Blood Endothelial-Cell Extracellular Vesicles as Potential Biomarkers for the Selection of Plasma in COVID-19 Convalescent Plasma Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Cell Culture
2.3. Measurement of Cellular Viability
2.4. Analyses of Extracellular Vesicles in Plasma
2.5. Messenger RNA Analysis by RT-qPCR
2.6. Immunoblotting
2.7. Statistical Analysis
3. Results
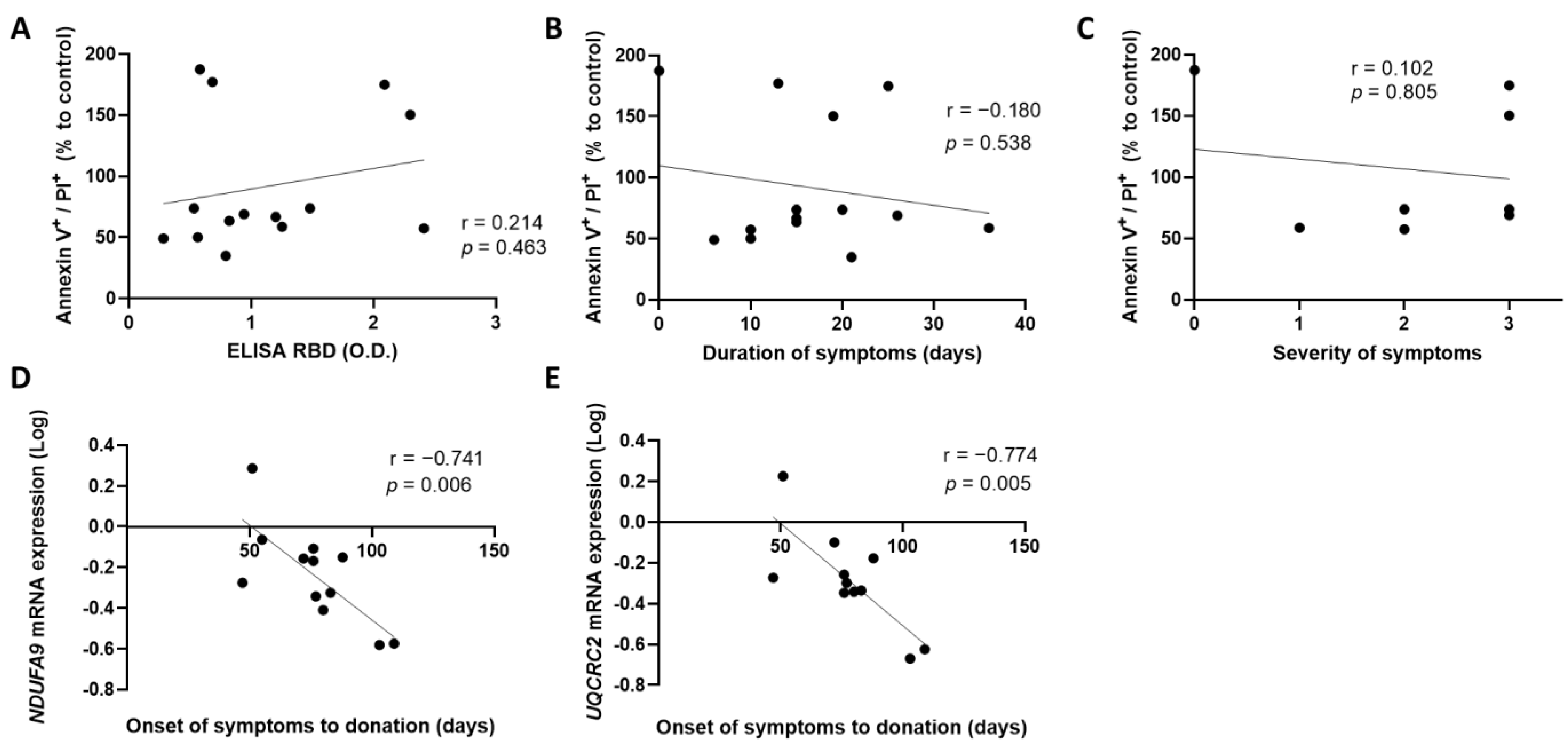
3.1. An Early Donation of Convalescent Plasma Increases the Expression of Mitochondrial Genes by Blood Endothelial Cells
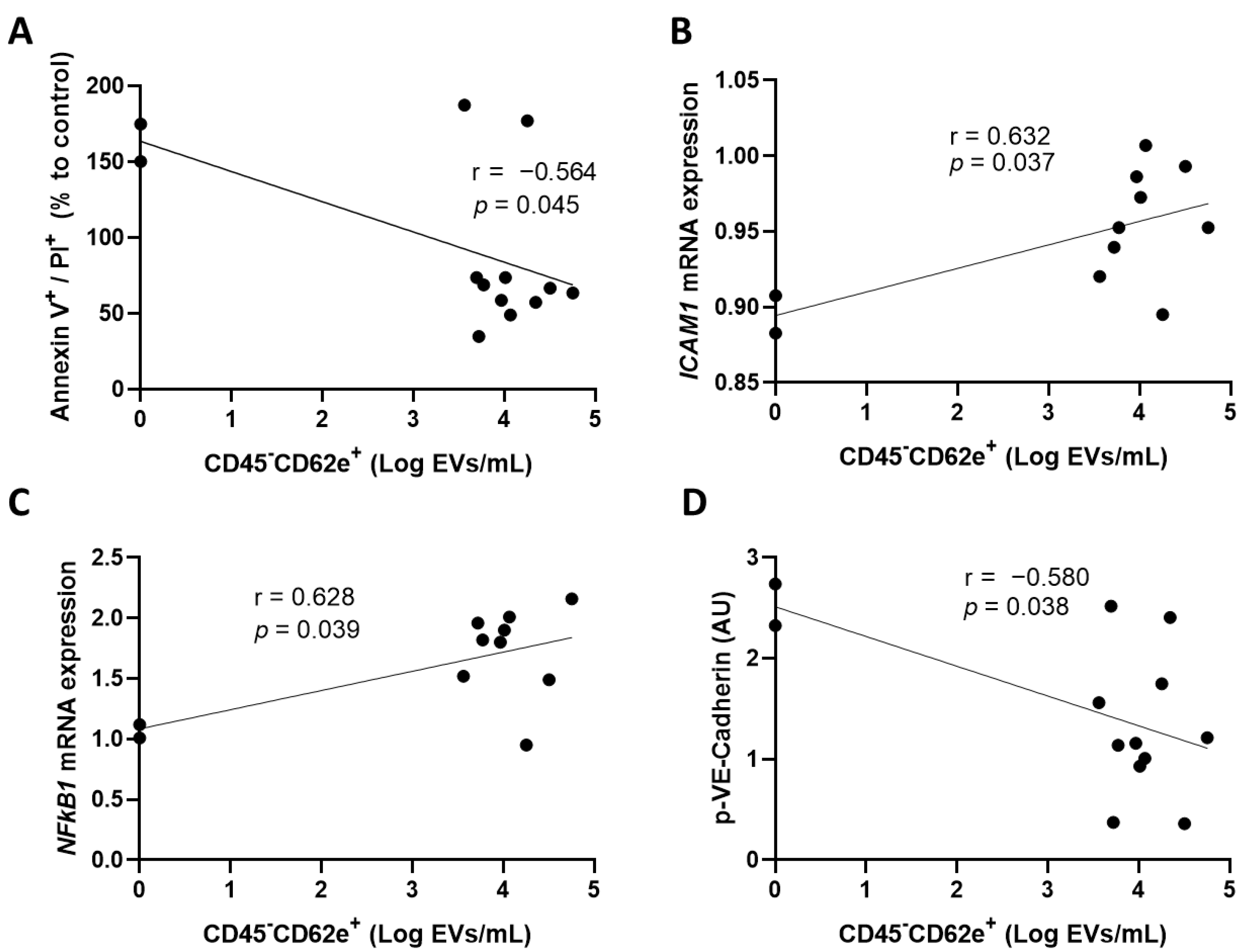
3.2. Convalescent Plasma with High Concentrations of Extracellular Vesicles Derived from Blood Endothelial Cells Increases the Activation of Human Coronary Artery Endothelial Cells
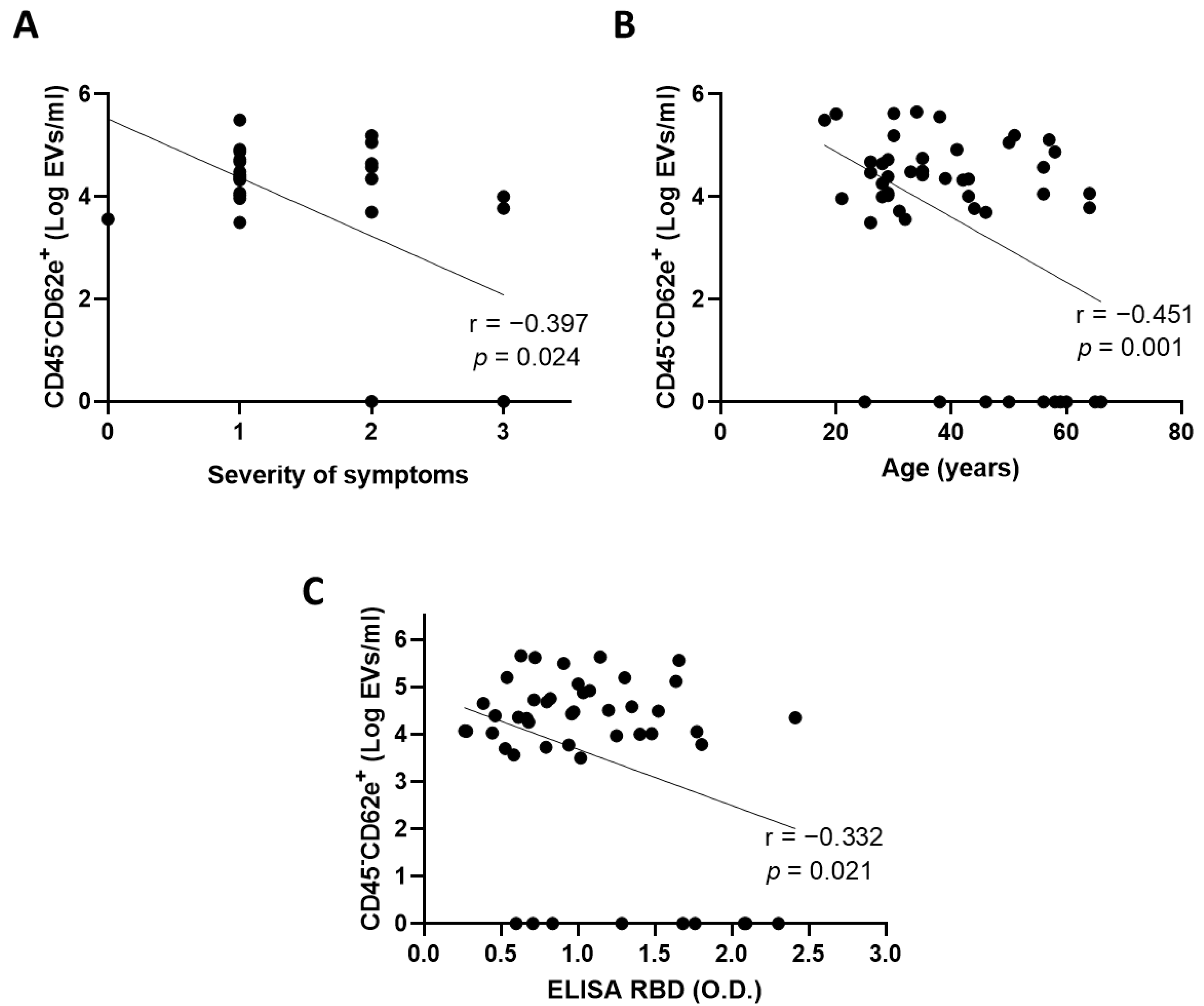
3.3. Extracellular Vesicles Derived from Blood Endothelial Cells Are More Abundant in Convalescent Plasma from Donors with Mild Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization: Geneva, 2020. 2021. Available online: https://www.who.int/health-topics/severe-acute-respiratory-syndrome#tab=tab_1 (accessed on 22 September 2022).
- Estcourt, L.J.; Turgeon, A.F.; McQuilten, Z.K.; McVerry, B.J.; Al-Beidh, F.; Annane, D.; Arabi, Y.M.; Arnold, D.M.; Beane, A.; Bégin, P.; et al. Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients with COVID-19: A Randomized Clinical Trial. JAMA 2021, 326, 1690–1702. [Google Scholar] [PubMed]
- Rauch, A.; Dupont, A.; Goutay, J.; Caplan, M.; Staessens, S.; Moussa, M.; Jeanpierre, E.; Corseaux, D.; Lefevre, G.; Lassalle, F.; et al. Endotheliopathy Is Induced by Plasma from Critically-Ill Patients and Associated with Organ Failure in Severe COVID-19. Circulation 2020, 142, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Bandopadhyay, P.; D’Rozario, R.; Lahiri, A.; Sarif, J.; Ray, Y.; Paul, S.R.; Roy, R.; Maiti, R.; Chaudhuri, K.; Bagchi, S.; et al. Nature and Dimensions of Systemic Hyperinflammation and Its Attenuation by Convalescent Plasma in Severe COVID-19. J. Infect. Dis. 2021, 224, 565–574. [Google Scholar] [CrossRef]
- Cowan, L.T.; Lutsey, P.L.; Pankow, J.S.; Matsushita, K.; Ishigami, J.; Lakshminarayan, K. Inpatient and Outpatient Infection as a Trigger of Cardiovascular Disease: The Aric Study. J. Am. Heart Assoc. 2018, 7, e009683. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Reed, H.O.; Wang, L.; Sonett, J.; Chen, M.; Yang, J.; Li, L.; Aradi, P.; Jakus, Z.; D’Armiento, J.; Hancock, W.W.; et al. Lymphatic Impairment Leads to Pulmonary Tertiary Lymphoid Organ Formation and Alveolar Damage. J. Clin. Investig. 2019, 129, 2514–2526. [Google Scholar] [CrossRef]
- Jurisic, G.; Sundberg, J.P.; Detmar, M. Blockade of Vegf Receptor-3 Aggravates Inflammatory Bowel Disease and Lymphatic Vessel Enlargement. Inflamm. Bowel Dis. 2013, 19, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Piechotta, V.; Iannizzi, C.; Chai, K.L.; Valk, S.J.; Kimber, C.; Dorando, E.; Monsef, I.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; et al. Convalescent Plasma or Hyperimmune Immunoglobulin for People with COVID-19: A Living Systematic Review. Cochrane Database Syst. Rev. 2021, 5, Cd013600. [Google Scholar] [PubMed]
- Sullivan, D.J.; Gebo, K.A.; Shoham, S.; Bloch, E.M.; Lau, B.; Shenoy, A.G.; Mosnaim, G.S.; Gniadek, T.J.; Fukuta, Y.; Patel, B.; et al. Early Outpatient Treatment for Covid-19 with Convalescent Plasma. N. Engl. J. Med. 2022, 386, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Amri, N.; Begin, R.; Tessier, N.; Vachon, L.; Villeneuve, L.; Begin, P.; Bazin, R.; Loubaki, L.; Martel, C. Use of Early Donated Covid-19 Convalescent Plasma Is Optimal to Preserve the Integrity of Lymphatic Endothelial Cells. Pharmaceuticals 2022, 15, 365. [Google Scholar] [CrossRef]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Jy, W.; Mauro, L.M.; Soderland, C.; Horstman, L.L.; Ahn, Y.S. Endothelial Cells Release Phenotypically and Quantitatively Distinct Microparticles in Activation and Apoptosis. Thromb. Res. 2003, 109, 175–180. [Google Scholar] [CrossRef]
- Bégin, P.; Callum, J.; Jamula, E.; Cook, R.; Heddle, N.M.; Tinmouth, A.; Zeller, M.P.; Beaudoin-Bussières, G.; Amorim, L.; Bazin, R.; et al. Convalescent Plasma for Hospitalized Patients with Covid-19: An Open-Label, Randomized Controlled Trial. Nat. Med. 2021, 27, 2012–2024. [Google Scholar] [CrossRef]
- Perreault, J.; Tremblay, T.; Fournier, M.J.; Drouin, M.; Beaudoin-Bussières, G.; Prévost, J.; Lewin, A.; Bégin, P.; Finzi, A.; Bazin, R. Waning of Sars-Cov-2 Rbd Antibodies in Longitudinal Convalescent Plasma Samples within 4 Months after Symptom Onset. Blood 2020, 136, 2588–2591. [Google Scholar] [CrossRef]
- Martel, C.; Granger, C.B.; Ghitescu, M.; Stebbins, A.; Fortier, A.; Armstrong, P.W.; Bonnefoy, A.; Theroux, P. Pexelizumab Fails to Inhibit Assembly of the Terminal Complement Complex in Patients with St-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Insight from a Substudy of the Assessment of Pexelizumab in Acute Myocardial Infarction (Apex-Ami) Trial. Am. Heart J. 2012, 164, 43–51. [Google Scholar]
- Vachon, L.; Smaani, A.; Tessier, N.; Jean, G.; Demers, A.; Milasan, A.; Ardo, N.; Jarry, S.; Villeneuve, L.; Alikashani, A.; et al. Downregulation of Low-Density Lipoprotein Receptor Mrna in Lymphatic Endothelial Cells Impairs Lymphatic Function through Changes in Intracellular Lipids. Theranostics 2021, 12, 1440–1458. [Google Scholar] [CrossRef]
- Milasan, A.; Tessandier, N.; Tan, S.; Brisson, A.; Boilard, E.; Martel, C. Extracellular Vesicles Are Present in Mouse Lymph and Their Level Differs in Atherosclerosis. J. Extracell. Vesicles 2016, 5, 31427. [Google Scholar] [CrossRef]
- Poncelet, P.; Robert, S.; Bailly, N.; Garnache-Ottou, F.; Bouriche, T.; Devalet, B.; Segatchian, J.H.; Saas, P.; Mullier, F. Tips and Tricks for Flow Cytometry-Based Analysis and Counting of Microparticles. Transfus. Apher. Sci. 2015, 53, 110–126. [Google Scholar] [CrossRef]
- Jones, D.; Min, W. An Overview of Lymphatic Vessels and Their Emerging Role in Cardiovascular Disease. J. Cardiovasc. Dis. Res. 2011, 2, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Hoefs, S.J.G.; Dieteren, C.E.J.; Distelmaier, F.; Janssen, R.J.R.J.; Epplen, A.; Swarts, H.G.P.; Forkink, M.; Rodenburg, R.J.; Nijtmans, L.G.; Willems, P.H.; et al. NDUFA2 complex I mutation leads to Leigh disease. Am J. Hum. Genet. 2008, 82, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Smeitink, J.; van den Heuvel, L.; DiMauro, S. The Genetics and Pathology of Oxidative Phosphorylation. Nat. Rev. Genet. 2001, 2, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Mandò, C.; Savasi, V.M.; Anelli, G.M.; Corti, S.; Serati, A.; Lisso, F.; Tasca, C.; Novielli, C.; Cetin, I. Mitochondrial and Oxidative Unbalance in Placentas from Mothers with Sars-Cov-2 Infection. Antioxidants 2021, 10, 1517. [Google Scholar] [CrossRef] [PubMed]
- Miyake, N.; Yano, S.; Sakai, C.; Hatakeyama, H.; Matsushima, Y.; Shiina, M.; Watanabe, Y.; Bartley, J.; Abdenur, J.E.; Wang, R.Y.; et al. Mitochondrial Complex Iii Deficiency Caused by a Homozygous Uqcrc2 Mutation Presenting with Neonatal-Onset Recurrent Metabolic Decompensation. Hum. Mutat. 2013, 34, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, P.; Eyer, D.; Lebigot, E.; Oliveira, C.; Therond, P.; Boutron, A.; Slama, A. Uqcrc2 Mutation in a Patient with Mitochondrial Complex Iii Deficiency Causing Recurrent Liver Failure, Lactic Acidosis and Hypoglycemia. J. Hum. Genet. 2017, 62, 729–731. [Google Scholar] [CrossRef]
- Chernyak, B.V.; Popova, E.N.; Zinovkina, L.A.; Lyamzaev, K.G.; Zinovkin, R.A. Mitochondria as Targets for Endothelial Protection in COVID-19. Front. Physiol. 2020, 11, 606170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed Cell Death and Its Role in Inflammation. Mil. Med. Res. 2015, 2, 12. [Google Scholar] [CrossRef]
- Krishnamachary, B.; Cook, C.; Kumar, A.; Spikes, L.; Chalise, P.; Dhillon, N.K. Extracellular Vesicle-Mediated Endothelial Apoptosis and Ev-Associated Proteins Correlate with Covid-19 Disease Severity. J. Extracell. Vesicles 2021, 10, e12117. [Google Scholar] [CrossRef]
- Barnes, B.J.; Somerville, C.C. Modulating Cytokine Production Via Select Packaging and Secretion from Extracellular Vesicles. Front. Immunol. 2020, 11, 1040. [Google Scholar] [CrossRef]
- Adams, D.H.; Shaw, S. Leucocyte-Endothelial Interactions and Regulation of Leucocyte Migration. Lancet 1994, 343, 831–836. [Google Scholar] [CrossRef]
- Gotsch, U.; Borges, E.; Bosse, R.; Boggemeyer, E.; Simon, M.; Mossmann, H.; Vestweber, D. Ve-Cadherin Antibody Accelerates Neutrophil Recruitment in Vivo. J. Cell Sci. 1997, 110, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Del Maschio, A.; Zanetti, A.; Corada, M.; Rival, Y.; Ruco, L.; Lampugnani, M.G.; Dejana, E. Polymorphonuclear Leukocyte Adhesion Triggers the Disorganization of Endothelial Cell-to-Cell Adherens Junctions. J. Cell Biol. 1996, 135, 497–510. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amri, N.; Tessier, N.; Bégin, R.; Vachon, L.; Bégin, P.; Bazin, R.; Loubaki, L.; Martel, C. Blood Endothelial-Cell Extracellular Vesicles as Potential Biomarkers for the Selection of Plasma in COVID-19 Convalescent Plasma Therapy. Cells 2022, 11, 3122. https://doi.org/10.3390/cells11193122
Amri N, Tessier N, Bégin R, Vachon L, Bégin P, Bazin R, Loubaki L, Martel C. Blood Endothelial-Cell Extracellular Vesicles as Potential Biomarkers for the Selection of Plasma in COVID-19 Convalescent Plasma Therapy. Cells. 2022; 11(19):3122. https://doi.org/10.3390/cells11193122
Chicago/Turabian StyleAmri, Nada, Nolwenn Tessier, Rémi Bégin, Laurent Vachon, Philippe Bégin, Renée Bazin, Lionel Loubaki, and Catherine Martel. 2022. "Blood Endothelial-Cell Extracellular Vesicles as Potential Biomarkers for the Selection of Plasma in COVID-19 Convalescent Plasma Therapy" Cells 11, no. 19: 3122. https://doi.org/10.3390/cells11193122
APA StyleAmri, N., Tessier, N., Bégin, R., Vachon, L., Bégin, P., Bazin, R., Loubaki, L., & Martel, C. (2022). Blood Endothelial-Cell Extracellular Vesicles as Potential Biomarkers for the Selection of Plasma in COVID-19 Convalescent Plasma Therapy. Cells, 11(19), 3122. https://doi.org/10.3390/cells11193122






