Lack of ZNF365 Drives Senescence and Exacerbates Experimental Lung Fibrosis
Abstract
:1. Introduction
2. Results
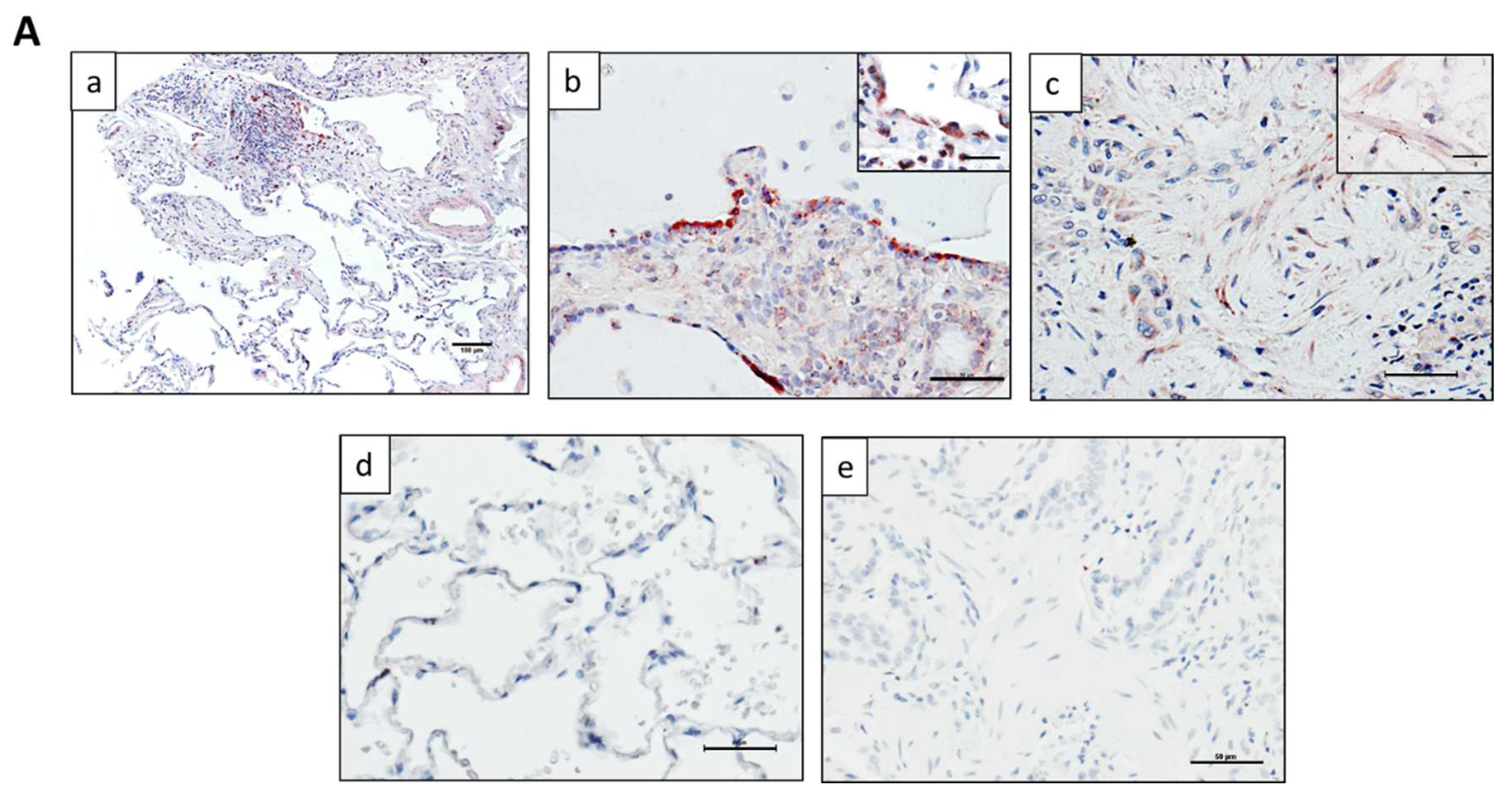
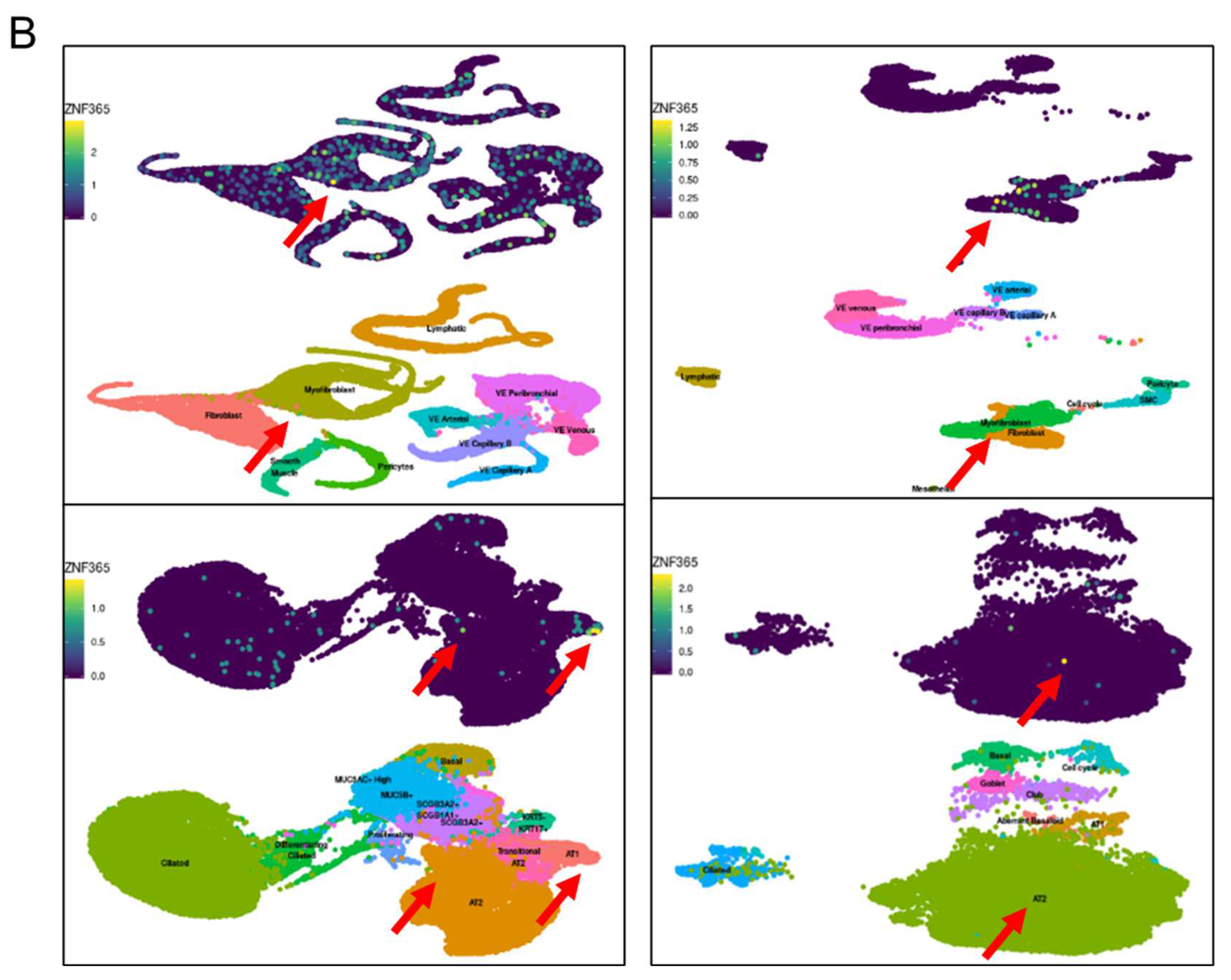
2.1. ZNF365 Is Increased in IPF Lung Tissue, and It Is Located in Fibroblastic Foci and Alveolar Epithelial Cells
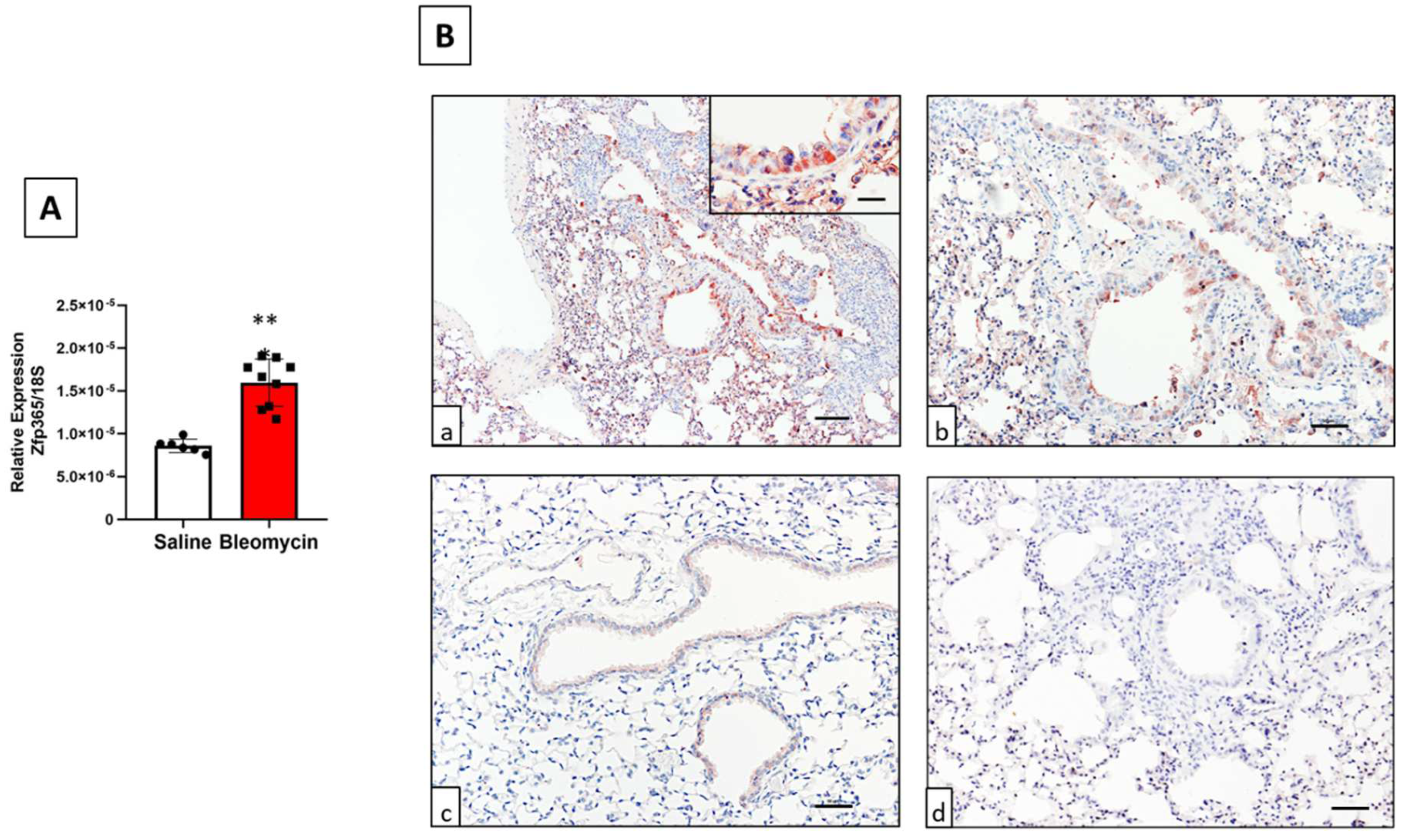
2.2. ZNF365 Mouse Orthologue, Zfp365, Is Overexpressed in Lungs from Mice Treated with Bleomycin
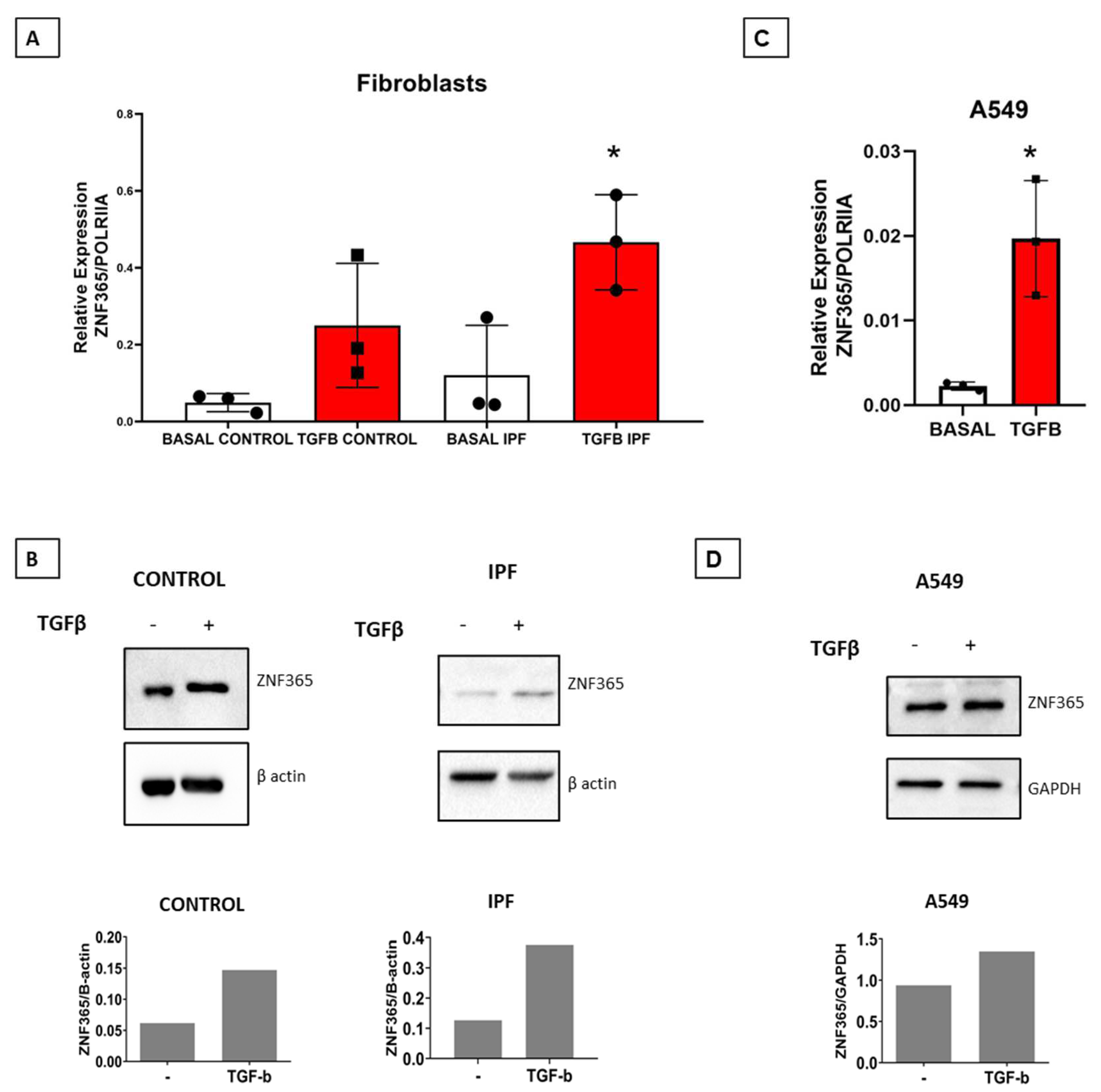
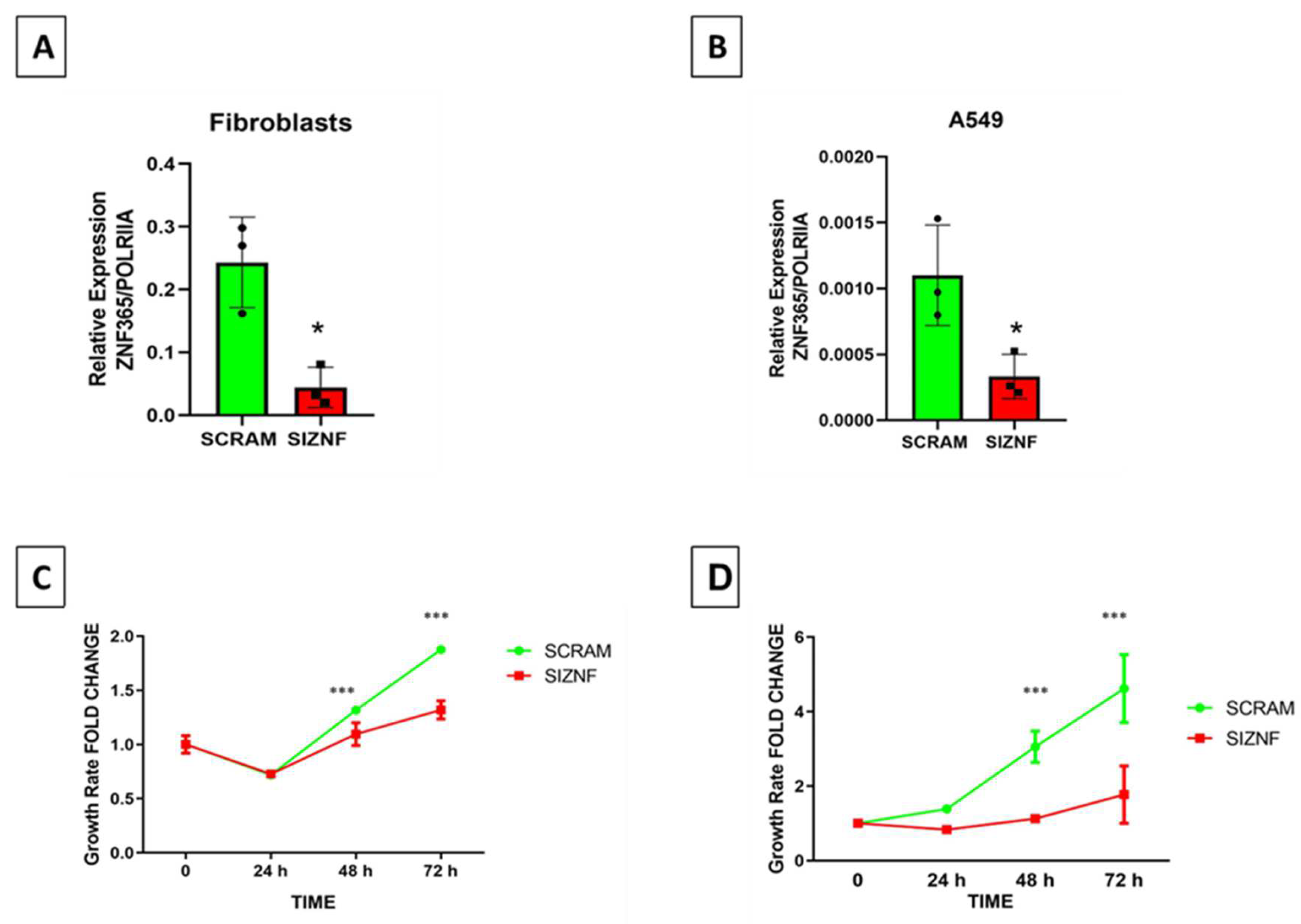
2.3. TGFβ-1 Stimulates the Expression of ZNF365 in Normal and IPF Lung Fibroblasts and in the A549 Epithelial Cell Line
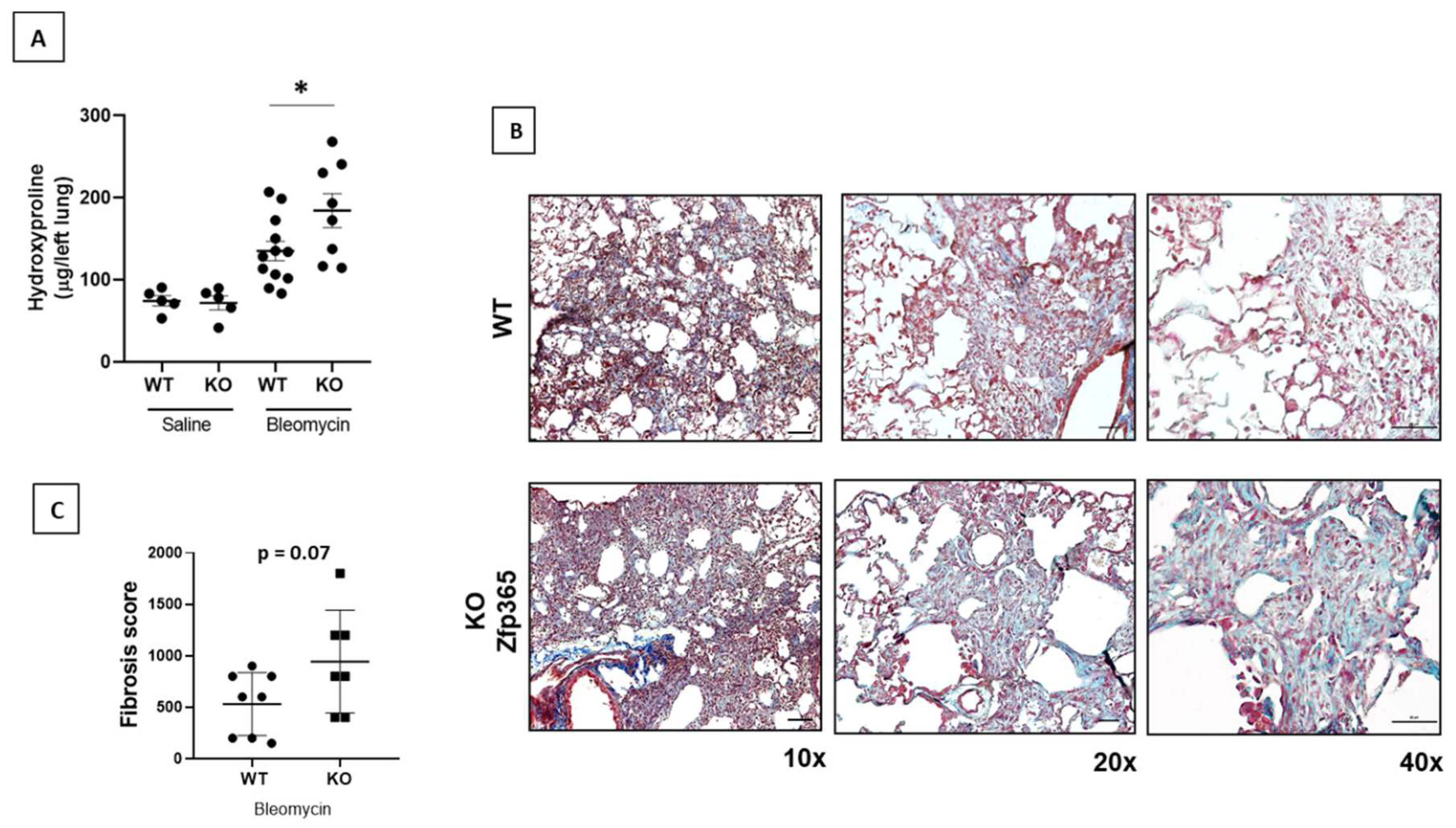
2.4. Zfp365 KO Mice Display an Exacerbated Fibrotic Response to Bleomycin
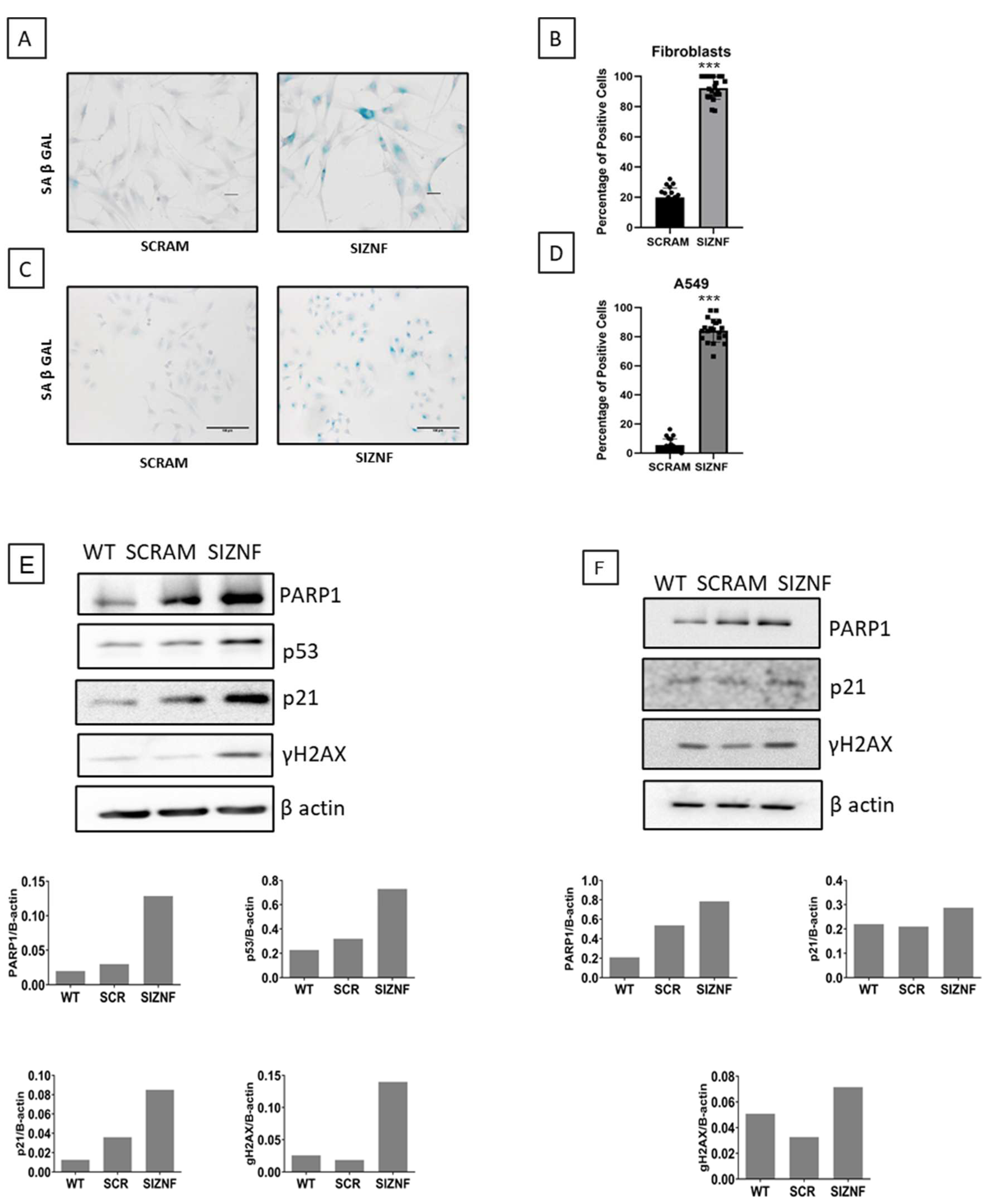
2.5. ZNF365 Silencing Induces a Senescent Phenotype in Fibroblasts and Epithelial Cells
2.6. Reduction of ZNF365 Expression in Normal Human Lung Fibroblasts and A549 Epithelial Cells Is Observed by Doxorubicin Treatment
3. Discussion
4. Material and Methods
4.1. Human Samples
4.2. Immunohistochemistry
4.3. Cell Culture
4.4. TGFβ-1 and Doxorubicin Stimulation
4.5. Real-Time Polymerase Chain Reaction (PCR)
4.6. Western Blot
4.7. ZNF365 Silencing
4.8. Growth Rate Experiments
4.9. Apoptosis
4.10. Senescence Associated-β Galactosidase Activity Assay
4.11. Murine Model of Pulmonary Fibrosis
4.12. Determination of Hydroxyproline
4.13. Semiquantitative Evaluation of Lung Morphological Lesions
4.14. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selman, M.; King, T.E., Jr.; Pardo, A. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 2001, 134, 136–151. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell. Signal. 2020, 66, 109482. [Google Scholar] [CrossRef]
- Negreros, M.; Hagood, J.S.; Espinoza, C.R.; Balderas-Martínez, Y.I.; Selman, M.; Pardo, A. Transforming growth factor beta 1 induces methylation changes in lung fibroblasts. PLoS ONE 2019, 14, e0223512. [Google Scholar] [CrossRef] [PubMed]
- Renzoni, E.A.; Abraham, D.J.; Howat, S.; Shi-Wen, X.; Sestini, P.; Bou-Gharios, G.; Wells, A.U.; Veeraraghavan, S.; Nicholson, A.G.; Denton, C.P.; et al. Gene expression profiling reveals novel TGFβ-1 targets in adult lung fibroblasts. Respir. Res. 2004, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, E.; Kim, C.S.; Paik, J.-H. ZNF365 promotes stalled replication forks recovery to maintain genome instability. Cell Cycle 2013, 12, 2817–2828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shin, S.J.; Liu, D.; Ivanova, E.; Foerster, F.; Ying, H.; Zheng, H.; Xiao, Y.; Chen, Z.; Protopopov, A.; et al. ZNF365 promotes stability of fragile sites and telomeres. Cancer Discov. 2013, 3, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Hattori, T.; Shimizu, S.; Taniguchi, M.; Yamada, K.; Takamura, H.; Kumamoto, M.; Matsuzaki, S.; Ito, A.; Katayama, T.; et al. DBZ (DISC1- binding zinc finger protein)-deficient mice display abnormalities in basket cells in the somatosensory cortices. J. Chem. Neuroanat. 2013, 53, 1–10. [Google Scholar] [CrossRef]
- Okamoto, M.; Iguchi, T.; Hattori, T.; Matsuzaki, S.; Koyama, Y.; Taniguchi, M.; Komada, M.; Xie, M.-J.; Yagi, H.; Shimizu, S.; et al. DBZ regulates cortical cell positioning and neurite development by sustaining the anterograde transport of Lis1 and DISC1 through control of Ndel1 dual phosphorylation. J. Neurosci. 2015, 35, 2942–2958. [Google Scholar] [CrossRef]
- Hirohashi, Y.; Wang, Q.; Liu, Q.; Li, B.; Du, X.; Zhang, H.; Furuuchi, K.; Masuda, K.; Sato, N.; Greene, M.I. Centrosomal proteins Nde1 and Su48 form a complex regulated by phosphorylation. Oncogene 2006, 25, 6048–6055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolaños, A.L.; Milla, C.M.; Lira, J.C.; Ramírez, R.; Checa, M.; Barrera, L.; García-Álvarez, J.; Carbajal, V.; Becerril, C.; Gaxiola, M.; et al. Role of Sonic hedgehog in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol 2012, 303, L978–L990. [Google Scholar] [CrossRef] [PubMed]
- Sanders, Y.Y.; Ambalavanan, N.; Halloran, B.; Zhang, X.; Liu, X.; Crossman, D.K.; Bray, M.; Zhang, K.; Thannickal, V.J.; Hagood, J.S. Altered DNA methylation profile in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 525–535. [Google Scholar] [CrossRef]
- Casella, G.; Munk, R.; Kim, K.M.; Piao, Y.; De, S.; Abdelmohsen, K.; Gorospe, M. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019, 47, 7294–7305. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.C.; Rogers, D.S.; Sharma, V.; Vittal, R.; White, E.S.; Cui, Z.; Thannickal, V.J. Combinatorial activation of FAK and AKT by transforming growth factor-β-1 confers and anoikis resistant phenotype to myofibroblasts. Cell. Signal. 2007, 19, 761–771. [Google Scholar] [CrossRef]
- Lee, J.S.; La, J.; Aziz, S.; Dobrinskikh, E.; Brownell, R.; Jones, K.D.; Achtar-Zadeh, N.; Green, G.; Elicker, B.M.; Golden, J.A.; et al. Molecular markers of telomere dysfunction and senescence are common findings in the usual interstitial pneumonia pattern of lung fibrosis. Histopathology 2021, 79, 67–76. [Google Scholar] [CrossRef]
- Álvarez, D.; Cárdenes, N.; Sellarés, J.; Bueno, M.; Corey, C.; Hanumanthu, V.S.; Peng, Y.; D’Cunha, H.; Sembrat, J.; Nouraie, M.; et al. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1164–L1173. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.-I.; Ren, Z.; et al. Single-Cell transcriptomic analysis of human lung reveals complex multicellular changes during pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Morse, C.; Tabib, T.; Sembrat, J.; Buschur, K.; Trejo Bittar, H.; Valenzi, E.; Jiang, Y.; Kass, D.J.; Gibson, K.; Chen, W.; et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. 2019, 54, 1802441. [Google Scholar] [CrossRef]
- Adams, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A.; Neumark, N.; Ahangari, F.; Chu, S.G.; Raby, B.A.; DeIuliis, G.; Janusyk, M.; et al. Single Cell RNA-seq reveals extopic and aberrant lung resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2019, 6, eaba1983. [Google Scholar] [CrossRef]
- Habermann, A.C.; Gutiérrez, A.J.; Bui, L.T.; Yahn, S.L.; Winters, N.I.; Calvi, C.L.; Peter, L.; Chung, M.-I.; Taylor, C.J.; Jetter, C.; et al. Single-Cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1972. [Google Scholar] [CrossRef]
- Yu, G.; Kovkarova-Naumovski, E.; Jara, P.; Parwani, A.; Kass, D.; Ruiz, V.; López-Otín, C.; Rosas, I.O.; Gibson, K.F.; Cabrera, S.; et al. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am. J. Respir. Crit. Care Med. 2012, 186, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Lin, F.; Martins, V.; Rangel, M.P.; Capelozzi, V.L. Immunohistochemical and morphometric evaluation of COX-1 and COX-2 in the remodeled lung in idiopathic pulmonary fibrosis and systemic sclerosis. J. Bras. Pneumol. 2013, 39, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Keerthisingam, C.B.; Jenkins, R.G.; Harrison, N.K.; Hernandez-Rodriguez, N.A.; Booth, H.; Laurent, G.J.; Hart, S.L.; Foster, M.L.; McAnulty, R.J. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 2001, 158, 1411–1422. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assess cellular senescence in vitro and in vivo. FEBS J. 2020, 288, 56–80. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; di Fagagna, F.D. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. The interplay of the genetic architecture, aging and environmental factors in the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 64, 163–172. [Google Scholar] [CrossRef]
- Yao, C.; Guan, X.; Carraro, G.; Parimon, T.; Liu, X.; Huang, G.; Mulay, A.; Soukiasian, H.J.; David, G.; Weigt, S.S.; et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am. J. Respir. Crit. Care. Med. 2021, 204, 113. [Google Scholar] [CrossRef]
- Arcamone, F.; Animati, F.; Cuprunico, G.; Lombardi, P. New developments in antitumor antracyclines. Pharmacol. Ther. 1997, 76, 117–124. [Google Scholar] [CrossRef]
- Bojko, A.; Czarnecka-Herok, J.; Charzynska, A.; Dabrowski, M.; Sikora, E. Diversity of the senescence phenotype of cancer cells treated with chemotherapeutic agents. Cells 2019, 8, 1501. [Google Scholar] [CrossRef]
- Da Silveira, W.A.; Renaud, L.; Hazard, E.S.; Hardiman, G. MiRNA and lncRNA expression networks modulate cell cycle and DNA repair inhibition in senescent prostate cells. Genes 2022, 13, 208. [Google Scholar] [CrossRef]
- White, J.K.; Gerdin, A.-K.; Karp, N.A.; Ryder, E.; Buljan, M.; Bussell, J.N.; Salisbury, J.; Clare, S.; Ingham, N.J.; Podrini, C.; et al. Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell 2013, 154, 452–464. [Google Scholar] [CrossRef]
- Skarnes, W.C.; Rosen, B.; West, A.P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A.O.; Thomas, M.; Harrow, J.; Cox, T.; et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011, 474, 337–342. [Google Scholar] [CrossRef]
- Bradley, A.; Anastassiadis, K.; Ayadi, A.; Battey, J.F.; Bell, C.; Birling, M.-C.; Bottomley, J.; Brown, S.D.; Bürger, A.; Bult, C.J.; et al. The mammalian gene function resource: The international knockout mouse consortium. Mamm. Genome 2012, 23, 580–586. [Google Scholar] [CrossRef]
- Pettitt, S.J.; Liang, Q.; Rairdan, X.Y.; Moran, J.L.; Prosser, H.M.; Beier, D.R.; Lloyd, K.C.; Bradley, A.; Skarnes, W.C. Agouti C57BL/6N embryonic stem cells for mouse genetic resources. Nat. Methods 2009, 6, 493–495. [Google Scholar] [CrossRef]
- Pardo, A.; Barrios, R.; Gaxiola, M.; Segura-Valdez, L.; Carrillo, G.; Estrada, A.; Mejía, M.; Selman, M. Increase of lung neutrophils and upregulation of neutrophil gelatinase B and collagenase in hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 2000, 161, 1698–1704. [Google Scholar] [CrossRef]
- Cabrera, S.; Maciel, M.; Herrera, I.; Nava, T.; Vergara, F.; Gaxiola, M.; López Otín, C.; Selman, M.; Pardo, A. Essential role for the ATG4B protease and autophagy in bleomycin-induced pulmonary fibrosis. Autophagy 2015, 11, 670–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams,, T.S.; Schupp, J.C.; Poli, S.; Ayaub, E.A. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1983. [Google Scholar]
- Team, R.C. Others. R: A language and environment for statistical computing. Sci. Adv. 2013. [Google Scholar]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar]
- Hao, Y. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3578.e29. [Google Scholar]
- Wickham, Y. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Available online: https://www.infrafrontier.eu/sites/infrafrontier.eu/files/upload/public/pdf/Resources%20and%20Services/eucomm-alleles-overview_infrafrontier-2016.pdf (accessed on 20 July 2022).
- Available online: https://www.infrafrontier.eu/sites/infrafrontier.eu/files/upload/public/pdf/Resources%20and%20Services/eucomm_komp-csd_allele_conversion_guide_v3a_2016.pdf (accessed on 20 July 2022).
- Available online: https://www.infrafrontier.eu/sites/infrafrontier.eu/files/upload/public/pdf/genotype_protocols/EM08383_geno.pdf (accessed on 20 July 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urista, J.; Maldonado, M.; Toscano-Marquez, F.; Ramírez, R.; Balderas-Martínez, Y.I.; Becerril, C.; Romero, Y.; Selman, M.; Pardo, A. Lack of ZNF365 Drives Senescence and Exacerbates Experimental Lung Fibrosis. Cells 2022, 11, 2848. https://doi.org/10.3390/cells11182848
Urista J, Maldonado M, Toscano-Marquez F, Ramírez R, Balderas-Martínez YI, Becerril C, Romero Y, Selman M, Pardo A. Lack of ZNF365 Drives Senescence and Exacerbates Experimental Lung Fibrosis. Cells. 2022; 11(18):2848. https://doi.org/10.3390/cells11182848
Chicago/Turabian StyleUrista, Juan, Mariel Maldonado, Fernanda Toscano-Marquez, Remedios Ramírez, Yalbi Itzel Balderas-Martínez, Carina Becerril, Yair Romero, Moisés Selman, and Annie Pardo. 2022. "Lack of ZNF365 Drives Senescence and Exacerbates Experimental Lung Fibrosis" Cells 11, no. 18: 2848. https://doi.org/10.3390/cells11182848
APA StyleUrista, J., Maldonado, M., Toscano-Marquez, F., Ramírez, R., Balderas-Martínez, Y. I., Becerril, C., Romero, Y., Selman, M., & Pardo, A. (2022). Lack of ZNF365 Drives Senescence and Exacerbates Experimental Lung Fibrosis. Cells, 11(18), 2848. https://doi.org/10.3390/cells11182848








