Reprogramming—Evolving Path to Functional Surrogate β-Cells
Abstract
1. Introduction
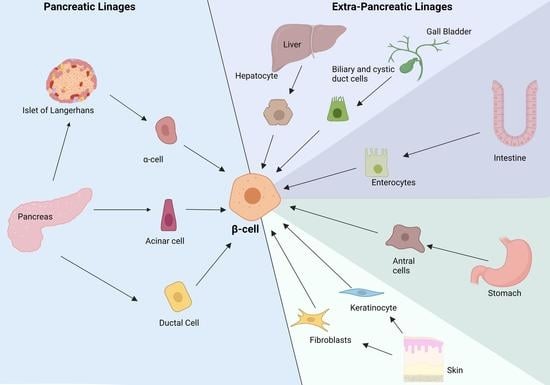
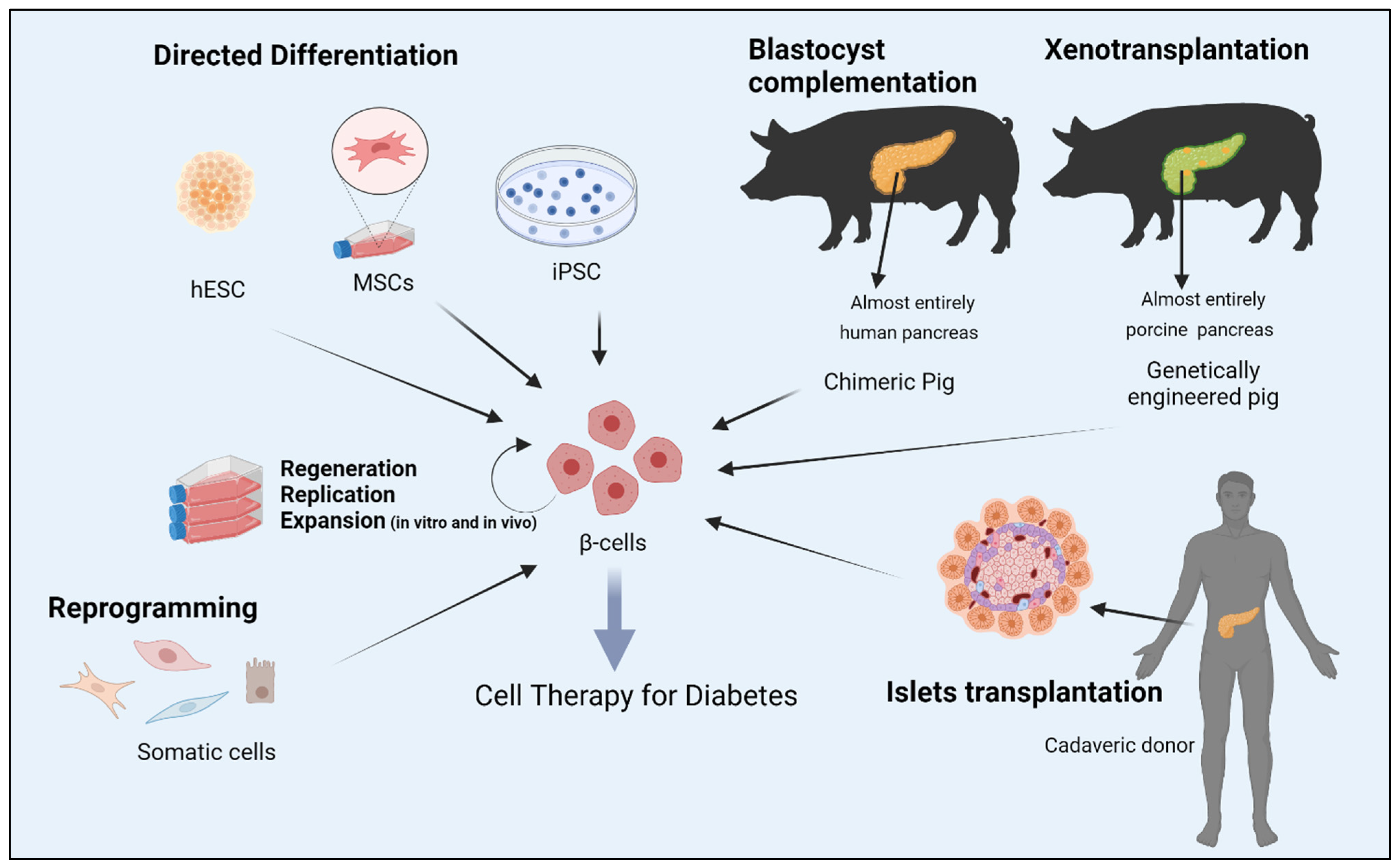
2. Reprogramming Cells to Make Insulin
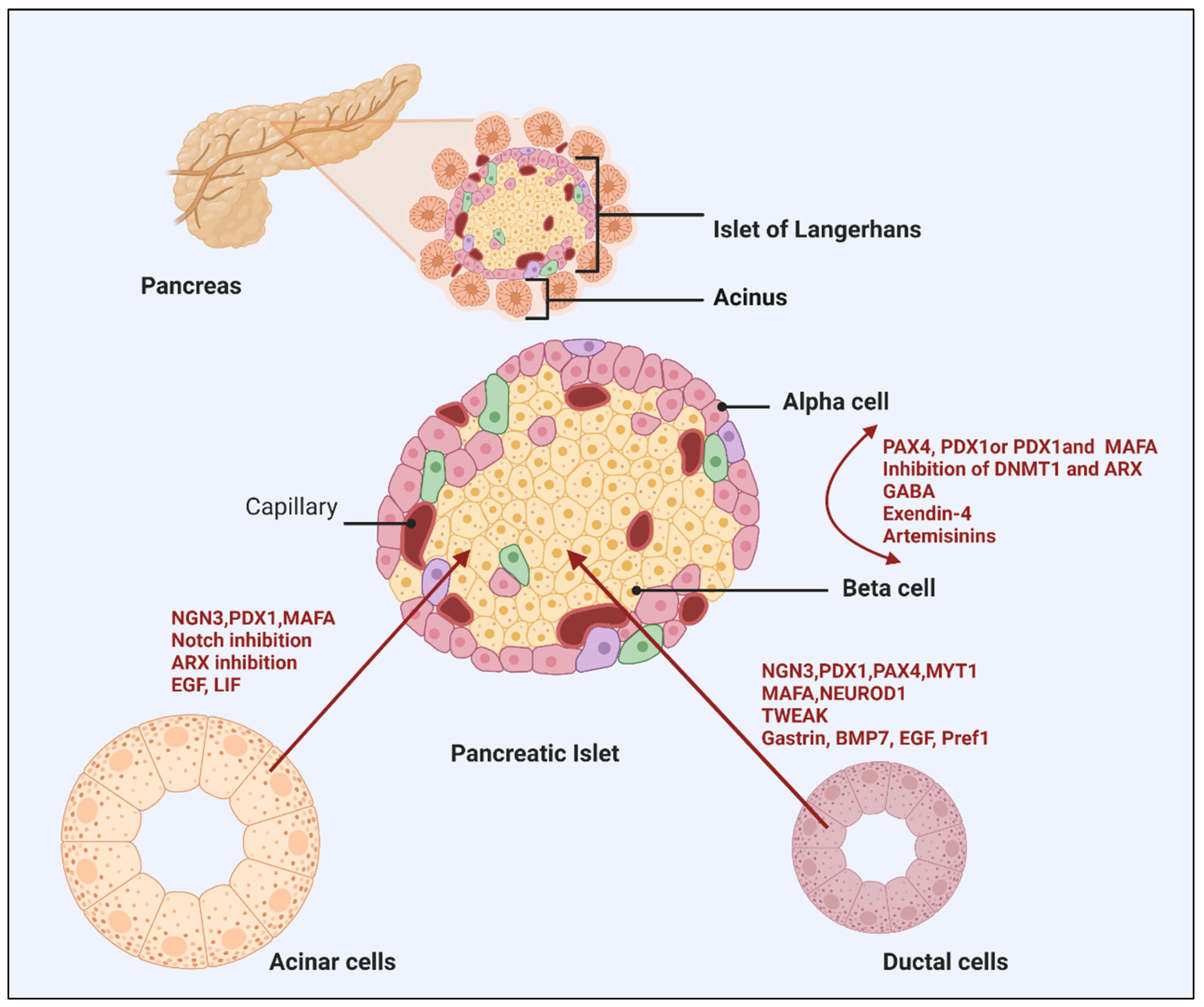
3. Alpha to β-cell Reprogramming
4. Pancreatic Non-Endocrine Cells
4.1. Acinar Cell Reprogramming
4.2. Duct Epithelial Cells
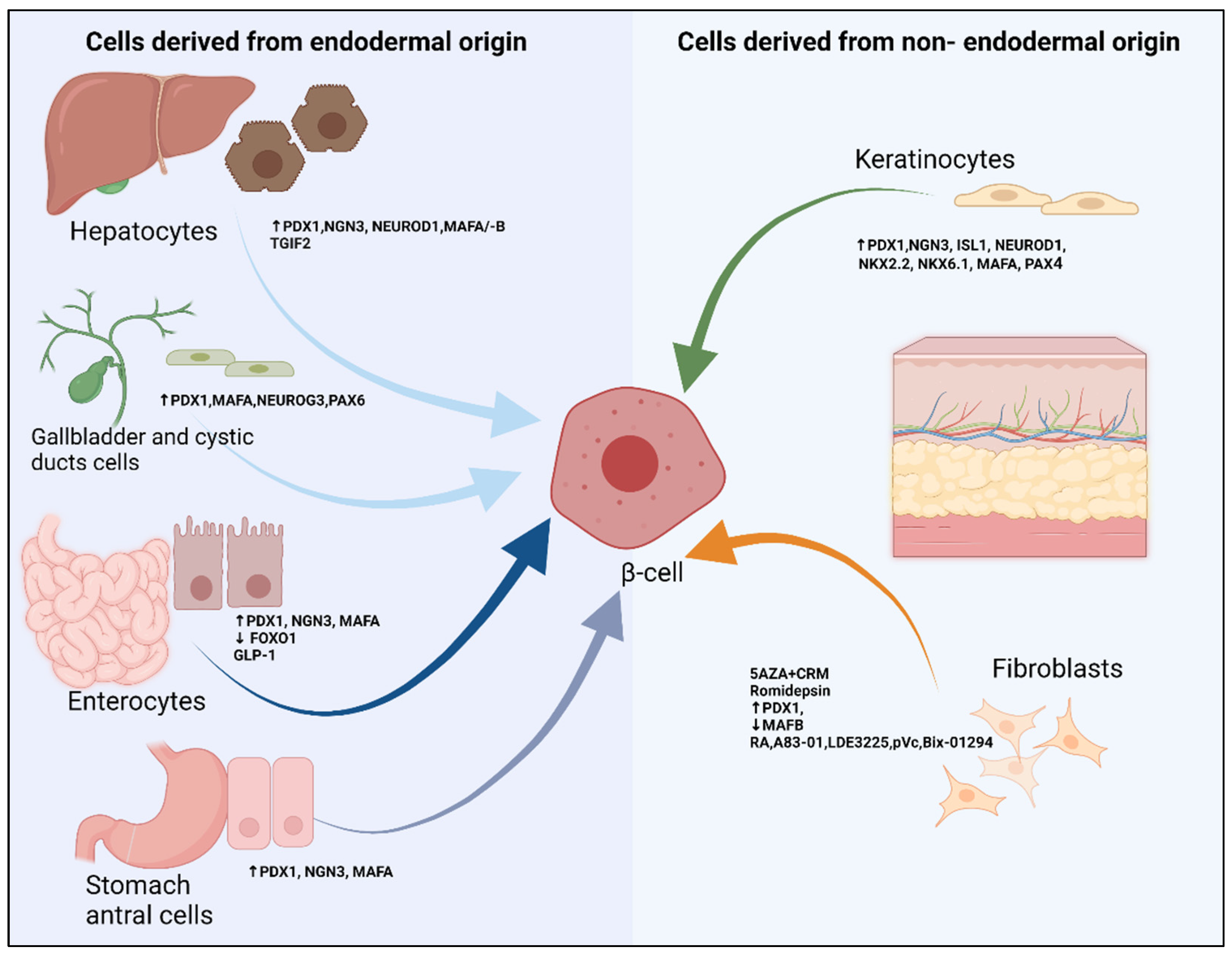
5. Extra Pancreatic Cell Sources
- (A)
- Cells derived from endodermal origin
5.1. Liver, Gallbladder and Cystic Derived Cells
5.2. Intestinal and Antral Stomach Cells
- (B)
- Cells derived from non-endodermal origin
5.3. Fibroblasts
5.4. Keratinocytes
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- International Diabetes Federation. Diabetes Facts & Figures; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 1 September 2022).
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Chen, H.; Chen, G.; Zheng, X.; Guo, Y. Contribution of specific diseases and injuries to changes in health adjusted life expectancy in 187 countries from 1990 to 2013: Retrospective observational study. BMJ 2019, 364, l969. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial (DCCT); Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015, 64, 631–642. [Google Scholar] [CrossRef]
- Zinman, B.; Genuth, S.; Nathan, D.M. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study: 30th Anniversary Presentations. Diabetes Care 2014, 37, 8. [Google Scholar] [CrossRef]
- Polonsky, K.S. The Past 200 Years in Diabetes. N. Engl. J. Med. 2012, 367, 1332–1340. [Google Scholar] [CrossRef]
- NICE-Sugar Study Investigators. Hypoglycemia and risk of death in critically ill patients. N. Engl. J. Med. 2012, 367, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef]
- Brown, S.A.; Kovatchev, B.P.; Raghinaru, D.; Lum, J.W.; Buckingham, B.A.; Kudva, Y.C.; Laffel, L.M.; Levy, C.J.; Pinsker, J.E.; Wadwa, R.P.; et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 1707–1717. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International Trial of the Edmonton Protocol for Islet Transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef]
- Oliver-Krasinski, J.M.; Stoffers, D.A. On the origin of the beta cell. Genes Dev. 2008, 22, 1998–2021. [Google Scholar] [CrossRef]
- Assady, S.; Maor, G.; Amit, M.; Itskovitz-Eldor, J.; Skorecki, K.L.; Tzukerman, M. Insulin Production by Human Embryonic Stem Cells. Diabetes 2001, 50, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Bang, A.G.; Eliazer, S.; Kelly, O.G.; Agulnick, A.D.; Smart, N.G.; Moorman, M.A.; Kroon, E.; Carpenter, M.K.; Baetge, E.E. Production of pancreatic hormone–expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006, 24, 1392–1401. [Google Scholar] [CrossRef]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Nair, G.G.; Liu, J.S.; Russ, H.A.; Tran, S.; Saxton, M.S.; Chen, R.; Juang, C.; Li, M.-I.; Nguyen, V.Q.; Giacometti, S.; et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nat. Cell Biol. 2019, 21, 263–274. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Augsornworawat, P.; Maxwell, K.G.; Velazco-Cruz, L.; Millman, J.R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 2020, 38, 460–470. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef]
- Russ, H.A.; Parent, A.V.; Ringler, J.J.; Hennings, T.G.; Nair, G.G.; Shveygert, M.; Guo, T.; Puri, S.; Haataja, L.; Cirulli, V.; et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015, 34, 1759–1772. [Google Scholar] [CrossRef]
- Naujok, O.; Burns, C.; Jones, P.M.; Lenzen, S. Insulin-producing surrogate beta-cells from embryonic stem cells: Are we there yet? Mol. Ther. 2011, 19, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Melton, D.A. How to make a functional beta-cell. Development 2013, 140, 2472–2483. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, K.; Utsunomiya, N.; Takaki, R.; Yoshimatsu, H.; Sakata, T. Cellular interaction between mouse pancreatic alpha-cell and beta-cell lines: Possible contact-dependent inhibition of insulin secretion. Exp. Biol. Med. 2003, 228, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Thorel, F.; Népote, V.; Avril, I.; Kohno, K.; Desgraz, R.; Chera, S.; Herrera, P.L. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010, 464, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Collombat, P.; Xu, X.; Ravassard, P.; Sosa-Pineda, B.; Dussaud, S.; Billestrup, N.; Madsen, O.D.; Serup, P.; Heimberg, H.; Mansouri, A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009, 138, 449–462. [Google Scholar] [CrossRef]
- Yang, Y.P.; Thorel, F.; Boyer, D.F.; Herrera, P.L.; Wright, C.V. Context-specific alpha- to-beta-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011, 25, 1680–1685. [Google Scholar] [CrossRef]
- Matsuoka, T.A.; Kawashima, S.; Miyatsuka, T.; Sasaki, S.; Shimo, N.; Katakami, N.; Kawamori, D.; Takebe, S.; Herrera, P.L.; Kaneto, H.; et al. Mafa Enables Pdx1 to Effectively Convert Pancreatic Islet Progenitors and Committed Islet alpha-Cells Into beta-Cells In Vivo. Diabetes 2017, 66, 1293–1300. [Google Scholar] [CrossRef]
- Chakravarthy, H.; Gu, X.; Enge, M.; Dai, X.; Wang, Y.; Damond, N.; Downie, C.; Liu, K.; Wang, J.; Xing, Y.; et al. Converting Adult Pancreatic Islet alpha Cells into beta Cells by Targeting Both Dnmt1 and Arx. Cell Metab. 2017, 25, 622–634. [Google Scholar] [CrossRef]
- Furuyama, K.; Chera, S.; Van Gurp, L.; Oropeza, D.; Ghila, L.; Damond, N.; Vethe, H.; Paulo, J.A.; Joosten, A.M.; Berney, T.; et al. Diabetes relief in mice by glucose-sensing insulin-secreting human alpha-cells. Nature 2019, 567, 43–48. [Google Scholar] [CrossRef]
- Xiao, X.; Guo, P.; Shiota, C.; Zhang, T.; Coudriet, G.M.; Fischbach, S.; Prasadan, K.; Fusco, J.; Ramachandran, S.; Witkowski, P.; et al. Endogenous Reprogramming of Alpha Cells into Beta Cells, Induced by Viral Gene Therapy, Reverses Autoimmune Diabetes. Cell Stem Cell 2018, 22, 78–90.e4. [Google Scholar] [CrossRef]
- Fomina-Yadlin, D.; Kubicek, S.; Walpita, D.; Dančik, V.; Hecksher-Sørensen, J.; Bittker, J.A.; Sharifnia, T.; Shamji, A.; Clemons, P.A.; Wagner, B.K.; et al. Small-molecule inducers of insulin expression in pancreatic α-cells. Proc. Natl. Acad. Sci. USA 2010, 107, 15099–15104. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, C.; Choung, J.S.; Jung, H.S.; Jun, H.S. Glucagon-Like Peptide 1 Increases beta-Cell Regeneration by Promoting alpha- to beta-Cell Transdifferentiation. Diabetes 2018, 67, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, N.; Vieira, A.; Courtney, M.; Record, F.; Gjernes, E.; Avolio, F.; Hadzic, B.; Druelle, N.; Napolitano, T.; Navarro-Sanz, S.; et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 2017, 168, 73–85.e11. [Google Scholar] [CrossRef] [PubMed]
- Menegaz, D.; Hagan, D.W.; Almaça, J.; Cianciaruso, C.; Rodriguez-Diaz, R.; Molina, J.; Dolan, R.M.; Becker, M.W.; Schwalie, P.C.; Nano, R.; et al. Mechanism and effects of pulsatile GABA secretion from cytosolic pools in the human beta cell. Nat. Metab. 2019, 1, 1110–1126. [Google Scholar] [CrossRef]
- Li, J.; Casteels, T.; Frogne, T.; Ingvorsen, C.; Honoré, C.; Courtney, M.; Huber, K.V.; Schmitner, N.; Kimmel, R.A.; Romanov, R.A.; et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Cell 2016, 168, 86–100.e15. [Google Scholar] [CrossRef]
- van der Meulen, T.L.S.; Noordeloos, E.; Donaldson, C.J.; Adams, M.W.; Noguchi, G.M.; Mawla, A.M.; Huising, M.O. Artemether Does Not Turn α Cells into β Cells. Cell Metab. 2018, 27, 218–225.e4. [Google Scholar] [CrossRef]
- Ackermann, A.M.; Moss, N.G.; Kaestner, K.H. GABA and Artesunate Do Not Induce Pancreatic alpha-to-beta Cell Transdifferentiation In Vivo. Cell Metab. 2018, 28, 787–792.e3. [Google Scholar] [CrossRef]
- Wang, M.Y.; Dean, E.D.; Quittner-Strom, E.; Zhu, Y.; Chowdhury, K.H.; Zhang, Z.; Zhao, S.; Li, N.; Ye, R.; Lee, Y.; et al. Glucagon blockade restores functional beta-cell mass in type 1 diabetic mice and enhances function of human islets. Proc. Natl. Acad. Sci. USA 2021, 118, e2022142118. [Google Scholar] [CrossRef]
- Houbracken, I.; de Waele, E.; Lardon, J.; Ling, Z.; Heimberg, H.; Rooman, I.; Bouwens, L. Lineage Tracing Evidence for Transdifferentiation of Acinar to Duct Cells and Plasticity of Human Pancreas. Gastroenterology 2011, 141, 731–741.e4. [Google Scholar] [CrossRef]
- Kuroda, A.; Yamasaki, Y.; Imagawa, A. Beta-cell regeneration in a patient with type 1 diabetes mellitus who was receiving immunosuppressive therapy. Ann. Intern. Med. 2003, 139, W81. [Google Scholar] [CrossRef]
- Bogdani, M.; Lefebvre, V.; Buelens, N.; Bock, T.; Pipeleers-Marichal, M.; Veld, P.I.; Pipeleers, D. Formation of insulin-positive cells in implants of human pancreatic duct cell preparations from young donors. Diabetologia 2003, 46, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.J.; Muir, K.R.; Docherty, H.M.; McGowan, N.W.A.; Forbes, S.; Heremans, Y.; Heimberg, H.; Casey, J.; Docherty, K. Generation of Functional Beta-Like Cells from Human Exocrine Pancreas. PLoS ONE 2016, 11, e0156204. [Google Scholar] [CrossRef]
- German, M.S. New beta-cells from old acini. Nat. Biotechnol. 2008, 26, 1092–1093. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.B.; Tonnesen, M.F.; Madsen, A.N.; Hagedorn, P.H.; Friberg, J.; Grunnet, L.G.; Heller, R.S.; Nielsen, A.Ø.; Størling, J.; Baeyens, L.; et al. Divalent metal transporter 1 regulates iron-mediated ROS and pancreatic beta cell fate in response to cytokines. Cell Metab. 2012, 16, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, L.; De Breuck, S.; Lardon, J.; Mfopou, J.K.; Rooman, I.; Bouwens, L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 2005, 48, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, M.; Finegood, D.T. Beta-cell neogenesis during prolonged hyperglycemia in rats. Diabetes 2002, 51, 1834–1841. [Google Scholar] [CrossRef][Green Version]
- Bonner-Weir, S.; Baxter, L.A.; Schuppin, G.T.; Smith, F.E. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 1993, 42, 1715–1720. [Google Scholar] [CrossRef]
- Bonner-Weir, S.; Toschi, E.; Inada, A.; Reitz, P.; Fonseca, S.Y.; Aye, T.; Sharma, A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr. Diabetes 2004, 5 (Suppl. 2), 16–22. [Google Scholar] [CrossRef]
- Inada, A.; Nienaber, C.; Katsuta, H.; Fujitani, Y.; Levine, J.; Morita, R.; Sharma, A.; Bonner-Weir, S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA 2008, 105, 19915–19919. [Google Scholar] [CrossRef]
- Liao, Y.H.T.; Verchere, C.B.; Warnock, G.L. Adult stem or progenitor cells in treatment for type 1 diabetes: Current progress. Can. J. Surg. 2007, 50, 137–142. [Google Scholar] [PubMed]
- Luo, H.; Wu, H.; Lu, J.; Lu, D.; Shen, K.; Jin, J. TAT-MafA Inducing Intestinal Epithelial Cells IEC-6 into Insulin Positive Cells. Biotechnol. Bull. 2008, 5, 140. [Google Scholar]
- Heimberg, H.; Bouwens, L.; Heremans, Y.; Van De Casteele, M.; Lefebvre, V.; Pipeleers, D. Adult human pancreatic duct and islet cells exhibit similarities in expression and differences in phosphorylation and complex formation of the homeodomain protein Ipf-1. Diabetes 2000, 49, 571–579. [Google Scholar] [CrossRef]
- Yatoh, S.; Dodge, R.; Akashi, T.; Omer, A.; Sharma, A.; Weir, G.C.; Bonner-Weir, S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes 2007, 56, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.L.; O’Driscoll, M.; Sheets, T.P.; Hruban, R.H.; Oberholzer, J.; McGarrigle, J.J.; Shamblott, M.J. Neurogenin 3 Expressing Cells in the Human Exocrine Pancreas Have the Capacity for Endocrine Cell Fate. PLoS ONE 2015, 10, e0133862. [Google Scholar] [CrossRef]
- Jin, L.; Gao, D.; Feng, T.; Tremblay, J.R.; Ghazalli, N.; Luo, A.; Rawson, J.; Quijano, J.C.; Chai, J.; Wedeken, L.; et al. Cells with surface expression of CD133highCD71low are enriched for tripotent colony-forming progenitor cells in the adult murine pancreas. Stem Cell Res. 2016, 16, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Rodriguez, R.T.; McLean, G.W.; Kim, S.K. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc. Natl. Acad. Sci. USA 2007, 104, 175–180. [Google Scholar] [CrossRef]
- Westphalen, C.B.; Takemoto, Y.; Tanaka, T.; Macchini, M.; Jiang, Z.; Renz, B.W.; Chen, X.; Ormanns, S.; Nagar, K.; Tailor, Y.; et al. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell 2016, 18, 441–455. [Google Scholar] [CrossRef]
- Rovira, M.; Scott, S.-G.; Liss, A.S.; Jensen, J.; Thayer, S.P.; Leach, S.D. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc. Natl. Acad. Sci. USA 2010, 107, 75–80. [Google Scholar] [CrossRef]
- Ziv, O.; Glaser, B.; Dor, Y. The plastic pancreas. Dev. Cell 2013, 26, 3–7. [Google Scholar] [CrossRef][Green Version]
- Van de Casteele, M.; Leuckx, G.; Baeyens, L.; Cai, Y.; Yuchi, Y.; Coppens, V.; De Groef, S.; Eriksson, M.; Svensson, C.; Ahlgren, U.; et al. Neurogenin 3+ cells contribute to beta-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis. 2013, 4, e523. [Google Scholar] [CrossRef]
- Swales, N.; Martens, G.A.; Bonné, S.; Heremans, Y.; Borup, R.; Van de Casteele, M.; Ling, Z.; Pipeleers, D.; Ravassard, P.; Nielsen, F.; et al. Plasticity of Adult Human Pancreatic Duct Cells by Neurogenin3-Mediated Reprogramming. PLoS ONE 2012, 7, e37055. [Google Scholar] [CrossRef] [PubMed]
- Tourrel, C.; Bailbé, D.; Meile, M.J.; Kergoat, M.; Portha, B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes 2001, 50, 1562–1570. [Google Scholar] [CrossRef]
- Wu, F.; Guo, L.; Jakubowski, A.; Su, L.; Li, W.-C.; Bonner-Weir, S.; Burkly, L.C. TNF-Like Weak Inducer of Apoptosis (TWEAK) Promotes Beta Cell Neogenesis from Pancreatic Ductal Epithelium in Adult Mice. PLoS ONE 2013, 8, e72132. [Google Scholar] [CrossRef]
- Valdez, I.A.; Dirice, E.; Gupta, M.K.; Shirakawa, J.; Teo, A.K.K.; Kulkarni, R.N. Proinflammatory Cytokines Induce Endocrine Differentiation in Pancreatic Ductal Cells via STAT3-Dependent NGN3 Activation. Cell Rep. 2016, 15, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Sancho, R.; Gruber, R.; Gu, G.; Behrens, A. Loss of Fbw7 reprograms adult pancreatic ductal cells into alpha, delta, and beta cells. Cell Stem Cell 2014, 15, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Pinzon, W.L.; Yan, Y.; Power, R.; Brand, S.J.; Rabinovitch, A. Combination therapy with epidermal growth factor and gastrin increases beta-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes 2005, 54, 2596–2601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tellez, N.; Montanya, E. Gastrin induces ductal cell dedifferentiation and beta-cell neogenesis after 90% pancreatectomy. J. Endocrinol. 2014, 223, 67–78. [Google Scholar] [CrossRef]
- Klein, D.; Álvarez-Cubela, S.; Lanzoni, G.; Vargas, N.; Prabakar, K.R.; Boulina, M.; Ricordi, C.; Inverardi, L.; Pastori, R.L.; Domínguez-Bendala, J. BMP-7 Induces Adult Human Pancreatic Exocrine-to-Endocrine Conversion. Diabetes 2015, 64, 4123–4134. [Google Scholar] [CrossRef]
- Rhee, M.; Lee, S.-H.; Kim, J.-W.; Ham, D.-S.; Park, H.-S.; Yang, H.K.; Shin, J.-Y.; Cho, J.-H.; Kim, Y.-B.; Youn, B.-S.; et al. Preadipocyte factor 1 induces pancreatic ductal cell differentiation into insulin-producing cells. Sci. Rep. 2016, 6, 23960. [Google Scholar] [CrossRef]
- Noguchi, H.; Xu, G.; Matsumoto, S.; Kaneto, H.; Kobayashi, N.; Bonner-Weir, S.; Hayashi, S. Induction of Pancreatic Stem/Progenitor Cells into Insulin-Producing Cells by Adenoviral-Mediated Gene Transfer Technology. Cell Transplant. 2006, 15, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Bonner-Weir, S.; Wei, F.Y.; Matsushita, M.; Matsumoto, S. BETA2/NeuroD protein can be transduced into cells due to an arginine- and lysine-rich sequence. Diabetes 2005, 54, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Liu, G.-H.; Belmonte, J.C.I. Rejuvenating liver and pancreas through cell transdifferentiation. Cell Res. 2012, 22, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Grompe, M. Pancreatic-hepatic switches in vivo. Mech. Dev. 2003, 120, 99–106. [Google Scholar] [CrossRef]
- Ferber, S.; Halkin, A.; Cohen, H.; Ber, I.; Einav, Y.; Goldberg, I.; Barshack, I.; Seijffers, R.; Kopolovic, J.; Kaiser, N.; et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 2000, 6, 568–572. [Google Scholar] [CrossRef]
- Wang, A.Y.; Ehrhardt, A.; Xu, H.; Kay, M.A. Adenovirus Transduction is Required for the Correction of Diabetes Using Pdx-1 or Neurogenin-3 in the Liver. Mol. Ther. 2007, 15, 255–263. [Google Scholar] [CrossRef]
- Kojima, H.; Fujimiya, M.; Matsumura, K.; Younan, P.; Imaeda, H.; Maeda, M.; Chan, L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat. Med. 2003, 9, 596–603. [Google Scholar] [CrossRef]
- Miyatsuka, T.; Kaneto, H.; Kajimoto, Y.; Hirota, S.; Arakawa, Y.; Fujitani, Y.; Umayahara, Y.; Watada, H.; Yamasaki, Y.; Magnuson, M.; et al. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem. Biophys. Res. Commun. 2003, 310, 1017–1025. [Google Scholar] [CrossRef]
- Cerdá-Esteban, N.; Naumann, H.; Ruzittu, S.; Mah, N.; Pongrac, I.M.; Cozzitorto, C.; Hommel, A.; Andrade-Navarro, M.A.; Bonifacio, E.; Spagnoli, F.M. Stepwise reprogramming of liver cells to a pancreas progenitor state by the transcriptional regulator Tgif2. Nat. Commun. 2017, 8, 14127. [Google Scholar] [CrossRef]
- Galivo, F.B.E.; Wang, Y.; Pelz, C.; Schug, J.; Kaestner, K.H.; Grompe, M. Reprogramming human gallbladder cells into insulin-producing β-like cells. PLoS ONE 2017, 12, e0181812. [Google Scholar]
- Cairnie, A.B.; Lamerton, L.F.; Steel, G.G. Cell proliferation studies in the intestinal epithelium of the rat. I. Determination of the kinetic parameters. Exp. Cell Res. 1965, 39, 528–538. [Google Scholar] [CrossRef]
- Grapin-Botton, A.; Melton, D.A. Endoderm development: From patterning to organogenesis. Trends Genet. 2000, 16, 124–130. [Google Scholar] [CrossRef]
- Rubino, F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 2008, 31 (Suppl. 2), S290–S296. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. New Therapeutic Strategies for the Treatment of Type 2 Diabetes Mellitus Based on Incretins. Rev. Diabet. Stud. 2005, 2, 61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyawaki, K.; Yamada, Y.; Yano, H.; Niwa, H.; Ban, N.; Ihara, Y.; Kubota, A.; Fujimoto, S.; Kajikawa, M.; Kuroe, A.; et al. Glucose intolerance caused by a defect in the entero-insular axis: A study in gastric inhibitory polypeptide receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 14843–14847. [Google Scholar] [CrossRef]
- Drucker, D.J. Glucagon-like peptide-1 and the islet beta-cell: Augmentation of cell proliferation and inhibition of apoptosis. Endocrinology 2003, 144, 5145–5148. [Google Scholar] [CrossRef]
- Drucker, D.J. The role of gut hormones in glucose homeostasis. J. Clin. Investig. 2007, 117, 24–32. [Google Scholar] [CrossRef]
- Garber, A.J. Incretin effects on beta-cell function, replication, and mass: The human perspective. Diabetes Care 2011, 34 (Suppl. 2), S258–S263. [Google Scholar] [CrossRef]
- Suzuki, A.; Nakauchi, H.; Taniguchi, H. Glucagon-like peptide 1 (1–37) converts intestinal epithelial cells into insulin-producing cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5034–5039. [Google Scholar] [CrossRef]
- Koizumi, M.; Nagai, K.; Kida, A.; Kami, K.; Ito, D.; Fujimoto, K.; Kawaguchi, Y.; Doi, R. Forced expression of PDX-1 induces insulin production in intestinal epithelia. Surgery 2006, 140, 273–280. [Google Scholar] [CrossRef]
- Chen, Y.J.; Finkbeiner, S.R.; Weinblatt, D.; Emmett, M.J.; Tameire, F.; Yousefi, M.; Yang, C.; Maehr, R.; Zhou, Q.; Shemer, R.; et al. De novo formation of insulin-producing “neo-beta cell islets” from intestinal crypts. Cell Rep. 2014, 6, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Talchai, C.; Xuan, S.; Kitamura, T.; DePinho, R.A.; Accili, D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat. Genet. 2012, 44, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Ariyachet, C.; Tovaglieri, A.; Xiang, G.; Lu, J.; Shah, M.S.; Richmond, C.A.; Verbeke, C.; Melton, D.A.; Stanger, B.Z.; Mooney, D.; et al. Reprogrammed Stomach Tissue as a Renewable Source of Functional beta Cells for Blood Glucose Regulation. Cell Stem Cell 2016, 18, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yoon, B.S.; Moon, J.-H.; Kim, J.; Jun, E.K.; Lee, J.H.; Kim, J.S.; Baik, C.S.; Kim, A.; Whang, K.Y.; et al. Differentiation of human labia minora dermis-derived fibroblasts into insulin-producing cells. Exp. Mol. Med. 2012, 44, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Chen, F.G.; Zhang, W.J.; Zhou, G.D.; Cui, L.; Liu, W.; Cao, Y. Differentiation of human multipotent dermal fibroblasts into islet-like cell clusters. BMC Cell Biol. 2010, 11, 46. [Google Scholar] [CrossRef]
- Peleg, S.; Sananbenesi, F.; Zovoilis, A.; Burkhardt, S.; Bahari-Javan, S.; Agis-Balboa, R.C.; Cota, P.; Wittnam, J.L.; Gogol-Doering, A.; Opitz, L.; et al. Altered Histone Acetylation Is Associated with Age-Dependent Memory Impairment in Mice. Science 2010, 328, 753–756. [Google Scholar] [CrossRef]
- Pennarossa, G.; Maffei, S.; Campagnol, M.; Tarantini, L.; Gandolfi, F.; Brevini, T.A.L. Brief demethylation step allows the conversion of adult human skin fibroblasts into insulin-secreting cells. Proc. Natl. Acad. Sci. USA 2013, 110, 8948–8953. [Google Scholar] [CrossRef]
- Pennarossa, G.; Maffei, S.; Campagnol, M.; Rahman, M.M.; Brevini, T.A.L.; Gandolfi, F. Reprogramming of Pig Dermal Fibroblast into Insulin Secreting Cells by a Brief Exposure to 5-aza-cytidine. Stem Cell Rev. Rep. 2014, 10, 31–43. [Google Scholar] [CrossRef]
- Abelev, B.; Adam, J.; Adamová, D.; Adare, A.M.; Aggarwal, M.M.; Rinella, G.A.; Agocs, A.G.; Agostinelli, A.; Salazar, S.A.; Ahammed, Z.; et al. Measurement of the cross section for electromagnetic dissociation with neutron emission in Pb-Pb collisions at sqrt[s(NN)] = 2.76 TeV. Phys. Rev. Lett. 2012, 109, 252302. [Google Scholar] [CrossRef]
- Li, K.; Zhu, S.; Russ, H.A.; Xu, S.; Xu, T.; Zhang, Y.; Ma, T.; Hebrok, M.; Ding, S. Small Molecules Facilitate the Reprogramming of Mouse Fibroblasts into Pancreatic Lineages. Cell Stem Cell 2014, 14, 228–236. [Google Scholar] [CrossRef]
- Zhu, S.; Russ, H.A.; Wang, X.; Zhang, M.; Ma, T.; Xu, T.; Tang, S.; Hebrok, M.; Ding, S. Human pancreatic beta-like cells converted from fibroblasts. Nat. Commun. 2016, 7, 10080. [Google Scholar] [CrossRef] [PubMed]
- Mauda-Havakuk, M.; Litichever, N.; Chernichovski, E.; Nakar, O.; Winkler, E.; Mazkereth, R.; Orenstein, A.; Bar-Meir, E.; Ravassard, P.; Meivar-Levy, I.; et al. Ectopic PDX-1 Expression Directly Reprograms Human Keratinocytes along Pancreatic Insulin-Producing Cells Fate. PLoS ONE 2011, 6, e26298. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Cell source | Origin | Reprogramming Tool | Model | Results | Reference |
|---|---|---|---|---|---|
| Pancreatic Cell Sources | |||||
| Alpha cells | Endoderm | Adeno-associated virus (AAV) carrying Pdx1 and MafA expression cassettes | ALX-induced diabetes and in autoimmune non obese diabetic (NOD) mice (in vivo) | -Alpha-cell-derived insulin+ cells have a similar expression profile to normal beta cells. -Prolonged normalization of blood glucose in hyperglycemic NOD mice for 4 months. | [34] |
| Acinar cells | Endoderm | Transcription factors overexpression Pdx1, MafA and Ngn3 (PMN cocktail) | Adult (Rag−/−), NOD mice (>2 month). (in vivo) | -Reprogrammed β-cells do not organize into islet structures. -New β-cells do not express exocrine genes. -Relatively fast speed direct conversion with the first insulin+ cells appear at day 3, and with efficiency of up to 20%. | [43] |
| Ductal epithelial cells | Endoderm | Ngn3 overexpression combined with modulation of the Delta-Notch signaling and addition of pancreatic endocrine transcription factors (Myt1, MafA and Pdx1) | Adult Human Duct Cells (in vitro) | -10% of full duct-to-endocrine reprogramming achieved. | [63] |
| Extra-pancreatic cell sources | |||||
| Hepatic cells | Endoderm | Expression of TGFβ-induced factor homeobox 2 (TGIF2), both ex-vivo and in-vivo | Murine adult primary or BAML hepatocytes (ex-vivo), mice models (in-vivo) | -Primary hepatocytes transduced with LV-TGIF2 formed pancreatic organoid structures. -AAV.TGIF2-injected mice displayed reduced blood glucose levels for 2 months. | [80] |
| Biliary tree, gallbladder, and cystic ducts | Endoderm | Adenoviral-mediated expression of transcription factors Pdx1, MafA, Neurog3, and Pax6 | Primary cultures of human gallbladder and cystic duct cells (in vitro) | -Scalable in vitro expansion. -Insulin protein production (as measured by C-peptide) was found on day 5 and lasted for 3 months. | [81] |
| Intestinal cells | Endoderm | Transient intestinal expression of Pdx1, MafA, and Ngn3 (PMN) in the intestinal crypts | Mice (in vivo), human intestinal organoids | -Intestinal neo-islets were generated which are glucose-responsive and able to ameliorate hyperglycaemia in mice model of diabetes. | [92] |
| Stomach antral cells | Endoderm | Reprogramming of antral gastric cells with the PMN cocktail (PNM) | Mice (in vivo), Antral stomach and duodenal organoids were derived from young adult mice (1–2 months) | -Suppressed hyperglycaemia in a diabetic mouse model for at least 6 months and can regenerate rapidly after ablation. | [94] |
| Fibroblasts | Mesoderm | 18 h of exposure of DNA methyltransferase inhibitor 5 azacytidine (5-AZA) followed by a three-step protocol for the induction of endocrine pancreatic differentiation that lasted 36 d | Adult human dermal fibroblasts (in vitro) | -Conversion of 35 ± 8.9% of fibroblasts into insulin producing cells at end of treatment protocol. -Converted cells were able to protect recipient mice against streptozotocin-induced diabetes, restoring a physiological response to glucose tolerance tests. | [98] |
| Keratinocytes | Ectoderm | Ectopic expression of Pdx1, NeuroD1, Ngn3 with high glucose concentration in culture | Cell culture of human keratinocytes (in vitro) | -Insulin production occurred in short time (5 to 7 days) with insulin pro-duction in 12 ± 8% of reprogrammed cells -More efficient than induced pluripotent cells (iPSC) which typically takes >20 days with an efficiency of 0.05–0.1%; or 3.3% in clonal cells | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalo, E.; Read, S.; Ahlenstiel, G. Reprogramming—Evolving Path to Functional Surrogate β-Cells. Cells 2022, 11, 2813. https://doi.org/10.3390/cells11182813
Kalo E, Read S, Ahlenstiel G. Reprogramming—Evolving Path to Functional Surrogate β-Cells. Cells. 2022; 11(18):2813. https://doi.org/10.3390/cells11182813
Chicago/Turabian StyleKalo, Eric, Scott Read, and Golo Ahlenstiel. 2022. "Reprogramming—Evolving Path to Functional Surrogate β-Cells" Cells 11, no. 18: 2813. https://doi.org/10.3390/cells11182813
APA StyleKalo, E., Read, S., & Ahlenstiel, G. (2022). Reprogramming—Evolving Path to Functional Surrogate β-Cells. Cells, 11(18), 2813. https://doi.org/10.3390/cells11182813