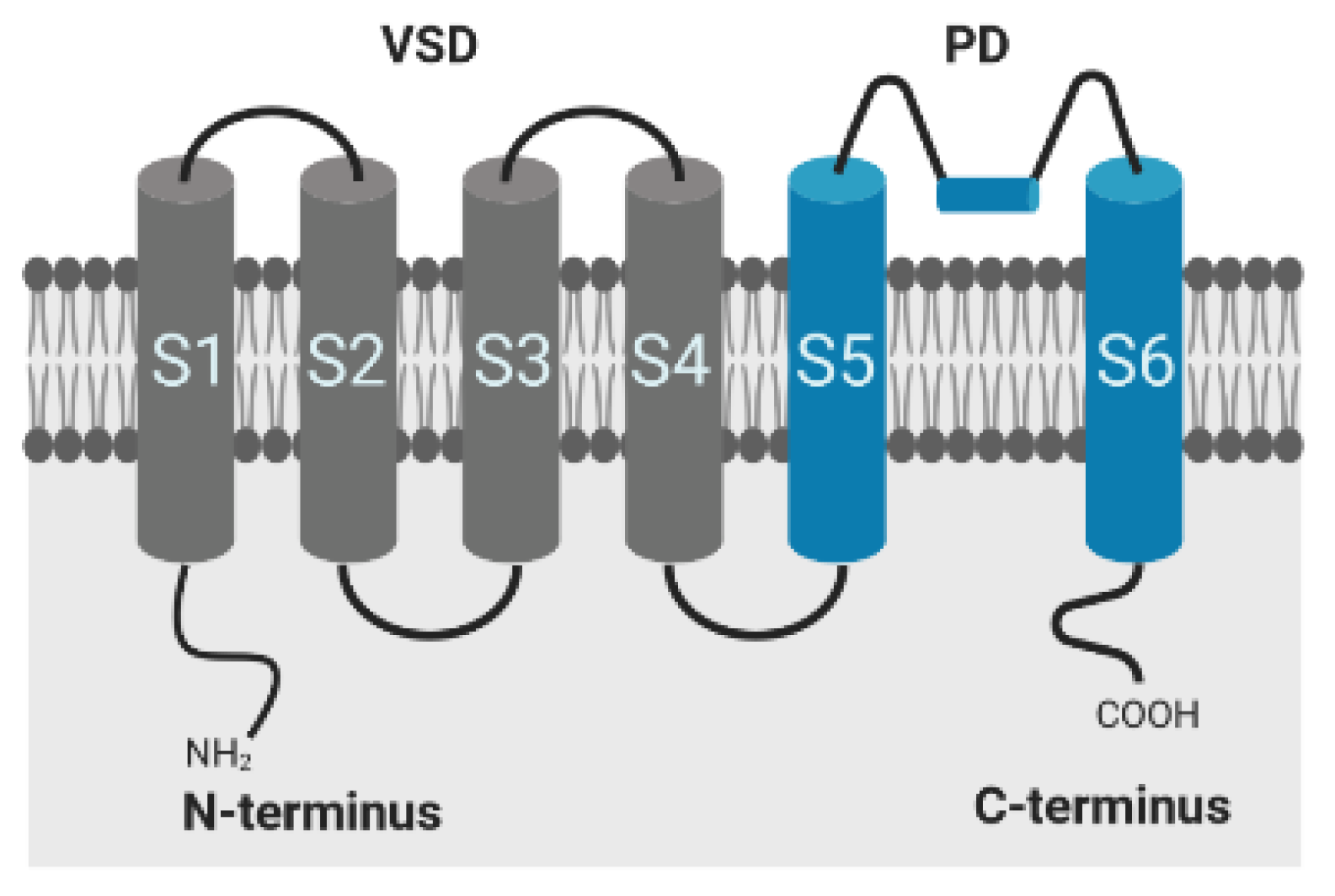
2.3. Available HH Model and HMM of the Ion Channel Kv1.1
2.4. Mathematical Concepts of Ion Channel Modelling
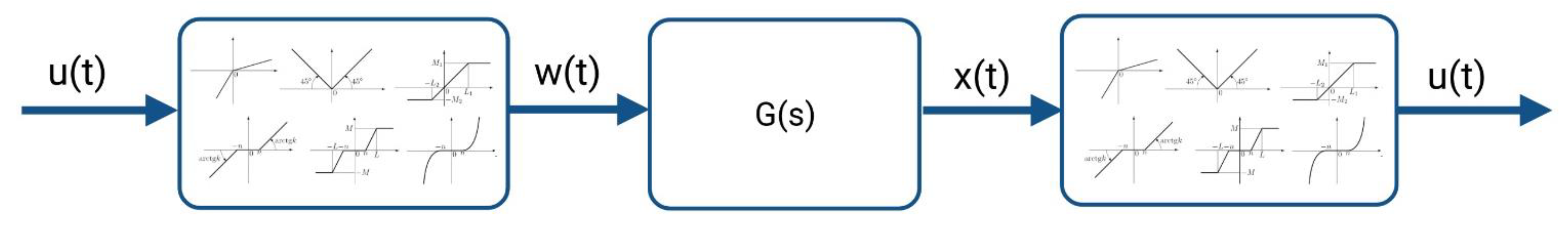
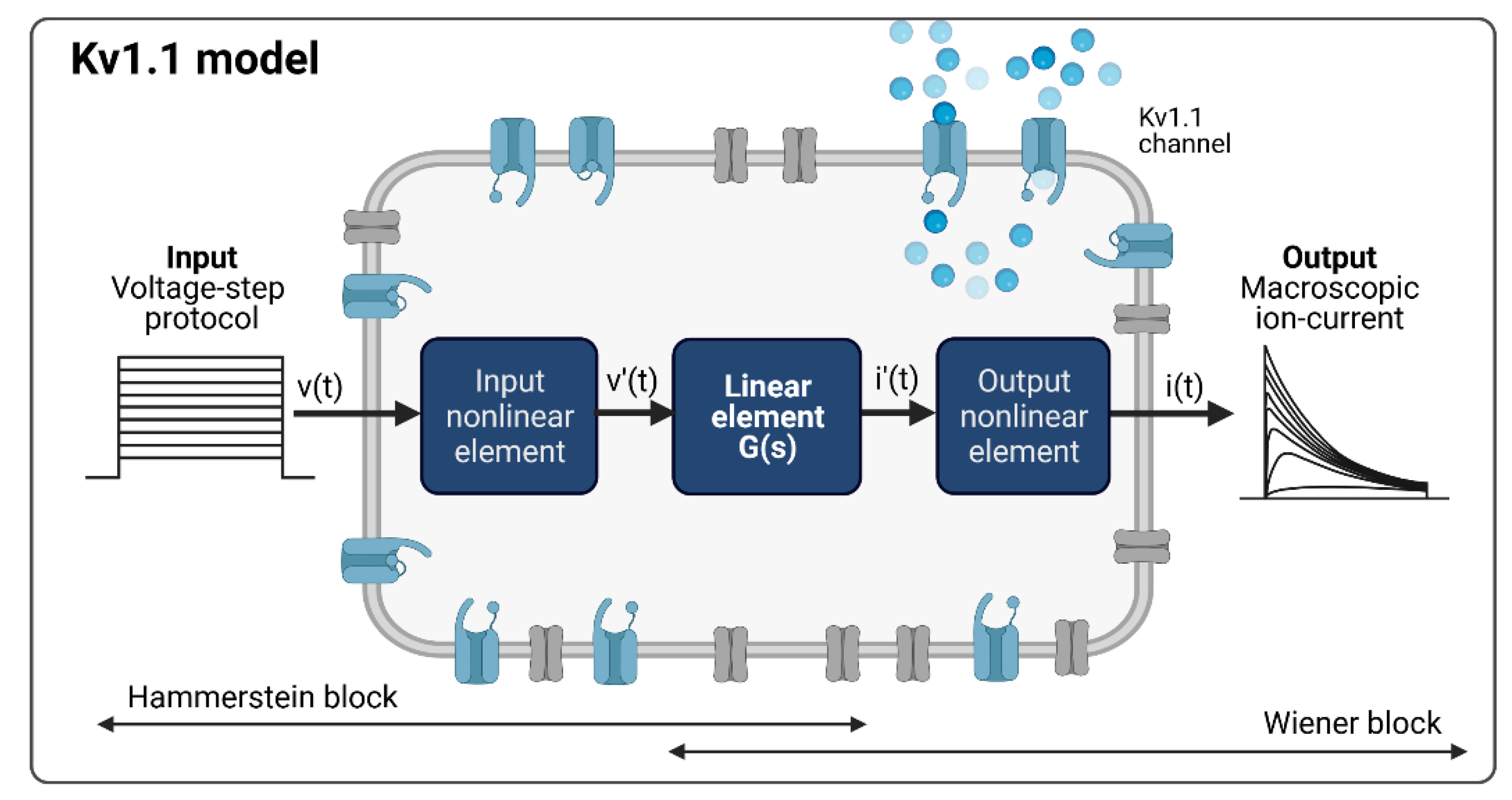
2.4.1. The System Theory-Based Modeling Approach for the Kv1.1 Channel
2.4.2. The HMM-Based Kv1.1 Model
2.4.3. The HH-Based Kv1.1 Model
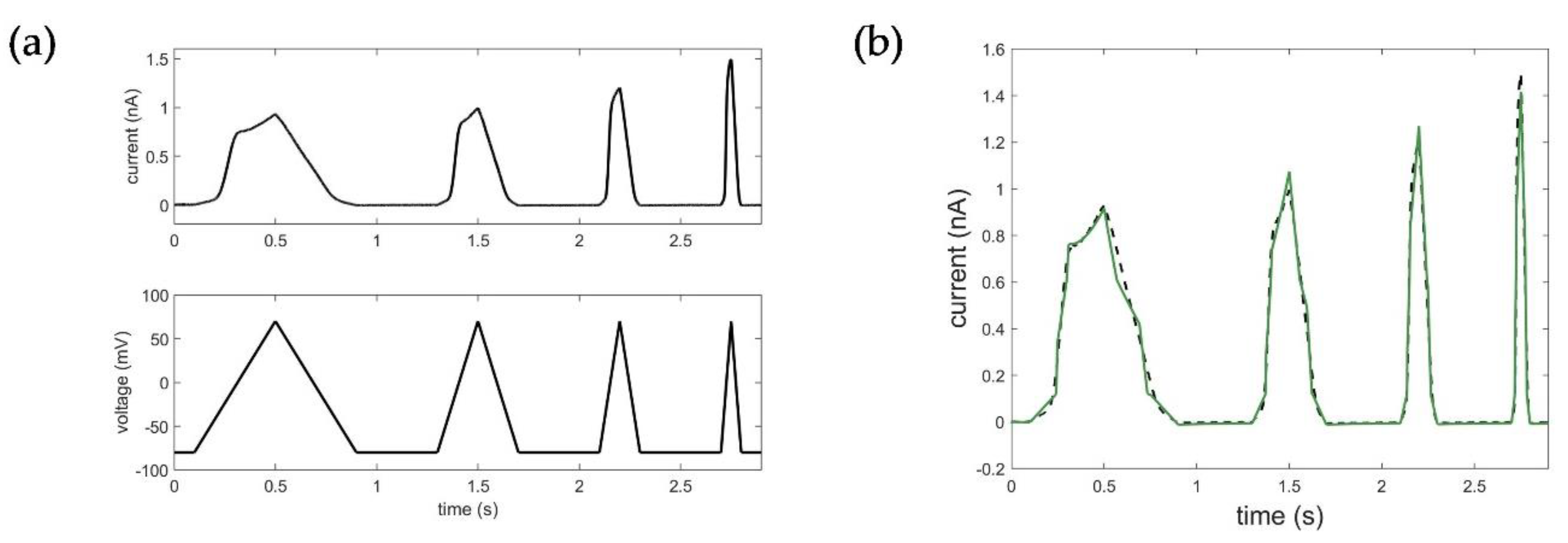
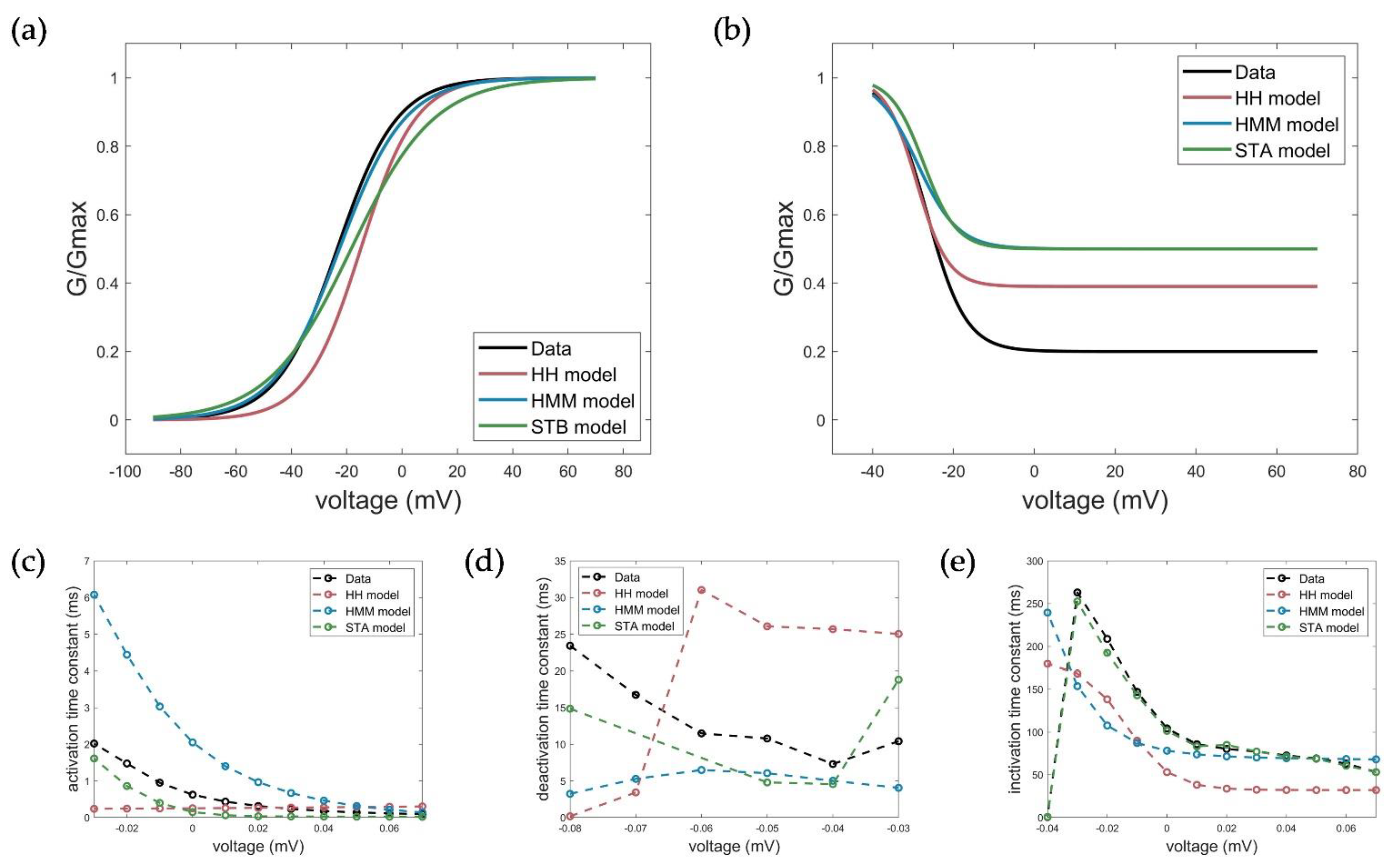
2.5. Evaluation, Verification, and Comparison of the Three Model Approaches
3. Discussion
3.1. Model Accuracy
3.2. Model Complexity, Explainability, and Adaptability
3.3. Computational Burden
3.4. Experimental Data for Model Parameterization
3.5. Which Method Should Now Be Chosen? When, How, and Why?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Computational Modeling and Parameterization
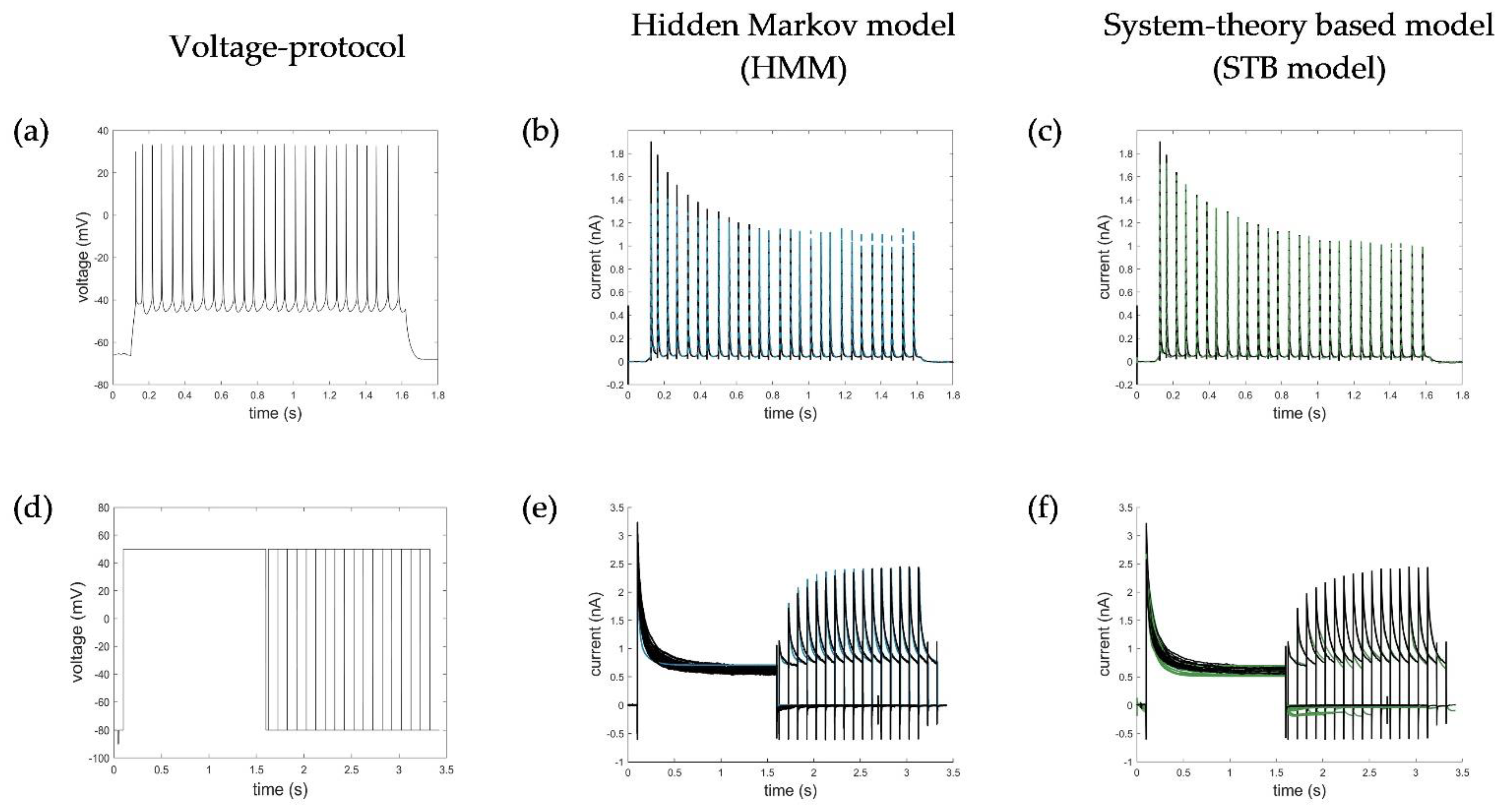
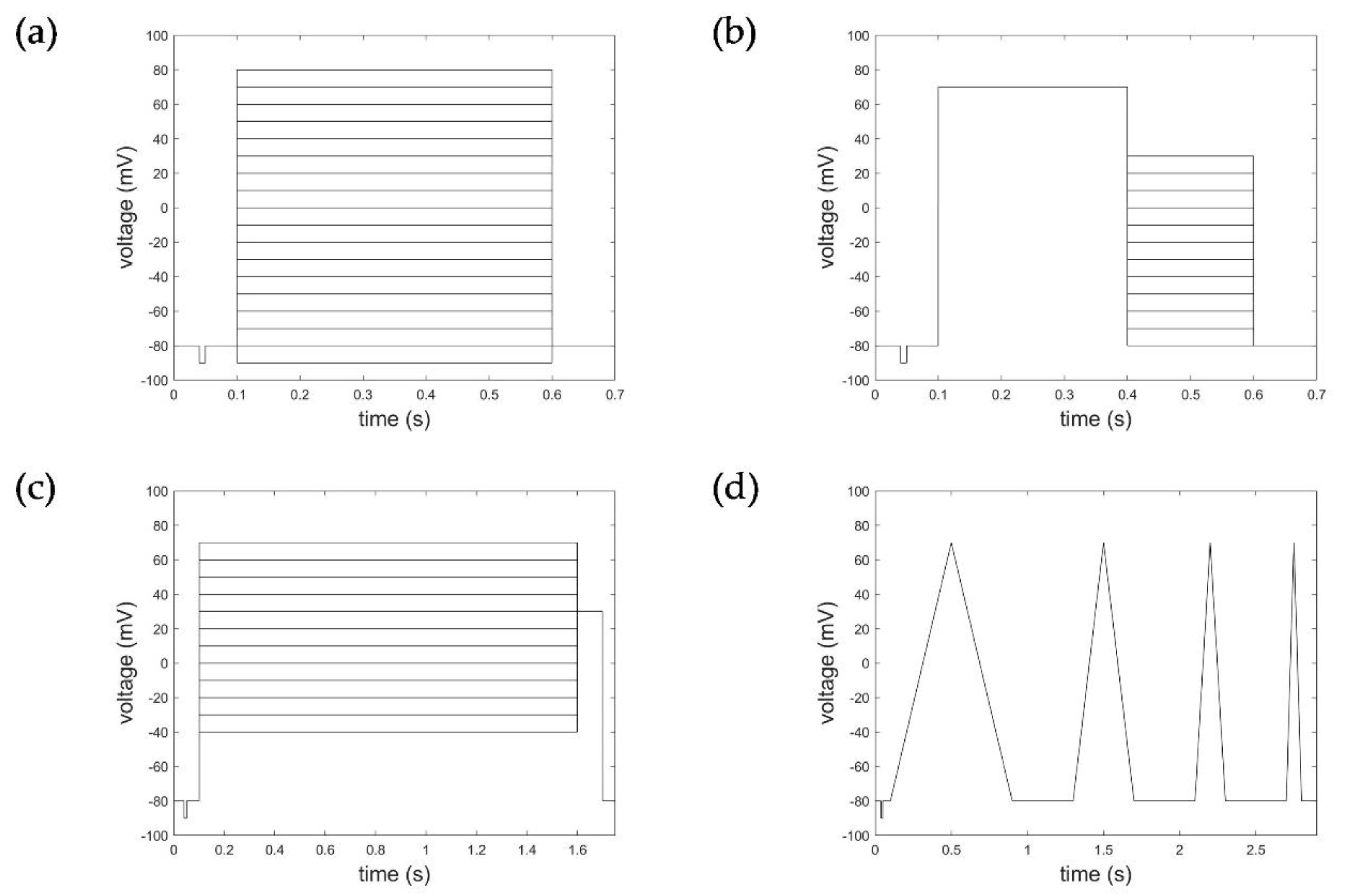
Appendix B. Simulation of AP and Recovery Voltage Protocols with the HMM and STB Models
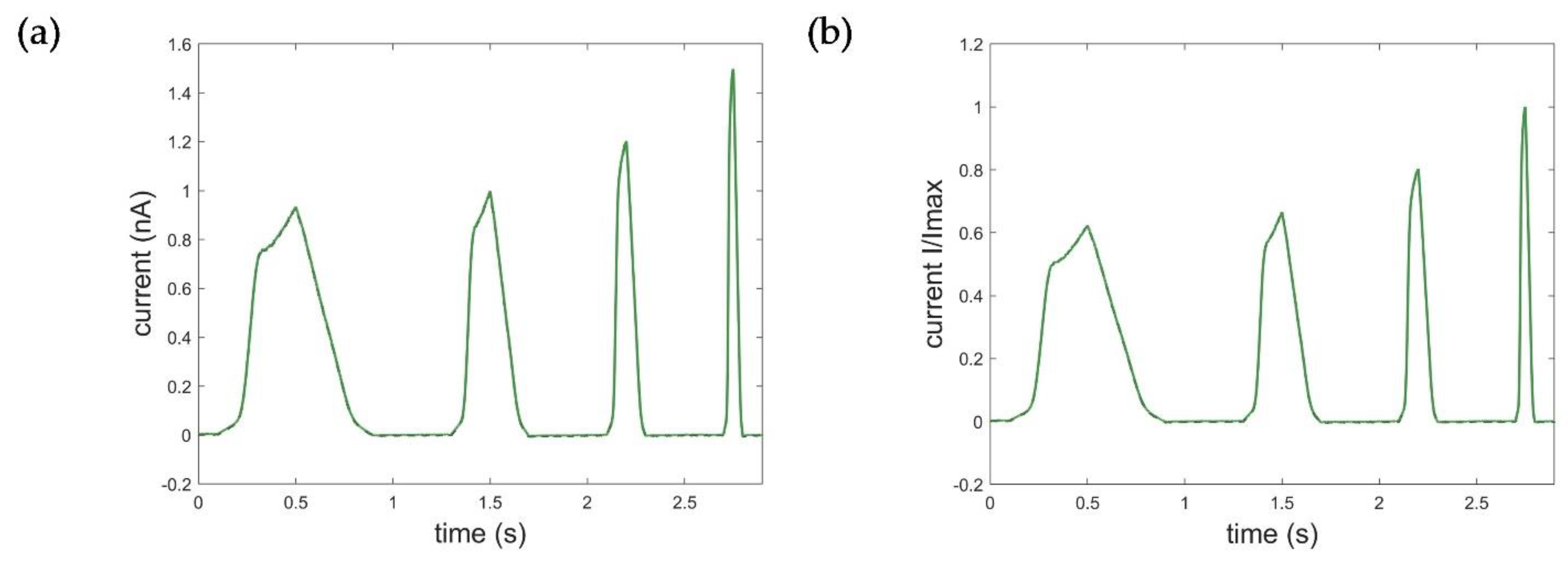
Appendix C. Additional Simulation of the Ramp Curve with the STB Model
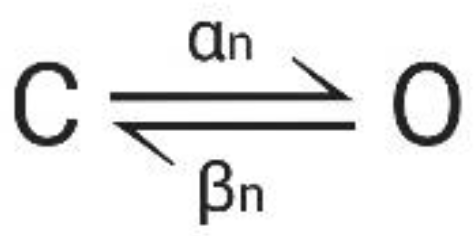
Appendix D. Original Hodgkin–Huxley Formalism of the Potassium Current
References
- Andreozzi, E.; Carannante, I.; D’Addio, G.; Cesarelli, M.; Balbi, P. Phenomenological Models of Na V 1.5. A Side by Side, Procedural, Hands-on Comparison between Hodgkin-Huxley and Kinetic Formalisms. Sci. Rep. 2019, 9, 17493. [Google Scholar] [CrossRef] [PubMed]
- Langthaler, S.; Rienmüller, T.; Scheruebel, S.; Pelzmann, B.; Shrestha, N.; Zorn-Pauly, K.; Schreibmayer, W.; Koff, A.; Baumgartner, C. A549 In-Silico 1.0: A First Computational Model to Simulate Cell Cycle Dependent Ion Current Modulation in the Human Lung Adenocarcinoma. PLoS Comput. Biol. 2021, 17, e1009091. [Google Scholar] [CrossRef]
- Ekeberg, O.; Wallén, P.; Lansner, A.; Tråvén, H.; Brodin, L.; Grillner, S. A Computer Based Model for Realistic Simulations of Neural Networks. I. The Single Neuron and Synaptic Interaction. Biol. Cybern. 1991, 65, 81–90. [Google Scholar] [CrossRef]
- Yarov-Yarovoy, V.; Allen, T.W.; Clancy, C.E. Computational Models for Predictive Cardiac Ion Channel Pharmacology. Drug Discov. Today Dis. Models 2014, 14, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Beheshti, M.; Umapathy, K.; Krishnan, S. Electrophysiological Cardiac Modeling: A Review. Crit. Rev. Biomed. Eng. 2016, 44, 99–122. [Google Scholar] [CrossRef]
- Dössel, O.; Sachse, F.B.; Seemann, G.; Werner, C.D. Computer models of the electrophysiological properties of the heart. Biomed. Technol. 2002, 47, 250–257. [Google Scholar]
- Nelson, M.E. Electrophysiological Models of Neural Processing. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Maffeo, C.; Bhattacharya, S.; Yoo, J.; Wells, D.; Aksimentiev, A. Modeling and Simulation of Ion Channels. Chem. Rev. 2012, 112, 6250–6284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigg, D. Modeling Ion Channels: Past, Present, and Future. J. Gen. Physiol. 2014, 144, 7–26. [Google Scholar] [CrossRef]
- Nelson, M. Electrophysiological Models. Available online: https://www.semanticscholar.org/paper/Electrophysiological-Models-Nelson/149a7a3c3c8c894a42e1f0978036a4f5ab5daf7a (accessed on 10 December 2020).
- Sterratt, D.; Graham, B.; Gillies, A.; Willshaw, D. Principles of Computational Modelling in Neuroscience; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Carbonell-Pascual, B.; Godoy, E.; Ferrer, A.; Romero, L.; Ferrero, J.M. Comparison between Hodgkin-Huxley and Markov Formulations of Cardiac Ion Channels. J. Theor. Biol. 2016, 399, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Noble, D. Markov Models for Ion Channels: Versatility versus Identifiability and Speed. Philos. Trans. Math. Phys. Eng. Sci. 2009, 367, 2161–2179. [Google Scholar] [CrossRef] [PubMed]
- Lampert, A.; Korngreen, A. Markov Modeling of Ion Channels: Implications for Understanding Disease. Prog. Mol. Biol. Transl. Sci. 2014, 123, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Logette, E.; Marani, M.; Herzog, M.; Tâche, V.; Scantamburlo, E.; Buchillier, V.; Markram, H. A Kinetic Map of the Homomeric Voltage-Gated Potassium Channel (Kv) Family. Front. Cell. Neurosci. 2019, 13, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akemann, W.; Knöpfel, T. Interaction of Kv3 Potassium Channels and Resurgent Sodium Current Influences the Rate of Spontaneous Firing of Purkinje Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 4602–4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, I.; Wang, H.-G.; Sutachan, J.J.; Zhu, J.; Recio-Pinto, E.; Thornhill, W.B. Glycosylation Affects Rat Kv1.1 Potassium Channel Gating by a Combined Surface Potential and Cooperative Subunit Interaction Mechanism. J. Physiol. 2003, 550, 51–66. [Google Scholar] [CrossRef]
- Ågren, R.; Nilsson, J.; Århem, P. Closed and Open State Dependent Block of Potassium Channels Cause Opposing Effects on Excitability—A Computational Approach. Sci. Rep. 2019, 9, 8175. [Google Scholar] [CrossRef] [PubMed]
- Zagotta, W.N.; Hoshi, T.; Aldrich, R.W. Shaker Potassium Channel Gating. III: Evaluation of Kinetic Models for Activation. J. Gen. Physiol. 1994, 103, 321–362. [Google Scholar] [CrossRef]
- Tytgat, J.; Maertens, C.; Daenens, P. Effect of Fluoxetine on a Neuronal, Voltage-Dependent Potassium Channel (Kv1.1). Br. J. Pharmacol. 1997, 122, 1417–1424. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Wang, W.; Fedchyshyn, M.J.; Zhou, Z.; Ding, J.; Wang, L.-Y. Enhancing the Fidelity of Neurotransmission by Activity-Dependent Facilitation of Presynaptic Potassium Currents. Nat. Commun. 2014, 5, 4564. [Google Scholar] [CrossRef] [PubMed]
- Michaelevski, I.; Korngreen, A.; Lotan, I. Interaction of Syntaxin with a Single Kv1.1 Channel: A Possible Mechanism for Modulating Neuronal Excitability. Pflug. Arch. 2007, 454, 477–494. [Google Scholar] [CrossRef]
- Hatton, W.J.; Mason, H.S.; Carl, A.; Doherty, P.; Latten, M.J.; Kenyon, J.L.; Sanders, K.M.; Horowitz, B. Functional and Molecular Expression of a Voltage-Dependent K+ Channel (Kv1.1) in Interstitial Cells of Cajal. J. Physiol. 2001, 533, 315–327. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, M.C.; Gallenmüller, C.; Servettini, I.; Hartl, E.; Tucker, S.J.; Arning, L.; Biskup, S.; Grottesi, A.; Guglielmi, L.; Imbrici, P.; et al. Novel Phenotype Associated with a Mutation in the KCNA1(Kv1.1) Gene. Front. Physiol. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bähring, R.; Vardanyan, V.; Pongs, O. Differential Modulation of Kv1 Channel-Mediated Currents by Co-Expression of Kvβ3 Subunit in a Mammalian Cell-Line. Mol. Membr. Biol. 2004, 21, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.H.; Rettig, J.; Graack, H.R.; Pongs, O. Functional Characterization of Kv Channel Beta-Subunits from Rat Brain. J. Physiol. 1996, 493, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Ljung, L. System Identification—Theory for the User, 2nd ed.; Prentice-Hall International: Hoboken, NJ, USA, 2002; ISBN 0136566952. [Google Scholar]
- Aryani, D.; Wang, L.; Patikirikorala, T. Control Oriented System Identification for Performance Management in Virtualized Software System. IFAC Proc. Vol. 2014, 47, 4122–4127. [Google Scholar] [CrossRef] [Green Version]
- Dorf, R.; Bishop, R. Modern Control Systems, 12th ed.; Prentice-Hall: Hoboken, NJ, USA, 2010; ISBN 9780136024583. [Google Scholar]
- Ogata, K. Modern Control Engineering, 5th ed.; Pearson: New York, NY, USA, 2009; ISBN 139780136156734. [Google Scholar]
- Ljung, L. System Identification ToolboxTM User’s Guide; The Math Works, Inc.: Natick, MA, USA, 2014. [Google Scholar]
- Hasan, S.; Megaro, A.; Cenciarini, M.; Coretti, L.; Botti, F.M.; Imbrici, P.; Steinbusch, H.W.M.; Hunter, T.; Hunter, G.; Pessia, M.; et al. Electromechanical Coupling of the Kv1.1 Voltage-Gated K+ Channel Is Fine-Tuned by the Simplest Amino Acid Residue in the S4-S5 Linker. Pflug. Arch. 2020, 472, 899–909. [Google Scholar] [CrossRef]
- D’Adamo, M.C.; Liantonio, A.; Rolland, J.-F.; Pessia, M.; Imbrici, P. Kv1.1 Channelopathies: Pathophysiological Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 2935. [Google Scholar] [CrossRef]
- Rudy, B.; Maffie, J.; Amarillo, Y.; Clark, B.; Goldberg, E.M.; Jeong, H.-Y.; Kruglikov, I.; Kwon, E.; Nadal, M.; Zagha, E. Voltage Gated Potassium Channels: Structure and Function of Kv1 to Kv9 Subfamilies. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 397–425. ISBN 9780080450469. [Google Scholar]
- Glasscock, E. Kv1.1 Channel Subunits in the Control of Neurocardiac Function. Channels 2019, 13, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Rettig, J.; Heinemann, S.H.; Wunder, F.; Lorra, C.; Parcej, D.N.; Dolly, J.O.; Pongs, O. Inactivation Properties of Voltage-Gated K+ Channels Altered by Presence of Beta-Subunit. Nature 1994, 369, 289–294. [Google Scholar] [CrossRef]
- Pongs, O.; Schwarz, J.R. Ancillary Subunits Associated with Voltage-Dependent K+ Channels. Physiol. Rev. 2010, 90, 755–796. [Google Scholar] [CrossRef] [Green Version]
- Gulbis, J.M.; Mann, S.; MacKinnon, R. Structure of a Voltage-Dependent K+ Channel β Subunit. Cell 1999, 97, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Bett, G.C.L.; Dinga-Madou, I.; Zhou, Q.; Bondarenko, V.E.; Rasmusson, R.L. A Model of the Interaction between N-Type and C-Type Inactivation in Kv1.4 Channels. Biophys. J. 2011, 100, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Destexhe, A.; Huguenard, J.R. Modeling Voltage-Dependent Channels. Computational Modeling Methods for Neuroscientists; The MIT Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Hou, P.; Zhang, R.; Liu, Y.; Feng, J.; Wang, W.; Wu, Y.; Ding, J. Physiological Role of Kv1.3 Channel in T Lymphocyte Cell Investigated Quantitatively by Kinetic Modeling. PLoS ONE 2014, 9, e89975. [Google Scholar] [CrossRef] [Green Version]
- Hodgkin, A.L.; Huxley, A.F. A Quantitative Description of Membrane Current and Its Application to Conduction and Excitation in Nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
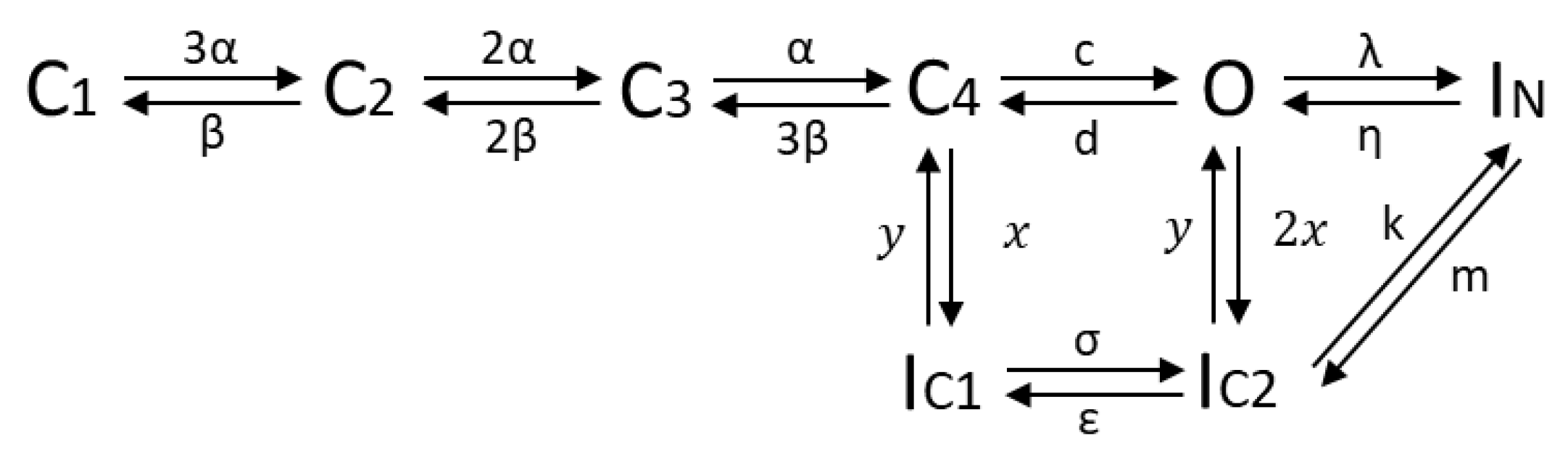
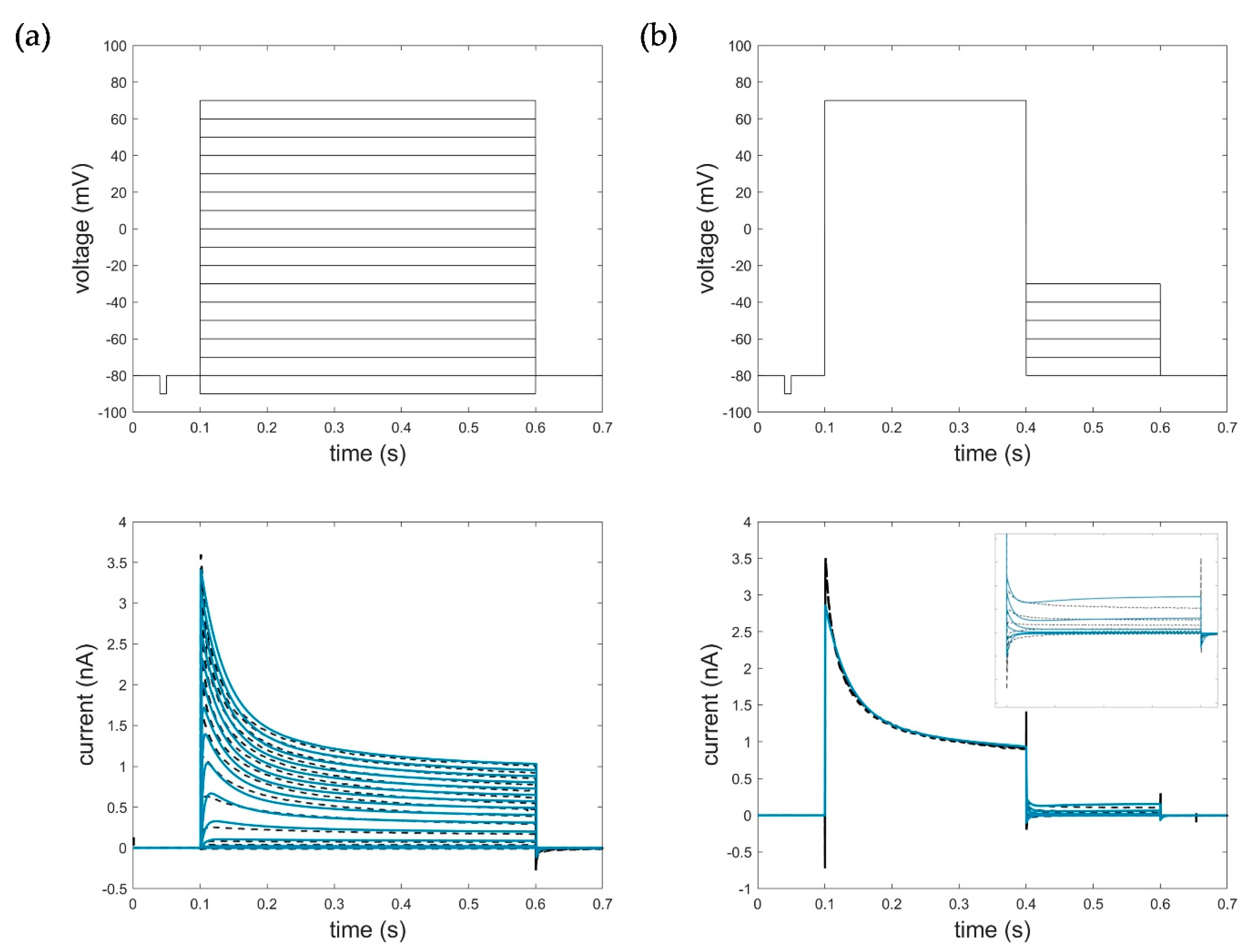
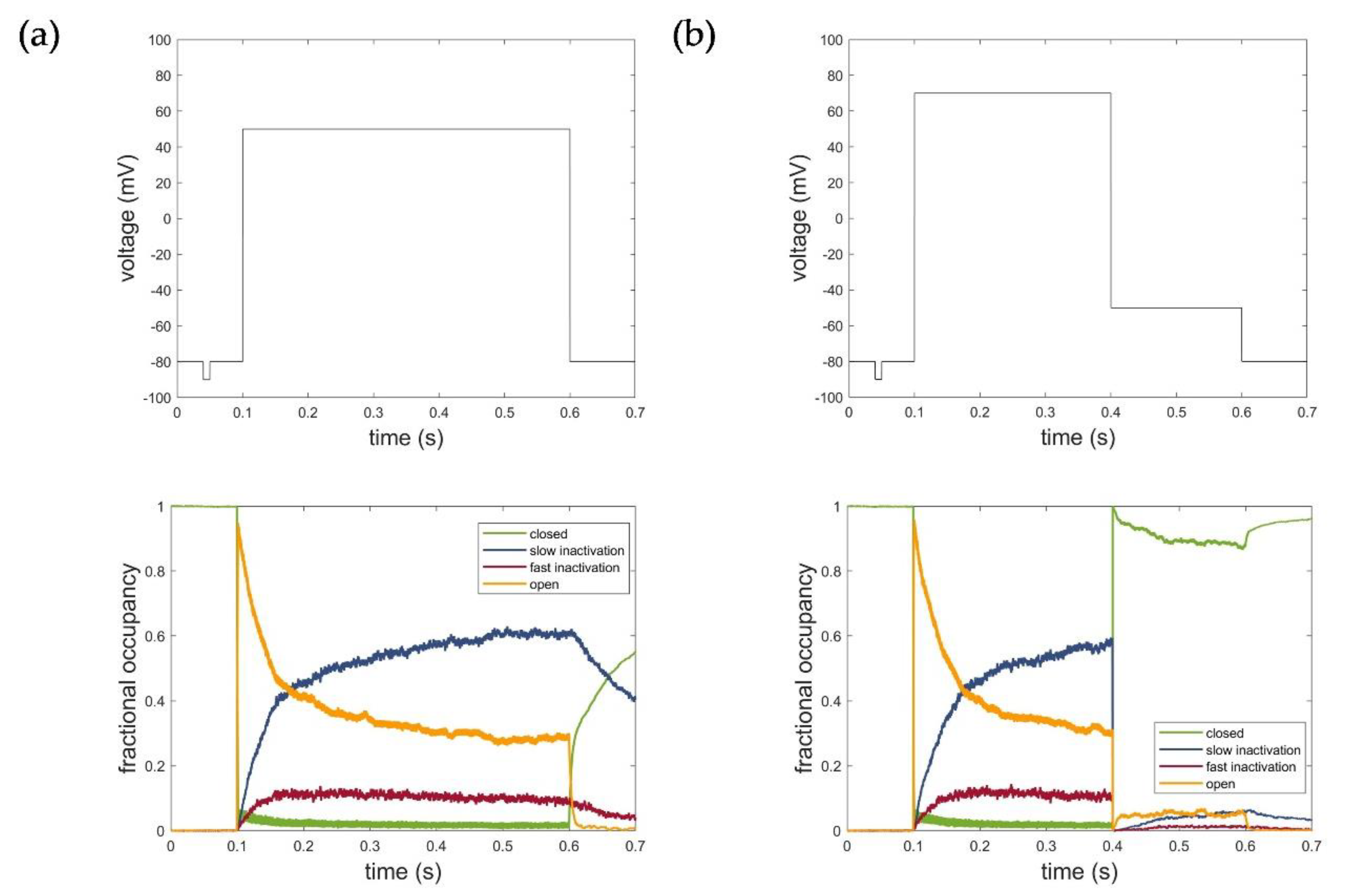
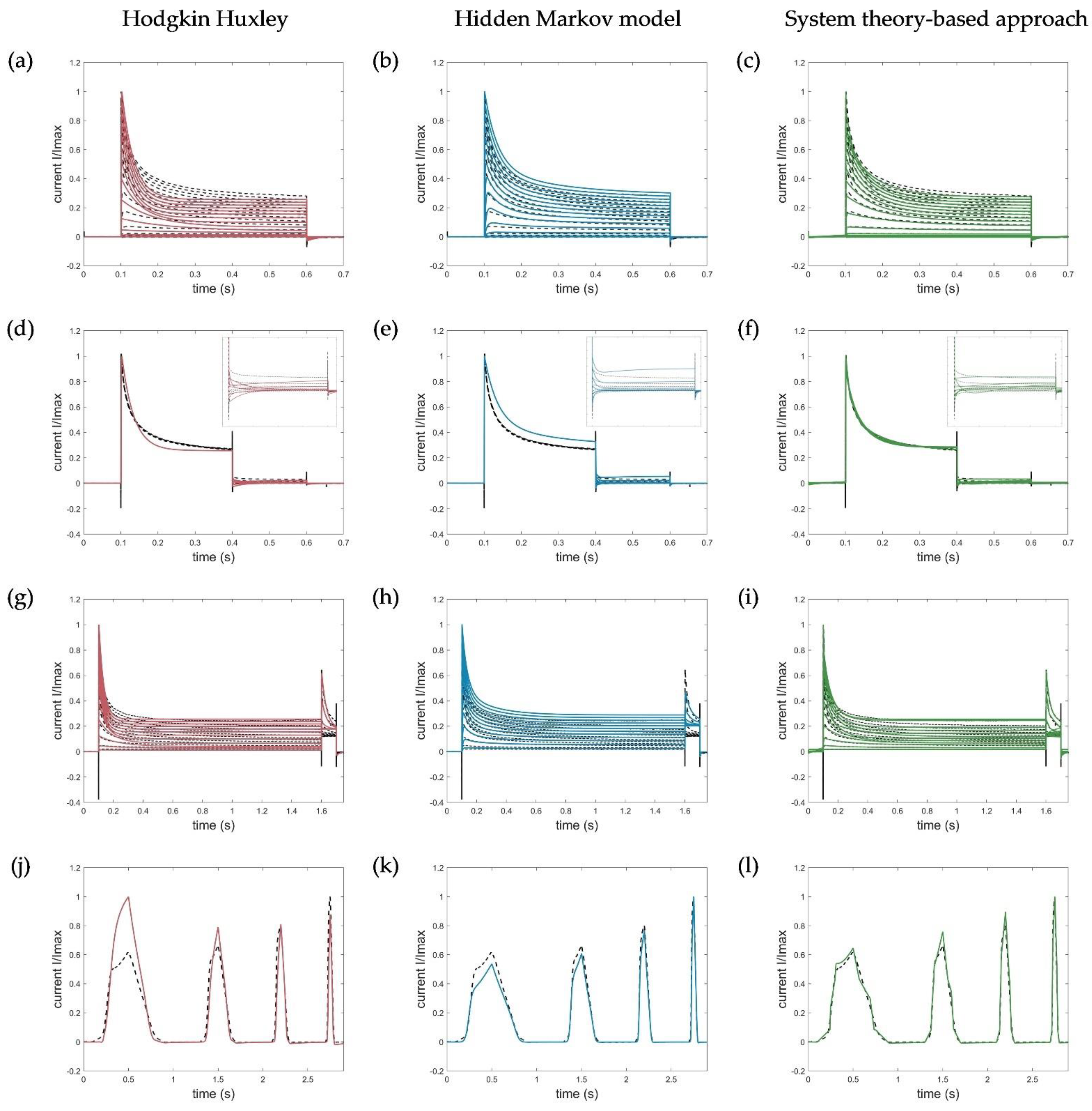
| Rate Constants and Parameters | |||||
|---|---|---|---|---|---|
| α1 | 951.2464 s−1 | λ1 | 14.1140 s−1 | σ1 | 3.8031 s−1 |
| α2 | 0.03 V | λ2 | 20.2499 V | σ2 | 11.8850 V |
| β1 | 395.7896 s−1 | η1 | 49.9528 s−1 | ε1 | 58.364 s−1 |
| β2 | 0.0501 V | η2 | 5 V | ε2 | 55.3568 V |
| c | 799,720 s−1 | k | 370.9594 s−1 | x | 1.6056 s−1 |
| d | 38,916 s−1 | m | 1199.6 s−1 | y | 0.0822 s−1 |
| EK | −0.065 V | gKv1.1 | 8.7 pS | ||
| Nc_act | 3088 | Nc_deact | 2588 | ||
| HH | HMM | STB | |
|---|---|---|---|
| unknown parameters | 22 | 20 | 7 |
| mathematical description of the model | 2 first-order differential equations | 8 first-order differential equations | 1 third-order differential equations |
| parameterization data | activation single-cell measurements | activation and deactivation average | ramp average |
| number of cells | 56 | 60 (activation) 37 (deactivation) | 54 |
| voltage range | −40 to +50 mV | −90 to +70 mV (activation) −80 to −30 mV (deactivation) | −80 to +70 mV |
| total sweep number considered | 10 | 13: −50 to +70 mV (activation) 6: −80 to −30 mV (deactivation) | 1 |
| time for parameterization/system identification | data not available | 30 h | 5–10 min |
| Temp 35 °C | Experimental Data | Simulated Data | |||
|---|---|---|---|---|---|
| HH | HMM | STA | |||
| activation | |||||
| V1/2_act | (mV) | −22.45 | −14.94 | −22.64 | −18.39 |
| kact | (mV) | 10.81 | 9.913 | 11.82 | 14.97 |
| (ms) | 0.5493 | 0.5766 | 2.2449 | 0.2706 | |
| (ms) | 0.09283 | 0.3036 | 0.1391 | 0.01875 | |
| (ms) | 0.1135 | 0.2949 | 0.2125 | 0.02084 | |
| (ms) | 0.1403 | 0.2865 | 0.3157 | 0.02339 | |
| (ms) | 0.1791 | 0.2783 | 0.4613 | 0.02634 | |
| (ms) | 0.2351 | 0.2703 | 0.668 | 0.02994 | |
| (ms) | 0.3148 | 0.2629 | 0.9654 | 0.03359 | |
| (ms) | 0.4343 | 0.2567 | 1.401 | 0.06175 | |
| (ms) | 0.6244 | 0.2524 | 2.052 | 0.1491 | |
| (ms) | 0.9504 | 0.2486 | 3.033 | 0.3984 | |
| (ms) | 1.476 | 0.2430 | 4.437 | 0.8607 | |
| (ms) | 2.02 | 0.2374 | 6.077 | 1.613 | |
| RMSEnorm RMSEabs | 0.0326 - | 0.0213 0.0714 * | 0.0138 0.0381 | ||
| deactivation | |||||
| (ms) | 13.3627 | 18.5689 | 5.0230 | 10.76 | |
| (ms) | 23.42 | 0.1704 | 3.236 | 14.86 | |
| (ms) | 16.75 | 3.433 | 5.282 | - | |
| (ms) | 11.49 | 31.03 | 6.491 | - | |
| (ms) | 10.79 | 26.07 | 6.058 | 4.793 | |
| (ms) | 7.306 | 25.68 | 5.019 | 4.564 | |
| (ms) | 10.42 | 25.03 | 4.052 | 18.82 | |
| RMSEnorm RMSEabs | 0.0429 - | 0.0627 0.1098 * | 0.0283 0.0985 | ||
| inactivation | |||||
| V1/2_inact | (mV) | −26.46 | −29.12 | −28.95 | −27.37 |
| kinact | (mV) | 4.755 | 3.882 | 5.04 | 4.074 |
| (ms) | 102.1077 | 71.9092 | 96.4150 | 99.1621 | |
| (ms) | 53.22 | 32.14 | 68.15 | 53.2 | |
| (ms) | 63.13 | 32.14 | 68.45 | 60.65 | |
| (ms) | 69.17 | 32.17 | 68.85 | 68.74 | |
| (ms) | 72.65 | 32.25 | 69.41 | 71.38 | |
| (ms) | 77.25 | 32.57 | 70.25 | 77.26 | |
| (ms) | 80.26 | 33.79 | 71.6 | 85.05 | |
| (ms) | 85.9 | 38.27 | 73.89 | 83.26 | |
| (ms) | 104.3 | 53.18 | 78.19 | 101.5 | |
| (ms) | 147.1 | 89.9 | 87.19 | 143.1 | |
| (ms) | 208.7 | 138.4 | 107.7 | 192.4 | |
| (ms) | 263.1 | 168.4 | 153.8 | 252.6 | |
| (ms) | 0.5125 | 179.7 | 239.5 | 0.8051 | |
| RMSEnorm RMSEabs | 0.0257 - | 0.0548 0.1297 * | 0.0146 0.0463 | ||
| ramp | |||||
| Vmax_cond | (mV) | 69.6 | 67.0 | 69.2 | 69.6 |
| RMSEnorm RMSEabs | 0.1098 - | 0.0396 0.0317 * | 0.0262 0.0364 | ||
| HH | HMM | STB | |
|---|---|---|---|
| explainability of channel gating | + | +++ | n.a. |
| flexibility and adaptability | + | +++ | + |
| model complexity | + | +++ | + |
| model accuracy | (<<) + | ++ (>>) | +++ |
| comp. burden optimization | ++ | +++ | + |
| comp. burden simulation | + | ++ | + |
| experimental data for model parameterization | +++ | +++ (>>) | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langthaler, S.; Lozanović Šajić, J.; Rienmüller, T.; Weinberg, S.H.; Baumgartner, C. Ion Channel Modeling beyond State of the Art: A Comparison with a System Theory-Based Model of the Shaker-Related Voltage-Gated Potassium Channel Kv1.1. Cells 2022, 11, 239. https://doi.org/10.3390/cells11020239
Langthaler S, Lozanović Šajić J, Rienmüller T, Weinberg SH, Baumgartner C. Ion Channel Modeling beyond State of the Art: A Comparison with a System Theory-Based Model of the Shaker-Related Voltage-Gated Potassium Channel Kv1.1. Cells. 2022; 11(2):239. https://doi.org/10.3390/cells11020239
Chicago/Turabian StyleLangthaler, Sonja, Jasmina Lozanović Šajić, Theresa Rienmüller, Seth H. Weinberg, and Christian Baumgartner. 2022. "Ion Channel Modeling beyond State of the Art: A Comparison with a System Theory-Based Model of the Shaker-Related Voltage-Gated Potassium Channel Kv1.1" Cells 11, no. 2: 239. https://doi.org/10.3390/cells11020239
APA StyleLangthaler, S., Lozanović Šajić, J., Rienmüller, T., Weinberg, S. H., & Baumgartner, C. (2022). Ion Channel Modeling beyond State of the Art: A Comparison with a System Theory-Based Model of the Shaker-Related Voltage-Gated Potassium Channel Kv1.1. Cells, 11(2), 239. https://doi.org/10.3390/cells11020239







