MYH9 Facilitates Cell Invasion and Radioresistance in Head and Neck Cancer via Modulation of Cellular ROS Levels by Activating the MAPK-Nrf2-GCLC Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Transfection
2.2. The Constructions of shRNA Plasmids
2.3. Analyses of Cell Migration
2.4. Analyses of Cell Invasion
2.5. Assessment of Radiosensitivity
2.6. RT-qPCR Method to Determine mRNA Expressions
2.7. Western Blot Method to Determine Protein Levels
2.8. Measurement of Cellular Reactive Oxygen Species (ROS) Level
2.9. Luciferase Report Assay for the Transcriptional Activity of Nrf2
2.10. Clinical Association and Prognostic Evaluation of MYH9 in HNC Patients
3. Results
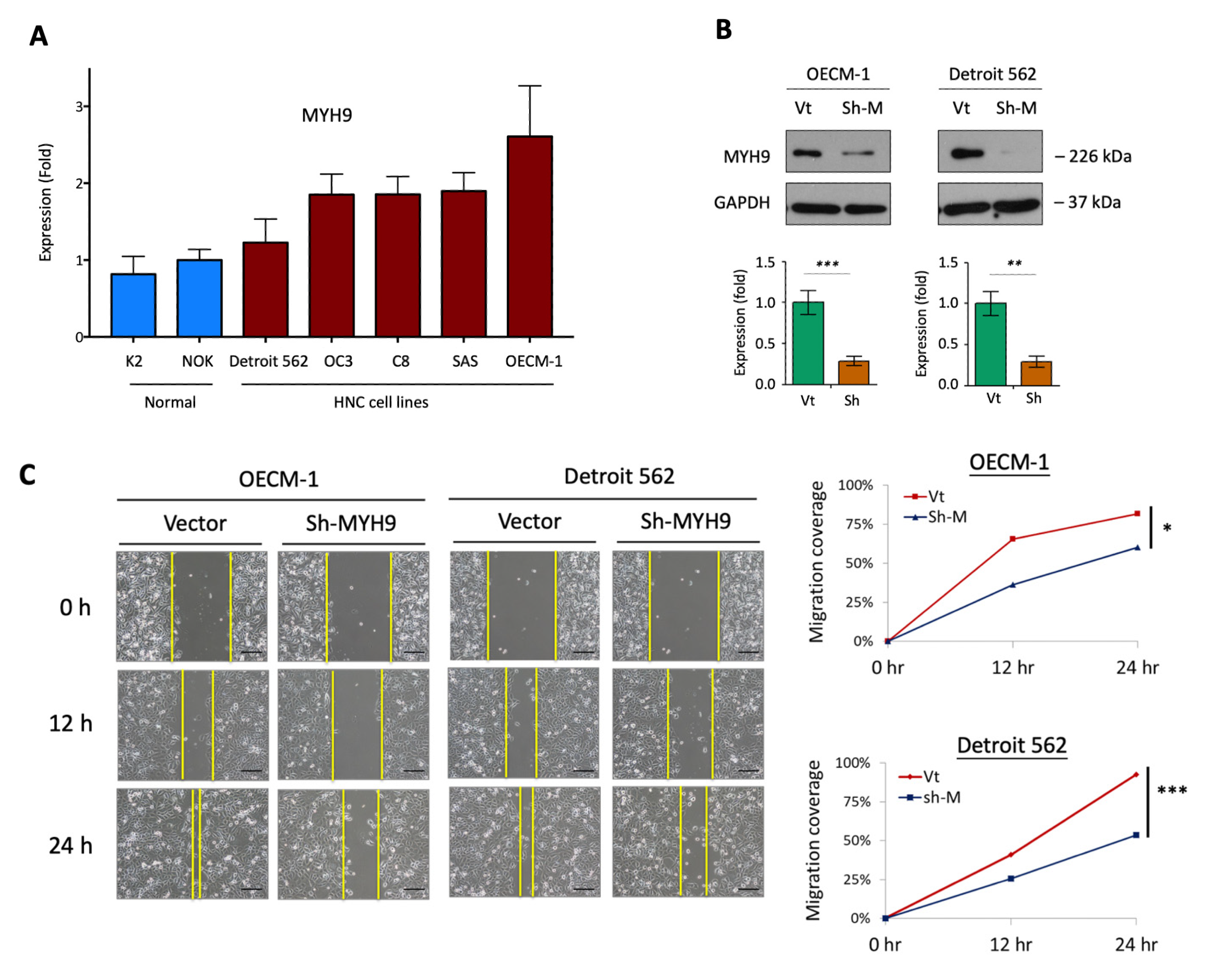
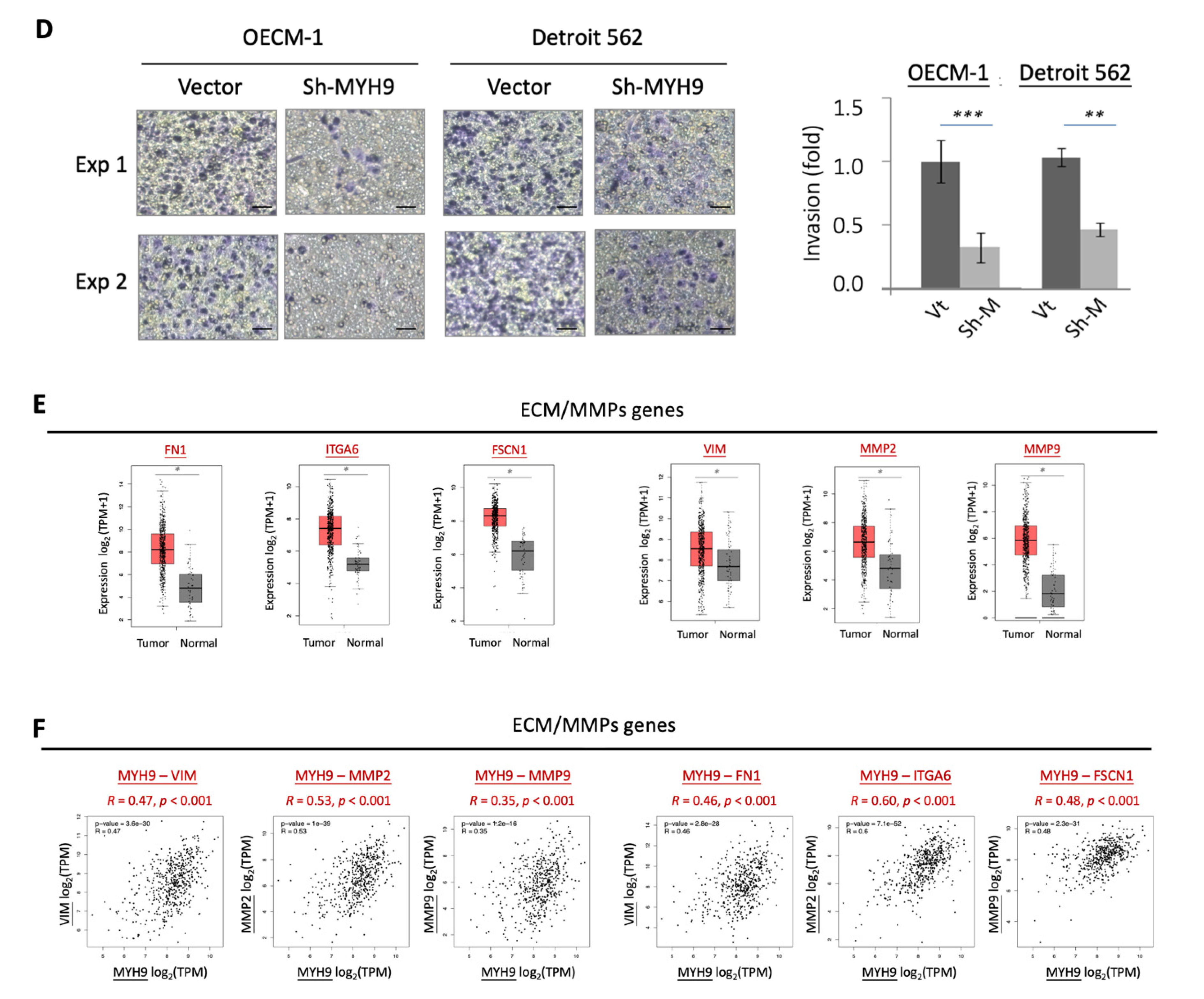
3.1. MYH9 Promotes Cell Motility along with the Modulation of the Extracellular Matrix
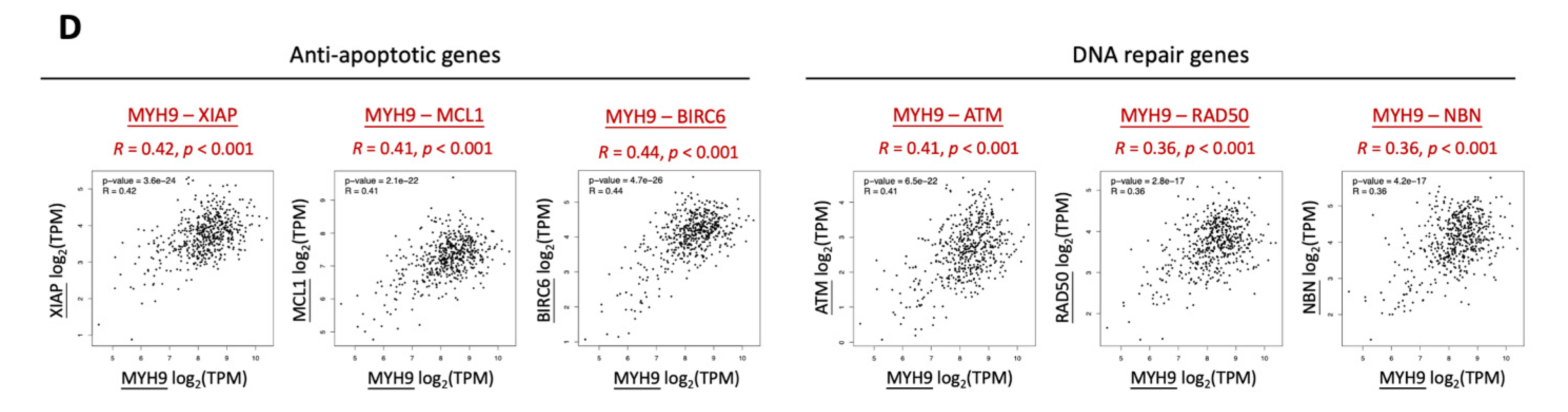
3.2. MYH9 Contributes to Radioresistance and Is Associated with the Anti-Apoptotic Mechanism
3.3. MYH9 Suppressed Cellular ROS Levels via Activation of Nrf2 and Up-Regulation of Antioxidant Enzymes
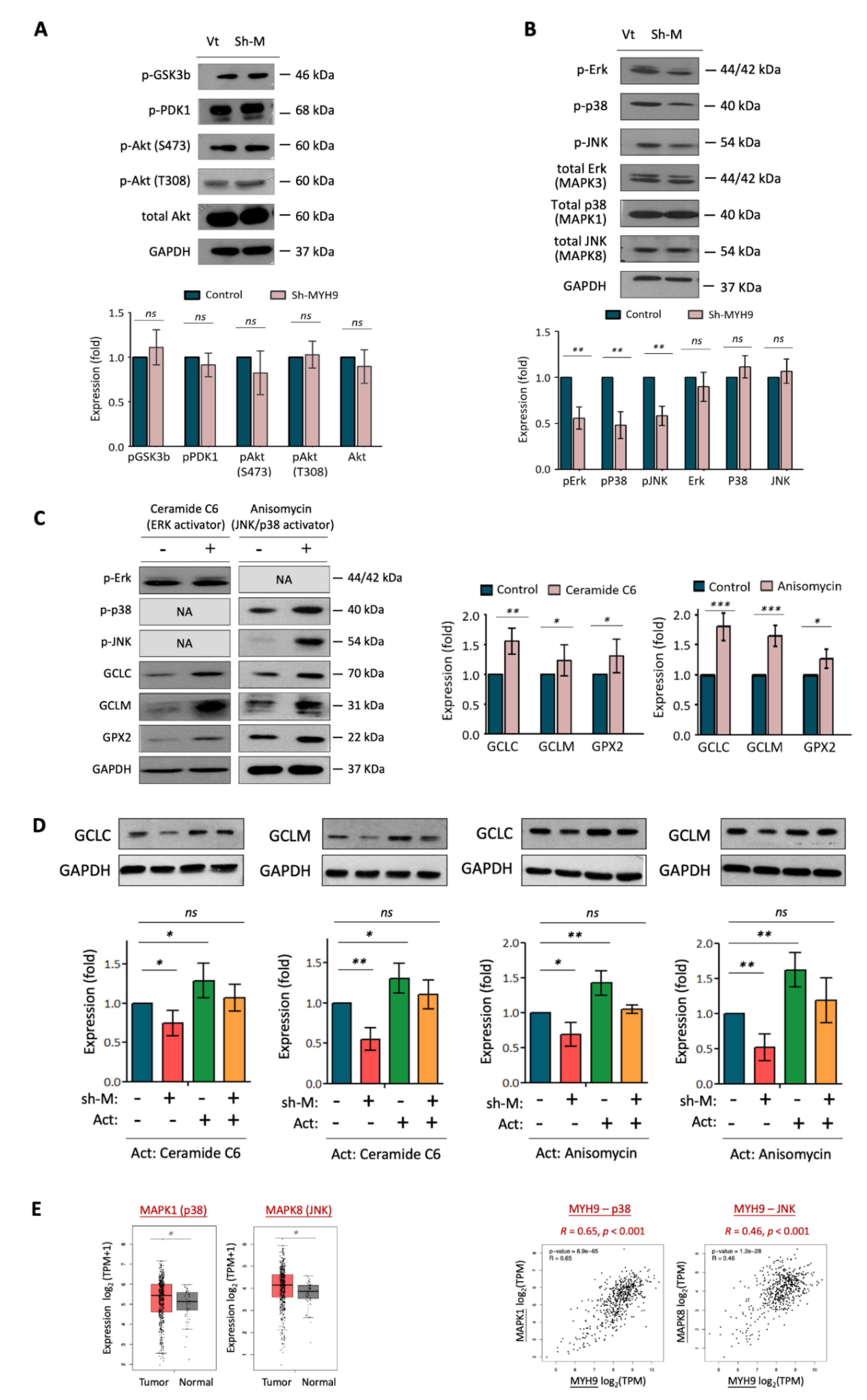
3.4. MYH9 Activates Pan-MAPK Signaling Pathways Leading to ROS Dysregulation
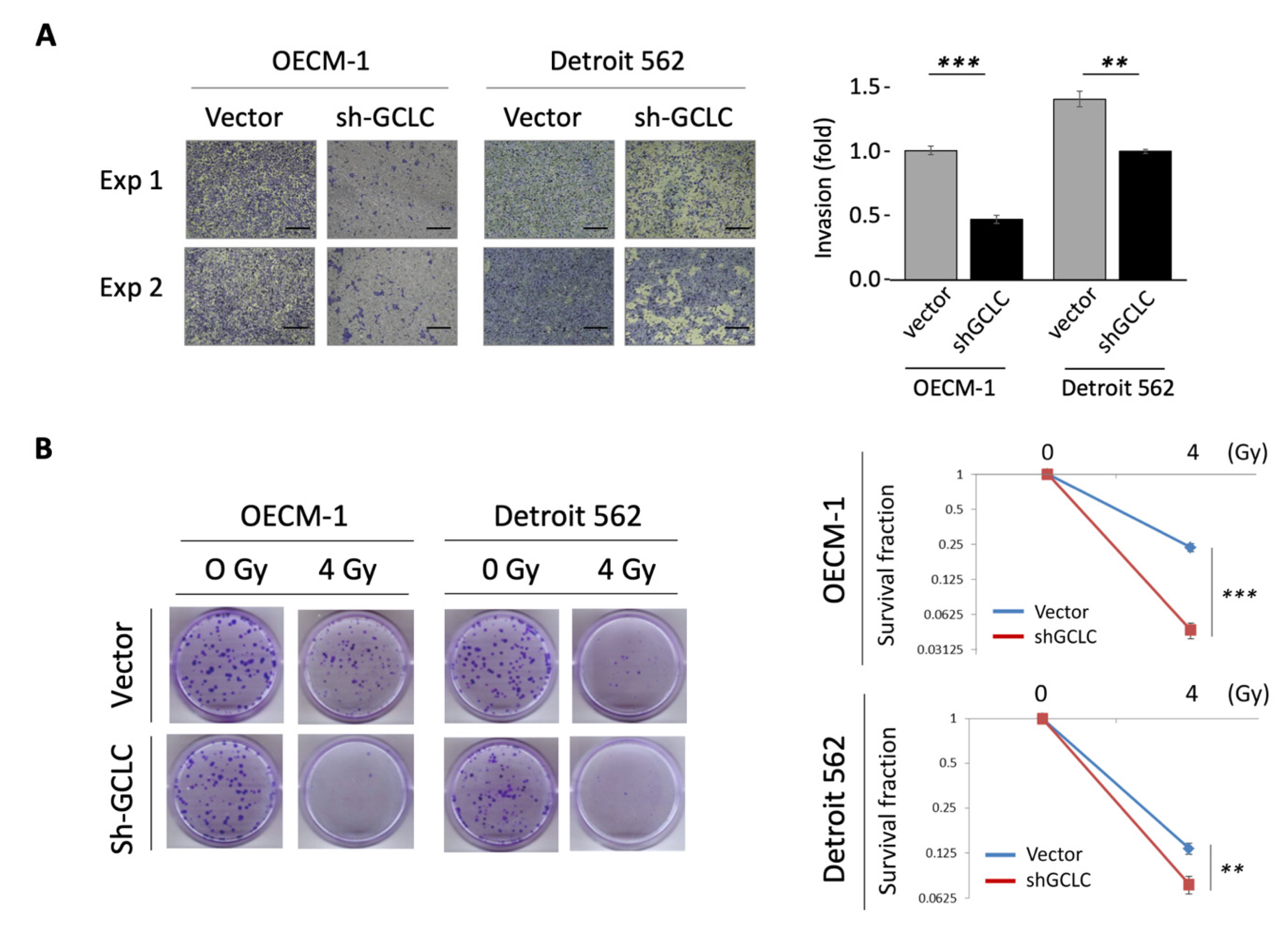
3.5. GCLC Modulated by MYH9 Facilitates Cell Invasion and Radioresistance
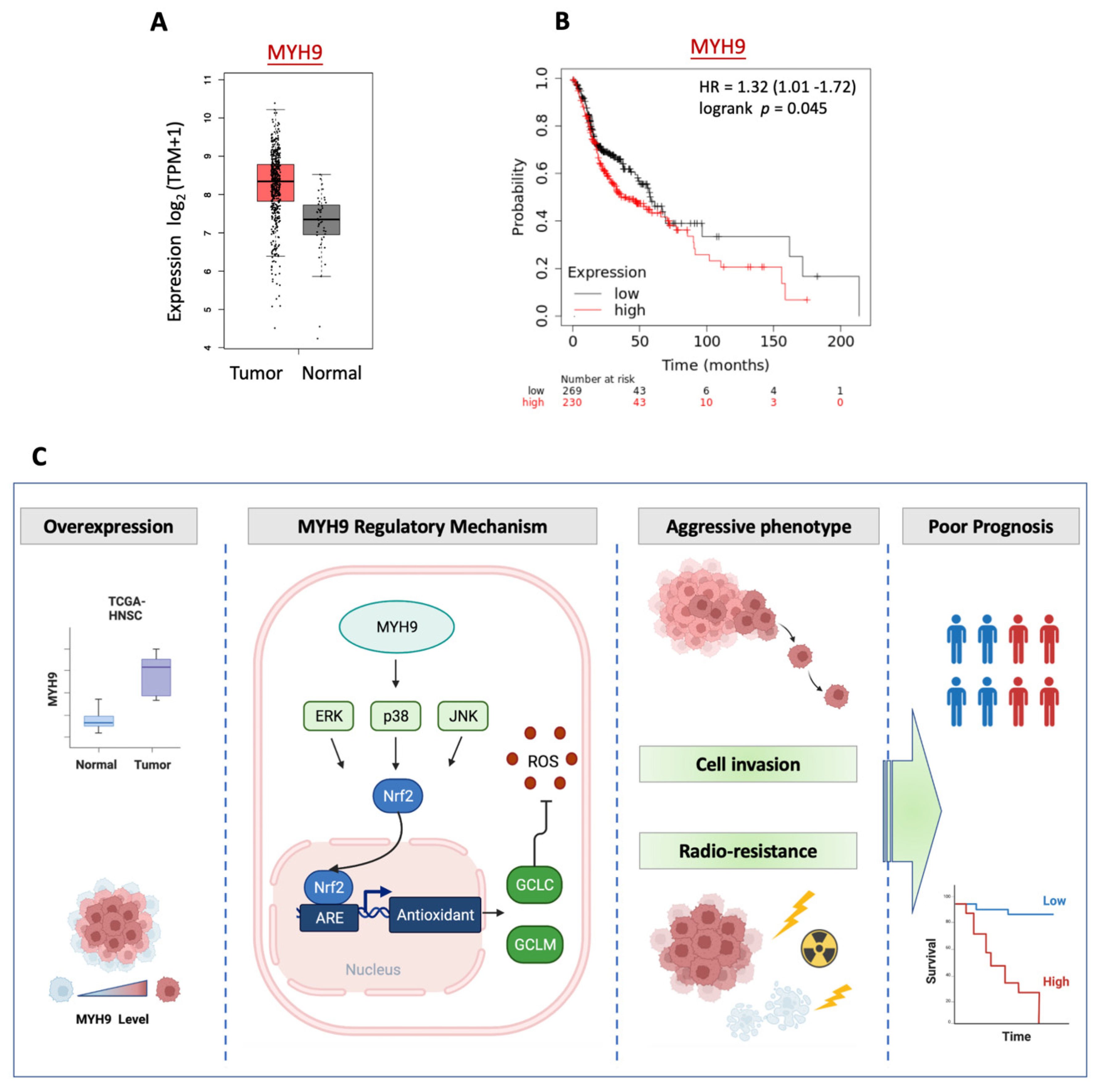
3.6. MYH9 Is Overexpressed in Tumors and Associated with Poor Prognosis in Patients with HNC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, e57. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, C.; Nguyen, N.T.; Zhang, J.; Aguilar, J.; Blatchins, M.A.; Quesenberry, C.P., Jr.; Wang, Y.; Sakoda, L.C. Survival Associated With Consolidated Multidisciplinary Care in Head and Neck Cancer: A Retrospective Cohort Study. Otolaryngol. Head Neck Surg. 2021, 01945998211057852. [Google Scholar] [CrossRef]
- Fan, K.H.; Yeh, C.H.; Hung, S.P.; Kang, C.J.; Huang, S.F.; Chang, K.P.; Wang, H.M.; Chia-Hsun Hsieh, J.; Lin, C.Y.; Cheng, A.J.; et al. Prognostic value of radiologic extranodal extension in patients with hypopharyngeal cancer treated with primary chemoradiation. Radiother. Oncol. 2021, 156, 217–222. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Q.; Zhang, Y.; Wei, J.; Wang, B.; Zheng, Z.; Liu, S.; Liu, Z.; Meng, L.; Xin, Y.; et al. Efficacy and safety of systemic treatments for patients with recurrent/metastatic head and neck squamous cell carcinoma: A systematic review and network meta-analysis. Pharm. Res. 2021, 173, 105866. [Google Scholar] [CrossRef]
- Lorini, L.; Ardighieri, L.; Bozzola, A.; Romani, C.; Bignotti, E.; Buglione, M.; Guerini, A.; Lombardi, D.; Deganello, A.; Tomasoni, M.; et al. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021, 115, 105213. [Google Scholar] [CrossRef]
- Tang, E.; Nguyen, T.V.; Clatot, F.; Rambeau, A.; Johnson, A.; Sun, X.S.; Tao, Y.; Thariat, J. Radiation therapy on primary tumour of synchronous metastatic head and neck squamous cell carcinomas. Cancer Radiother. 2020, 24, 559–566. [Google Scholar] [CrossRef]
- You, G.R.; Chang, J.T.; Li, Y.L.; Chen, Y.J.; Huang, Y.C.; Fan, K.H.; Chen, Y.C.; Kang, C.J.; Cheng, A.J. Molecular Interplays Between Cell Invasion and Radioresistance That Lead to Poor Prognosis in Head-Neck Cancer. Front. Oncol. 2021, 11, 681717. [Google Scholar] [CrossRef]
- Brito, C.; Sousa, S. Non-Muscle Myosin 2A (NM2A): Structure, Regulation and Function. Cells 2020, 9, 1590. [Google Scholar] [CrossRef]
- Asensio-Juarez, G.; Llorente-Gonzalez, C.; Vicente-Manzanares, M. Linking the Landscape of MYH9-Related Diseases to the Molecular Mechanisms that Control Non-Muscle Myosin II-A Function in Cells. Cells 2020, 9, 1458. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, L.; Shen, Z.; Tan, F.; Hu, Y.; Yu, J.; Li, G. Clinicopathological significance of NMIIA overexpression in human gastric cancer. Int. J. Mol. Sci. 2012, 13, 15291–15304. [Google Scholar] [CrossRef]
- Xia, Z.K.; Yuan, Y.C.; Yin, N.; Yin, B.L.; Tan, Z.P.; Hu, Y.R. Nonmuscle myosin IIA is associated with poor prognosis of esophageal squamous cancer. Dis. Esophagus 2012, 25, 427–436. [Google Scholar] [CrossRef]
- Katono, K.; Sato, Y.; Jiang, S.X.; Kobayashi, M.; Nagashio, R.; Ryuge, S.; Fukuda, E.; Goshima, N.; Satoh, Y.; Saegusa, M.; et al. Prognostic significance of MYH9 expression in resected non-small cell lung cancer. PLoS ONE 2015, 10, e0121460. [Google Scholar] [CrossRef]
- Chen, M.; Sun, L.X.; Yu, L.; Liu, J.; Sun, L.C.; Yang, Z.H.; Shu, X.; Ran, Y.L. MYH9 is crucial for stem cell-like properties in non-small cell lung cancer by activating mTOR signaling. Cell Death Discov. 2021, 7, 282. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, D.; Liu, D.; Wu, W.; Dou, X.; Ji, X.; Li, J.; Zhang, X. The Overexpression of NMHC IIA Promoted Invasion and Metastasis of Nasopharyngeal Carcinoma Cells. J. Cancer 2021, 12, 4218–4228. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Z.; Li, C.; Zhang, Y.; Li, Z.; Sun, S. NMIIA promotes tumorigenesis and prevents chemosensitivity in colorectal cancer by activating AMPK/mTOR pathway. Exp. Cell Res. 2021, 398, 112387. [Google Scholar] [CrossRef]
- Wang, B.; Qi, X.; Liu, J.; Zhou, R.; Lin, C.; Shangguan, J.; Zhang, Z.; Zhao, L.; Li, G. MYH9 Promotes Growth and Metastasis via Activation of MAPK/AKT Signaling in Colorectal Cancer. J. Cancer 2019, 10, 874–884. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Y.; Li, B.; Zhang, M.; Liu, Y.; Yao, Y.; Li, D. NMIIA promotes tumor growth and metastasis by activating the Wnt/beta-catenin signaling pathway and EMT in pancreatic cancer. Oncogene 2019, 38, 5500–5515. [Google Scholar] [CrossRef]
- Zhong, Y.; Long, T.; Gu, C.S.; Tang, J.Y.; Gao, L.F.; Zhu, J.X.; Hu, Z.Y.; Wang, X.; Ma, Y.D.; Ding, Y.Q.; et al. MYH9-dependent polarization of ATG9B promotes colorectal cancer metastasis by accelerating focal adhesion assembly. Cell Death Differ. 2021, 28, 3251–3269. [Google Scholar] [CrossRef]
- Que, T.; Zheng, H.; Zeng, Y.; Liu, X.; Qi, G.; La, Q.; Liang, T.; Li, Z.; Yi, G.; Zhang, S.; et al. HMGA1 stimulates MYH9-dependent ubiquitination of GSK-3beta via PI3K/Akt/c-Jun signaling to promote malignant progression and chemoresistance in gliomas. Cell Death Dis. 2021, 12, 1147. [Google Scholar] [CrossRef]
- Du, H.; Huang, Y.; Hou, X.; Yu, X.; Lin, S.; Wei, X.; Li, R.; Khan, G.J.; Yuan, S.; Sun, L. DT-13 inhibits cancer cell migration by regulating NMIIA indirectly in the tumor microenvironment. Oncol. Rep. 2016, 36, 721–728. [Google Scholar] [CrossRef]
- Schramek, D.; Sendoel, A.; Segal, J.P.; Beronja, S.; Heller, E.; Oristian, D.; Reva, B.; Fuchs, E. Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas. Science 2014, 343, 309–313. [Google Scholar] [CrossRef]
- Singh, S.K.; Sinha, S.; Padhan, J.; Jangde, N.; Ray, R.; Rai, V. MYH9 suppresses melanoma tumorigenesis, metastasis and regulates tumor microenvironment. Med. Oncol. 2020, 37, 88. [Google Scholar] [CrossRef]
- Yang, C.Y.; Meng, C.L. Regulation of PG synthase by EGF and PDGF in human oral, breast, stomach, and fibrosarcoma cancer cell lines. J. Dent. Res. 1994, 73, 1407–1415. [Google Scholar] [CrossRef]
- Li, Y.L.; Chang, J.T.; Lee, L.Y.; Fan, K.H.; Lu, Y.C.; Li, Y.C.; Chiang, C.H.; You, G.R.; Chen, H.Y.; Cheng, A.J. GDF15 contributes to radioresistance and cancer stemness of head and neck cancer by regulating cellular reactive oxygen species via a SMAD-associated signaling pathway. Oncotarget 2017, 8, 1508–1528. [Google Scholar] [CrossRef]
- Lu, Y.C.; Cheng, A.J.; Lee, L.Y.; You, G.R.; Li, Y.L.; Chen, H.Y.; Chang, J.T. MiR-520b as a novel molecular target for suppressing stemness phenotype of head-neck cancer by inhibiting CD44. Sci. Rep. 2017, 7, 2042. [Google Scholar] [CrossRef]
- Wang, X.J.; Hayes, J.D.; Wolf, C.R. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res. 2006, 66, 10983–10994. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Ho, V.; Chung, L.; Singh, A.; Lea, V.; Abubakar, A.; Lim, S.H.; Ng, W.; Lee, M.; de Souza, P.; Shin, J.S.; et al. Overexpression of the MRE11-RAD50-NBS1 (MRN) complex in rectal cancer correlates with poor response to neoadjuvant radiotherapy and prognosis. BMC Cancer 2018, 18, 869. [Google Scholar] [CrossRef]
- Raimondi, C.; Falasca, M. Targeting PDK1 in cancer. Curr. Med. Chem. 2011, 18, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal. Transduct. Target. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Munoz, N.; Robles-Flores, M. Glycogen synthase kinase 3 in Wnt signaling pathway and cancer. IUBMB Life 2015, 67, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.J.; Sharma, B.; Chawla, P.A. Recent developments in mitogen activated protein kinase inhibitors as potential anticancer agents. Bioorganic Chem. 2021, 114, 105161. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [PubMed]
- Coaxum, S.D.; Tiedeken, J.; Garrett-Mayer, E.; Myers, J.; Rosenzweig, S.A.; Neskey, D.M. The tumor suppressor capability of p53 is dependent on non-muscle myosin IIA function in head and neck cancer. Oncotarget 2017, 8, 22991–23007. [Google Scholar] [CrossRef] [PubMed]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Fox, D.B.; Garcia, N.M.G.; McKinney, B.J.; Lupo, R.; Noteware, L.C.; Newcomb, R.; Liu, J.; Locasale, J.W.; Hirschey, M.D.; Alvarez, J.V. NRF2 activation promotes the recurrence of dormant tumour cells through regulation of redox and nucleotide metabolism. Nat. Metab. 2020, 2, 318–334. [Google Scholar] [CrossRef]
- Panieri, E.; Buha, A.; Telkoparan-Akillilar, P.; Cevik, D.; Kouretas, D.; Veskoukis, A.; Skaperda, Z.; Tsatsakis, A.; Wallace, D.; Suzen, S.; et al. Potential Applications of NRF2 Modulators in Cancer Therapy. Antioxidants 2020, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Telkoparan-Akillilar, P.; Panieri, E.; Cevik, D.; Suzen, S.; Saso, L. Therapeutic Targeting of the NRF2 Signaling Pathway in Cancer. Molecules 2021, 26, 1417. [Google Scholar] [CrossRef]
- Kang, J.S.; Nam, L.B.; Yoo, O.K.; Keum, Y.S. Molecular mechanisms and systemic targeting of NRF2 dysregulation in cancer. Biochem Pharm. 2020, 177, 114002. [Google Scholar] [CrossRef]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef]
- Emami, M.H.; Sereshki, N.; Malakoutikhah, Z.; Dehkordi, S.A.E.; Fahim, A.; Mohammadzadeh, S.; Maghool, F. Nrf2 signaling pathway in trace metal carcinogenesis: A cross-talk between oxidative stress and angiogenesis. Comp. Biochem. Physiol. C Toxicol. Pharm. 2022, 254, 109266. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar] [CrossRef]
- Lin, C.H.; Vu, J.P.; Yang, C.Y.; Sirisawad, M.; Chen, C.T.; Dao, H.; Liu, J.; Ma, X.; Pan, C.; Cefalu, J.; et al. Glutamate-cysteine ligase catalytic subunit as a therapeutic target in acute myeloid leukemia and solid tumors. Am. J. Cancer Res. 2021, 11, 2911–2927. [Google Scholar]
- Sun, J.; Zhou, C.; Ma, Q.; Chen, W.; Atyah, M.; Yin, Y.; Fu, P.; Liu, S.; Hu, B.; Ren, N.; et al. High GCLC level in tumor tissues is associated with poor prognosis of hepatocellular carcinoma after curative resection. J. Cancer 2019, 10, 3333–3343. [Google Scholar] [CrossRef]
- Hiyama, N.; Ando, T.; Maemura, K.; Sakatani, T.; Amano, Y.; Watanabe, K.; Kage, H.; Yatomi, Y.; Nagase, T.; Nakajima, J.; et al. Glutamate-cysteine ligase catalytic subunit is associated with cisplatin resistance in lung adenocarcinoma. Jpn. J. Clin. Oncol. 2018, 48, 303–307. [Google Scholar] [CrossRef]
- Liu, C.W.; Hua, K.T.; Li, K.C.; Kao, H.F.; Hong, R.L.; Ko, J.Y.; Hsiao, M.; Kuo, M.L.; Tan, C.T. Histone Methyltransferase G9a Drives Chemotherapy Resistance by Regulating the Glutamate-Cysteine Ligase Catalytic Subunit in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2017, 16, 1421–1434. [Google Scholar] [CrossRef]
- Jardim, B.V.; Moschetta, M.G.; Leonel, C.; Gelaleti, G.B.; Regiani, V.R.; Ferreira, L.C.; Lopes, J.R.; Zuccari, D.A. Glutathione and glutathione peroxidase expression in breast cancer: An immunohistochemical and molecular study. Oncol. Rep. 2013, 30, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Tomisawa, M.; Yamazaki, H.; Abe, Y.; Suemizu, H.; Tsukamoto, H.; Tomii, Y.; Kawamura, M.; Kijima, H.; Hatanaka, H.; et al. The modifier subunit of glutamate cysteine ligase (GCLM) is a molecular target for amelioration of cisplatin resistance in lung cancer. Int. J. Oncol. 2003, 23, 1333–1339. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, G.-R.; Chang, J.T.; Li, Y.-L.; Huang, C.-W.; Tsai, Y.-L.; Fan, K.-H.; Kang, C.-J.; Huang, S.-F.; Chang, P.-H.; Cheng, A.-J. MYH9 Facilitates Cell Invasion and Radioresistance in Head and Neck Cancer via Modulation of Cellular ROS Levels by Activating the MAPK-Nrf2-GCLC Pathway. Cells 2022, 11, 2855. https://doi.org/10.3390/cells11182855
You G-R, Chang JT, Li Y-L, Huang C-W, Tsai Y-L, Fan K-H, Kang C-J, Huang S-F, Chang P-H, Cheng A-J. MYH9 Facilitates Cell Invasion and Radioresistance in Head and Neck Cancer via Modulation of Cellular ROS Levels by Activating the MAPK-Nrf2-GCLC Pathway. Cells. 2022; 11(18):2855. https://doi.org/10.3390/cells11182855
Chicago/Turabian StyleYou, Guo-Rung, Joseph T. Chang, Yan-Liang Li, Chi-Wei Huang, Yu-Liang Tsai, Kang-Hsing Fan, Chung-Jan Kang, Shiang-Fu Huang, Po-Hung Chang, and Ann-Joy Cheng. 2022. "MYH9 Facilitates Cell Invasion and Radioresistance in Head and Neck Cancer via Modulation of Cellular ROS Levels by Activating the MAPK-Nrf2-GCLC Pathway" Cells 11, no. 18: 2855. https://doi.org/10.3390/cells11182855
APA StyleYou, G.-R., Chang, J. T., Li, Y.-L., Huang, C.-W., Tsai, Y.-L., Fan, K.-H., Kang, C.-J., Huang, S.-F., Chang, P.-H., & Cheng, A.-J. (2022). MYH9 Facilitates Cell Invasion and Radioresistance in Head and Neck Cancer via Modulation of Cellular ROS Levels by Activating the MAPK-Nrf2-GCLC Pathway. Cells, 11(18), 2855. https://doi.org/10.3390/cells11182855







