HIV-Related Myocardial Fibrosis: Inflammatory Hypothesis and Crucial Role of Immune Cells Dysregulation
Abstract
1. Introduction
2. HIV Treatment and Cardiovascular Complications
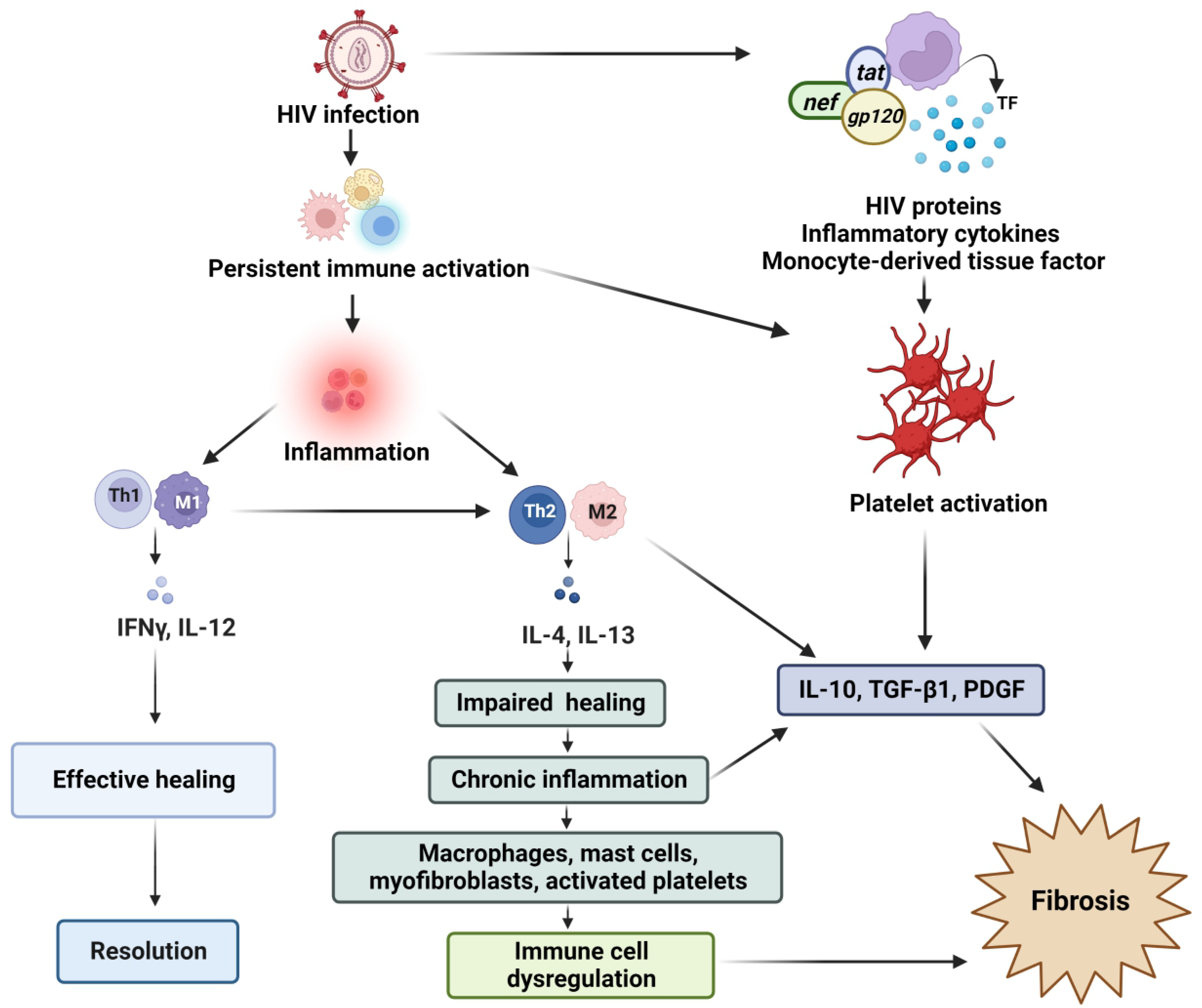
3. Immune Activation and Chronic Inflammation
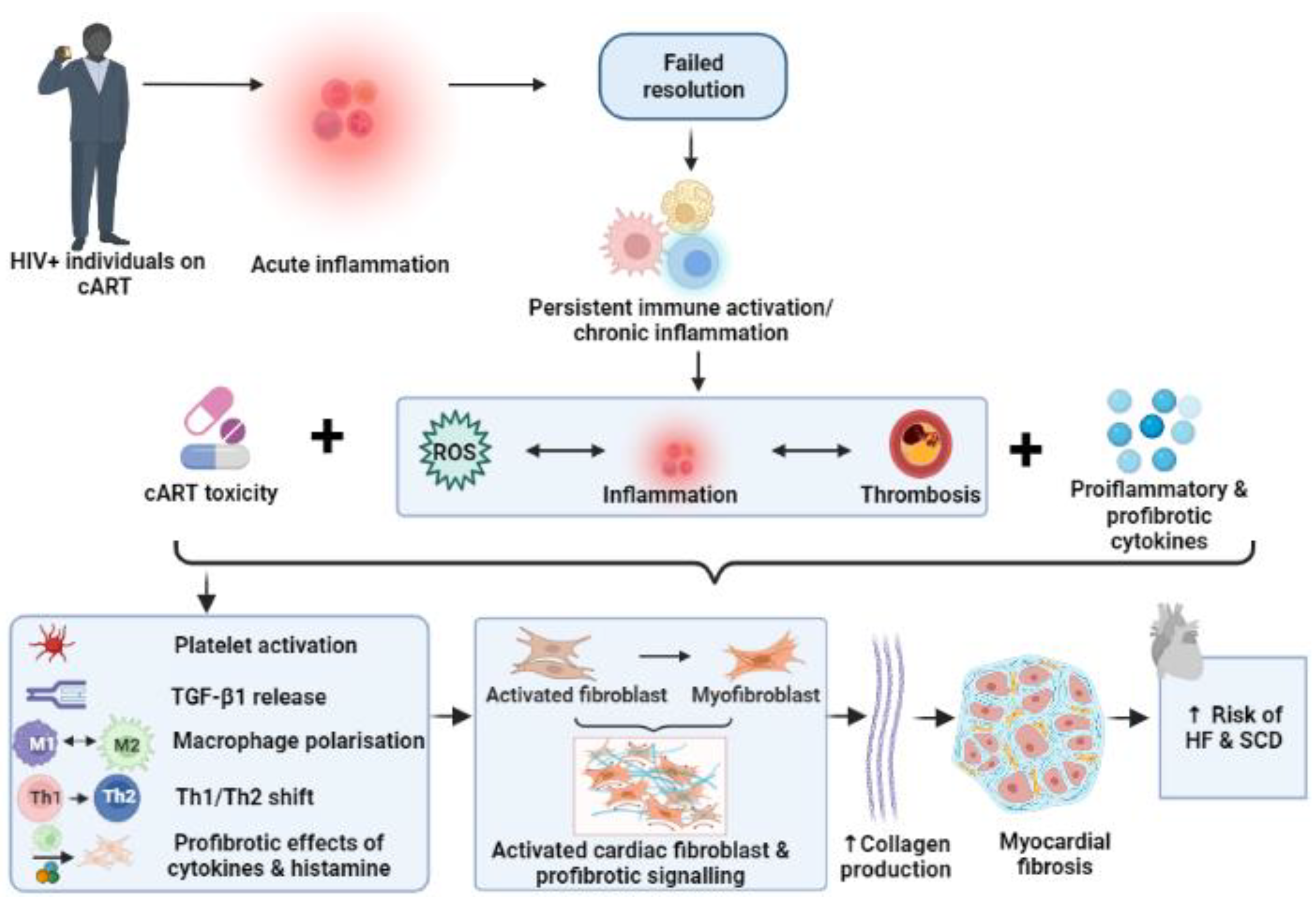
4. Persistent Immune Activation, Chronic Inflammation, and Cardiac Fibrosis
5. Myocardial Fibrosis: Role in the Pathogenesis of Heart Failure and Sudden Cardiac Death
6. Monocytes/Macrophages
7. Mast Cells
8. Lymphocytes
9. HIV-Related Myocardial Fibrosis: Role of Platelets
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS. Global HIV Statistics [Fact Sheet] 2022. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 19 August 2022).
- Hileman, C.O.; Funderburg, N.T. Inflammation, Immune Activation, and Antiretroviral Therapy in HIV. Curr. HIV/AIDS Rep. 2017, 14, 93–100. [Google Scholar] [CrossRef]
- Alonso, A.; Barnes, A.E.; Guest, J.L.; Shah, A.; Shao, I.Y.; Marconi, V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J. Am. Heart Assoc. 2019, 8, e012241. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, M.J.; Bogorodskaya, M.; Bloomfield, G.S.; Vedanthan, R.; Siedner, M.J.; Kwan, G.F.; Longenecker, C.T. Cardiovascular Complications of HIV in Endemic Countries. Curr. Cardiol. Rep. 2016, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Friis-Moller, N.; Ryom, L.; Smith, C.; Weber, R.; Reiss, P.; Dabis, F.; De Wit, S.; Monforte, A.D.; Kirk, O.; Fontas, E.; et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur. J. Prev. Cardiol. 2016, 23, 214–223. [Google Scholar] [CrossRef]
- Gopal, M.; Bhaskaran, A.; Khalife, W.I.; Barbagelata, A. Heart Disease in Patients with HIV/AIDS-An Emerging Clinical Problem. Curr. Cardiol. Rev. 2009, 5, 149–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bloomfield, G.S.; Hogan, J.W.; Keter, A.; Holland, T.L.; Sang, E.; Kimaiyo, S.; Velazquez, E.J. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: A retrospective analysis of electronic health records. BMC Infect. Dis. 2014, 14, 284. [Google Scholar] [CrossRef] [PubMed]
- Dominick, L.; Midgley, N.; Swart, L.M.; Sprake, D.; Deshpande, G.; Laher, I.; Joseph, D.; Teer, E.; Essop, M.F. HIV-related cardiovascular diseases: The search for a unifying hypothesis. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H731–H746. [Google Scholar] [CrossRef] [PubMed]
- Teer, E.; Joseph, D.E.; Driescher, N.; Nell, T.A.; Dominick, L.; Midgley, N.; Deshpande, G.; Page, M.J.; Pretorius, E.; Woudberg, N.J.; et al. HIV and cardiovascular diseases risk: Exploring the interplay between T-cell activation, coagulation, monocyte subsets, and lipid subclass alterations. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1146–H1157. [Google Scholar] [CrossRef]
- Yuyun, M.F.; Sliwa, K.; Kengne, A.P.; Mocumbi, A.O.; Bukhman, G. Cardiovascular Diseases in Sub-Saharan Africa Compared to High-Income Countries: An Epidemiological Perspective. Glob. Heart 2020, 15, 15. [Google Scholar] [CrossRef]
- Triant, V.A. Cardiovascular disease and HIV infection. Curr. HIV/AIDS Rep. 2013, 10, 199–206. [Google Scholar] [CrossRef]
- Longenecker, C.T.; Sullivan, C.; Baker, J.V. Immune activation and cardiovascular disease in chronic HIV infection. Curr. Opin. HIV AIDS 2016, 11, 216–225. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Tawakol, A. Inflammation and Fibrosis in HIV: Getting to the Heart of the Matter. Circ. Cardiovasc. Imaging 2016, 9, e004427. [Google Scholar] [CrossRef]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef]
- Musselwhite, L.W.; Sheikh, V.; Norton, T.D.; Rupert, A.; Porter, B.O.; Penzak, S.R.; Skinner, J.; Mican, J.M.; Hadigan, C.; Sereti, I. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS 2011, 25, 787–795. [Google Scholar] [CrossRef]
- Teer, E.; Essop, M.F. HIV and Cardiovascular Disease: Role of Immunometabolic Perturbations. Physiology 2018, 33, 74–82. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Hunt, P.W.; Ho, J.E.; Farah, H.H.; Schnell, A.; Hoh, R.; Martin, J.N.; Deeks, S.G.; Bolger, A.F. Impact of HIV infection on diastolic function and left ventricular mass. Circ. Heart Fail. 2010, 3, 132–139. [Google Scholar] [CrossRef]
- de Leuw, P.; Arendt, C.T.; Haberl, A.E.; Froadinadl, D.; Kann, G.; Wolf, T.; Stephan, C.; Schuettfort, G.; Vasquez, M.; Arcari, L.; et al. Myocardial Fibrosis and Inflammation by CMR Predict Cardiovascular Outcome in People Living with HIV. JACC Cardiovasc. Imaging 2021, 14, 1548–1557. [Google Scholar] [CrossRef]
- Shuldiner, S.R.; Wong, L.Y.; Peterson, T.E.; Wolfson, J.; Jermy, S.; Saad, H.; Lumbamba, M.A.J.; Singh, A.; Shey, M.; Meintjes, G.; et al. Myocardial Fibrosis Among Antiretroviral Therapy-Treated Persons with Human Immunodeficiency Virus in South Africa. Open Forum Infect. Dis. 2021, 8, ofaa600. [Google Scholar] [CrossRef]
- Bloomfield, G.S.; Barasa, F.A.; Doll, J.A.; Velazquez, E.J. Heart failure in sub-Saharan Africa. Curr. Cardiol. Rev. 2013, 9, 157–173. [Google Scholar] [CrossRef]
- Acierno, L.J. Cardiac complications in acquired immunodeficiency syndrome (AIDS): A review. J. Am. Coll. Cardiol. 1989, 13, 1144–1154. [Google Scholar] [CrossRef][Green Version]
- Filardi, P.P.; Paolillo, S.; Marciano, C.; Iorio, A.; Losco, T.; Marsico, F.; Scala, O.; Ruggiero, D.; Ferraro, S.; Chiariello, M. Cardiovascular effects of antiretroviral drugs: Clinical review. Cardiovasc. Hematol. Disord. Drug Targets 2008, 8, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Montessori, V.; Press, N.; Harris, M.; Akagi, L.; Montaner, J.S. Adverse effects of antiretroviral therapy for HIV infection. CMAJ 2004, 170, 229–238. [Google Scholar]
- Carr, A.; Samaras, K.; Burton, S.; Law, M.; Freund, J.; Chisholm, D.J.; Cooper, D.A. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998, 12, F51–F58. [Google Scholar] [CrossRef]
- Matoga, M.M.; Hosseinipour, M.C.; Aga, E.; Ribaudo, H.J.; Kumarasamy, N.; Bartlett, J.; Hughes, M.D.; Team, A.A.S. Hyperlipidaemia in HIV-infected patients on lopinavir/ritonavir monotherapy in resource-limited settings. Antivir. Ther. 2017, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dorjee, K.; Choden, T.; Baxi, S.M.; Steinmaus, C.; Reingold, A.L. Risk of cardiovascular disease associated with exposure to abacavir among individuals with HIV: A systematic review and meta-analyses of results from 17 epidemiologic studies. Int. J. Antimicrob. Agents 2018, 52, 541–553. [Google Scholar] [CrossRef]
- Abebe, M.; Kinde, S.; Belay, G.; Gebreegziabxier, A.; Challa, F.; Gebeyehu, T.; Nigussie, P.; Tegbaru, B. Antiretroviral treatment associated hyperglycemia and dyslipidemia among HIV infected patients at Burayu Health Center, Addis Ababa, Ethiopia: A cross-sectional comparative study. BMC Res. Notes 2014, 7, 380. [Google Scholar] [CrossRef]
- van Oosterhout, J.J.; Mallewa, J.; Kaunda, S.; Chagoma, N.; Njalale, Y.; Kampira, E.; Mukaka, M.; Heyderman, R.S. Stavudine toxicity in adult longer-term ART patients in Blantyre, Malawi. PLoS ONE 2012, 7, e42029. [Google Scholar] [CrossRef][Green Version]
- Gelpi, M.; Afzal, S.; Fuchs, A.; Lundgren, J.; Knudsen, A.D.; Drivsholm, N.; Mocroft, A.; Lebech, A.M.; Lindegaard, B.; Kuhl, J.T.; et al. Prior exposure to thymidine analogs and didanosine is associated with long-lasting alterations in adipose tissue distribution and cardiovascular risk factors. AIDS 2019, 33, 675–683. [Google Scholar] [CrossRef]
- Mavroudis, C.A.; Majumder, B.; Loizides, S.; Christophides, T.; Johnson, M.; Rakhit, R.D. Coronary artery disease and HIV; getting to the HAART of the matter. Int. J. Cardiol. 2013, 167, 1147–1153. [Google Scholar] [CrossRef]
- Vos, A.G.; Venter, W.D.F. Cardiovascular toxicity of contemporary antiretroviral therapy. Curr. Opin. HIV AIDS 2021, 16, 286–291. [Google Scholar] [CrossRef]
- Thiara, D.K.; Liu, C.Y.; Raman, F.; Mangat, S.; Purdy, J.B.; Duarte, H.A.; Schmidt, N.; Hur, J.; Sibley, C.T.; Bluemke, D.A.; et al. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J. Infect. Dis. 2015, 212, 1544–1551. [Google Scholar] [CrossRef]
- Cerrato, E.; D’Ascenzo, F.; Biondi-Zoccai, G.; Calcagno, A.; Frea, S.; Grosso Marra, W.; Castagno, D.; Omede, P.; Quadri, G.; Sciuto, F.; et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: A meta-analysis in the highly active antiretroviral therapy era. Eur. Heart J. 2013, 34, 1432–1436. [Google Scholar] [CrossRef]
- Savvoulidis, P.; Butler, J.; Kalogeropoulos, A. Cardiomyopathy and Heart Failure in Patients with HIV Infection. Can. J. Cardiol. 2019, 35, 299–309. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Chang, C.H.; Skanderson, M.; Patterson, O.V.; DuVall, S.L.; Brandt, C.A.; So-Armah, K.A.; Vasan, R.S.; Oursler, K.A.; Gottdiener, J.; et al. Association Between HIV Infection and the Risk of Heart Failure with Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017, 2, 536–546. [Google Scholar] [CrossRef]
- Alvi, R.M.; Neilan, A.M.; Tariq, N.; Hassan, M.O.; Awadalla, M.; Zhang, L.; Afshar, M.; Rokicki, A.; Mulligan, C.P.; Triant, V.A.; et al. The Risk for Sudden Cardiac Death Among Patients Living with Heart Failure and Human Immunodeficiency Virus. JACC Heart Fail 2019, 7, 759–767. [Google Scholar] [CrossRef]
- Yan, C.; Li, R.; Guo, X.; Yu, H.; Li, W.; Li, W.; Ren, M.; Yang, M.; Li, H. Cardiac Involvement in Human Immunodeficiency Virus Infected Patients: An Observational Cardiac Magnetic Resonance Study. Front. Cardiovasc. Med. 2021, 8, 756162. [Google Scholar] [CrossRef]
- Zanni, M.V.; Awadalla, M.; Toribio, M.; Robinson, J.; Stone, L.A.; Cagliero, D.; Rokicki, A.; Mulligan, C.P.; Ho, J.E.; Neilan, A.M.; et al. Immune Correlates of Diffuse Myocardial Fibrosis and Diastolic Dysfunction Among Aging Women with Human Immunodeficiency Virus. J. Infect. Dis. 2020, 221, 1315–1320. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution? Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Pereyra, F.; Lo, J.; Triant, V.A.; Wei, J.; Buzon, M.J.; Fitch, K.V.; Hwang, J.; Campbell, J.H.; Burdo, T.H.; Williams, K.C.; et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012, 26, 2409–2412. [Google Scholar] [CrossRef]
- Liu, Z.; Cumberland, W.G.; Hultin, L.E.; Prince, H.E.; Detels, R.; Giorgi, J.V. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997, 16, 83–92. [Google Scholar] [CrossRef]
- Lohman-Payne, B.; Koster, J.; Gabriel, B.; Chilengi, R.; Forman, L.S.; Heeren, T.; Duffy, C.R.; Herlihy, J.; Crimaldi, S.; Gill, C.; et al. Persistent Immune Activation in Human Immunodeficiency Virus-Infected Pregnant Women Starting Combination Antiretroviral Therapy After Conception. J. Infect. Dis. 2022, 225, 1162–1167. [Google Scholar] [CrossRef]
- Teer, E.; Joseph, D.E.; Glashoff, R.H.; Faadiel Essop, M. Monocyte/Macrophage-Mediated Innate Immunity in HIV-1 Infection: From Early Response to Late Dysregulation and Links to Cardiovascular Diseases Onset. Virol. Sin. 2021, 36, 565–576. [Google Scholar] [CrossRef]
- Appay, V.; Sauce, D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. J. Pathol. 2008, 214, 231–241. [Google Scholar] [CrossRef]
- Paiardini, M.; Muller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101. [Google Scholar] [CrossRef]
- Chu, A.J. Tissue factor mediates inflammation. Arch. Biochem. Biophys. 2005, 440, 123–132. [Google Scholar] [CrossRef]
- Sinha, A.; Ma, Y.; Scherzer, R.; Hur, S.; Li, D.; Ganz, P.; Deeks, S.G.; Hsue, P.Y. Role of T-Cell Dysfunction, Inflammation, and Coagulation in Microvascular Disease in HIV. J. Am. Heart Assoc. 2016, 5, e004243. [Google Scholar] [CrossRef]
- Vachiat, A.; McCutcheon, K.; Tsabedze, N.; Zachariah, D.; Manga, P. HIV and Ischemic Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 73–82. [Google Scholar] [CrossRef]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H.; Melchjorsen, J.; Larsen, C.S.; Paludan, S.R. Innate immune recognition and activation during HIV infection. Retrovirology 2010, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.; Tracy, R.; Osler, T.; Grace, C. Elevated Biomarkers of Inflammation and Coagulation in Patients with HIV Are Associated with Higher Framingham and VACS Risk Index Scores. PLoS ONE 2015, 10, e0144312. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Arildsen, H.; Sorensen, K.E.; Ingerslev, J.M.; Ostergaard, L.J.; Laursen, A.L. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV Med. 2013, 14, 1–9. [Google Scholar] [CrossRef]
- Marincowitz, C.; Genis, A.; Goswami, N.; De Boever, P.; Nawrot, T.S.; Strijdom, H. Vascular endothelial dysfunction in the wake of HIV and ART. FEBS J. 2019, 286, 1256–1270. [Google Scholar] [CrossRef]
- Nou, E.; Lo, J.; Grinspoon, S.K. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 2016, 30, 1495–1509. [Google Scholar] [CrossRef]
- Sager, H.B.; Kessler, T.; Schunkert, H. Monocytes and macrophages in cardiac injury and repair. J. Thorac. Dis. 2017, 9, S30–S35. [Google Scholar] [CrossRef]
- Butler, J.; Kalogeropoulos, A.P.; Anstrom, K.J.; Hsue, P.Y.; Kim, R.J.; Scherzer, R.; Shah, S.J.; Shah, S.H.; Velazquez, E.J.; Hernandez, A.F.; et al. Diastolic Dysfunction in Individuals with Human Immunodeficiency Virus Infection: Literature Review, Rationale and Design of the Characterizing Heart Function on Antiretroviral Therapy (CHART) Study. J. Card. Fail. 2018, 24, 255–265. [Google Scholar] [CrossRef]
- Holloway, C.J.; Ntusi, N.; Suttie, J.; Mahmod, M.; Wainwright, E.; Clutton, G.; Hancock, G.; Beak, P.; Tajar, A.; Piechnik, S.K.; et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013, 128, 814–822. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, J.; Terry, H.; Choi, M.E.; Laurence, J. Transforming growth factor-β1-mediated cardiac fibrosis: Potential role in HIV and HIV/antiretroviral therapy-linked cardiovascular disease. AIDS 2016, 30, 535–542. [Google Scholar] [CrossRef]
- Utay, N.; Ananworanich, J.; Pinyakorn, S.; Rupert, A.; Sutthichom, D.; Puttamaswin, S. Inflammation persists despite early initiation of ART in acute HIV infection. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, DC, USA, 23–26 February 2015; pp. 123–128. [Google Scholar]
- Tseng, Z.H.; Moffatt, E.; Kim, A.; Vittinghoff, E.; Ursell, P.; Connolly, A.; Olgin, J.E.; Wong, J.K.; Hsue, P.Y. Sudden Cardiac Death and Myocardial Fibrosis, Determined by Autopsy, in Persons with HIV. N. Engl. J. Med. 2021, 384, 2306–2316. [Google Scholar] [CrossRef]
- Tseng, Z.H.; Secemsky, E.A.; Dowdy, D.; Vittinghoff, E.; Moyers, B.; Wong, J.K.; Havlir, D.V.; Hsue, P.Y. Sudden cardiac death in patients with human immunodeficiency virus infection. J. Am. Coll. Cardiol. 2012, 59, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Haberlen, S.A.; Plankey, M.W.; Palella, F.J.; Piggott, D.A.; Kirk, G.D.; Margolick, J.B.; Post, W.S. Human immunodeficiency viral infection and differences in interstitial ventricular fibrosis and left atrial size. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Hill, M.A.; Sowers, J.R. Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension 2018, 72, 537–548. [Google Scholar] [CrossRef]
- Smaill, B.H.; Zhao, J.; Trew, M.L. Three-dimensional impulse propagation in myocardium: Arrhythmogenic mechanisms at the tissue level. Circ. Res. 2013, 112, 834–848. [Google Scholar] [CrossRef]
- Weber, K.T. Cardiac interstitium in health and disease: The fibrillar collagen network. J. Am. Coll. Cardiol. 1989, 13, 1637–1652. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Jung, M.; Hall, M.E.; DeLeon-Pennell, K.Y. Proteomic analysis of the cardiac extracellular matrix: Clinical research applications. Expert Rev. Proteom. 2018, 15, 105–112. [Google Scholar] [CrossRef]
- Silva, A.C.; Pereira, C.; Fonseca, A.; Pinto-do, O.P.; Nascimento, D.S. Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response. Front. Cell Dev. Biol. 2020, 8, 621644. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current Understanding of the Pathophysiology of Myocardial Fibrosis and Its Quantitative Assessment in Heart Failure. Front. Physiol. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Janicki, J.S.; Brower, G.L. The role of myocardial fibrillar collagen in ventricular remodeling and function. J. Card. Fail. 2002, 8, S319–S325. [Google Scholar] [CrossRef] [PubMed]
- Toribio, M.; Neilan, T.G.; Zanni, M.V. Heart failure among people with HIV: Evolving risks, mechanisms, and preventive considerations. Curr. HIV/AIDS Rep. 2019, 16, 371–380. [Google Scholar] [CrossRef]
- Beltrami, C.A.; Finato, N.; Rocco, M.; Feruglio, G.A.; Puricelli, C.; Cigola, E.; Quaini, F.; Sonnenblick, E.H.; Olivetti, G.; Anversa, P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 1994, 89, 151–163. [Google Scholar] [CrossRef]
- Khan, R.; Sheppard, R. Fibrosis in heart disease: Understanding the role of transforming growth factor-β1 in cardiomyopathy, valvular disease and arrhythmia. Immunology 2006, 118, 10–24. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Hulsmans, M.; Sam, F.; Nahrendorf, M. Monocyte and macrophage contributions to cardiac remodeling. J. Mol. Cell. Cardiol. 2016, 93, 149–155. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.; Miao, Y.; Wang, Y.; Cui, W.; Guo, J.; Qiu, S.; Han, Y.; Jia, L.; Li, H. Serum-Glucocorticoid Regulated Kinase 1 Regulates Alternatively Activated Macrophage Polarization Contributing to Angiotensin II–Induced Inflammation and Cardiac Fibrosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1675–1686. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A.; Beck, G.A.; Campbell, J.H.; Miller, A.D.; Burdo, T.H.; Williams, K.C. Anti-alpha4 Integrin Antibody Blocks Monocyte/Macrophage Traffic to the Heart and Decreases Cardiac Pathology in a SIV Infection Model of AIDS. J. Am. Heart Assoc. 2015, 4, e001932. [Google Scholar] [CrossRef] [PubMed]
- Levick, S.P.; Melendez, G.C.; Plante, E.; McLarty, J.L.; Brower, G.L.; Janicki, J.S. Cardiac mast cells: The centrepiece in adverse myocardial remodelling. Cardiovasc. Res. 2011, 89, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Levi-Schaffer, F.; Rubinchik, E. Mast cell/fibroblast interactions. Clin. Exp. Allergy 1994, 24, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Hatamochi, A.; Fujiwara, K.; Ueki, H. Effects of histamine on collagen synthesis by cultured fibroblasts derived from guinea pig skin. Arch. Dermatol. Res. 1984, 277, 60–64. [Google Scholar] [CrossRef]
- Kim, J.; Washio, T.; Asakura, M.; Asanuma, H.; Kitakaze, M. Impact of Blockade of Histamine H2 Receptors on Chronic Heart Failure. J. Card. Fail. 2007, 13, S25. [Google Scholar] [CrossRef]
- Kanellakis, P.; Ditiatkovski, M.; Kostolias, G.; Bobik, A. A pro-fibrotic role for interleukin-4 in cardiac pressure overload. Cardiovasc. Res. 2012, 95, 77–85. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Wei, L. Immunological aspect of cardiac remodeling: T lymphocyte subsets in inflammation-mediated cardiac fibrosis. Exp. Mol. Pathol. 2011, 90, 74–78. [Google Scholar] [CrossRef]
- Tang, T.T.; Yuan, J.; Zhu, Z.F.; Zhang, W.C.; Xiao, H.; Xia, N.; Yan, X.X.; Nie, S.F.; Liu, J.; Zhou, S.F.; et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 2012, 107, 232. [Google Scholar] [CrossRef] [PubMed]
- Laurence, J.; Elhadad, S.; Robison, T.; Terry, H.; Varshney, R.; Woolington, S.; Ghafoory, S.; Choi, M.E.; Ahamed, J. HIV protease inhibitor-induced cardiac dysfunction and fibrosis is mediated by platelet-derived TGF-beta1 and can be suppressed by exogenous carbon monoxide. PLoS ONE 2017, 12, e0187185. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, J.; Laurence, J. Role of platelet-derived transforming growth factor-β1 and reactive oxygen species in radiation-induced organ fibrosis. Antioxid. Redox Signal. 2017, 27, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Wang, W.; Qu, J.; Croft, L.; Degen, J.L.; Coller, B.S.; Ahamed, J. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood 2012, 119, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Nkambule, B.B.; Mxinwa, V.; Mkandla, Z.; Mutize, T.; Mokgalaboni, K.; Nyambuya, T.M.; Dludla, P.V. Platelet activation in adult HIV-infected patients on antiretroviral therapy: A systematic review and meta-analysis. BMC Med. 2020, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Lindsey, M.L.; Michael, L.H.; Youker, K.A.; Bressler, R.B.; Mendoza, L.H.; Spengler, R.N.; Smith, C.W.; Entman, M.L. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 1998, 98, 699–710. [Google Scholar] [CrossRef]
- Varshney, R.; Murphy, B.; Woolington, S.; Ghafoory, S.; Chen, S.; Robison, T.; Ahamed, J. Inactivation of platelet-derived TGF-β1 attenuates aortic stenosis progression in a robust murine model. Blood Adv. 2019, 3, 777–788. [Google Scholar] [CrossRef]
- Ask, K.; Bonniaud, P.; Maass, K.; Eickelberg, O.; Margetts, P.J.; Warburton, D.; Groffen, J.; Gauldie, J.; Kolb, M. Progressive pulmonary fibrosis is mediated by TGF-β isoform 1 but not TGF-β3. Int. J. Biochem. Cell Biol. 2008, 40, 484–495. [Google Scholar] [CrossRef]
- Greene, R.M.; Nugent, P.; Mukhopadhyay, P.; Warner, D.R.; Pisano, M.M. Intracellular dynamics of Smad-mediated TGFβ signaling. J. Cell. Physiol. 2003, 197, 261–271. [Google Scholar] [CrossRef]
- Lijnen, P.; Petrov, V. Transforming growth factor-beta 1-induced collagen production in cultures of cardiac fibroblasts is the result of the appearance of myofibroblasts. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 333–344. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Luangmonkong, T.; Mangmool, S.; Kurose, H. Therapeutic Targets for the Treatment of Cardiac Fibrosis and Cancer: Focusing on TGF-beta Signaling. Front. Cardiovasc. Med. 2020, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Assoian, R.K.; Sporn, M.B. Type beta transforming growth factor in human platelets: Release during platelet degranulation and action on vascular smooth muscle cells. J. Cell Biol. 1986, 102, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A. Platelets and infection–an emerging role of platelets in viral infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef] [PubMed]
- Funderburg, N.T.; Mayne, E.; Sieg, S.F.; Asaad, R.; Jiang, W.; Kalinowska, M.; Luciano, A.A.; Stevens, W.; Rodriguez, B.; Brenchley, J.M.; et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: Relationship to in vivo coagulation and immune activation. Blood 2010, 115, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Loelius, S.G.; Lannan, K.L.; Casey, A.E.; Spinelli, S.L.; Phipps, R.P. Antiretroviral drugs and tobacco smoke dysregulate human platelets: A novel investigation into the etiology of HIV co-morbid cardiovascular disease. J. Immunol. 2017, 198, 125.10. [Google Scholar]
- van der Heijden, W.A.; van Crevel, R.; de Groot, P.G.; Urbanus, R.T.; Koenen, H.; Bosch, M.; Keuter, M.; van der Ven, A.J.; de Mast, Q. A switch to a raltegravir containing regimen does not lower platelet reactivity in HIV-infected individuals. AIDS 2018, 32, 2469–2475. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teer, E.; Dominick, L.; Mukonowenzou, N.C.; Essop, M.F. HIV-Related Myocardial Fibrosis: Inflammatory Hypothesis and Crucial Role of Immune Cells Dysregulation. Cells 2022, 11, 2825. https://doi.org/10.3390/cells11182825
Teer E, Dominick L, Mukonowenzou NC, Essop MF. HIV-Related Myocardial Fibrosis: Inflammatory Hypothesis and Crucial Role of Immune Cells Dysregulation. Cells. 2022; 11(18):2825. https://doi.org/10.3390/cells11182825
Chicago/Turabian StyleTeer, Eman, Leanne Dominick, Nyasha C. Mukonowenzou, and M. Faadiel Essop. 2022. "HIV-Related Myocardial Fibrosis: Inflammatory Hypothesis and Crucial Role of Immune Cells Dysregulation" Cells 11, no. 18: 2825. https://doi.org/10.3390/cells11182825
APA StyleTeer, E., Dominick, L., Mukonowenzou, N. C., & Essop, M. F. (2022). HIV-Related Myocardial Fibrosis: Inflammatory Hypothesis and Crucial Role of Immune Cells Dysregulation. Cells, 11(18), 2825. https://doi.org/10.3390/cells11182825





