Transient Receptor Potential (TRP) Channels in Airway Toxicity and Disease: An Update
Abstract
1. Introduction
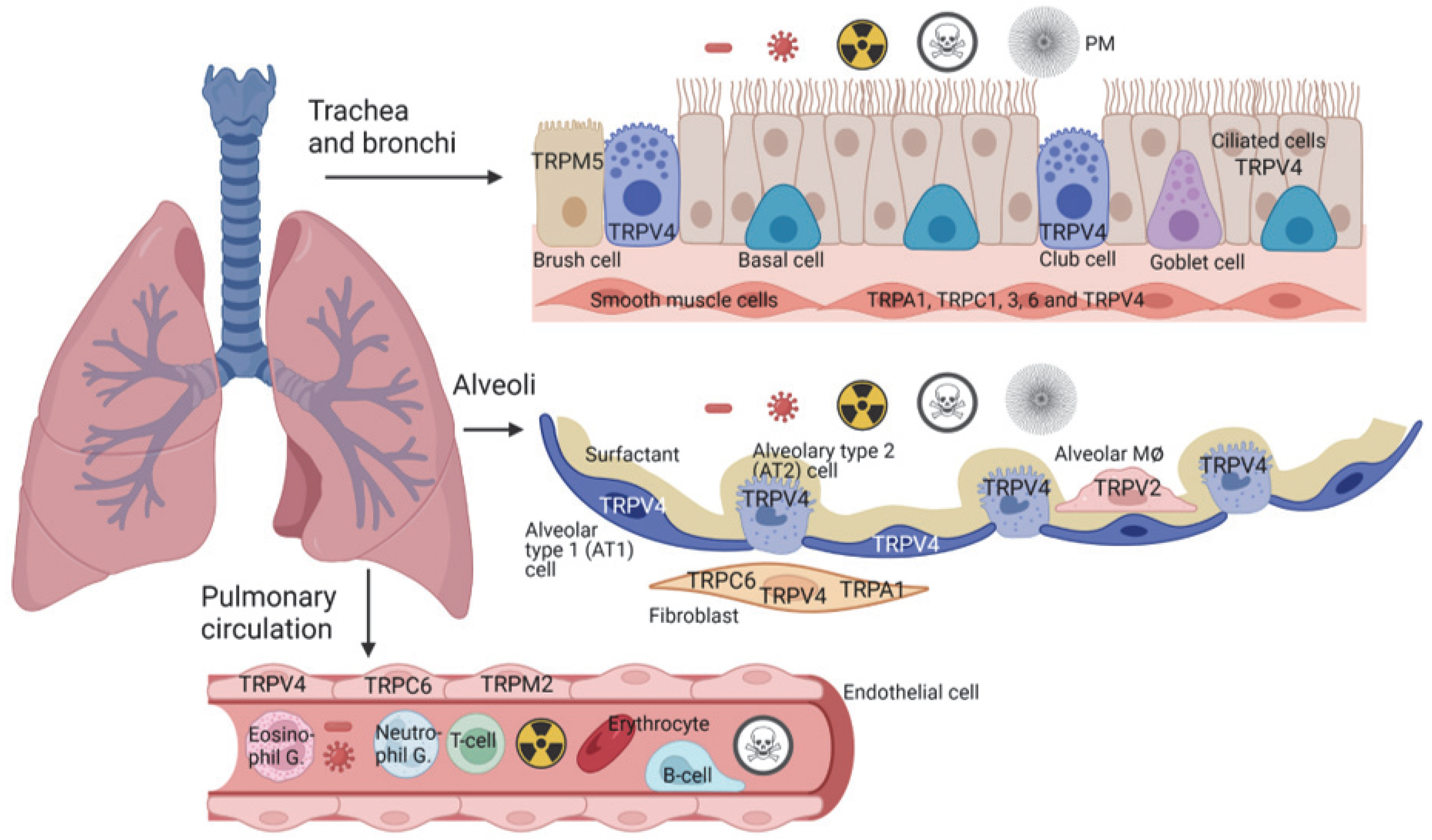
2. Cells and Their TRP Expression in the Trachea and Bronchi
2.1. TRP Channels and Cystic Fibrosis (CF)
2.2. Non-Neuronal TRP Channels in Asthma and Airway Inflammation
2.3. Lung Toxicity and TRP Channels in the Trachea and Bronchi
3. TRP Expression and Function in Alveoli
3.1. Expression of TRP Channels in Alveolar Cells and Their Role in Barrier Function
3.2. TRP Channels and Alveolar Toxicity
4. TRP Expression in Fibroblasts and Their Involvement in the Development of Lung Fibrosis
5. Roles for TRP Channels in Barrier Function of the Pulmonary Endothelium
6. TRP Channels and the Development of Pulmonary Arterial Hypertension
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L709–L725. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Nilius, B.; Szallasi, A. Transient Receptor Potential Channels as Drug Targets: From the Science of Basic Research to the Art of Medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef] [PubMed]
- Zergane, M.; Kuebler, W.; Michalick, L. Heteromeric TRP Channels in Lung Inflammation. Cells 2021, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Steinritz, D.; Gudermann, T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium 2017, 67, 123–137. [Google Scholar] [CrossRef]
- Steinritz, D.; Stenger, B.; Dietrich, A.; Gudermann, T.; Popp, T. TRPs in Tox: Involvement of Transient Receptor Potential-Channels in Chemical-Induced Organ Toxicity—A Structured Review. Cells 2018, 7, 98. [Google Scholar] [CrossRef]
- Dietrich, A. Modulators of Transient Receptor Potential (TRP) Channels as Therapeutic Options in Lung Disease. Pharmaceuticals 2019, 12, 23. [Google Scholar] [CrossRef]
- Rajan, S.; Schremmer, C.; Weber, J.; Alt, P.; Geiger, F.; Dietrich, A. Ca2+ Signaling by TRPV4 Channels in Respiratory Function and Disease. Cells 2021, 10, 822. [Google Scholar] [CrossRef]
- Hogan, B.; Tata, P.R. Cellular organization and biology of the respiratory system. Nat. Cell Biol. 2019. [Google Scholar] [CrossRef]
- Evans, M.J.; Van Winkle, L.S.; Fanucchi, M.V.; Plopper, C.G. Cellular and molecular characteristics of basal cells in airway epithelium. Exp. Lung Res. 2001, 27, 401–415. [Google Scholar] [CrossRef]
- Rokicki, W.; Rokicki, M.; Wojtacha, J.; Dżeljijli, A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Pol. J. Cardio.-Thoracic Surg. 2016, 1, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hijiya, K.; Okada, Y.; Tankawa, H. Ultrastructural Study of the Alveolar Brush Cell. QJM Int. J. Med. 1977, 26, 321–329. [Google Scholar] [CrossRef]
- Rawlins, E.L.; Hogan, B.L.M. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L231–L234. [Google Scholar] [CrossRef]
- Rose, M.C.; Voynow, J.A. Respiratory Tract Mucin Genes and Mucin Glycoproteins in Health and Disease. Physiol. Rev. 2006, 86, 245–278. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Garcia-Elias, A.; Mrkonjic, S.; Jung, C.; Pardo-Pastor, C.; Vicente, R.; Valverde, M.A. The TRPV4 channel. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 293–319. [Google Scholar] [CrossRef]
- Toft-Bertelsen, T.; MacAulay, N. TRPing to the Point of Clarity: Understanding the Function of the Complex TRPV4 Ion Channel. Cells 2021, 10, 165. [Google Scholar] [CrossRef]
- Toft-Bertelsen, T.L.; MacAulay, N. TRPing on Cell Swelling—TRPV4 Senses It. Front. Immunol. 2021, 12, 730982. [Google Scholar] [CrossRef]
- Lorenzo, I.M.; Liedtke, W.; Sanderson, M.J.; Valverde, M.A. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 12611–12616. [Google Scholar] [CrossRef]
- Alenmyr, L.; Uller, L.; Greiff, L.; Högestätt, E.D.; Zygmunt, P.M. TRPV4-Mediated Calcium Influx and Ciliary Activity in Human Native Airway Epithelial Cells. Basic Clin. Pharmacol. Toxicol. 2013, 114, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Alpizar, Y.A.; Boonen, B.; Sanchez, A.; Jung, C.; López-Requena, A.; Naert, R.; Steelant, B.; Luyts, K.; Plata, C.; De Vooght, V.; et al. TRPV4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells. Nat. Commun. 2017, 8, 1059. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.; Kung, J.W.; Bhatti, F. Structure, genetics and function of the pulmonary associated surfactant proteins A and D: The extra-pulmonary role of these C type lectins. Ann. Anat.-Anat. Anz. 2017, 211, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Haagsman, H.P.; Diemel, R.V. Surfactant-associated proteins: Functions and structural variation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 129, 91–108. [Google Scholar] [CrossRef]
- Wright, J.R. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 2005, 5, 58–68. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Wert, S.E.; Weaver, T.E. Alveolar Surfactant Homeostasis and the Pathogenesis of Pulmonary Disease. Annu. Rev. Med. 2010, 61, 105–119. [Google Scholar] [CrossRef]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Januszyk, M.; et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar] [CrossRef]
- Wiesner, D.L.; Merkhofer, R.; Ober, C.; Kujoth, G.C.; Niu, M.; Keller, N.P.; Gern, J.E.; Brockman-Schneider, R.A.; Evans, M.; Jackson, D.J.; et al. Club Cell TRPV4 Serves as a Damage Sensor Driving Lung Allergic Inflammation. Cell Host Microbe 2020, 27, 614–628.e6. [Google Scholar] [CrossRef]
- Liman, E.R. Trpm5. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 489–502. [Google Scholar] [CrossRef]
- Lin, W.; Ogura, T.; Margolskee, R.F.; Finger, T.E.; Restrepo, D. TRPM5-Expressing Solitary Chemosensory Cells Respond to Odorous Irritants. J. Neurophysiol. 2008, 99, 1451–1460. [Google Scholar] [CrossRef]
- Hansen, A.; E Finger, T. Is TrpM5 a reliable marker for chemosensory cells? Multiple types of microvillous cells in the main olfactory epithelium of mice. BMC Neurosci. 2008, 9, 115. [Google Scholar] [CrossRef]
- Kaske, S.; Krasteva, G.; König, P.; Kummer, W.; Hofmann, T.; Gudermann, T.; Chubanov, V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Ualiyeva, S.; Hallen, N.; Kanaoka, Y.; Ledderose, C.; Matsumoto, I.; Junger, W.G.; Barrett, N.A.; Bankova, L.G. Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Sci. Immunol. 2020, 5, eaax7224. [Google Scholar] [CrossRef] [PubMed]
- Bankova, L.G.; Dwyer, D.F.; Yoshimoto, E.; Ualiyeva, S.; McGinty, J.W.; Raff, H.; von Moltke, J.; Kanaoka, Y.; Austen, K.F.; Barrett, N.A. The cysteinyl leukotriene 3 receptor regulates expansion of IL-25–producing airway brush cells leading to type 2 inflammation. Sci. Immunol. 2018, 3, eaat9453. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, M.I.; Jurastow, I.; Nandigama, R.; Appenzeller, S.; Li, L.; Vogel, J.; Wiederhold, S.; Althaus, M.; Empting, M.; Altmüller, J.; et al. Tracheal brush cells release acetylcholine in response to bitter tastants for paracrine and autocrine signaling. FASEB J. 2019, 34, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Perniss, A.; Liu, S.; Boonen, B.; Keshavarz, M.; Ruppert, A.-L.; Timm, T.; Pfeil, U.; Soultanova, A.; Kusumakshi, S.; Delventhal, L.; et al. Chemosensory Cell-Derived Acetylcholine Drives Tracheal Mucociliary Clearance in Response to Virulence-Associated Formyl Peptides. Immunity 2020, 52, 683–699.e11. [Google Scholar] [CrossRef]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Investig. 2014, 124, 1393–1405. [Google Scholar] [CrossRef]
- Hollenhorst, M.I.; Nandigama, R.; Evers, S.B.; Gamayun, I.; Wadood, N.A.; Salah, A.; Pieper, M.; Wyatt, A.; Stukalov, A.; Gebhardt, A.; et al. Bitter taste signaling in tracheal epithelial brush cells elicits innate immune responses to bacterial infection. J. Clin. Investig. 2022, 132, e150951. [Google Scholar] [CrossRef]
- Hofmann, T.; Chubanov, V.; Gudermann, T.; Montell, C. TRPM5 Is a Voltage-Modulated and Ca2+-Activated Monovalent Selective Cation Channel. Curr. Biol. 2003, 13, 1153–1158. [Google Scholar] [CrossRef]
- Saunders, C.J.; Christensen, M.; Finger, T.E.; Tizzano, M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc. Natl. Acad. Sci. USA 2014, 111, 6075–6080. [Google Scholar] [CrossRef]
- Baxter, B.D.; Larson, E.D.; Merle, L.; Feinstein, P.; Polese, A.G.; Bubak, A.N.; Niemeyer, C.S.; Hassell, J.; Shepherd, D.; Ramakrishnan, V.R.; et al. Transcriptional profiling reveals potential involvement of microvillous TRPM5-expressing cells in viral infection of the olfactory epithelium. BMC Genom. 2021, 22, 224. [Google Scholar] [CrossRef] [PubMed]
- Rane, C.K.; Jackson, S.R.; Pastore, C.F.; Zhao, G.; Weiner, A.I.; Patel, N.N.; Herbert, D.R.; Cohen, N.A.; Vaughan, A.E. Development of solitary chemosensory cells in the distal lung after severe influenza injury. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L1141–L1149. [Google Scholar] [CrossRef] [PubMed]
- Sel, S.; Rost, B.R.; Yildirim, A.Ö.; Sel, B.; Kalwa, H.; Fehrenbach, H.; Renz, H.; Gudermann, T.; Dietrich, A. Loss of classical transient receptor potential 6 channel reduces allergic airway response. Clin. Exp. Allergy 2008, 38, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Kalwa, H.; Rost, B.R.; Gudermann, T. The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: Functional characterization and physiological relevance. Pflugers Arch. 2005, 451, 72–80. [Google Scholar] [CrossRef]
- Schnitzler, M.M.Y.; Gudermann, T.; Storch, U. Emerging Roles of Diacylglycerol-Sensitive TRPC4/5 Channels. Cells 2018, 7, 218. [Google Scholar] [CrossRef]
- Borowitz, D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr. Pulmonol. 2015, 50 (Suppl. S40), S24–S30. [Google Scholar] [CrossRef]
- Riordan, J.R. The Cystic Fibrosis Transmembrane Conductance Regulator. Annu. Rev. Physiol. 1993, 55, 609–630. [Google Scholar] [CrossRef]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C.; et al. Transient Receptor Potential Ankyrin 1 Channel Localized to Non-Neuronal Airway Cells Promotes Non-Neurogenic Inflammation. PLoS ONE 2012, 7, e42454. [Google Scholar] [CrossRef]
- Prandini, P.; De Logu, F.; Fusi, C.; Provezza, L.; Nassini, R.; Montagner, G.; Materazzi, S.; Munari, S.; Gilioli, E.; Bezzerri, V.; et al. Transient Receptor Potential Ankyrin 1 Channels Modulate Inflammatory Response in Respiratory Cells from Patients with Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 645–656. [Google Scholar] [CrossRef]
- Grebert, C.; Becq, F.; Vandebrouck, C. Focus on TRP channels in cystic fibrosis. Cell Calcium 2019, 81, 29–37. [Google Scholar] [CrossRef]
- Antigny, F.; Norez, C.; Dannhoffer, L.; Bertrand, J.; Raveau, D.; Corbi, P.; Jayle, C.; Becq, F.; Vandebrouck, C. Transient Receptor Potential Canonical Channel 6 Links Ca2+ Mishandling to Cystic Fibrosis Transmembrane Conductance Regulator Channel Dysfunction in Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 44, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fleig, A.; Chubanov, V. Trpm7. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 521–546. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Gudermann, T. Mapping TRPM7 Function by NS8593. Int. J. Mol. Sci. 2020, 21, 7017. [Google Scholar] [CrossRef] [PubMed]
- Huguet, F.; Calvez, M.L.; Benz, N.; Le Hir, S.; Mignen, O.; Buscaglia, P.; Horgen, F.D.; Férec, C.; Kerbiriou, M.; Trouvé, P. Function and regulation of TRPM7, as well as intracellular magnesium content, are altered in cells expressing ΔF508-CFTR and G551D-CFTR. Experientia 2016, 73, 3351–3373. [Google Scholar] [CrossRef] [PubMed]
- Arniges, M.; Vázquez, E.; Fernández-Fernández, J.M.; Valverde, M.A. Swelling-activated Ca2+ Entry via TRPV4 Channel Is Defective in Cystic Fibrosis Airway Epithelia. J. Biol. Chem. 2004, 279, 54062–54068. [Google Scholar] [CrossRef] [PubMed]
- Fecher-Trost, C.; Weissgerber, P.; Wissenbach, U. TRPV6 channels. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 359–384. [Google Scholar] [CrossRef]
- Vachel, L.; Norez, C.; Jayle, C.; Becq, F.; Vandebrouck, C. The low PLC-δ1 expression in cystic fibrosis bronchial epithelial cells induces upregulation of TRPV6 channel activity. Cell Calcium 2015, 57, 38–48. [Google Scholar] [CrossRef]
- Del Porto, P.; Cifani, N.; Guarnieri, S.; Di Domenico, E.G.; Mariggiò, M.A.; Spadaro, F.; Guglietta, S.; Anile, M.; Venuta, F.; Quattrucci, S.; et al. Dysfunctional CFTR Alters the Bactericidal Activity of Human Macrophages against Pseudomonas aeruginosa. PLoS ONE 2011, 6, e19970. [Google Scholar] [CrossRef]
- Hayes, E.; Pohl, K.; McElvaney, N.G.; Reeves, E.P. The Cystic Fibrosis Neutrophil: A Specialized Yet Potentially Defective Cell. Arch. Immunol. Ther. Exp. 2011, 59, 97–112. [Google Scholar] [CrossRef]
- Norez, C.; Vandebrouck, C.; Bertrand, J.; Noel, S.; Durieu, E.; Oumata, N.; Galons, H.; Antigny, F.; Chatelier, A.; Bois, P.; et al. Roscovitine is a proteostasis regulator that corrects the trafficking defect of F508del-CFTR by a CDK-independent mechanism. Br. J. Cereb. Blood Flow Metab. 2014, 171, 4831–4849. [Google Scholar] [CrossRef]
- Riazanski, V.; Gabdoulkhakova, A.G.; Boynton, L.S.; Eguchi, R.R.; Deriy, L.V.; Hogarth, D.K.; Loaëc, N.; Oumata, N.; Galons, H.; Brown, M.E.; et al. TRPC6 channel translocation into phagosomal membrane augments phagosomal function. Proc. Natl. Acad. Sci. USA 2015, 112, E6486–E6495. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.; Hery-Arnaud, G.; Leven, C.; Nowak, E.; Hillion, S.; Renaudineau, Y.; Durieu, I.; Chiron, R.; Prevotat, A.; Fajac, I.; et al. Safety and pharmacokinetics of Roscovitine (Seliciclib) in cystic fibrosis patients chronically infected with Pseudomonas aeruginosa, a randomized, placebo-controlled study. J. Cyst. Fibros. 2021, 21, 529–536. [Google Scholar] [CrossRef]
- Lemanske, R.F., Jr.; Busse, W.W. 6. Asthma. J. Allergy Clin. Immunol. 2003, 111, S502–S519. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2014, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.N.J. Type-2 Innate Lymphoid Cells in Asthma and Allergy. Ann. Am. Thorac. Soc. 2014, 11, S263–S270. [Google Scholar] [CrossRef]
- Bateman, E.D.; Hurd, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008, 31, 143–178. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013, 131, 636–645. [Google Scholar] [CrossRef]
- Balestrini, A.; Joseph, V.; Dourado, M.; Reese, R.M.; Shields, S.D.; Rougé, L.; Bravo, D.D.; Chernov-Rogan, T.; Austin, C.D.; Chen, H.; et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J. Exp. Med. 2021, 218, e20201637. [Google Scholar] [CrossRef]
- Yildirim, E.; Carey, M.A.; Card, J.W.; Dietrich, A.; Flake, G.P.; Zhang, Y.; Bradbury, J.A.; Rebolloso, Y.; Germolec, D.R.; Morgan, D.L.; et al. Severely blunted allergen-induced pulmonary Th2 cell response and lung hyperresponsiveness in type 1 transient receptor potential channel-deficient mice. Am. J. Physiol. Cell. Mol. Physiol. 2012, 303, L539–L549. [Google Scholar] [CrossRef]
- Dietrich, A.; Fahlbusch, M.; Gudermann, T. Classical Transient Receptor Potential 1 (TRPC1): Channel or Channel Regulator? Cells 2014, 3, 939–962. [Google Scholar] [CrossRef]
- Storch, U.; Forst, A.-L.; Philipp, M.; Gudermann, T.; Schnitzler, M.M.Y. Transient Receptor Potential Channel 1 (TRPC1) Reduces Calcium Permeability in Heteromeric Channel Complexes. J. Biol. Chem. 2012, 287, 3530–3540. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zheng, Y.-M.; Vincent, P.A.; Cai, D.; Rosenberg, P.; Wang, Y.-X. Canonical transient receptor potential 3 channels activate NF-κB to mediate allergic airway disease via PKC-α/IκB-α and calcineurin/IκB-β pathways. FASEB J. 2015, 30, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, Y.-C.; Wang, J.-F.; Wang, Q.; Gao, J.; Fu, W.-L.; Zhu, Z.-Y.; Wang, Y.-Y.; Zou, M.-J.; Wang, J.-X.; et al. Transient Receptor Potential Vanilloid 2 (TRPV2), a Potential Novel Biomarker in Childhood Asthma. J. Asthma 2013, 50, 209–214. [Google Scholar] [CrossRef]
- Li, M.; Fan, X.; Ji, L.; Fan, Y.; Xu, L. Exacerbating effects of trimellitic anhydride in ovalbumin-induced asthmatic mice and the gene and protein expressions of TRPA1, TRPV1, TRPV2 in lung tissue. Int. Immunopharmacol. 2019, 69, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Reyes-García, J.; Carbajal-García, A.; Montaño, L.M. Transient receptor potential cation channel subfamily V (TRPV) and its importance in asthma. Eur. J. Pharmacol. 2021, 915, 174692. [Google Scholar] [CrossRef] [PubMed]
- Gombedza, F.; Kondeti, V.; Al-Azzam, N.; Koppes, S.; Duah, E.; Patil, P.; Hexter, M.; Phillips, D.; Thodeti, C.K.; Paruchuri, S. Mechanosensitive transient receptor potential vanilloid 4 regulates Dermatophagoides farinae–induced airway remodeling via 2 distinct pathways modulating matrix synthesis and degradation. FASEB J. 2016, 31, 1556–1570. [Google Scholar] [CrossRef]
- Lee, K.; Byun, J.; Kim, B.; Yeon, J.; Tai, J.; Lee, S.H.; Kim, T.H. TRPV4-Mediated Epithelial Junction Disruption in Allergic Rhinitis Triggered by House Dust Mites. Am. J. Rhinol. Allergy 2020, 35, 432–440. [Google Scholar] [CrossRef]
- Bonvini, S.J.; Birrell, M.A.; Dubuis, E.; Adcock, J.J.; Wortley, M.A.; Flajolet, P.; Bradding, P.; Belvisi, M.G. Novel airway smooth muscle–mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma. Eur. Respir. J. 2020, 56, 1901458. [Google Scholar] [CrossRef]
- Basu, T.; Seyedmousavi, S.; Sugui, J.A.; Balenga, N.; Zhao, M.; Chung, K.J.K.; Biardel, S.; Laviolette, M.; Druey, K.M. Aspergillus fumigatus alkaline protease 1 (Alp1/Asp f13) in the airways correlates with asthma severity. J. Allergy Clin. Immunol. 2017, 141, 423–425.e7. [Google Scholar] [CrossRef]
- Schiffers, C.; Hristova, M.; Habibovic, A.; Dustin, C.M.; Danyal, K.; Reynaert, N.L.; Wouters, E.F.M.; Van Der Vliet, A. The Transient Receptor Potential Channel Vanilloid 1 Is Critical in Innate Airway Epithelial Responses to Protease Allergens. Am. J. Respir. Cell Mol. Biol. 2020, 63, 198–208. [Google Scholar] [CrossRef]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front. Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Lee, L.-Y. TRP channels in airway sensory nerves. Neurosci. Lett. 2021, 748, 135719. [Google Scholar] [CrossRef] [PubMed]
- Bessac, B.F.; Sivula, M.; von Hehn, C.A.; Escalera, J.; Cohn, L.; Jordt, S.-E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Investig. 2008, 118, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Achanta, S.; Jordt, S. Transient receptor potential channels in pulmonary chemical injuries and as countermeasure targets. Ann. N. Y. Acad. Sci. 2020, 1480, 73–103. [Google Scholar] [CrossRef]
- Kannler, M.; Lüling, R.; Yildirim, A.Ö.; Gudermann, T.; Steinritz, D.; Dietrich, A. TRPA1 channels: Expression in non-neuronal murine lung tissues and dispensability for hyperoxia-induced alveolar epithelial hyperplasia. Pflugers Arch. Eur. J. Physiol. 2018, 470, 1231–1241. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Hogestatt, E.D. Trpa1. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 583–630. [Google Scholar] [CrossRef]
- Cai, J.; Bhatnagar, A.; Pierce, J.W.M. Protein Modification by Acrolein: Formation and Stability of Cysteine Adducts. Chem. Res. Toxicol. 2009, 22, 708–716. [Google Scholar] [CrossRef]
- Conklin, D.J.; Haberzettl, P.; Jagatheesan, G.; Kong, M.; Hoyle, G.W. Role of TRPA1 in acute cardiopulmonary toxicity of inhaled acrolein. Toxicol. Appl. Pharmacol. 2016, 324, 61–72. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Xu, M.; Zhang, H.; Chen, Y.; Chung, K.F.; Adcock, I.M.; Li, F. Roles of TRPA1 and TRPV1 in cigarette smoke-induced airway epithelial cell injury model. Free Radic. Biol. Med. 2019, 134, 229–238. [Google Scholar] [CrossRef]
- Balakrishna, S.; Song, W.; Achanta, S.; Doran, S.F.; Liu, B.; Kaelberer, M.M.; Yu, Z.; Sui, A.; Cheung, M.; Leishman, E.; et al. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2014, 307, L158–L172. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Michalick, L.; Tang, C.; Tabuchi, A.; Goldenberg, N.; Dan, Q.; Awwad, K.; Wang, L.; Erfinanda, L.; Nouailles, G.; et al. Role of Transient Receptor Potential Vanilloid 4 in Neutrophil Activation and Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2016, 54, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Büch, T.; Schäfer, E.; Steinritz, D.; Dietrich, A.; Gudermann, T. Chemosensory TRP Channels in the Respiratory Tract: Role in Toxic Lung Injury and Potential as “Sweet Spots” for Targeted Therapies. Rev. Physiol. Biochem. Pharmacol. 2013, 165, 31–65. [Google Scholar] [CrossRef] [PubMed]
- Milici, A.; Talavera, K. TRP Channels as Cellular Targets of Particulate Matter. Int. J. Mol. Sci. 2021, 22, 2783. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.; Wang, M.; Zhang, H.; Chen, Y.; Adcock, I.M.; Chung, K.F.; Mo, J.; Zhang, Y.; Li, F. TRPV1 and TRPA1 in Lung Inflammation and Airway Hyperresponsiveness Induced by Fine Particulate Matter (PM2.5). Oxidative Med. Cell. Longev. 2019, 2019, 7450151. [Google Scholar] [CrossRef]
- Sanchez, A.; Alvarez, J.L.; Demydenko, K.; Jung, C.; Alpizar, Y.A.; Alvarez-Collazo, J.; Cokic, S.M.; Valverde, M.A.; Hoet, P.H.; Talavera, K. Silica nanoparticles inhibit the cation channel TRPV4 in airway epithelial cells. Part. Fibre Toxicol. 2017, 14, 43. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Yazdimamaghani, M.; Reilly, C.A.; Ghandehari, H.; Ghandehari, H. Transient Receptor Potential Ion Channel–Dependent Toxicity of Silica Nanoparticles and Poly(amido amine) Dendrimers. J. Pharmacol. Exp. Ther. 2018, 370, 751–760. [Google Scholar] [CrossRef]
- Dubes, V.; Parpaite, T.; Ducret, T.; Quignard, J.-F.; Mornet, S.; Reinhardt, N.; Baudrimont, I.; Dubois, M.; Freund-Michel, V.; Marthan, R.; et al. Calcium signalling induced by in vitro exposure to silicium dioxide nanoparticles in rat pulmonary artery smooth muscle cells. Toxicology 2017, 375, 37–47. [Google Scholar] [CrossRef]
- Chen, E.Y.T.; Garnica, M.; Wang, Y.-C.; Chen, C.-S.; Chin, W.-C. Mucin Secretion Induced by Titanium Dioxide Nanoparticles. PLoS ONE 2011, 6, e16198. [Google Scholar] [CrossRef]
- Kim, B.-G.; Park, M.-K.; Lee, P.-H.; Lee, S.-H.; Hong, J.; Aung, M.M.M.; Moe, K.T.; Han, N.Y.; Jang, A.-S. Effects of nanoparticles on neuroinflammation in a mouse model of asthma. Respir. Physiol. Neurobiol. 2019, 271, 103292. [Google Scholar] [CrossRef]
- Li, J.; Kanju, P.; Patterson, M.; Chew, W.L.; Cho, S.-H.; Gilmour, I.; Oliver, T.; Yasuda, R.; Ghio, A.; Simon, S.A.; et al. TRPV4-Mediated Calcium Influx into Human Bronchial Epithelia upon Exposure to Diesel Exhaust Particles. Environ. Health Perspect. 2011, 119, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Gulsvik, A.; Bakke, P.; Ghatta, S.; Anderson, W.; Lomas, D.A.; Silverman, E.K.; Pillai, S.G. ICGN Investigators Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum. Mol. Genet. 2009, 18, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and Lung Cancer: Roles of Reactive Oxygen/Nitrogen Species. J. Toxicol. Environ. Health Part B 2008, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Emmendoerffer, A.; Hecht, M.; Boeker, T.; Mueller, M.; Heinrich, U. Role of inflammation in chemical-induced lung cancer. Toxicol. Lett. 2000, 112–113, 185–191. [Google Scholar] [CrossRef]
- Cook, J.A.; Gius, D.; Wink, D.A.; Krishna, M.C.; Russo, A.; Mitchell, J.B. Oxidative stress, redox, and the tumor microenvironment. Semin. Radiat. Oncol. 2004, 14, 259–266. [Google Scholar] [CrossRef]
- Yoo, J.A.; Yu, E.; Park, S.-H.; Oh, S.W.; Kwon, K.; Park, S.J.; Kim, H.; Yang, S.; Park, J.Y.; Cho, J.Y.; et al. Blue Light Irradiation Induces Human Keratinocyte Cell Damage via Transient Receptor Potential Vanilloid 1 (TRPV1) Regulation. Oxidative Med. Cell. Longev. 2020, 2020, 8871745. [Google Scholar] [CrossRef]
- Camponogara, C.; Brum, E.S.; Pegoraro, N.S.; Brusco, I.; Brucker, N.; Oliveira, S.M. Diosmetin, a novel transient receptor potential vanilloid 1 antagonist, alleviates the UVB radiation-induced skin inflammation in mice. Inflammopharmacology 2021, 29, 879–895. [Google Scholar] [CrossRef]
- Gao, H.; Chen, X.; Du, X.; Guan, B.; Liu, Y.; Zhang, H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium 2011, 50, 559–568. [Google Scholar] [CrossRef]
- Tajeddine, N.; Gailly, P. TRPC1 Protein Channel Is Major Regulator of Epidermal Growth Factor Receptor Signaling. J. Biol. Chem. 2012, 287, 16146–16157. [Google Scholar] [CrossRef]
- Jiang, H.-N.; Zeng, B.; Zhang, Y.; Daskoulidou, N.; Fan, H.; Qu, J.-M.; Xu, S.-Z. Involvement of TRPC Channels in Lung Cancer Cell Differentiation and the Correlation Analysis in Human Non-Small Cell Lung Cancer. PLoS ONE 2013, 8, e67637. [Google Scholar] [CrossRef]
- Ke, C.; Long, S. Dysregulated transient receptor potential channel 1 expression and its correlation with clinical features and survival profile in surgical non-small-cell lung cancer patients. J. Clin. Lab. Anal. 2022, 36, e24229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Fan, K.; Li, B.; Li, H.; Qi, H.; Guo, J.; Cao, Y.; Sun, H. Overexpression of TRPV3 Correlates with Tumor Progression in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2016, 17, 437. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.A.; Stohr, S.; Meister, M.; Aigner, A.; Gudermann, T.; Buech, T.R. Stimulation of the chemosensory TRPA1 cation channel by volatile toxic substances promotes cell survival of small cell lung cancer cells. Biochem. Pharmacol. 2013, 85, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, C.; Xiang, Q.; Fan, S.; Xiao, T.; Chen, Y.; Zheng, D. Transient Receptor Potential Cation Channel Subfamily V Member 1 Expression Promotes Chemoresistance in Non-Small-Cell Lung Cancer. Front. Oncol. 2022, 12, 773654. [Google Scholar] [CrossRef]
- Wang, S.; Hubmayr, R.D. Type I Alveolar Epithelial Phenotype in Primary Culture. Am. J. Respir. Cell Mol. Biol. 2011, 44, 692–699. [Google Scholar] [CrossRef]
- Fehrenbach, H. Alveolar epithelial type II cell: Defender of the alveolus revisited. Respir. Res. 2001, 2, 33–46. [Google Scholar] [CrossRef]
- Halliday, H.L. Surfactants: Past, present and future. J. Perinatol. 2008, 28, S47–S56. [Google Scholar] [CrossRef]
- Weber, J.; Rajan, S.; Schremmer, C.; Chao, Y.-K.; Krasteva-Christ, G.; Kannler, M.; Yildirim, A.; Brosien, M.; Schredelseker, J.; Weissmann, N.; et al. TRPV4 channels are essential for alveolar epithelial barrier function as protection from lung edema. JCI Insight 2020, 5, e134464. [Google Scholar] [CrossRef]
- Akazawa, Y.; Yuki, T.; Yoshida, H.; Sugiyama, Y.; Inoue, S. Activation of TRPV4 Strengthens the Tight-Junction Barrier in Human Epidermal Keratinocytes. Ski. Pharmacol. Physiol. 2012, 26, 15–21. [Google Scholar] [CrossRef]
- Janssen, D.A.; Jansen, C.J.; Hafmans, T.G.; Verhaegh, G.W.; Hoenderop, J.G.; Heesakkers, J.P.; Schalken, J.A. TRPV4 channels in the human urogenital tract play a role in cell junction formation and epithelial barrier. Acta Physiol. 2016, 218, 38–48. [Google Scholar] [CrossRef]
- Martínez-Rendón, J.; Sánchez-Guzmán, E.; Rueda, A.; González, J.; Gulias-Cañizo, R.; Aquino-Jarquín, G.; Castro-Muñozledo, F.; García-Villegas, R. TRPV4 Regulates Tight Junctions and Affects Differentiation in a Cell Culture Model of the Corneal Epithelium. J. Cell. Physiol. 2016, 232, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.T. Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart 2014, 101, 253–256. [Google Scholar] [CrossRef]
- Kojima, I.; Nagasawa, M. Trpv2. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 247–272. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K. Physiological significance of TRPV2 as a mechanosensor, thermosensor and lipid sensor. J. Physiol. Sci. 2016, 66, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, H.; Ueno, M.; Maeno, T.; Yamaguchi, K.; Hara, K.; Sunaga, H.; Matsui, H.; Nagasawa, M.; Kojima, I.; Iwata, Y.; et al. Reduced transient receptor potential vanilloid 2 expression in alveolar macrophages causes COPD in mice through impaired phagocytic activity. BMC Pulm. Med. 2019, 19, 70. [Google Scholar] [CrossRef]
- Spix, B.; Butz, E.S.; Chen, C.-C.; Rosato, A.S.; Tang, R.; Jeridi, A.; Kudrina, V.; Plesch, E.; Wartenberg, P.; Arlt, E.; et al. Lung emphysema and impaired macrophage elastase clearance in mucolipin 3 deficient mice. Nat. Commun. 2022, 13, 318. [Google Scholar] [CrossRef]
- Spix, B.; Jeridi, A.; Ansari, M.; Yildirim, A.; Schiller, H.B.; Grimm, C. Endolysosomal Cation Channels and Lung Disease. Cells 2022, 11, 304. [Google Scholar] [CrossRef]
- Selvaggio, A.S.; Noble, P.W. Pirfenidone Initiates a New Era in the Treatment of Idiopathic Pulmonary Fibrosis. Annu. Rev. Med. 2016, 67, 487–495. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017, 3, 17074. [Google Scholar] [CrossRef]
- Yu, M.; Huang, C.; Huang, Y.; Wu, X.; Li, X.; Li, J. Inhibition of TRPM7 channels prevents proliferation and differentiation of human lung fibroblasts. Agents Actions 2013, 62, 961–970. [Google Scholar] [CrossRef]
- Rahaman, S.O.; Grove, L.M.; Paruchuri, S.; Southern, B.D.; Abraham, S.; Niese, K.A.; Scheraga, R.G.; Ghosh, S.; Thodeti, C.K.; Zhang, D.X.; et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J. Clin. Investig. 2014, 124, 5225–5238. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Kugler, M.C.; Wolters, P.J.; Robillard, L.; Galvez, M.G.; Brumwell, A.N.; Sheppard, D.; Chapman, H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. USA 2006, 103, 13180–13185. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Fiedler, S.; Vierkotten, S.; Weber, J.; Klee, S.; Jia, J.; Zwickenpflug, W.; Flockerzi, V.; Storch, U.; Yildirim, A.Ö.; et al. Classical transient receptor potential 6 (TRPC6) channels support myofibroblast differentiation and development of experimental pulmonary fibrosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Burr, A.R.; Davis, G.F.; Birnbaumer, L.; Molkentin, J.D. A TRPC6-Dependent Pathway for Myofibroblast Transdifferentiation and Wound Healing In Vivo. Dev. Cell 2012, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.M.G.; Ueda, T.; Kanemitsu, Y.; Takeda, N.; Fukumitsu, K.; Fukuda, S.; Uemura, T.; Tajiri, T.; Ohkubo, H.; Maeno, K.; et al. AITC inhibits fibroblast-myofibroblast transition via TRPA1-independent MAPK and NRF2/HO-1 pathways and reverses corticosteroids insensitivity in human lung fibroblasts. Respir. Res. 2021, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Virk, H.S.; Biddle, M.S.; Smallwood, D.T.; Weston, C.A.; Castells, E.; Bowman, V.W.; McCarthy, J.; Amrani, Y.; Duffy, S.M.; Bradding, P.; et al. TGFβ1 induces resistance of human lung myofibroblasts to cell death via down-regulation of TRPA1 channels. J. Cereb. Blood Flow Metab. 2021, 178, 2948–2962. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.; Barton, M.; Jacob, S.; Jalaludin, B. A model for decision making for the use of radiotherapy in lung cancer. Lancet Oncol. 2003, 4, 120–128. [Google Scholar] [CrossRef]
- Keffer, S.; Guy, C.L.; Weiss, E. Fatal Radiation Pneumonitis: Literature Review and Case Series. Adv. Radiat. Oncol. 2019, 5, 238–249. [Google Scholar] [CrossRef]
- Käsmann, L.; Dietrich, A.; Staab-Weijnitz, C.A.; Manapov, F.; Behr, J.; Rimner, A.; Jeremic, B.; Senan, S.; De Ruysscher, D.; Lauber, K.; et al. Radiation-induced lung toxicity—Cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat. Oncol. 2020, 15, 214. [Google Scholar] [CrossRef]
- Faouzi, M.; Penner, R. Trpm2. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 222, pp. 403–426. [Google Scholar] [CrossRef]
- Li, J.; Sun, L.-Q. Molecular Mechanisms and Treatment of Radiation-Induced Lung Fibrosis. Curr. Drug Targets 2013, 14, 1347–1356. [Google Scholar] [CrossRef]
- Liu, X.; Cotrim, A.P.; Teos, L.Y.; Zheng, C.; Swaim, W.D.; Mitchell, J.B.; Mori, Y.; Ambudkar, I.S. Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat. Commun. 2013, 4, 1515. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. Acute Pulmonary Edema. N. Engl. J. Med. 2005, 353, 2788–2796. [Google Scholar] [CrossRef]
- Simmons, S.; Erfinanda, L.; Bartz, C.; Kuebler, W.M. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J. Physiol. 2018, 597, 997–1021. [Google Scholar] [CrossRef]
- Wu, S.; Jian, M.-Y.; Xu, Y.-C.; Zhou, C.; Al-Mehdi, A.-B.; Liedtke, W.; Shin, H.-S.; Townsley, M.I. Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L650–L657. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Grace, M.S.; Gondin, A.B.; Retamal, J.S.; Dill, L.; Darby, W.; Bunnett, N.W.; Abogadie, F.C.; Carbone, S.E.; Tigani, T.; et al. The transient receptor potential vanilloid 4 (TRPV4) ion channel mediates protease activated receptor 1 (PAR1)-induced vascular hyperpermeability. Lab. Investig. 2020, 100, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Thorneloe, K.S.; Cheung, M.; Bao, W.; Alsaid, H.; Lenhard, S.; Jian, M.-Y.; Costell, M.; Maniscalco-Hauk, K.; Krawiec, J.A.; Olzinski, A.; et al. An Orally Active TRPV4 Channel Blocker Prevents and Resolves Pulmonary Edema Induced by Heart Failure. Sci. Transl. Med. 2012, 4, 159ra148. [Google Scholar] [CrossRef]
- Kuebler, W.M.; Jordt, S.-E.; Liedtke, W.B. Urgent reconsideration of lung edema as a preventable outcome in COVID-19: Inhibition of TRPV4 represents a promising and feasible approach. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L1239–L1243. [Google Scholar] [CrossRef]
- Hecquet, C.M.; Ahmmed, G.U.; Vogel, S.M.; Malik, A.B. Role of TRPM2 Channel in Mediating H2O2-Induced Ca2+ Entry and Endothelial Hyperpermeability. Circ. Res. 2008, 102, 347–355. [Google Scholar] [CrossRef]
- Dietrich, A.; Gudermann, T. Another TRP to endothelial dysfunction: TRPM2 and endothelial permeability. Circ. Res. 2008, 102, 275–277. [Google Scholar] [CrossRef]
- Zielińska, W.; Zabrzyński, J.; Gagat, M.; Grzanka, A. The Role of TRPM2 in Endothelial Function and Dysfunction. Int. J. Mol. Sci. 2021, 22, 7635. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Nepal, S.; Tsukasaki, Y.; Hecquet, C.M.; Soni, D.; Rehman, J.; Tiruppathi, C.; Malik, A.B. Neutrophil Activation of Endothelial Cell-Expressed TRPM2 Mediates Transendothelial Neutrophil Migration and Vascular Injury. Circ. Res. 2017, 121, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Tauseef, M.; Knezevic, N.N.; Chava, K.R.; Smith, M.; Sukriti, S.; Gianaris, N.; Obukhov, A.G.; Vogel, S.M.; Schraufnagel, D.; Dietrich, A.; et al. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 2012, 209, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Göggel, R.; Engel, S. Mechanisms of platelet-activating factor (PAF)-mediated responses in the lung. Pharmacol. Rep. 2005, 57, 206–221. [Google Scholar]
- Samapati, R.; Yang, Y.; Yin, J.; Stoerger, C.; Arenz, C.; Dietrich, A.; Gudermann, T.; Adam, D.; Wu, S.; Freichel, M.; et al. Lung Endothelial Ca2+ and Permeability Response to Platelet-Activating Factor Is Mediated by Acid Sphingomyelinase and Transient Receptor Potential Classical 6. Am. J. Respir. Crit. Care Med. 2012, 185, 160–170. [Google Scholar] [CrossRef]
- Jiang, T.; Samapati, R.; Klassen, S.; Lei, D.; Erfinanda, L.; Jankowski, V.; Simmons, S.; Yin, J.; Arenz, C.; Dietrich, A.; et al. Stimulation of the EP3 receptor causes lung edema by activation of TRPC6 in pulmonary endothelial cells. Eur. Respir. J. 2022, 60, 2102635. [Google Scholar] [CrossRef]
- de Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia–Reperfusion–induced Lung Injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef]
- Schremmer, C.; Steinritz, D.; Gudermann, T.; Beech, D.J.; Dietrich, A. An ex vivo perfused ventilated murine lung model suggests lack of acute pulmonary toxicity of the potential novel anticancer agent (−)-englerin A. Arch. Toxicol. 2022, 96, 1055–1063. [Google Scholar] [CrossRef]
- Weissmann, N.; Sydykov, A.; Kalwa, H.; Storch, U.; Fuchs, B.; Mederos y Schnitzler, M.; Brandes, R.P.; Grimminger, F.; Meissner, M.; Freichel, M.; et al. Activation of TRPC6 channels is essential for lung ischaemia–reperfusion induced oedema in mice. Nat. Commun. 2012, 3, 649. [Google Scholar] [CrossRef]
- Häfner, S.; Burg, F.; Kannler, M.; Urban, N.; Mayer, P.; Dietrich, A.; Trauner, D.; Broichhagen, J.; Schaefer, M. A (+)-Larixol Congener with High Affinity and Subtype Selectivity toward TRPC6. ChemMedChem 2018, 13, 1028–1035. [Google Scholar] [CrossRef]
- Santos-Gomes, J.; Le Ribeuz, H.; Brás-Silva, C.; Antigny, F.; Adão, R. Role of Ion Channel Remodeling in Endothelial Dysfunction Induced by Pulmonary Arterial Hypertension. Biomolecules 2022, 12, 484. [Google Scholar] [CrossRef]
- Negri, S.; Faris, P.; Berra-Romani, R.; Guerra, G.; Moccia, F. Endothelial transient receptor potential channels and vascular remodeling: Extracellular Ca2+ entry for angiogenesis, arteriogenesis and vasculogenesis. Front. Physiol. 2020, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Malczyk, M.; Erb, A.; Veith, C.; Ghofrani, A.; Schermuly, R.; Gudermann, T.; Dietrich, A.; Weissmann, N.; Sydykov, A. The Role of Transient Receptor Potential Channel 6 Channels in the Pulmonary Vasculature. Front. Immunol. 2017, 8, 707. [Google Scholar] [CrossRef]
- Jain, P.P.; Lai, N.; Xiong, M.; Chen, J.; Babicheva, A.; Zhao, T.; Parmisano, S.; Zhao, M.; Paquin, C.; Matti, M.; et al. TRPC6, a therapeutic target for pulmonary hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2021, 321, L1161–L1182. [Google Scholar] [CrossRef]
- Malczyk, M.; Veith, C.; Fuchs, B.; Hofmann, K.; Storch, U.; Schermuly, R.T.; Witzenrath, M.; Ahlbrecht, K.; Fecher-Trost, C.; Flockerzi, V.; et al. Classical Transient Receptor Potential Channel 1 in Hypoxia-induced Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2013, 188, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fantozzi, I.; Remillard, C.V.; Landsberg, J.W.; Kunichika, N.; Platoshyn, O.; Tigno, D.D.; Thistlethwaite, P.A.; Rubin, L.J.; Yuan, J.X.-J. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA 2004, 101, 13861–13866. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Francis, C.M.; Xu, N.; Stevens, T. The role of endothelial leak in pulmonary hypertension (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045894018798569. [Google Scholar] [CrossRef]
- Song, J.-L.; Zheng, S.-Y.; He, R.-L.; Gui, L.-X.; Lin, M.-J.; Sham, J.S. Serotonin and chronic hypoxic pulmonary hypertension activate a NADPH oxidase 4 and TRPM2 dependent pathway for pulmonary arterial smooth muscle cell proliferation and migration. Vasc. Pharmacol. 2021, 138, 106860. [Google Scholar] [CrossRef]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Yun, X.; Kliment, C.; Huetsch, J.C.; Damarla, M.; Shimoda, L.A. Reactive oxygen species induced Ca2+ influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L893–L907. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, L.; Huang, Y.; Ding, X.; Wang, L. The potential role of TRPV1 in pulmonary hypertension: Angel or demon? Channels 2019, 13, 235–246. [Google Scholar] [CrossRef]
- Lodola, F.; Rosti, V.; Tullii, G.; Desii, A.; Tapella, L.; Catarsi, P.; Lim, D.; Moccia, F.; Antognazza, M.R. Conjugated polymers optically regulate the fate of endothelial colony-forming cells. Sci. Adv. 2019, 5, eaav4620. [Google Scholar] [CrossRef] [PubMed]
- Bonezzi, C.; Costantini, A.; Cruccu, G.; Fornasari, D.M.; Guardamagna, V.; Palmieri, V.; Polati, E.; Zini, P.; Dickenson, A.H. Capsaicin 8% dermal patch in clinical practice: An expert opinion. Expert Opin. Pharmacother. 2020, 21, 1377–1387. [Google Scholar] [CrossRef]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2021, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Santana, L.F.; Lederer, W.J. The physiological sensor channels TRP and piezo: Nobel Prize in Physiology or Medicine 2021. Physiol. Rev. 2022, 102, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | TRP Channel | nTPM |
|---|---|---|
| AT2 cells | TRPC6 | 2.6 |
| TRPM2 | 0.5 | |
| B cells | TRPM2 | 2.0 |
| TRPV2 | 11.1 | |
| TRPV4 | 0.7 | |
| Basal cells | TRPV4 | 17.1 |
| Ciliated cells | TRPC6 | 0.8 |
| TRPV2 | 0.8 | |
| TRPV4 | 28.2 | |
| Club Cells | TRPC6 | 10.5 |
| TRPV2 | 0.6 | |
| TRPV4 | 12.0 | |
| Endothelial cells | TRPC6 | 1.6 |
| TRPM2 | 2.7 | |
| TRPV2 | 12.3 | |
| TRPV4 | 1.4 | |
| Fibroblasts | TRPA1 | 8.1 |
| TRPC6 | 3.1 | |
| TRPM2 | 0.5 | |
| TRPV2 | 11.0 | |
| TRPV4 | 1.2 | |
| Macrophages | TRPC6 | 1.8 |
| TRPM2 | 16.7 | |
| TRPV2 | 28.7 | |
| TRPV4 | 2.6 | |
| Smooth muscle cells | TRPA1 | 0.8 |
| TRPC6 | 20.2 | |
| TRPM2 | 0.4 | |
| TRPV2 | 23.6 | |
| TRPV4 | 1.1 | |
| T cells | TRPC6 | 0.1 |
| TRPM2 | 4.2 | |
| TRPV2 | 26.8 | |
| TRPV4 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, I.; Alt, P.; Rajan, S.; Schaller, L.; Geiger, F.; Dietrich, A. Transient Receptor Potential (TRP) Channels in Airway Toxicity and Disease: An Update. Cells 2022, 11, 2907. https://doi.org/10.3390/cells11182907
Müller I, Alt P, Rajan S, Schaller L, Geiger F, Dietrich A. Transient Receptor Potential (TRP) Channels in Airway Toxicity and Disease: An Update. Cells. 2022; 11(18):2907. https://doi.org/10.3390/cells11182907
Chicago/Turabian StyleMüller, Isabel, Philipp Alt, Suhasini Rajan, Lena Schaller, Fabienne Geiger, and Alexander Dietrich. 2022. "Transient Receptor Potential (TRP) Channels in Airway Toxicity and Disease: An Update" Cells 11, no. 18: 2907. https://doi.org/10.3390/cells11182907
APA StyleMüller, I., Alt, P., Rajan, S., Schaller, L., Geiger, F., & Dietrich, A. (2022). Transient Receptor Potential (TRP) Channels in Airway Toxicity and Disease: An Update. Cells, 11(18), 2907. https://doi.org/10.3390/cells11182907







