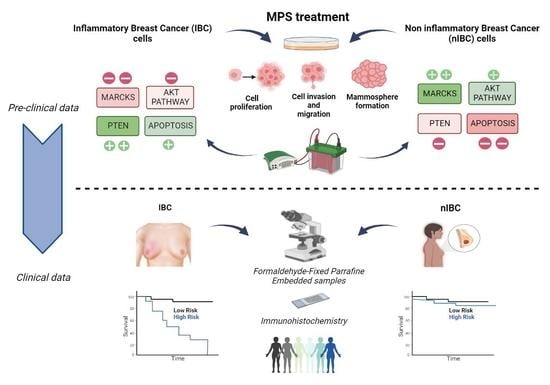
MARCKS as a Potential Therapeutic Target in Inflammatory Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and MARCKS Inhibitor Treatment
2.2. Cell Viability, Proliferation, and Colony Formation Assays
2.3. Scratch Wound-Healing Assay
2.4. Transwell Migration and Invasion Assay
2.5. Mammosphere Formation Assay
2.6. Western Blot Analysis
2.7. Patients and Samples
2.8. Immunohistochemistry (IHC) Analysis
2.9. Statistical Analysis
3. Results
3.1. MPS Inhibits Proliferation of IBC Cells at Lower Doses Compared to nIBC
3.2. MPS Inhibits the Invasiveness and Cell Motility in IBC Cells
3.3. MPS Impairs the Mammosphere Formation in IBC Cells
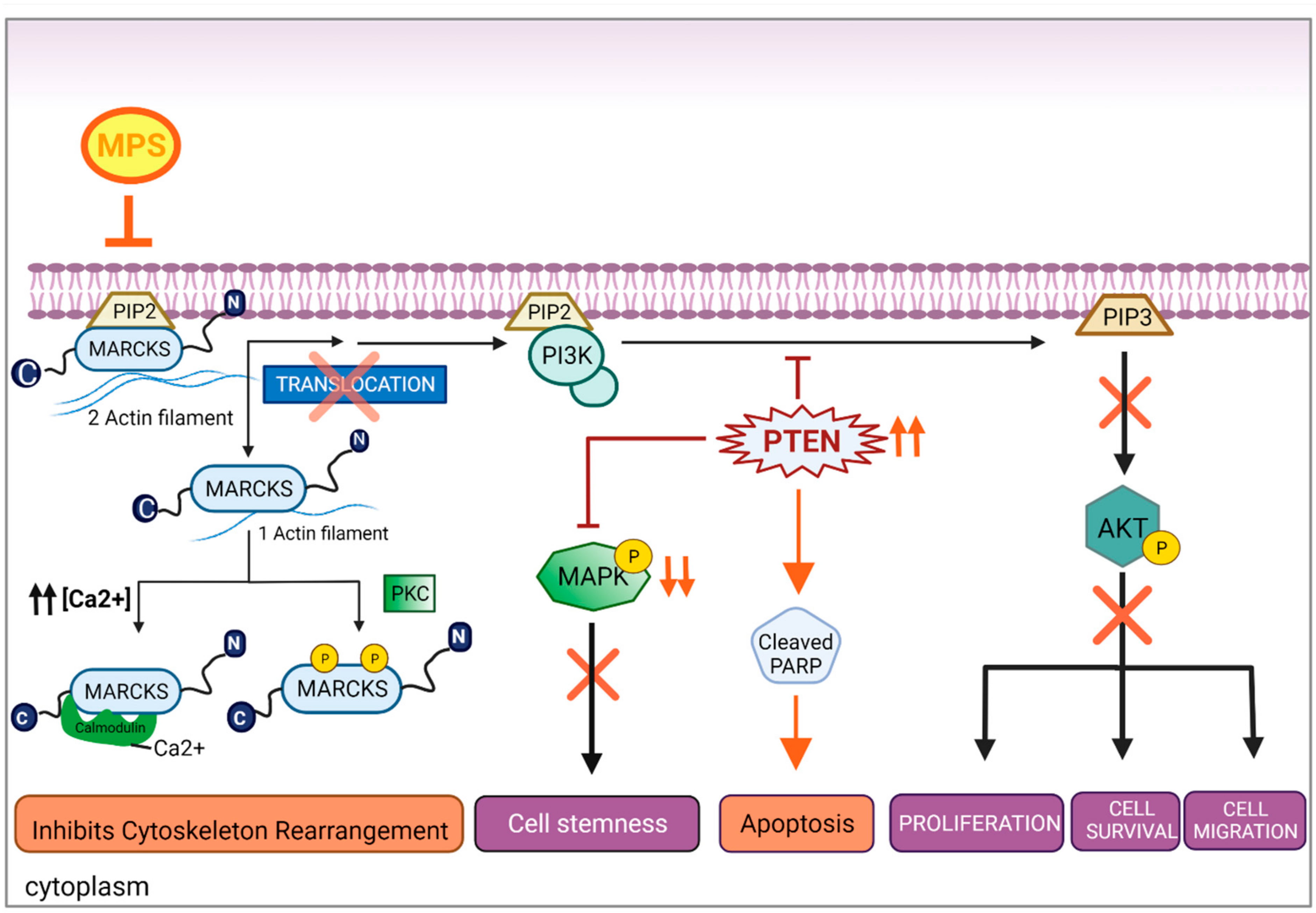
3.4. MPS Downregulates MAPK in IBC
3.5. Inhibition of MARCKS and Its Phosphorylated Form by MPS Upregulates PTEN and Downregulates AKT Pathway in IBC Cells
3.6. MARCKS and PTEN Protein Expressions Correlate with Survival in Patients with IBC, but Not in Patients with nIBC
3.7. Negative Correlation between MARCKS and PTEN Protein Expression in IBC Patients and Prognostic Value
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, F.M.; Bondy, M.; Yang, W.; Yamauchi, H.; Wiggins, S.; Kamrudin, S.; Krishnamurthy, S.; Le-Petross, H.; Bidaut, L.; Player, A.N.; et al. Inflammatory Breast Cancer: The Disease, the Biology, the Treatment. CA Cancer J. Clin. 2010, 60, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Boussen, H.; Bouzaiene, H.; Ben Hassouna, J.; Dhiab, T.; Khomsi, F.; Benna, F.; Gamoudi, A.; Mourali, N.; Hechiche, M.; Rahal, K.; et al. Inflammatory Breast Cancer in Tunisia: Epidemiological and Clinical Trends. Cancer 2010, 116, 2730–2735. [Google Scholar] [CrossRef] [PubMed]
- Levine, P.H.; Veneroso, C. The Epidemiology of Inflammatory Breast Cancer. Semin. Oncol. 2008, 35, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Manai, M.; Finetti, P.; Mejri, N.; Athimni, S.; Birnbaum, D.; Bertucci, F.; Rahal, K.; Gamoudi, A.; Chaffanet, M.; Manai, M.; et al. Inflammatory Breast Cancer in 210 Patients: A Retrospective Study on Epidemiological, Anatomo-Clinical Features and Therapeutic Results. Mol. Clin. Oncol. 2019, 10, 223–230. [Google Scholar] [CrossRef]
- Dawood, S.; Merajver, S.D.; Viens, P.; Vermeulen, P.B.; Swain, S.M.; Buchholz, T.A.; Dirix, L.Y.; Levine, P.H.; Lucci, A.; Krishnamurthy, S.; et al. International Expert Panel on Inflammatory Breast Cancer: Consensus Statement for Standardized Diagnosis and Treatment. Ann. Oncol. 2011, 22, 515–523. [Google Scholar] [CrossRef]
- Van Laere, S.J.; Ueno, N.T.; Finetti, P.; Vermeulen, P.; Lucci, A.; Robertson, F.M.; Marsan, M.; Iwamoto, T.; Krishnamurthy, S.; Masuda, H.; et al. Uncovering the Molecular Secrets of Inflammatory Breast Cancer Biology: An Integrated Analysis of Three Distinct Affymetrix Gene Expression Datasets. Clin. Cancer Res. 2013, 19, 4685–4696. [Google Scholar] [CrossRef]
- Manai, M.; Thomassin-Piana, J.; Gamoudi, A.; Finetti, P.; Lopez, M.; Eghozzi, R.; Ayadi, S.; Lamine, O.B.; Manai, M.; Rahal, K.; et al. MARCKS Protein Overexpression in Inflammatory Breast Cancer. Oncotarget 2017, 8, 6246–6257. [Google Scholar] [CrossRef]
- Ramsden, J.J. MARCKS: A Case of Molecular Exaptation? Int. J. Biochem. Cell Biol. 2000, 32, 475–479. [Google Scholar] [CrossRef]
- Li, Y.; Martin, L.D.; Spizz, G.; Adler, K.B. MARCKS Protein Is a Key Molecule Regulating Mucin Secretion by Human Airway Epithelial Cells in Vitro. J. Biol. Chem. 2001, 276, 40982–40990. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Selmi, C.; Ridgway, W.M.; Leung, P.S.C.; Zhang, F.; Gershwin, M.E. The Myristoylated Alanine-Rich C-Kinase Substrates (MARCKS): A Membrane-Anchored Mediator of the Cell Function. Autoimmun. Rev. 2021, 20, 102942. [Google Scholar] [CrossRef]
- Tatsumi, S.; Mabuchi, T.; Katano, T.; Matsumura, S.; Abe, T.; Hidaka, H.; Suzuki, M.; Sasaki, Y.; Minami, T.; Ito, S. Involvement of Rho-Kinase in Inflammatory and Neuropathic Pain through Phosphorylation of Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS). Neuroscience 2005, 131, 491–498. [Google Scholar] [CrossRef]
- Hasegawa, H.; Nakai, M.; Tanimukai, S.; Taniguchi, T.; Terashima, A.; Kawamata, T.; Fukunaga, K.; Miyamoto, E.; Misaki, K.; Mukai, H.; et al. Microglial Signaling by Amyloid Beta Protein through Mitogen-Activated Protein Kinase Mediating Phosphorylation of MARCKS. Neuroreport 2001, 12, 2567–2571. [Google Scholar] [CrossRef]
- Chen, C.-H.; Statt, S.; Chiu, C.-L.; Thai, P.; Arif, M.; Adler, K.B.; Wu, R. Targeting Myristoylated Alanine-Rich C Kinase Substrate Phosphorylation Site Domain in Lung Cancer. Mechanisms and Therapeutic Implications. Am. J. Respir. Crit. Care Med. 2014, 190, 1127–1138. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Saha, M.N.; Chen, J.; Evans, K.; Qiu, L.; Reece, D.; Chen, G.A.; Chang, H. Targeting Phospho-MARCKS Overcomes Drug-Resistance and Induces Antitumor Activity in Preclinical Models of Multiple Myeloma. Leukemia 2015, 29, 715–726. [Google Scholar] [CrossRef]
- Ginestier, C.; Charafe-Jauffret, E.; Bertucci, F.; Eisinger, F.; Geneix, J.; Bechlian, D.; Conte, N.; Adélaïde, J.; Toiron, Y.; Nguyen, C.; et al. Distinct and Complementary Information Provided by Use of Tissue and DNA Microarrays in the Study of Breast Tumor Markers. Am. J. Pathol. 2002, 161, 1223–1233. [Google Scholar] [CrossRef]
- Taube, J.H.; Herschkowitz, J.I.; Komurov, K.; Zhou, A.Y.; Gupta, S.; Yang, J.; Hartwell, K.; Onder, T.T.; Gupta, P.B.; Evans, K.W.; et al. Correction for Taube et al., Core Epithelial-to-Mesenchymal Transition Interactome Gene-Expression Signature Is Associated with Claudin-Low and Metaplastic Breast Cancer Subtypes. Proc. Natl. Acad. Sci. USA 2010, 107, 19132. [Google Scholar] [CrossRef]
- van Golen, K.L.; Bao, L.W.; Pan, Q.; Miller, F.R.; Wu, Z.F.; Merajver, S.D. Mitogen Activated Protein Kinase Pathway Is Involved in RhoC GTPase Induced Motility, Invasion and Angiogenesis in Inflammatory Breast Cancer. Clin. Exp. Metastasis 2002, 19, 301–311. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Tarpin, C.; Diebel, M.; Esterni, B.; Houvenaeghel, G.; Extra, J.-M.; Bertucci, F.; Jacquemier, J.; et al. Aldehyde Dehydrogenase 1-Positive Cancer Stem Cells Mediate Metastasis and Poor Clinical Outcome in Inflammatory Breast Cancer. Clin. Cancer Res. 2010, 16, 45–55. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Lim, E.; Vaillant, F.; Wu, D.; Forrest, N.C.; Pal, B.; Hart, A.H.; Asselin-Labat, M.-L.; Gyorki, D.E.; Ward, T.; Partanen, A.; et al. Aberrant Luminal Progenitors as the Candidate Target Population for Basal Tumor Development in BRCA1 Mutation Carriers. Nat. Med. 2009, 15, 907–913. [Google Scholar] [CrossRef]
- Lee, S.-M.; Suk, K.; Lee, W.-H. Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) Regulates the Expression of Proinflammatory Cytokines in Macrophages through Activation of P38/JNK MAPK and NF-ΚB. Cell Immunol. 2015, 296, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.; Bryant, A.; Frankel, P.; Wooden, R.; Kerkhoff, E.; Rapp, U.R.; Foster, D.A. Phospholipase D Overcomes Cell Cycle Arrest Induced by High-Intensity Raf Signaling. Oncogene 2002, 21, 3651–3658. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Cheng, C.-T.; Yuan, Y.; Zhai, J.; Arif, M.; Fong, L.W.R.; Wu, R.; Ann, D.K. Elevated MARCKS Phosphorylation Contributes to Unresponsiveness of Breast Cancer to Paclitaxel Treatment. Oncotarget 2015, 6, 15194–15208. [Google Scholar] [CrossRef] [PubMed]
- Jin Cho, S.; La, M.; Keun Ahn, J.; Meadows, G.G.; Joe, C.O. Tob-Mediated Cross-Talk between MARCKS Phosphorylation and ErbB-2 Activation. Biochem. Biophys. Res. Commun. 2001, 283, 273–277. [Google Scholar] [CrossRef]
- Techasen, A.; Loilome, W.; Namwat, N.; Takahashi, E.; Sugihara, E.; Puapairoj, A.; Miwa, M.; Saya, H.; Yongvanit, P. Myristoylated Alanine-Rich C Kinase Substrate Phosphorylation Promotes Cholangiocarcinoma Cell Migration and Metastasis via the Protein Kinase C-Dependent Pathway. Cancer Sci. 2010, 101, 658–665. [Google Scholar] [CrossRef]
- Song, J.; Wang, Q.; Luo, Y.; Yuan, P.; Tang, C.; Hui, Y.; Wang, Z. MiR-34c-3p Inhibits Cell Proliferation, Migration and Invasion of Hepatocellular Carcinoma by Targeting MARCKS. Int. J. Clin. Exp. Pathol. 2015, 8, 12728–12737. [Google Scholar]
- Dorris, E.; O’Neill, A.; Hanrahan, K.; Treacy, A.; Watson, R.W. MARCKS Promotes Invasion and Is Associated with Biochemical Recurrence in Prostate Cancer. Oncotarget 2017, 8, 72021–72030. [Google Scholar] [CrossRef]
- Van Laere, S.; Limame, R.; Van Marck, E.A.; Vermeulen, P.B.; Dirix, L.Y. Is There a Role for Mammary Stem Cells in Inflammatory Breast Carcinoma?: A Review of Evidence from Cell Line, Animal Model, and Human Tissue Sample Experiments. Cancer 2010, 116, 2794–2805. [Google Scholar] [CrossRef]
- Lacerda, L.; Debeb, B.G.; Smith, D.; Larson, R.; Solley, T.; Xu, W.; Krishnamurthy, S.; Gong, Y.; Levy, L.B.; Buchholz, T.; et al. Mesenchymal Stem Cells Mediate the Clinical Phenotype of Inflammatory Breast Cancer in a Preclinical Model. Breast Cancer Res. 2015, 17, 42. [Google Scholar] [CrossRef]
- Manuel Iglesias, J.; Beloqui, I.; Garcia-Garcia, F.; Leis, O.; Vazquez-Martin, A.; Eguiara, A.; Cufi, S.; Pavon, A.; Menendez, J.A.; Dopazo, J.; et al. Mammosphere Formation in Breast Carcinoma Cell Lines Depends upon Expression of E-Cadherin. PLoS ONE 2013, 8, e77281. [Google Scholar] [CrossRef]
- Chen, C.-H.; Thai, P.; Yoneda, K.; Adler, K.B.; Yang, P.-C.; Wu, R. A Peptide That Inhibits Function of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) Reduces Lung Cancer Metastasis. Oncogene 2014, 33, 3696–3706. [Google Scholar] [CrossRef]
- Jarboe, J.S.; Anderson, J.C.; Duarte, C.W.; Mehta, T.; Nowsheen, S.; Hicks, P.H.; Whitley, A.C.; Rohrbach, T.D.; McCubrey, R.O.; Chiu, S.; et al. MARCKS Regulates Growth and Radiation Sensitivity and Is a Novel Prognostic Factor for Glioma. Clin. Cancer Res. 2012, 18, 3030–3041. [Google Scholar] [CrossRef]
- Sundaram, M.; Cook, H.W.; Byers, D.M. The MARCKS Family of Phospholipid Binding Proteins: Regulation of Phospholipase D and Other Cellular Components. Biochem. Cell Biol. 2004, 82, 191–200. [Google Scholar] [CrossRef]
- Ziemba, B.P.; Burke, J.E.; Masson, G.; Williams, R.L.; Falke, J.J. Regulation of PI3K by PKC and MARCKS: Single-Molecule Analysis of a Reconstituted Signaling Pathway. Biophys. J. 2016, 110, 1811–1825. [Google Scholar] [CrossRef]
- Beckmann, L.; Berg, V.; Dickhut, C.; Sun, C.; Merkel, O.; Bloehdorn, J.; Robrecht, S.; Seifert, M.; da Palma Guerreiro, A.; Claasen, J.; et al. MARCKS Affects Cell Motility and Response to BTK Inhibitors in CLL. Blood 2021, 138, 544–556. [Google Scholar] [CrossRef]
- Courtney, K.D.; Corcoran, R.B.; Engelman, J.A. The PI3K Pathway as Drug Target in Human Cancer. J. Clin. Oncol. 2010, 28, 1075–1083. [Google Scholar] [CrossRef]
- Lehman, H.L.; Van Laere, S.J.; van Golen, C.M.; Vermeulen, P.B.; Dirix, L.Y.; van Golen, K.L. Regulation of Inflammatory Breast Cancer Cell Invasion through Akt1/PKBα Phosphorylation of RhoC GTPase. Mol. Cancer Res. 2012, 10, 1306–1318. [Google Scholar] [CrossRef]
- Rosenthal, D.T.; Zhang, J.; Bao, L.; Zhu, L.; Wu, Z.; Toy, K.; Kleer, C.G.; Merajver, S.D. RhoC Impacts the Metastatic Potential and Abundance of Breast Cancer Stem Cells. PLoS ONE 2012, 7, e40979. [Google Scholar] [CrossRef]
- Allensworth, J.L.; Evans, M.K.; Bertucci, F.; Aldrich, A.J.; Festa, R.A.; Finetti, P.; Ueno, N.T.; Safi, R.; McDonnell, D.P.; Thiele, D.J.; et al. Disulfiram (DSF) Acts as a Copper Ionophore to Induce Copper-Dependent Oxidative Stress and Mediate Anti-Tumor Efficacy in Inflammatory Breast Cancer. Mol. Oncol. 2015, 9, 1155–1168. [Google Scholar] [CrossRef]
- Ebbesen, S.H.; Scaltriti, M.; Bialucha, C.U.; Morse, N.; Kastenhuber, E.R.; Wen, H.Y.; Dow, L.E.; Baselga, J.; Lowe, S.W. Pten Loss Promotes MAPK Pathway Dependency in HER2/Neu Breast Carcinomas. Proc. Natl. Acad. Sci. USA 2016, 113, 3030–3035. [Google Scholar] [CrossRef]


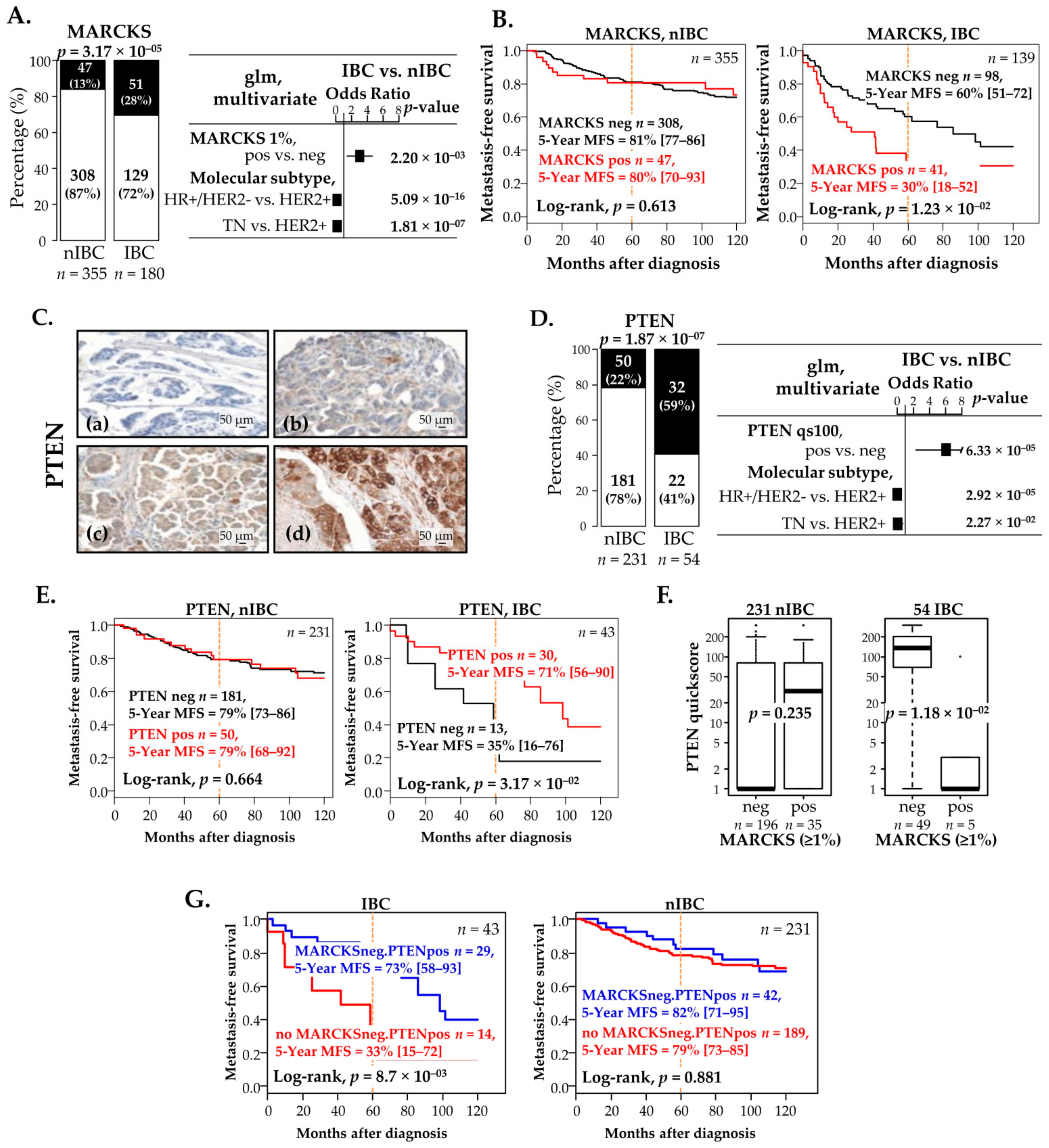
| Characteristics | n | Global | MARCKS, IBC | p-Value | n | Global | MARCKS, nIBC | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||||||||
| Age | 145 | 50 (15–81) | 51 (27–80) | 51 (15–81) | 0.966 | 355 | 58 (25–94) | 59 (25–94) | 58 (37–87) | 0.642 | |
| Histology | 0.795 | 0.126 | |||||||||
| ductal | 151 | 151 (89%) | 108 (90%) | 43 (88%) | 247 | 247 (70%) | 216 (70%) | 31 (66%) | |||
| lobular | 13 | 13 (8%) | 8 (7%) | 5 (10%) | 47 | 47 (13%) | 41 (13%) | 6 (13%) | |||
| mixed | 1 | 1 (1%) | 1 (1%) | 0 (0%) | 16 | 16 (5%) | 16 (5%) | 0 (0%) | |||
| other | 4 | 4 (2%) | 3 (2%) | 1 (2%) | 45 | 45 (13%) | 35 (11%) | 10 (21%) | |||
| Grade | 0.460 | <1.0 × 10−5 | |||||||||
| 1 | 10 | 10 (6%) | 6 (5%) | 4 (9%) | 119 | 119 (34%) | 111 (36%) | 8 (17%) | |||
| 2 | 52 | 52 (32%) | 40 (35%) | 12 (26%) | 144 | 144 (41%) | 131 (43%) | 13 (28%) | |||
| 3 | 99 | 99 (61%) | 69 (60%) | 30 (65%) | 91 | 91 (26%) | 66 (21%) | 25 (54%) | |||
| pT | 0.091 | ||||||||||
| pT1 | --- | --- | --- | --- | 147 | 147 (41%) | 130 (42%) | 17 (36%) | |||
| pT2 | --- | --- | --- | --- | 149 | 149 (42%) | 123 (40%) | 26 (55%) | |||
| pT3 | --- | --- | --- | --- | 59 | 59 (17%) | 55 (18%) | 4 (9%) | |||
| pN | 1.000 | ||||||||||
| 0 | --- | --- | --- | --- | 177 | 177 (50%) | 154 (50%) | 23 (50%) | |||
| 1 | --- | --- | --- | --- | 175 | 175 (50%) | 152 (50%) | 23 (50%) | |||
| Molecular subtype | 0.204 | 2.04 × 10−8 | |||||||||
| HER2+ | 54 | 54 (47%) | 37 (49%) | 17 (45%) | 22 | 22 (7%) | 14 (5%) | 8 (20%) | |||
| HR+/HER2− | 45 | 45 (39%) | 32 (42%) | 13 (34%) | 250 | 250 (79%) | 232 (84%) | 18 (44%) | |||
| TN | 15 | 15 (13%) | 7 (9%) | 8 (21%) | 44 | 44 (14%) | 29 (11%) | 15 (37%) | |||
| Follow-up median, months (range) | 139 | 42 (1–160) | 43 (1–159) | 23 (1–160) | 0.777 | 355 | 91 (4–231) | 90 (4–231) | 101 (5–216) | 0.698 | |
| MFS event, n(%) | 139 | 68 (33%) | 44 (45%) | 24 (59%) | 0.193 | 355 | 94 (21%) | 83 (27%) | 11 (23%) | 0.724 | |
| 5-year MFS [95%CI] | 139 | 52% [43–62] | 60% [51–72] | 30% [18–52] | 1.23× 10−2 | 355 | 81% [77–85] | 81% [77–86] | 80% [70–93] | 0.613 | |
| Characteristics | n | Global | PTEN, IBC | p-Value | n | Global | PTEN, nIBC | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||||||||
| Age | 32 | 55 (33.25) | 53 (33–80) | 57 (37–78) | 0.385 | 231 | 58 (25–94) | 60 (25–94) | 57 (35–87) | 0.125 | |
| Histology | 0.235 | 0.941 | |||||||||
| ductal | 46 | 46 (90%) | 17 (85%) | 29 (94%) | 172 | 172 (74%) | 134 (74%) | 38 (76%) | |||
| lobular | 4 | 4 (8%) | 3 (15%) | 1 (3%) | 21 | 21 (9%) | 17 (9%) | 4 (8%) | |||
| mixed | 1 | 1 (2%) | 0 (0%) | 1 (3%) | 7 | 7 (3%) | 5 (3%) | 2 (4%) | |||
| other | 0 | 0 (0%) | 0 (0%) | 0 (0%) | 31 | 31 (13%) | 25 (14%) | 6 (12%) | |||
| Grade | 0.229 | 0.239 | |||||||||
| 1 | 3 | 3 (6%) | 0 (0%) | 3 (10%) | 67 | 67 (29%) | 56 (31%) | 11 (22%) | |||
| 2 | 13 | 13 (25%) | 4 (20%) | 9 (29%) | 98 | 98 (43%) | 72 (40%) | 26 (53%) | |||
| 3 | 35 | 35 (69%) | 16 (80%) | 19 (61%) | 65 | 65 (28%) | 53 (29%) | 12 (24%) | |||
| pT | 0.143 | ||||||||||
| pT1 | --- | --- | --- | --- | 88 | 88 (38%) | 72 (40%) | 16 (32%) | |||
| pT2 | --- | --- | --- | --- | 104 | 104 (45%) | 83 (46%) | 21 (42%) | |||
| pT3 | --- | --- | --- | --- | 39 | 39 (17%) | 26 (14%) | 13 (26%) | |||
| pN | 0.759 | ||||||||||
| 0 | --- | --- | --- | --- | 113 | 113 (49%) | 90 (50%) | 23 (46%) | |||
| 1 | --- | --- | --- | --- | 118 | 118 (51%) | 91 (50%) | 27 (54%) | |||
| Molecular subtype | 0.962 | 0.571 | |||||||||
| HER2+ | 12 | 12 (43%) | 5 (45%) | 7 (41%) | 19 | 19 (9%) | 15 (9%) | 4 (9%) | |||
| HR+/HER2− | 13 | 13 (46%) | 5 (45%) | 8 (47%) | 165 | 165 (77%) | 129 (76%) | 36 (82%) | |||
| TN | 3 | 3 (11%) | 1 (9%) | 2 (12%) | 30 | 30 (14%) | 26 (15%) | 4 (9%) | |||
| Follow-up median, months (range) | 43 | 58 (1–159) | 35 (9–159) | 67 (1–143) | 0.223 | 231 | 85 (4–231) | 82 (4–206) | 104 (5–231) | 3.35 × 10−2 | |
| MFS event, n(%) | 43 | 25 (37%) | 10 (77%) | 14 (48%) | 0.104 | 231 | 64 (22%) | 48 (27%) | 16 (32%) | 0.477 | |
| 5-year MFS [95%CI] | 43 | 60% [46–78] | 35% [16–76] | 73% [58–93] | 1.85 × 10−2 | 231 | 79% [74–85] | 79% [73–86] | 79% [68–92] | 0.664 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manai, M.; ELBini-Dhouib, I.; Finetti, P.; Bichiou, H.; Reduzzi, C.; Aissaoui, D.; Ben-Hamida, N.; Agavnian, E.; Srairi-Abid, N.; Lopez, M.; et al. MARCKS as a Potential Therapeutic Target in Inflammatory Breast Cancer. Cells 2022, 11, 2926. https://doi.org/10.3390/cells11182926
Manai M, ELBini-Dhouib I, Finetti P, Bichiou H, Reduzzi C, Aissaoui D, Ben-Hamida N, Agavnian E, Srairi-Abid N, Lopez M, et al. MARCKS as a Potential Therapeutic Target in Inflammatory Breast Cancer. Cells. 2022; 11(18):2926. https://doi.org/10.3390/cells11182926
Chicago/Turabian StyleManai, Maroua, Ines ELBini-Dhouib, Pascal Finetti, Haifa Bichiou, Carolina Reduzzi, Dorra Aissaoui, Naziha Ben-Hamida, Emilie Agavnian, Najet Srairi-Abid, Marc Lopez, and et al. 2022. "MARCKS as a Potential Therapeutic Target in Inflammatory Breast Cancer" Cells 11, no. 18: 2926. https://doi.org/10.3390/cells11182926
APA StyleManai, M., ELBini-Dhouib, I., Finetti, P., Bichiou, H., Reduzzi, C., Aissaoui, D., Ben-Hamida, N., Agavnian, E., Srairi-Abid, N., Lopez, M., Amri, F., Guizani-Tabbane, L., Rahal, K., Mrad, K., Manai, M., Birnbaum, D., Mamessier, E., Cristofanilli, M., Boussen, H., ... Bertucci, F. (2022). MARCKS as a Potential Therapeutic Target in Inflammatory Breast Cancer. Cells, 11(18), 2926. https://doi.org/10.3390/cells11182926