The Role of Neutrophils in Oncolytic Orf Virus-Mediated Cancer Immunotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Biohazard Certification
2.2. Mice
2.3. Virus
2.4. In Vivo Tumor Model and Oncolytic Virotherapy
2.5. Tissue Processing
2.6. Analysis via Flow Cytometry
2.7. Antibody-Mediated Depletion Studies
2.8. Virus Growth Curve
2.9. Neutrophil Cytotoxicity Assays
2.10. Statistical Analyses
3. Results
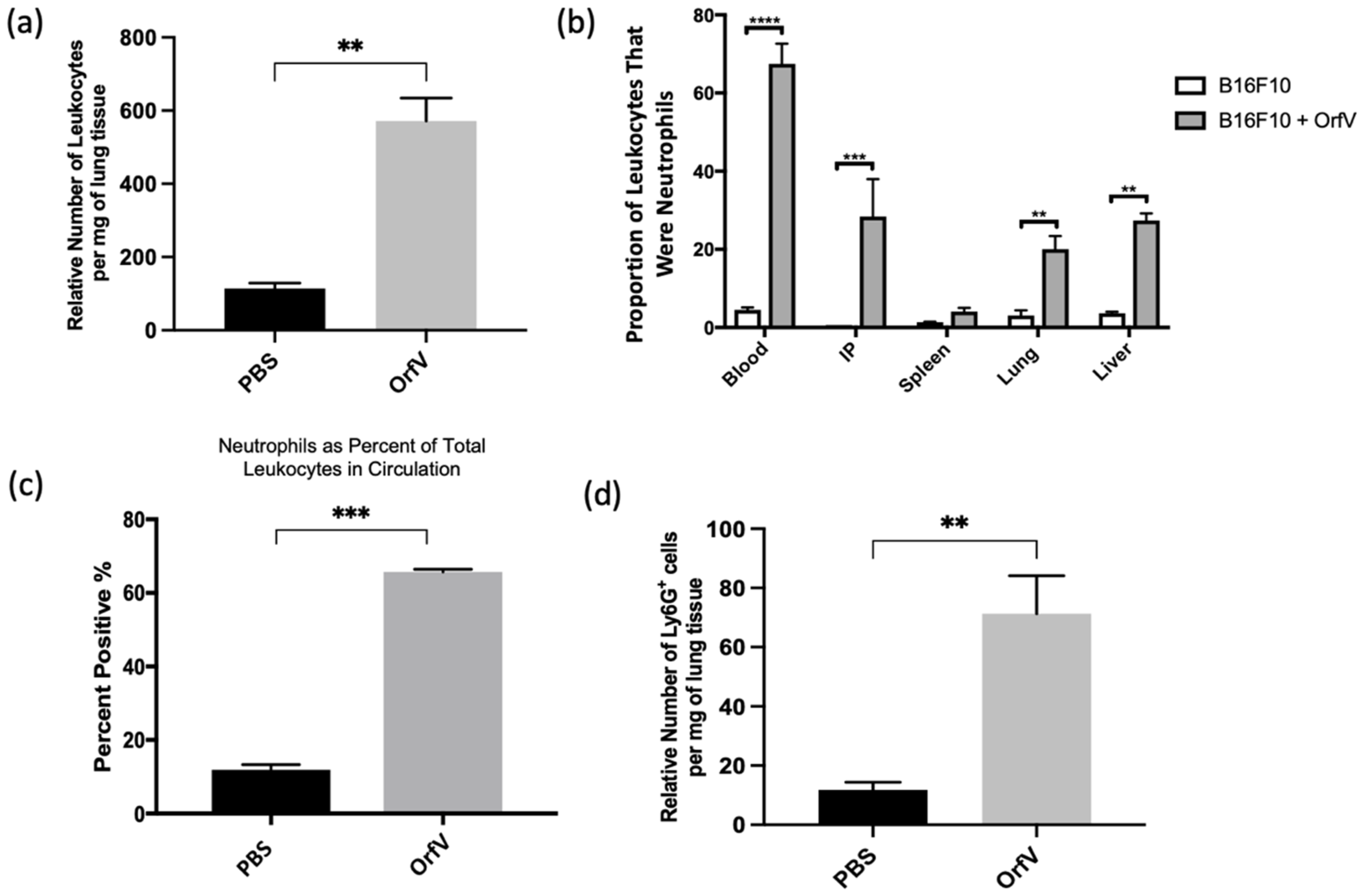
3.1. Intravenous Delivery of OrfV Recruits Leukocytes to the Tumor Microenvironment and Activates a Systemic Neutrophil Response in Tumor-Bearing Mice
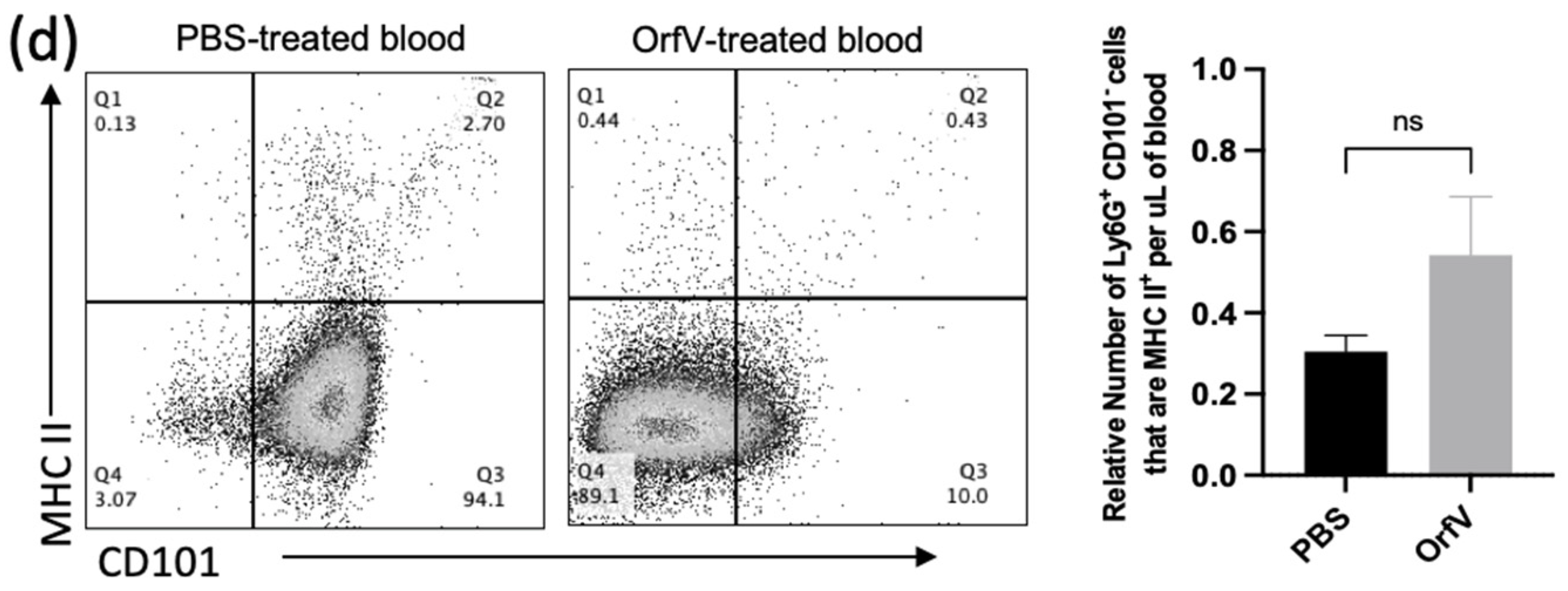
3.2. Phenotypically Immature Neutrophils with Increased Expression of Migratory and Activation Molecules Respond to Infection with OrfV to Colonize the Tumor Microenvironment of the Lungs
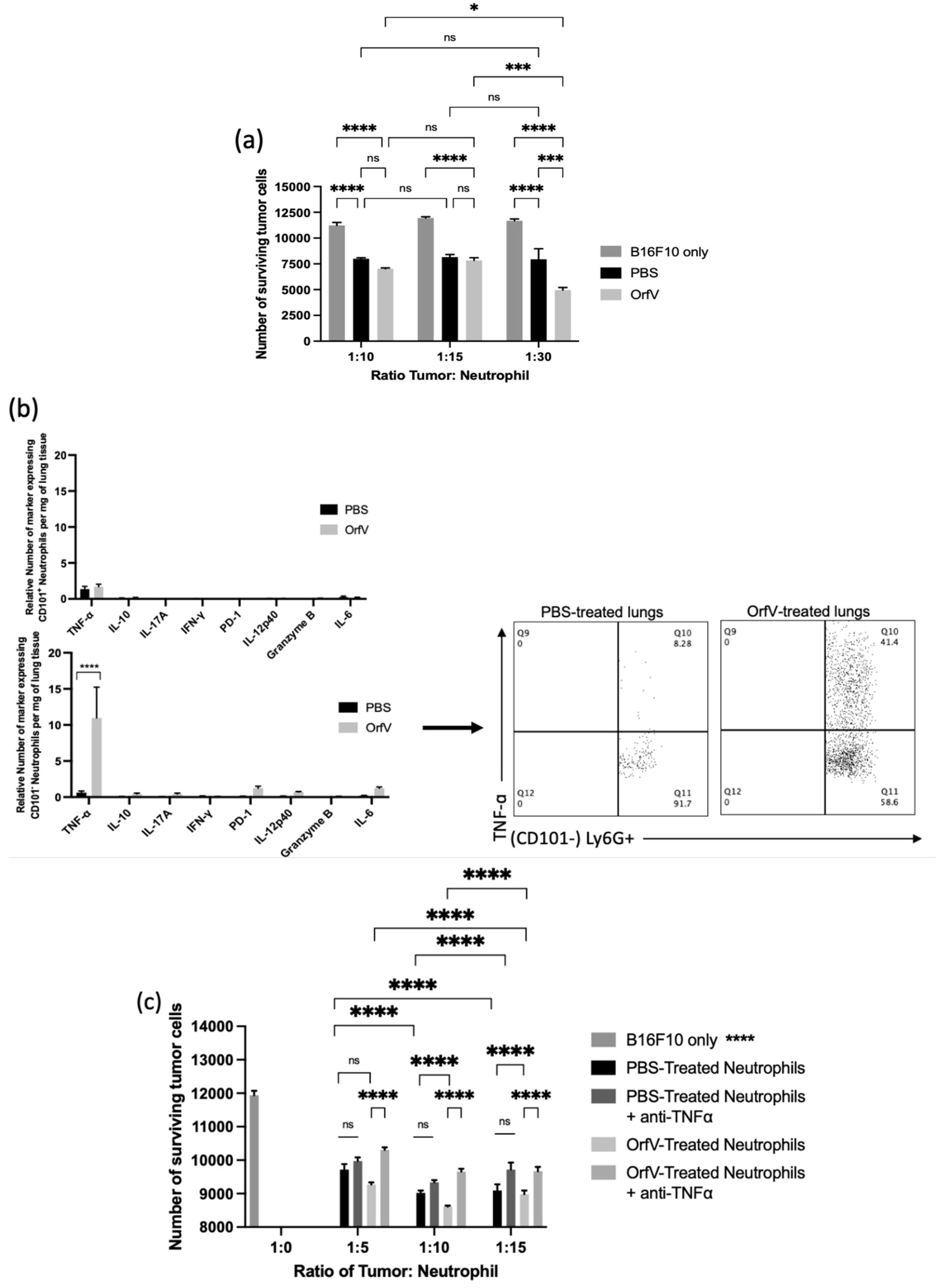
3.3. Neutrophils Activated by OrfV Exhibited Enhanced TNF-ɑ-Mediated Cytotoxicity against Target Tumor Cells
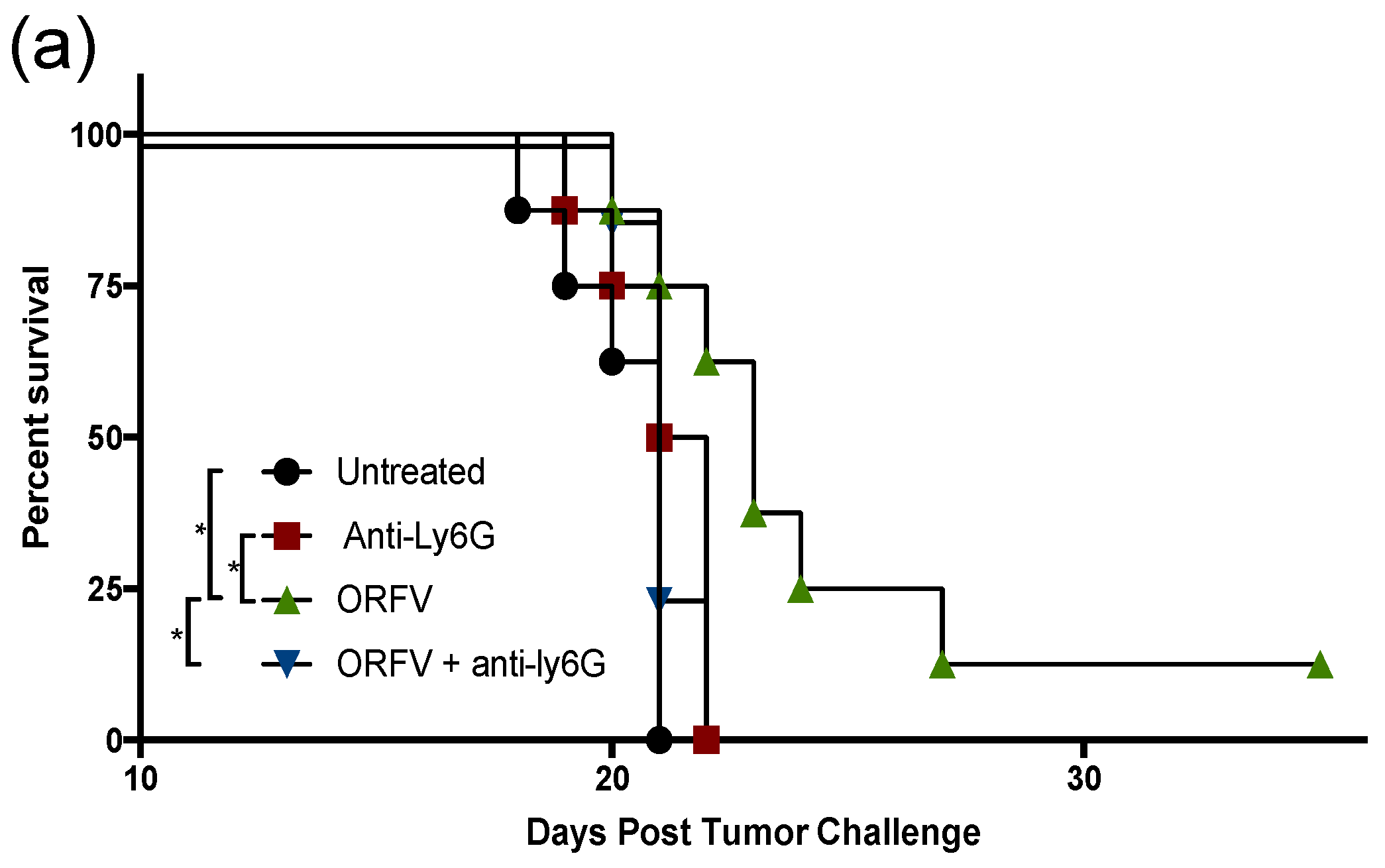
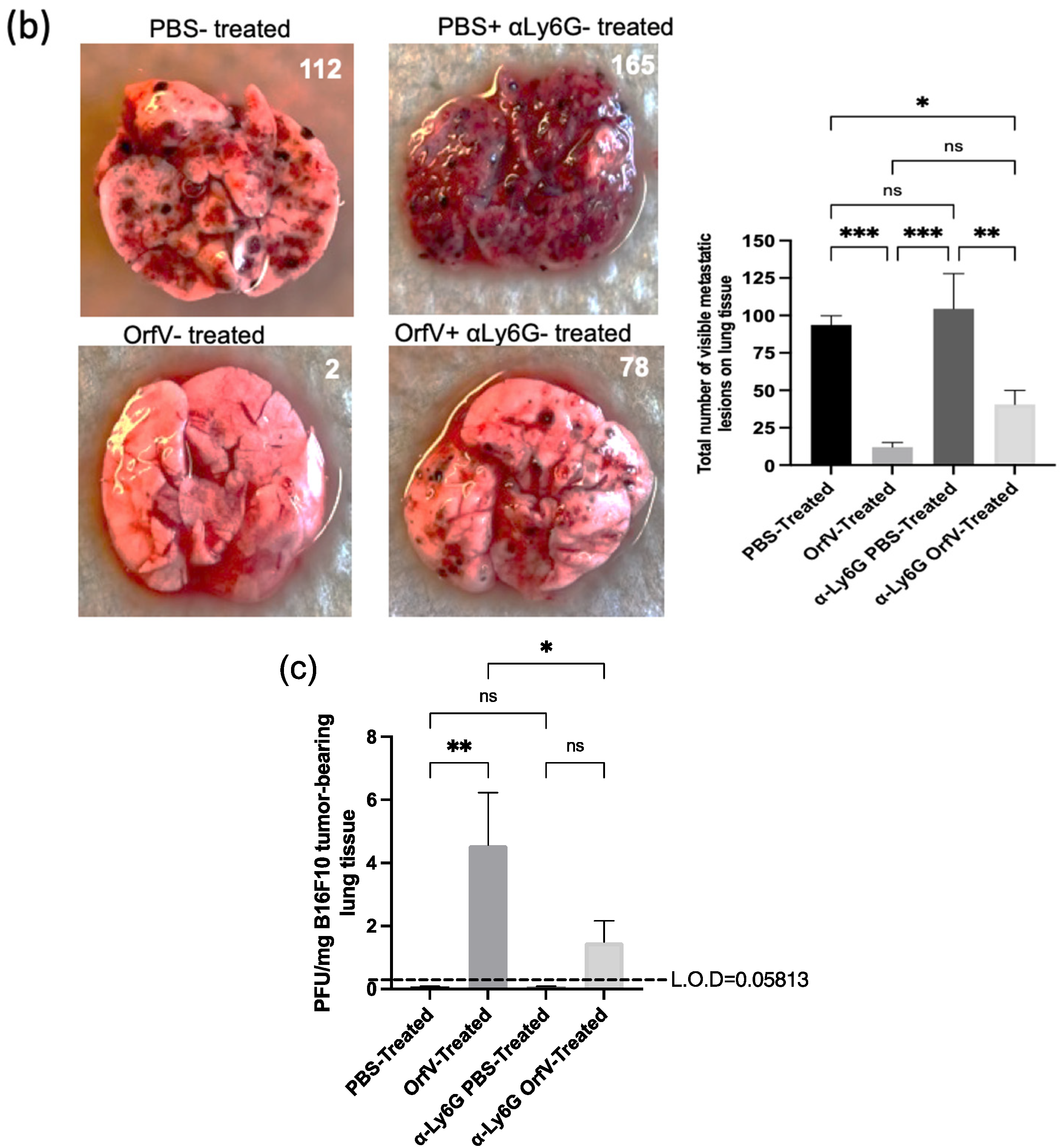
3.4. Depletion of Neutrophils Implicated them in OrfV-Mediated Antitumor Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raff, M.; Alberts, B.; Lewis, J.; Johnson, A.; Roberts, K. Molecular Biology of the Cell, 4th ed.; Garland, S., Ed.; National Center for Biotechnology Information: New York, NY, USA, 2002.
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.R.; Levy, O. Innate immunity. In Clinical Immunology; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Elsevier Health Sciences: London, UK, 2019; pp. 39–53. [Google Scholar]
- Naumenk, V.; Turk, M.; Jenne, C.N.; Kim, S.J. Neutrophils in Viral Infection. Cell Tissue Res. 2018, 371, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.W. Current Understanding in Neutrophil Differentiation and Heterogeneity. Immune Netw. 2017, 17, 298–306. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Hagerling, C.; Werb, Z. Neutrophils: Critical Components in Experimental Animal Models of Cancer. Semin. Immunol. 2016, 28, 197–204. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-Associated Neutrophils in Patients with Cancer. Nat. Rev. Clin. Oncol 2019, 16, 601–620. [Google Scholar] [CrossRef]
- van Vloten, J.P.; Workenhe, S.T.; Wootton, S.K.; Mossman, K.L.; Bridle, B.W. Critical Interactions between Immunogenic Cancer Cell Death, Oncolytic Viruses, and the Immune System Define the Rational Design of Combination Immunotherapies. J. Immunol. 2018, 200, 450–458. [Google Scholar] [CrossRef]
- Russell, S.J.; Barber, G.N. Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines. Cancer Cell 2018, 33, 599–605. [Google Scholar] [CrossRef]
- Lawler, S.E.; Speranza, M.C.; Cho, C.F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment a Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- Engeland, C.E. Oncolytic viruses. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020. [Google Scholar]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Rintoul, J.L.; Lemay, C.G.; Tai, L.-H.; Stanford, M.M.; Falls, T.J.; de Souza, C.T.; Bridle, W.; Daneshmand, M.; Ohashi, P.S.; Wan, Y.; et al. ORFV: A Novel Oncolytic and Immune Stimulating Parapoxvirus Therapeutic. Mol. Ther. 2012, 20, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- van Vloten, J.P.; Matuszewska, K.; Minow, M.A.; Minott, J.A.; Santry, L.A.; Pereira, M.; Stegelmeier, A.A.; McAusland, T.M.; Klafuric, E.M.; Karimi, K.; et al. Oncolytic Orf Virus Licenses NK Cells via CDC1 to Activate Innate and Adaptive Antitumor Mechanisms and Extends Survival in a Murine Model of Late-Stage Ovarian Cancer. J. Immunother. Cancer 2022, 10, e004335. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, H.H.; Siegling, A.; Volk, H.D.; Friebe, A.; Knolle, P.; Limmer, A.; Weber, O. Inactivated Orf Virus (Parapoxvirus Ovis) Induces Antitumoral Activity in Transplantable Tumor Models. Anticancer. Res. 2011, 31, 4185–4190. [Google Scholar]
- Tai, L.H.; De Souza, C.T.; Bélanger, S.; Ly, L.; Alkayyal, A.A.; Zhang, J.; Rintoul, J.L.; Ananth, A.A.; Lam, T.; Breitbach, C.J.; et al. Preventing Postoperative Metastatic Disease by Inhibiting Surgery-Induced Dysfunction in Natural Killer Cells. Cancer Res. 2013, 73, 97–107. [Google Scholar] [CrossRef]
- van Vloten, J.P.; Minott, J.A.; McAusland, T.M.; Ingrao, J.C.; Santry, L.A.; McFadden, G.D.; Petrik, J.J.; Bridle, B.W.; Wootton, S.K. Production and Purification of High-Titer OrfV for Preclinical Studies in Vaccinology and Cancer Therapy. Mol. Ther. Methods Clin. Dev. 2021, 23, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Bryan, W.R. Interpretation of Host Response in Quantitative Studies on Animal Viruses. Ann. N. Y. Acad. Sci. 1957, 69, 698–728. [Google Scholar] [CrossRef] [PubMed]
- Wulff, N.H.; Tzatzaris, M.; Young, P.J. Monte Carlo Simulation of the Spearman-Kaerber TCID50. J. Clin. Bioinforma. 2012, 2, 5. [Google Scholar] [CrossRef]
- Hayashi, K.; Hooper, L.C.; Okuno, T.; Takada, Y.; Hooks, J.J. Inhibition of HSV-1 by Chemoattracted Neutrophils: Supernatants of Corneal Epithelial Cells (HCE) and Macrophages (THP-1) Treated with Virus Components Chemoattract Neutrophils (PMN), and Supernatants of PMN Treated with These Conditioned Media Inhibit Vi. Arch. Virol. 2012, 157, 1377–1381. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; Chan, L.; Mehrani, Y.; Petrik, J.J.; Wootton, S.K.; Bridle, B.; Karimi, K. Characterization of the Impact of Oncolytic Vesicular Stomatitis Virus on the Trafficking, Phenotype, and Antigen Presentation Potential of Neutrophils and Their Ability to Acquire a Non-Structural Viral Protein. Int. J. Mol. Sci. 2020, 21, 6347. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; van Vloten, J.P.; Mould, R.C.; Klafuric, E.M.; Minott, J.A.; Wootton, S.K.; Bridle, B.W.; Karimi, K. Myeloid Cells during Viral Infections and Inflammation. Viruses 2019, 11, 168. [Google Scholar] [CrossRef]
- Fu, X.; Tao, L.; Rivera, A.; Xu, H.; Zhang, C. Virotherapy Induces Massive Infiltration of Neutrophils in a Subset of Tumors Defined by a Strong Endogenous Interferon Response Activity. Cancer Gene Ther. 2011, 18, 785–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, A.; Zhang, Y.; Castleton, A.Z.; Bailey, K.; Beaton, B.; Patel, B. The Role of Neutrophils in Measles Virus-Mediated Oncolysis Differs between B-Cell Malignancies and Is Not Always Enhanced by GCSF. Mol. Ther 2016, 24, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; Von Kockritz-Blickwede, M. Type I IFNs Induce Anti-Tumor Polarization of Tumor Associated Neutrophils in Mice and Human. Int J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Grote, D.; Cattaneo, R.; Fielding, A.K. Neutrophils Contribute to the Measles Virus-Induced Antitumor Effect: Enhancement by Granulocyte Macrophage Colony-Stimulating Factor Expression. Cancer Res. 2003, 63, 6463–6468. [Google Scholar] [PubMed]
- Zhang, Y.; Patel, B.; Dey, A.; Ghorani, E.; Rai, L.; Elham, M. Attenuated, Oncolytic, but Not Wild-Type Measles Virus Infection Has Pleiotropic Effects on Human Neutrophil Function. J. Immunol. 2012, 188, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Mackey, J.B.G.; Coffelt, S.B.; Carlin, L.M. Neutrophil Maturity in Cancer. Front. Immunol. 2019, 10, 1912. [Google Scholar] [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.W. A Subset of Neutrophils in Human Systemic Inflammation Inhibits T Cell Responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Rahman, S.; Sagar, D.; Hanna, R.N.; Lightfoot, Y.L.; Mistry, P.; Smith, C.K. Low-Density Granulocytes Activate T Cells and Demonstrate a Non-Suppressive Role in Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 957–966. [Google Scholar] [CrossRef]
- Manz, M.G.; Boettcher, S. Emergency Granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef]
- Seo, S.H.; Webster, R.G. Tumor Necrosis Factor Alpha Exerts Powerful Anti-Influenza Virus Effects in Lung Epithelial Cells. J. Virol. 2002, 76, 1071–1076. [Google Scholar] [CrossRef]
- Zimmermann, M.; Arruda-Silva, F.; Bianchetto-Aguilera, F. IFNα Enhances the Production of IL-6 by Human Neutrophils Activated via TLR8. Sci. Rep. 2016, 6, 19674. [Google Scholar] [CrossRef] [Green Version]
- Holl, E.K.; Brown, M.C.; Boczkowski, D.; McNamara, M.A.; George, D.J.; Bigner, D.D.; Gromeier, M.; Nair, S.K. Recombinant Oncolytic Poliovirus, PVSRIPO, Has Potent Cytotoxic and Innate Inflammatory Effects, Mediating Therapy in Human Breast and Prostate Cancer Xenograft Models. Oncotarget 2016, 7, 79828–79841. [Google Scholar] [CrossRef]
- Delwar, Z.M.; Kuo, Y.; Wen, Y.H.; Rennie, P.S.; Jia, W. Oncolytic Virotherapy Blockade by Microglia and Macrophages Requires STAT1/3. Cancer Res. 2018, 78, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Frenzke, M.; Myers, R.; Soeffker, D.; Harvey, M.; Greiner, S. Biodistribution of Oncolytic Measles Virus after Intraperitoneal Administration into Ifnar-CD46Ge Transgenic Mice. Hum. Gene Ther. 2003, 14, 1565–1577. [Google Scholar] [CrossRef]
- Bai, F.; Kong, K.F.; Dai, J.; Qian, F.; Zhang, L.; Brown, C.R.; Fikrig, E.; Montgomery, R.R. A Paradoxical Role for Neutrophils in the Pathogenesis of West Nile Virus. J. Infect. Dis. 2010, 202, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Dogra, P.; Masi, T.J.; Pitt, E.A.; de Kruijf, P.; Smit, M.J.; Sparer, T.E. Novel Human Cytomegalovirus Viral Chemokines, VCXCL-1s, Display Functional Selectivity for Neutrophil Signaling and Function. J. Immunol. 2015, 195, 227–236. [Google Scholar] [CrossRef]
- Beaulieu, A.D.; Paquin, R.; Gosselin, J. Epstein-Barr Virus Modulates de Novo Protein Synthesis in Human Neutrophils. Blood 1995, 86, 2789–2798. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, M.; Lau, L.T.; Lu, J.; Gao, Z.; Liu, J.; Yu, A.C.H.; Cao, Q.; Ye, J.; McNutt, M.A.; et al. Neutrophils May Be a Vehicle for Viral Replication and Dissemination in Human H5N1 Avian Influenza. Clin. Infect. Dis. 2008, 47, 1575–1578. [Google Scholar] [CrossRef]
- Haranaka, K. Antitumor Activities and Tumor Necrosis Factor Producibility of Traditional Chinese Medicines and Crude Drugs. Cancer Immunol. Immunother. 1985, 20, 1–5. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Mejías-Pérez, E.; Carreño-Fuentes, L.; Esteban, M. Development of a Safe and Effective Vaccinia Virus Oncolytic Vector WR-Δ4 with a Set of Gene Deletions on Several Viral Pathways. Mol. Ther.-Oncolytics 2018, 8, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minott, J.A.; van Vloten, J.P.; Chan, L.; Mehrani, Y.; Bridle, B.W.; Karimi, K. The Role of Neutrophils in Oncolytic Orf Virus-Mediated Cancer Immunotherapy. Cells 2022, 11, 2858. https://doi.org/10.3390/cells11182858
Minott JA, van Vloten JP, Chan L, Mehrani Y, Bridle BW, Karimi K. The Role of Neutrophils in Oncolytic Orf Virus-Mediated Cancer Immunotherapy. Cells. 2022; 11(18):2858. https://doi.org/10.3390/cells11182858
Chicago/Turabian StyleMinott, Jessica A., Jacob P. van Vloten, Lily Chan, Yeganeh Mehrani, Byram W. Bridle, and Khalil Karimi. 2022. "The Role of Neutrophils in Oncolytic Orf Virus-Mediated Cancer Immunotherapy" Cells 11, no. 18: 2858. https://doi.org/10.3390/cells11182858
APA StyleMinott, J. A., van Vloten, J. P., Chan, L., Mehrani, Y., Bridle, B. W., & Karimi, K. (2022). The Role of Neutrophils in Oncolytic Orf Virus-Mediated Cancer Immunotherapy. Cells, 11(18), 2858. https://doi.org/10.3390/cells11182858









