Preliminary Development and Testing of C595 Radioimmunoconjugates for Targeting MUC1 Cancer Epitopes in Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibody, Chelator, Peptides and Other Reagents
2.2. Conjugation of p-SCN-Bn-DOTA to C595 mAb
2.3. Determination of Number of Chelators per Antibody
2.4. Location of p-SCN-Bn-DOTA Binding on C595 Antibody Chains
2.5. Stability of Immunoconjugate Using Mass Spectrometry
2.6. Comparison of Binding Affinity
2.7. Radiolabelling of DOTA-C595
2.8. Determination of Radiolabelling Yield and Purity
2.9. Cell Cultures
2.10. Cell Binding Assays
2.11. Influence of Storage on Radiolabelling and Cell Binding
2.12. Statistical Analysis
2.13. Facilities
3. Results
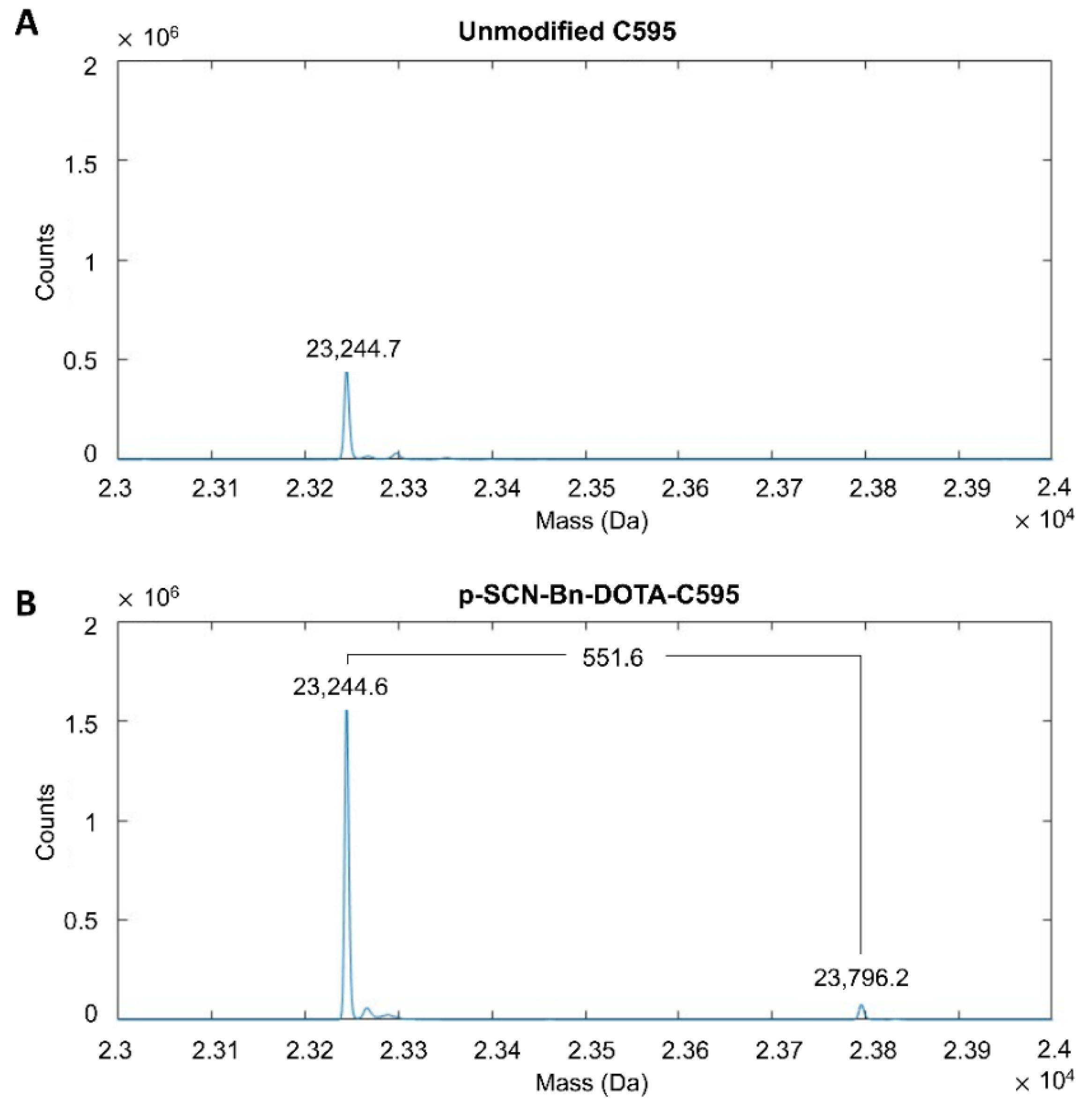
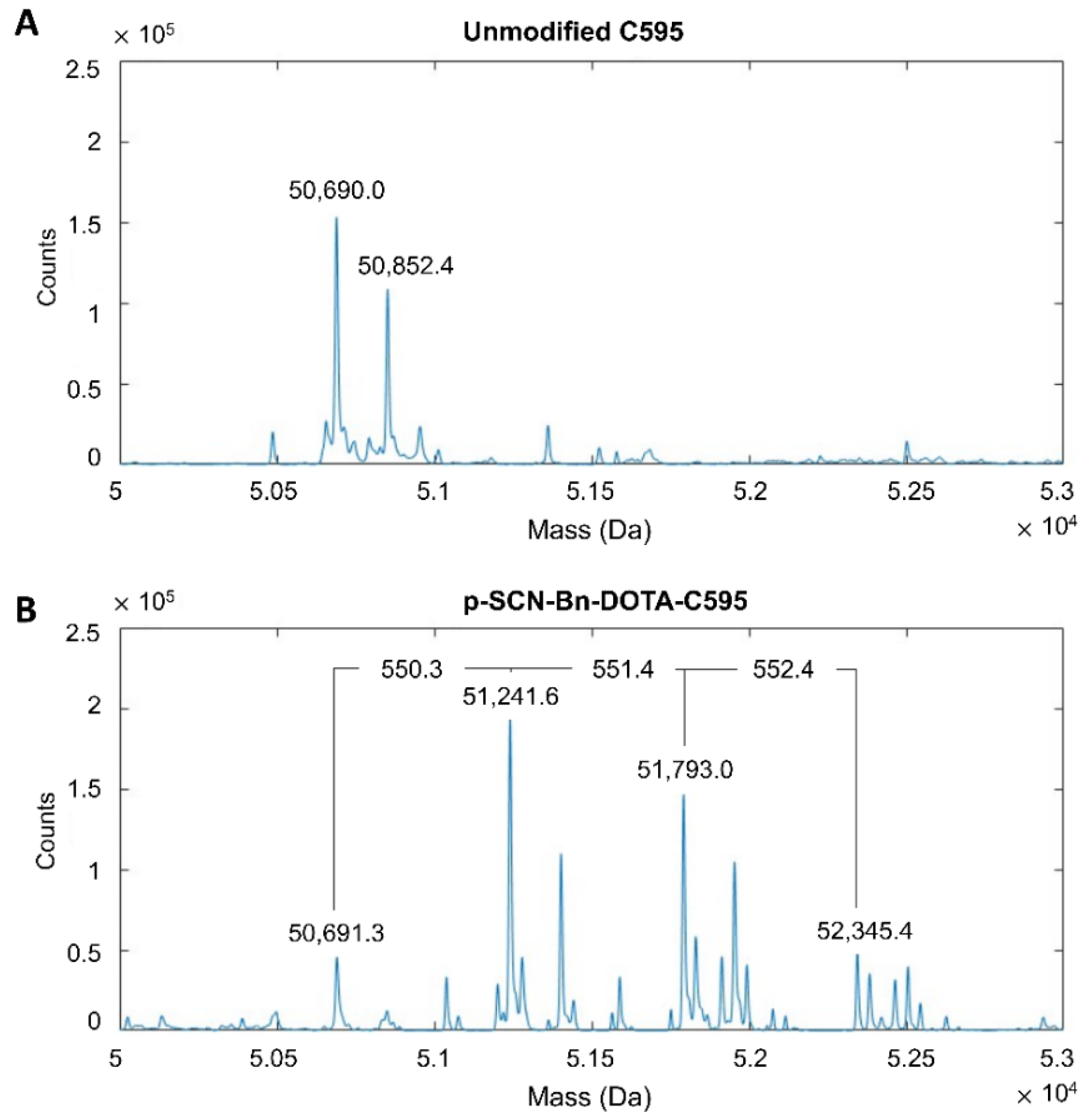
3.1. Conjugation of p-SCN-Bn-DOTA to C595
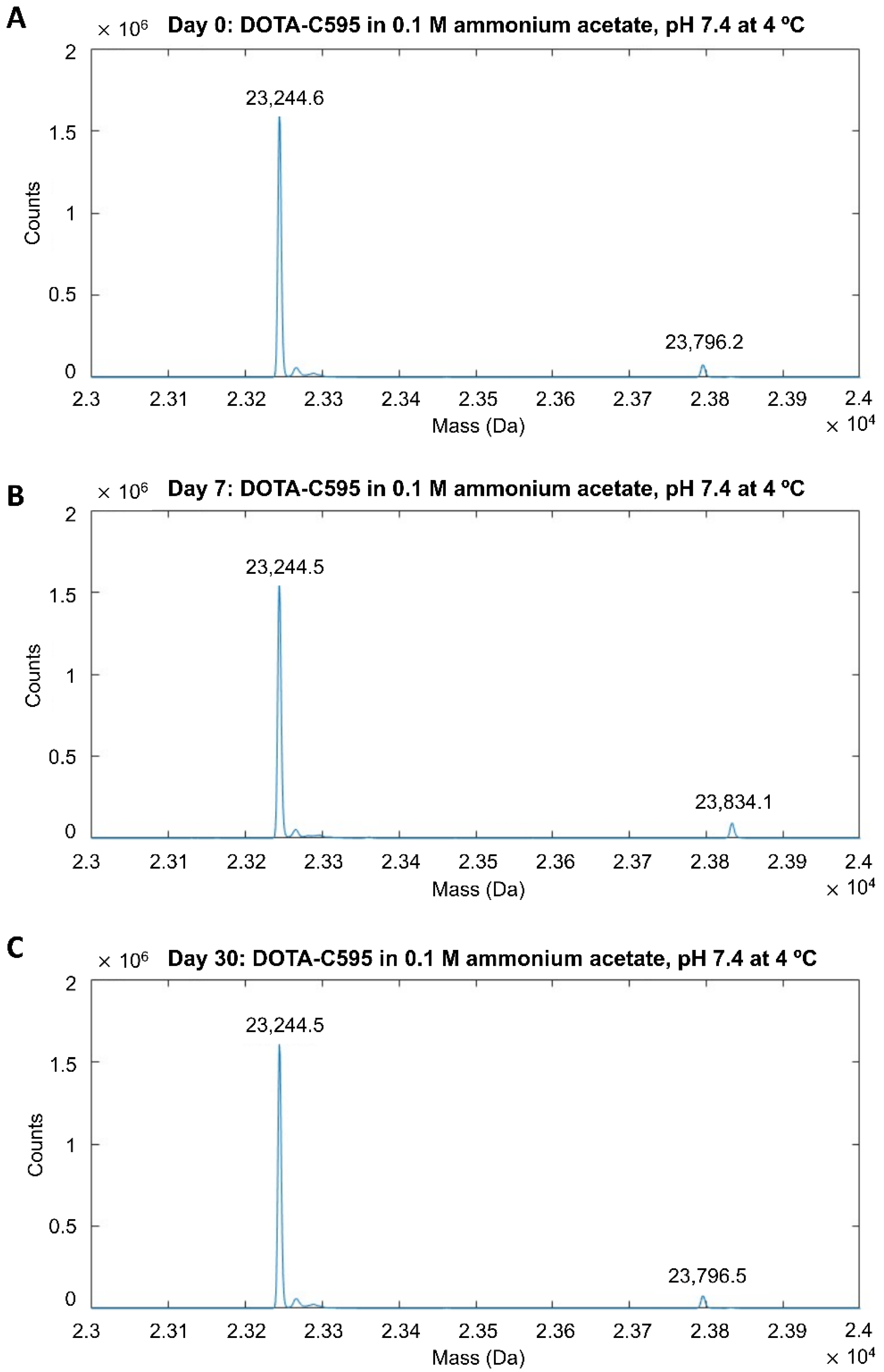
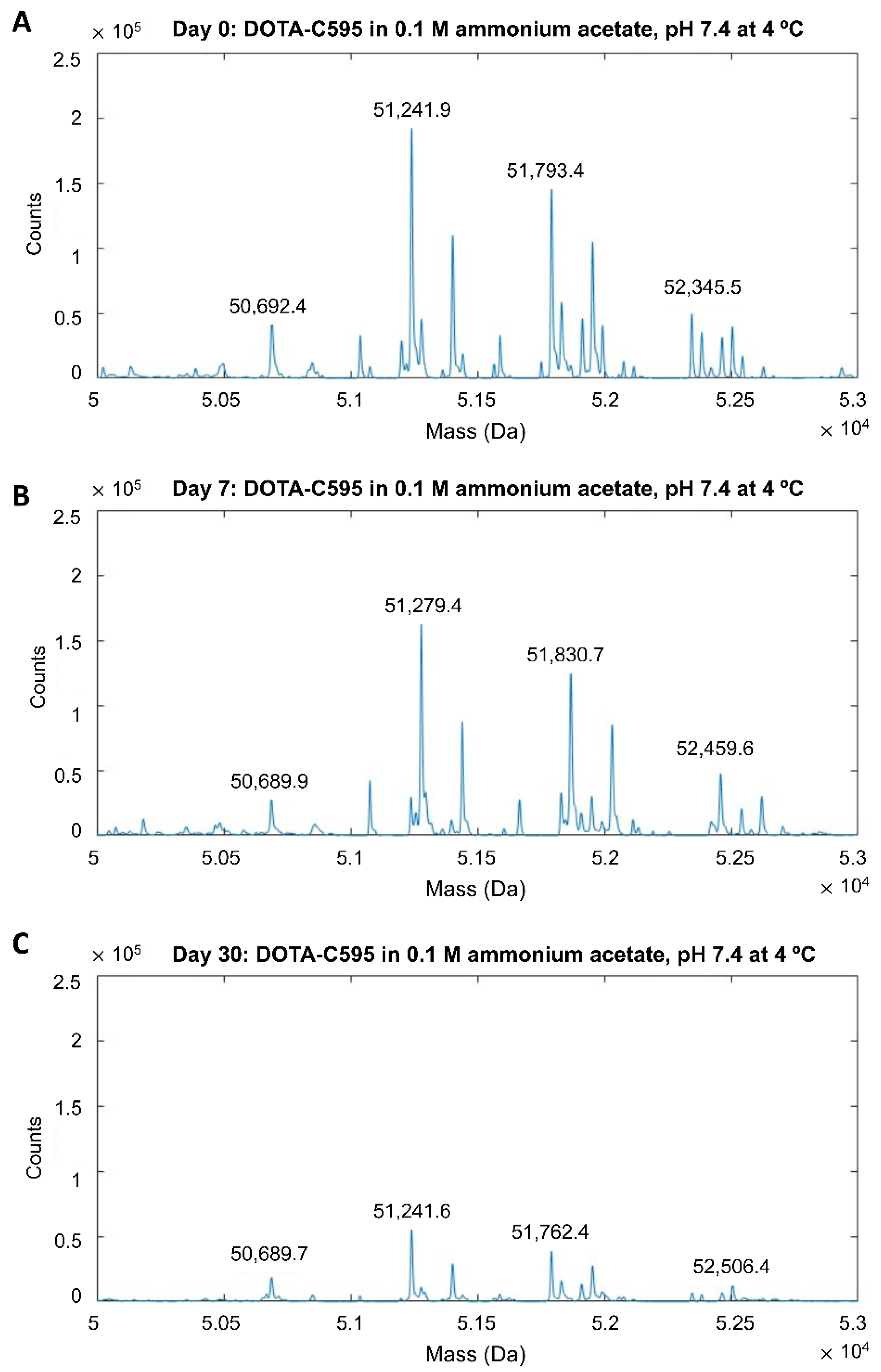
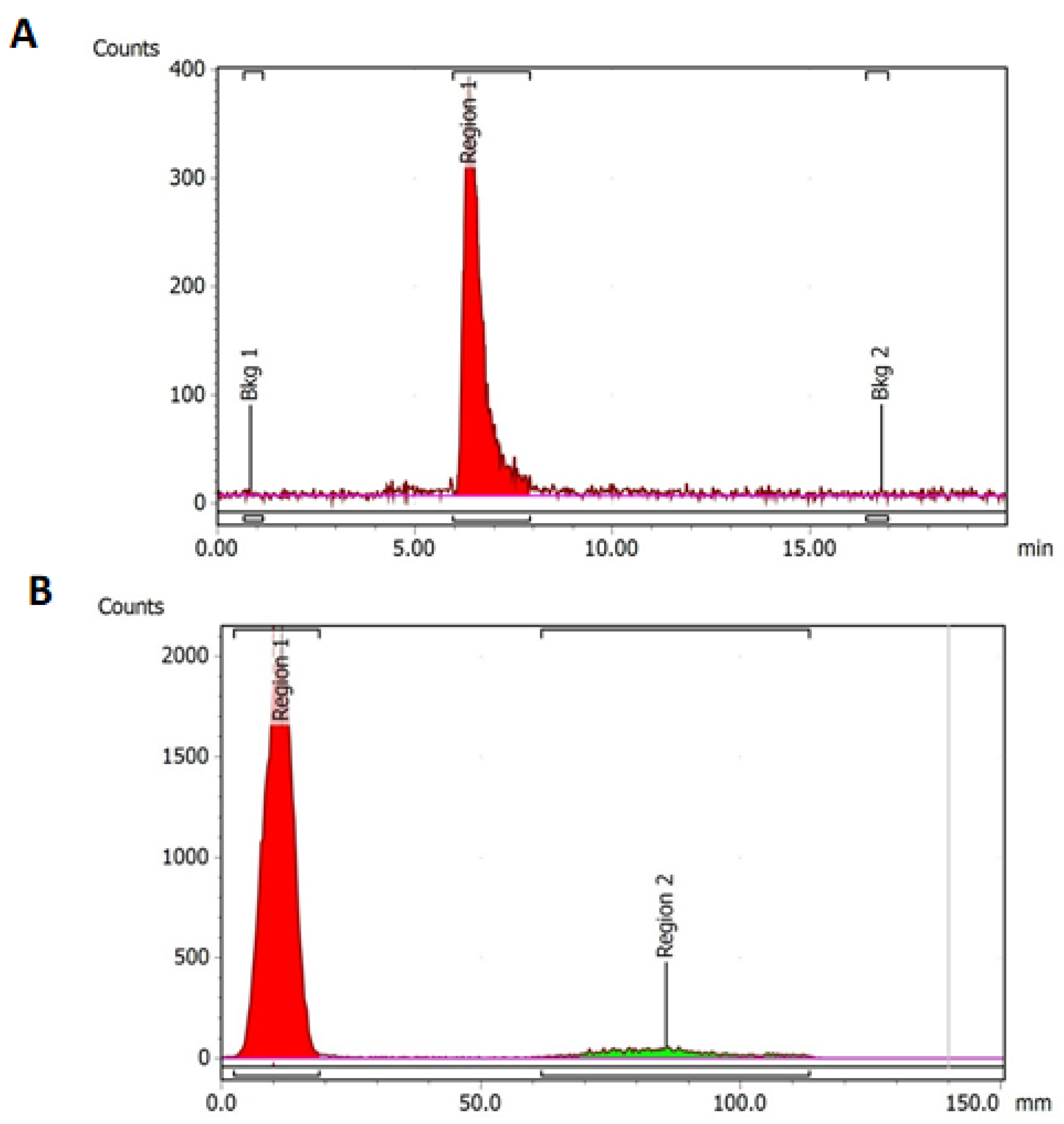
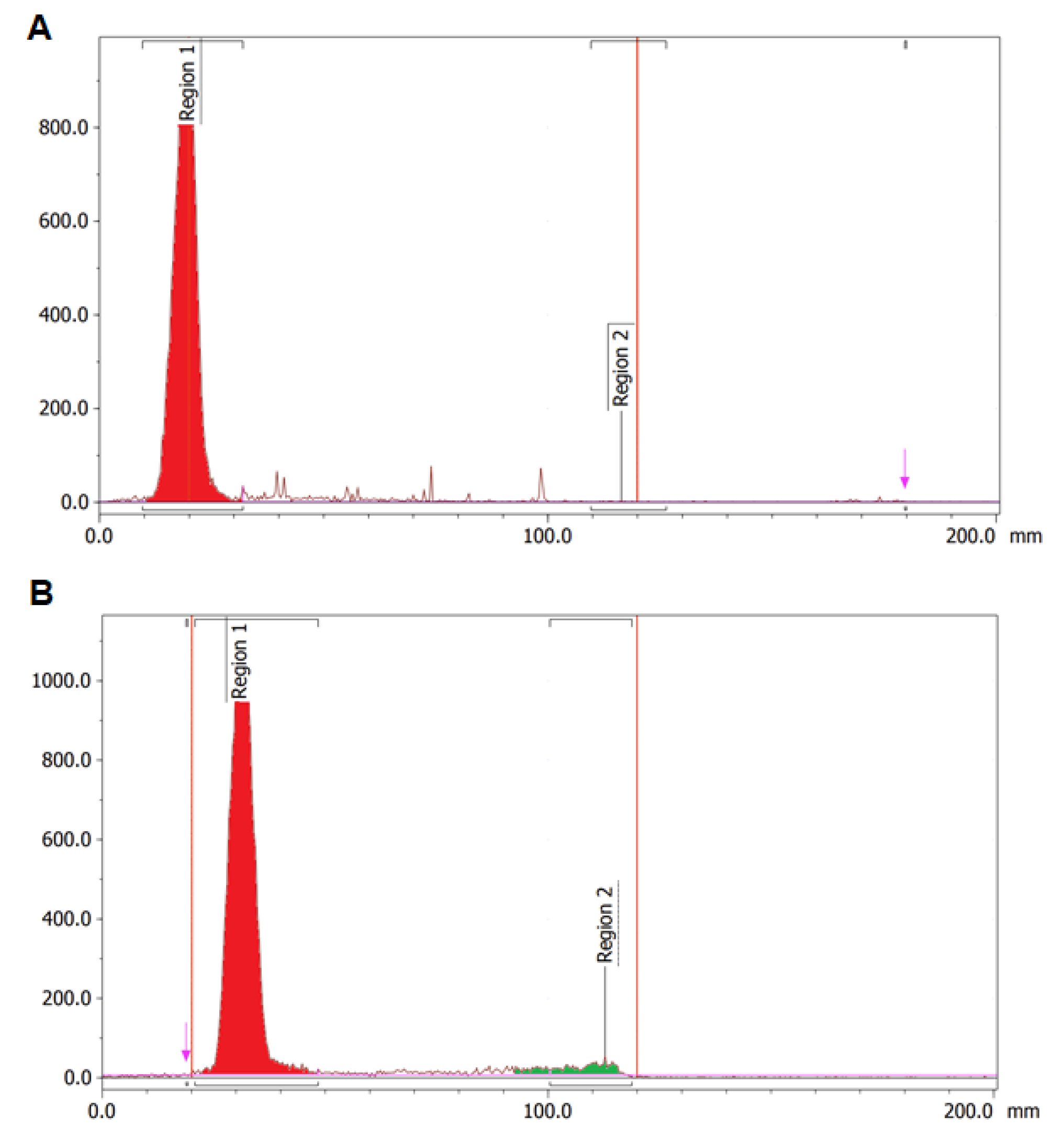
3.2. Stability of DOTA-C595 Immunoconjugate
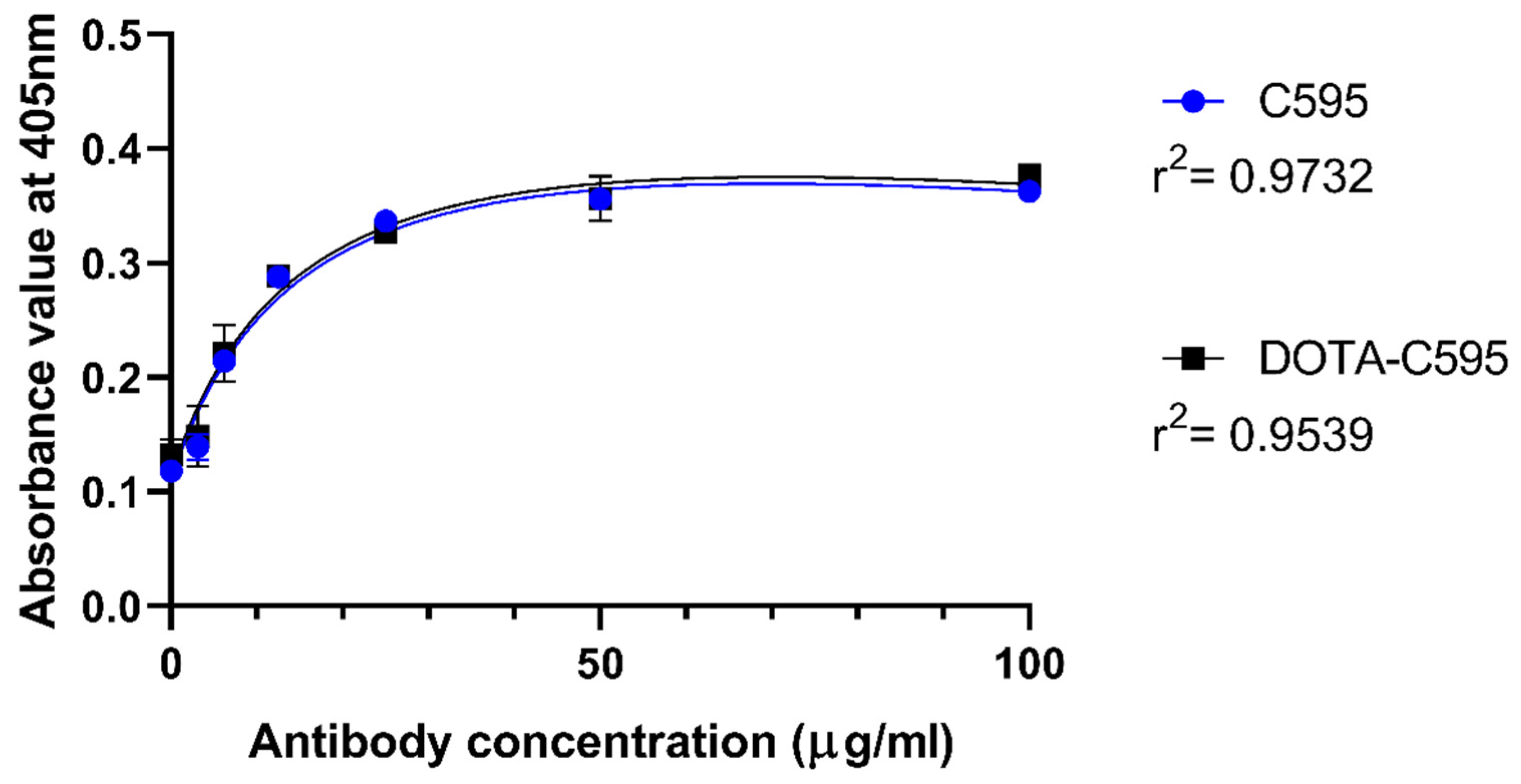
3.3. Effect of p-SCN-Bn-DOTA Conjugation to C595 Binding Affinity
3.4. Radioimmunoconjugate Production
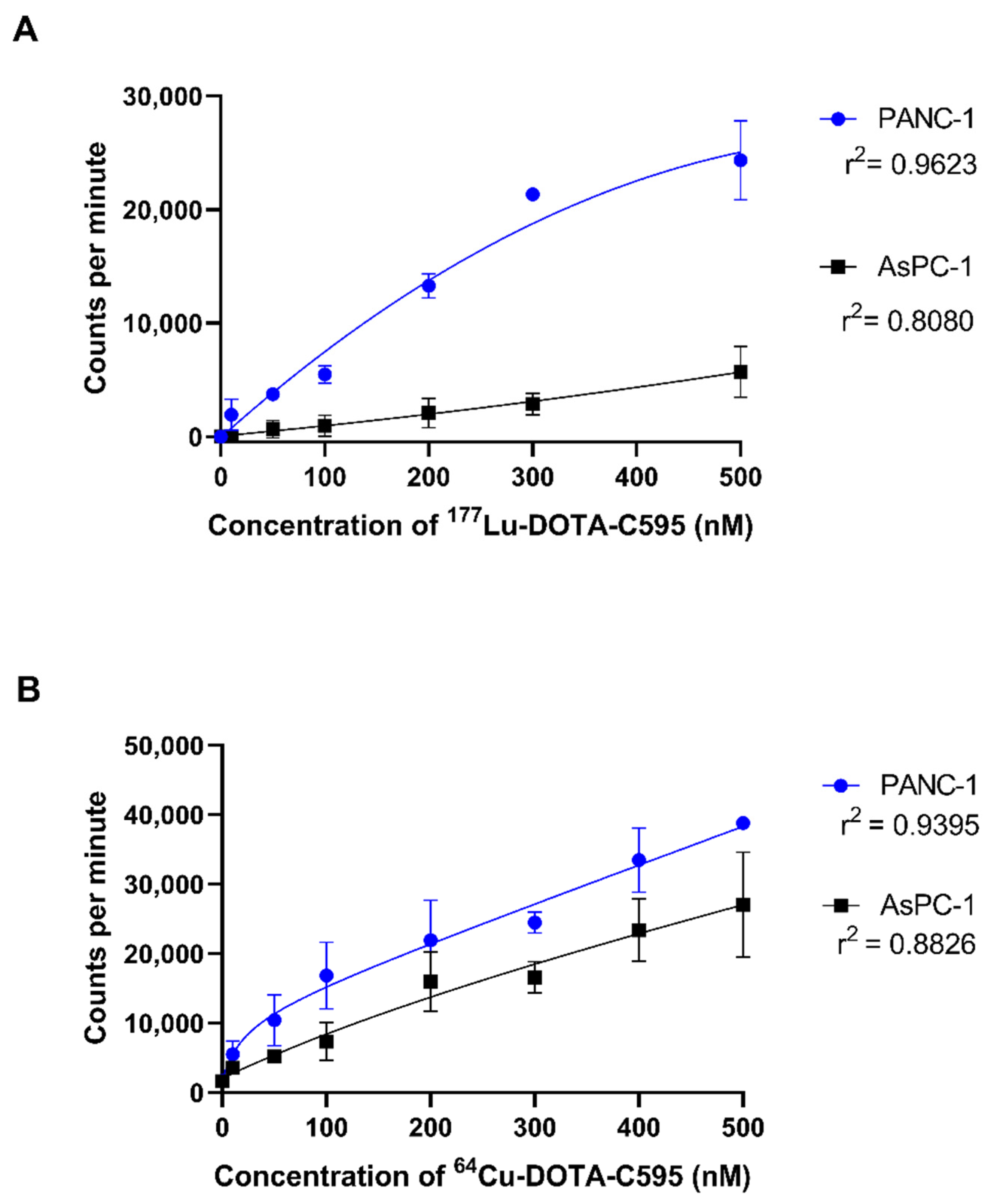
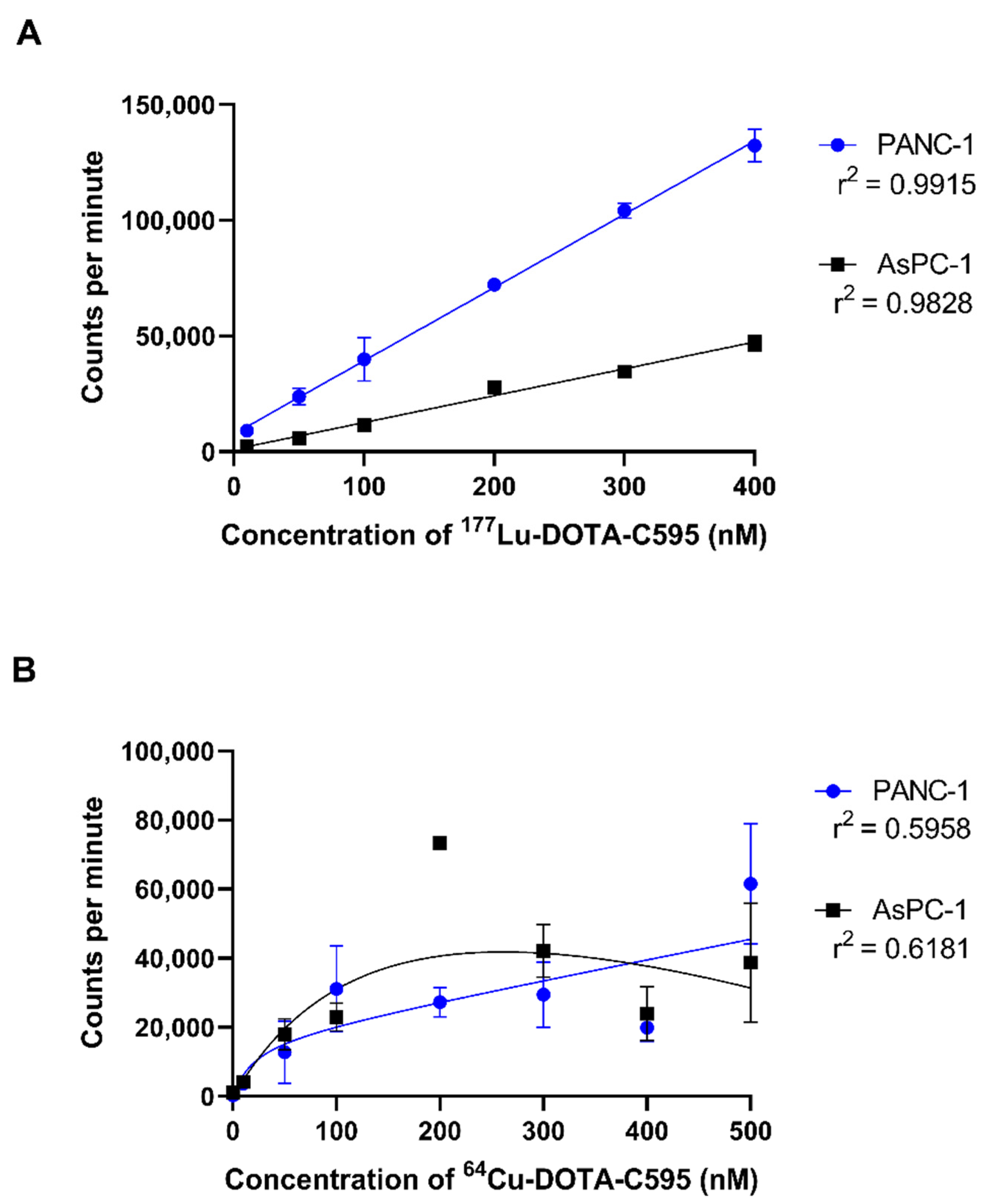
3.5. Cell Binding Assays
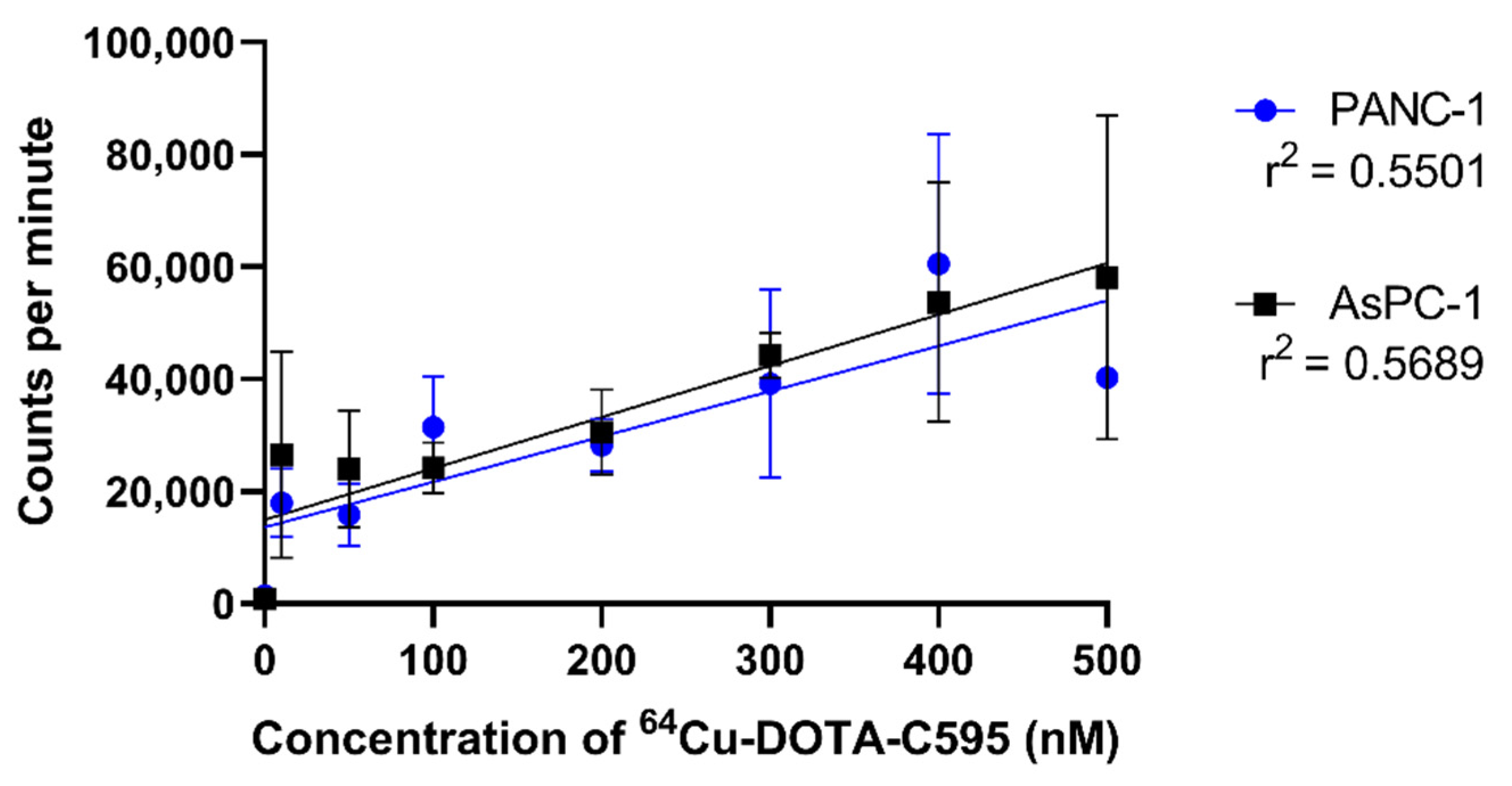
3.6. Influence of Storage on Radiolabelling and Cell Binding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Australian Institute of Health and Welfare. Cancer Data in Australia; AIHW: Canberra, Australia, 2020.
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Baine, M.J.; Jain, M.; Sasson, A.R.; Batra, S.K. Early diagnosis of pancreatic cancer: Challenges and new developments. Biomark Med. 2012, 6, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Oberstein, P.E.; Olive, K.P. Pancreatic cancer: Why is it so hard to treat? Ther. Adv. Gastroenterol. 2013, 6, 321–337. [Google Scholar] [CrossRef]
- Yoshii, Y.; Matsumoto, H.; Yoshimoto, M.; Oe, Y.; Zhang, M.R.; Nagatsu, K.; Sugyo, A.; Tsuji, A.B.; Higashi, T. 64Cu-intraperitoneal radioimmunotherapy: A novel approach for adjuvant treatment in a clinically relevant preclinical model of pancreatic cancer. J. Nucl. Med. 2019, 60, 1437–1443. [Google Scholar] [CrossRef]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef]
- Bourgeois, M.; Bailly, C.; Frindel, M.; Guerard, F.; Chérel, M.; Faivre-Chauvet, A.; Kraeber-Bodéré, F.; Bodet-Milin, C. Radioimmunoconjugates for treating cancer: Recent advances and current opportunities. Expert Opin. Biol. Ther. 2017, 17, 813–819. [Google Scholar] [CrossRef]
- Lee, D.Y.; Li, K.C.P. Molecular theranostics: A primer for the imaging professional. AJR Am. J. Roentgenol. 2011, 197, 318–324. [Google Scholar] [CrossRef]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA Theranostics: Review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef]
- Werner, R.A.; Weich, A.; Kircher, M.; Solnes, L.B.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; Pomper, M.G.; Rowe, S.P.; Lapa, C. The theranostic promise for neuroendocrine tumors in the late 2010s—Where do we stand, where do we go? Theranostics 2018, 8, 6088–6100. [Google Scholar] [CrossRef]
- Turner, J.H. Recent advances in theranostics and challenges for the future. Br. J. Radiol. 2018, 91, 20170893. [Google Scholar] [CrossRef] [PubMed]
- Bavelaar, B.M.; Lee, B.Q.; Gill, M.R.; Falzone, N.; Vallis, K.A. Subcellular targeting of theranostic radionuclides. Front. Pharmacol. 2018, 9, 996. [Google Scholar] [CrossRef]
- Suh, H.; Pillai, K.; Morris, D.L. Mucins in pancreatic cancer: Biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am. J. Cancer Res. 2017, 7, 1372–1383. [Google Scholar]
- van Putten, J.P.M.; Strijbis, K. Transmembrane mucins: Signaling receptors at the intersection of inflammation and cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, P.E.; Danysh, B.P.; Dharmaraj, N.; Carson, D.D. Transmembrane mucins as novel therapeutic targets. Expert Rev. Endocrinol. Metab. 2011, 6, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Zotter, S. Tissue and tumor distribution of human polymorphic eptithelial mucin. Cancer Rev. 1988, 11, 55. [Google Scholar]
- Hanisch, F.-G.; Müller, S. MUC1: The polymorphic appearance of a human mucin. Glycobiology 2000, 10, 439–449. [Google Scholar] [CrossRef]
- Gendler, S.J. MUC1, the renaissance molecule. J. Mammary Gland Biol. Neoplasia 2001, 6, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, Y.; Song, Y.; Rizvi, S.; Raja, C.; Zhang, D.; Samra, J.; Smith, R.; Perkins, A.C.; Apostolidis, C.; et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by 213Bi-C595 radioimmunoconjugate. Br. J. Cancer 2004, 91, 2086–2093. [Google Scholar] [CrossRef]
- Qu, C.; Songl, Y.J.; Rizvi, S.M.A.; Li, Y.; Smith, R.; Perkins, A.C.; Morgenstern, A.; Brechbiel, M.W.; Allen, B.J. In vivo and in vitro inhibition of pancreatic cancer growth by targeted alpha therapy using 213Bi-CHX.A”-C595. Cancer Biol. Ther. 2005, 4, 848–853. [Google Scholar] [CrossRef]
- Hull, A.; Li, Y.; Bartholomeusz, D.; Hsieh, W.; Escarbe, S.; Ruszkiewicz, A.; Bezak, E. The expression profile and textural characteristics of C595-reactive MUC1 in pancreatic ductal adenocarcinoma for targeted radionuclide therapy. Cancers 2020, 13, 61. [Google Scholar] [CrossRef]
- Gold, D.V.; Karanjawala, Z.; Modrak, D.E.; Goldenberg, D.M.; Hruban, R.H. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin. Cancer Res. 2007, 13, 7380–7387. [Google Scholar] [CrossRef]
- Tinder, T.L.; Subramani, D.B.; Basu, G.D.; Bradley, J.M.; Schettini, J.; Million, A.; Skaar, T.; Mukherjee, P. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J. Immunol. 2008, 181, 3116–3125. [Google Scholar] [CrossRef]
- Gendler, S.; Taylor-Papadimitriou, J.; Duhig, T.; Rothbard, J.; Burchell, J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J. Biol. Chem. 1988, 263, 12820–12823. [Google Scholar] [CrossRef] [PubMed]
- Price, M.R.; Pugh, J.A.; Hudecz, F.; Griffiths, W.; Jacobs, E.; Symonds, I.M.; Clarke, A.J.; Chan, W.; Baldwin, R.W. C595—A monoclonal antibody against the protein core of human urinary epithelial mucin commonly expressed in breast carcinomas. Br. J. Cancer 1990, 61, 681–686. [Google Scholar] [CrossRef]
- Price, M.R.; Hudecz, F.; O’Sullivan, C.; Baldwin, R.W.; Edwards, P.M.; Tendler, S.J. Immunological and structural features of the protein core of human polymorphic epithelial mucin. Mol. Immunol. 1990, 27, 795–802. [Google Scholar] [CrossRef]
- Smaglo, B.G.; Aldeghaither, D.; Weiner, L.M. The development of immunoconjugates for targeted cancer therapy. Nat. Rev. Clin. Oncol. 2014, 11, 637–648. [Google Scholar] [CrossRef]
- Al-Ejeh, F.; Darby, J.M.; Thierry, B.; Brown, M.P. A simplified suite of methods to evaluate chelator conjugation of antibodies: Effects on hydrodynamic radius and biodistribution. Nucl. Med. Biol. 2009, 36, 395–402. [Google Scholar] [CrossRef]
- Delage, J.A.; Faivre-Chauvet, A.; Barbet, J.; Fierle, J.K.; Schaefer, N.; Coukos, G.; Viertl, D.; Dunn, S.M.; Gnesin, S.; Prior, J.O. Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [177Lu]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model. Pharmaceutics 2021, 13, 96. [Google Scholar] [CrossRef]
- De León-Rodríguez, L.M.; Kovacs, Z. The synthesis and chelation chemistry of DOTA-peptide conjugates. Bioconjugate Chem. 2008, 19, 391–402. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.M.; Wrasidlo, W.A.; Reisfeld, R.A. Determination of the number of e-amino groups available for conjugation of effector molecules to monoclonal antibodies. Hybridoma 1988, 7, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Dennler, P.; Fischer, E.; Schibli, R. Antibody conjugates: From heterogeneous populations to defined reagents. Antibodies 2015, 4, 197–224. [Google Scholar] [CrossRef]
- Thakral, P.; Singla, S.; Yadav, M.P.; Vasisht, A.; Sharma, A.; Gupta, S.K.; Bal, C.; Snehlata; Malhotra, A. An approach for conjugation of (177) Lu- DOTA-SCN- Rituximab (BioSim) & its evaluation for radioimmunotherapy of relapsed & refractory B-cell non Hodgkins lymphoma patients. Indian J. Med. Res. 2014, 139, 544–554. [Google Scholar]
- Konermann, L. Addressing a common misconception: Ammonium acetate as neutral pH “buffer” for native electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 1827–1835. [Google Scholar] [CrossRef]
- Mitchell, B.L.; Yasui, Y.; Li, C.I.; Fitzpatrick, A.L.; Lampe, P.D. Impact of freeze-thaw cycles and storage time on plasma samples used in mass spectrometry based biomarker discovery projects. Cancer Inform. 2005, 1, 98–104. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Lewis, J.S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [Google Scholar] [CrossRef]
- Kilian, K. 68Ga-DOTA and analogs: Current status and future perspectives. Rep. Pract. Oncol. Radiother. 2014, 19, S13–S21. [Google Scholar] [CrossRef]
- Liu, S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv. Drug Deliv. Rev. 2008, 60, 1347–1370. [Google Scholar] [CrossRef]
- Spang, P.; Herrmann, C.; Roesch, F. Bifunctional gallium-68 chelators: Past, present, and future. Semin. Nucl. Med. 2016, 46, 373–394. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Lamsa, E.; Woods, M.; Duan, Y.; Fernando, P.; Bensimon, C.; Kordos, M.; Guenther, K.; Jurek, P.; Kiefer, G.E. Evaluation of bifunctional chelates for the development of gallium-based radiopharmaceuticals. Bioconjugate Chem. 2010, 21, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.S.; Ma, M.T.; Sunassee, K.; Shaw, K.P.; Williams, J.D.; Paul, R.L.; Donnelly, P.S.; Blower, P.J. Comparison of 64Cu-complexing bifunctional chelators for radioimmunoconjugation: Labeling efficiency, specific activity and in vitro/in vivo stability. Bioconjugate Chem. 2012, 23, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
| Radionuclide | Half-Life (T1/2) | Primary Decay Mode and Maximum Energy (MeV) | Clinical Application |
|---|---|---|---|
| 99mTc | 6 h | γ = 0.140 | Diagnostic SPECT |
| 68Ga | 68 min | β+ = 1.89 | Diagnostic PET |
| 177Lu | 6.7 d | γ = 0.208; β− = 0.497 | Therapeutic |
| 64Cu | 12.1 h | β+ = 0.653; β− = 0.578 | Diagnostic PET; Therapeutic |
| 225Ac | 9.9 d | α = 8.40 | Therapeutic |
| Molar Excess of p-SCN-Bn-DOTA | Average Number of Chelators Attached per Antibody |
|---|---|
| 20-fold | 3.00 |
| 40-fold | 3.83 |
| 50-fold | 4.50 |
| 100-fold | 6.17 |
| Radioimmunoconjugate | Radiolabelling Yield (%) (ITLC) | Radiochemical Purity (%) (HPLC) |
|---|---|---|
| 99mTc-DOTA-C595 | 93 | - |
| 68Ga-DOTA-C595 | 50 | - |
| 177Lu-DOTA-C595 | 93 | 96 |
| 64Cu-DOTA-C595 | >99 | >99 |
| Radioimmunoconjugate Concentration (nM) | 177Lu-DOTA-C595 | 64Cu-DOTA-C595 | ||||
|---|---|---|---|---|---|---|
| PANC-1 CPM (Mean ± S.D.) | AsPC-1 CPM (Mean ± S.D.) | p-Value | PANC-1 CPM (Mean ± S.D.) | AsPC-1 CPM (Mean ± S.D.) | p-Value | |
| 0 | 7.033 ± 39.11 | −12.33 ± 16.70 | 0.474343 | 1704 ± 15.81 | 1644 ± 47.37 | 0.103428 |
| 10 | 1916 ± 1390 | 47.33 ± 14.30 | 0.080383 | 5515 ± 1880 | 3571 ± 560.0 | 0.160980 |
| 50 | 3723 ± 206.5 | 656.7 ± 767.9 | 0.013368 * | 10,433 ± 3655 | 5167 ± 895.5 | 0.072480 |
| 100 | 5493 ± 771.7 | 962.7 ± 905.4 | 0.002738 ** | 16,846 ± 4819 | 7363 ± 2735 | 0.041383 |
| 200 | 13,293 ± 1078 | 2068 ± 1306 | 0.000328 *** | 21,949 ± 5776 | 15,969 ± 4246 | 0.221960 |
| 300 | 21,337 ± 237.6 | 2867 ± 933.4 | 0.001357 ** | 24,477 ± 1505 | 16,613 ± 2259 | 0.007389 |
| 400 | - | - | - | 33,484 ± 4618 | 23,377 ± 4506 | 0.053370 |
| 500 | 24,354 ± 3485 | 5723 ± 2242 | 0.004905 ** | 38,833 ± 454.4 | 27,083 ± 7548 | 0.054576 |
| Radionuclide | Charge | Coordination Number/Geometry | Ionic Radii (Å) |
|---|---|---|---|
| 99mTc | +4 | VI, distorted octahedral | 0.65 |
| 68Ga | +3 | VI, distorted octahedral | 0.62 |
| 177Lu | +3 | VIII, square antiprism | 0.98 |
| 64Cu | +2 | VI, distorted octahedral | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hull, A.; Li, Y.; Bartholomeusz, D.; Hsieh, W.; Tieu, W.; Pukala, T.L.; Staudacher, A.H.; Bezak, E. Preliminary Development and Testing of C595 Radioimmunoconjugates for Targeting MUC1 Cancer Epitopes in Pancreatic Ductal Adenocarcinoma. Cells 2022, 11, 2983. https://doi.org/10.3390/cells11192983
Hull A, Li Y, Bartholomeusz D, Hsieh W, Tieu W, Pukala TL, Staudacher AH, Bezak E. Preliminary Development and Testing of C595 Radioimmunoconjugates for Targeting MUC1 Cancer Epitopes in Pancreatic Ductal Adenocarcinoma. Cells. 2022; 11(19):2983. https://doi.org/10.3390/cells11192983
Chicago/Turabian StyleHull, Ashleigh, Yanrui Li, Dylan Bartholomeusz, William Hsieh, William Tieu, Tara L. Pukala, Alexander H. Staudacher, and Eva Bezak. 2022. "Preliminary Development and Testing of C595 Radioimmunoconjugates for Targeting MUC1 Cancer Epitopes in Pancreatic Ductal Adenocarcinoma" Cells 11, no. 19: 2983. https://doi.org/10.3390/cells11192983
APA StyleHull, A., Li, Y., Bartholomeusz, D., Hsieh, W., Tieu, W., Pukala, T. L., Staudacher, A. H., & Bezak, E. (2022). Preliminary Development and Testing of C595 Radioimmunoconjugates for Targeting MUC1 Cancer Epitopes in Pancreatic Ductal Adenocarcinoma. Cells, 11(19), 2983. https://doi.org/10.3390/cells11192983






