Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Histochemistry and Immunohistochemistry
2.1.1. 2D Cultures
2.1.2. 3D Cultures
3. Results
3.1. Biomineralization in Spheroids
3.1.1. Osteogenic Differentiation of Spheroids
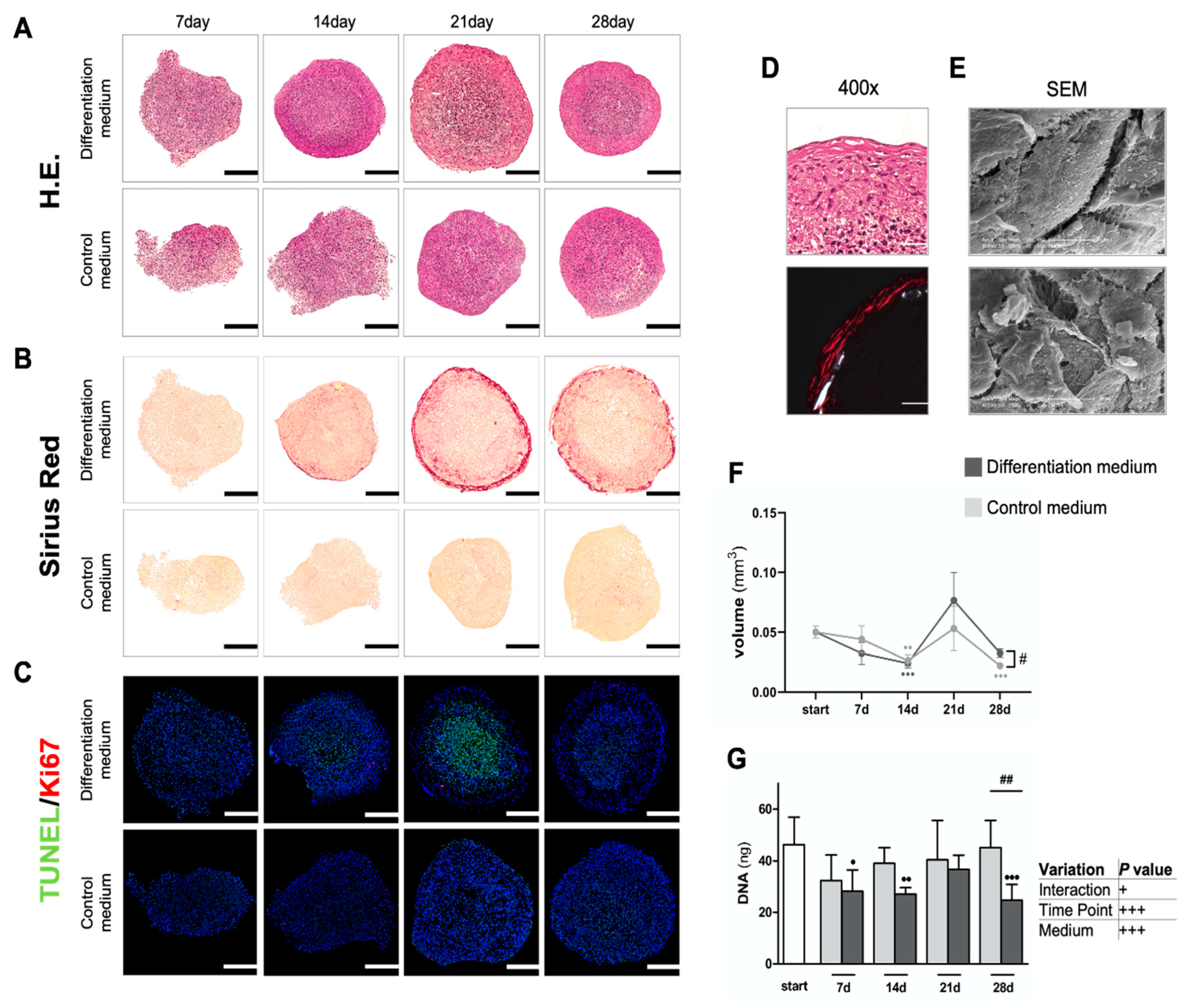
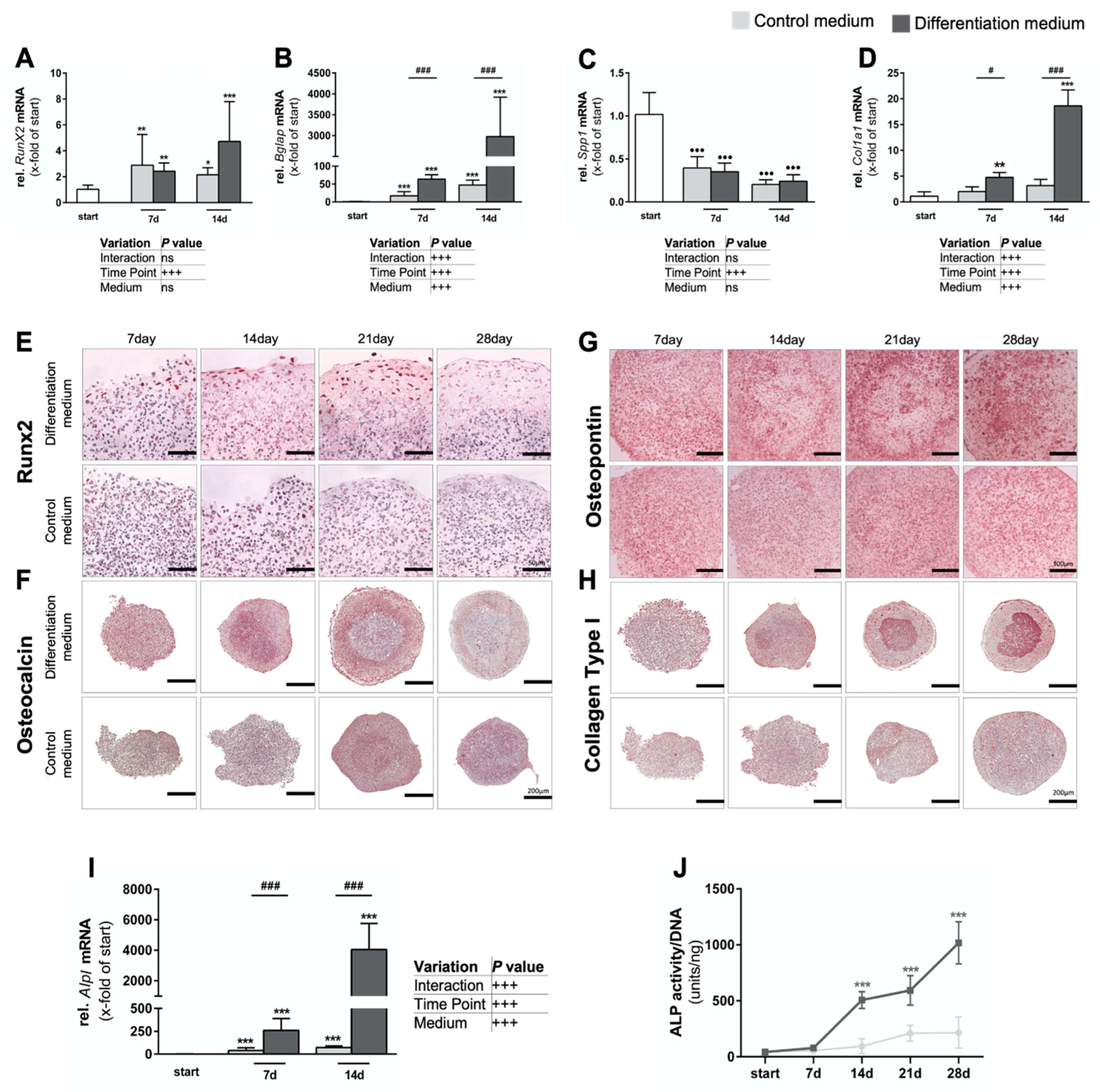
3.1.2. Intense Biomineralization in Osteogenic Spheroid Cultures
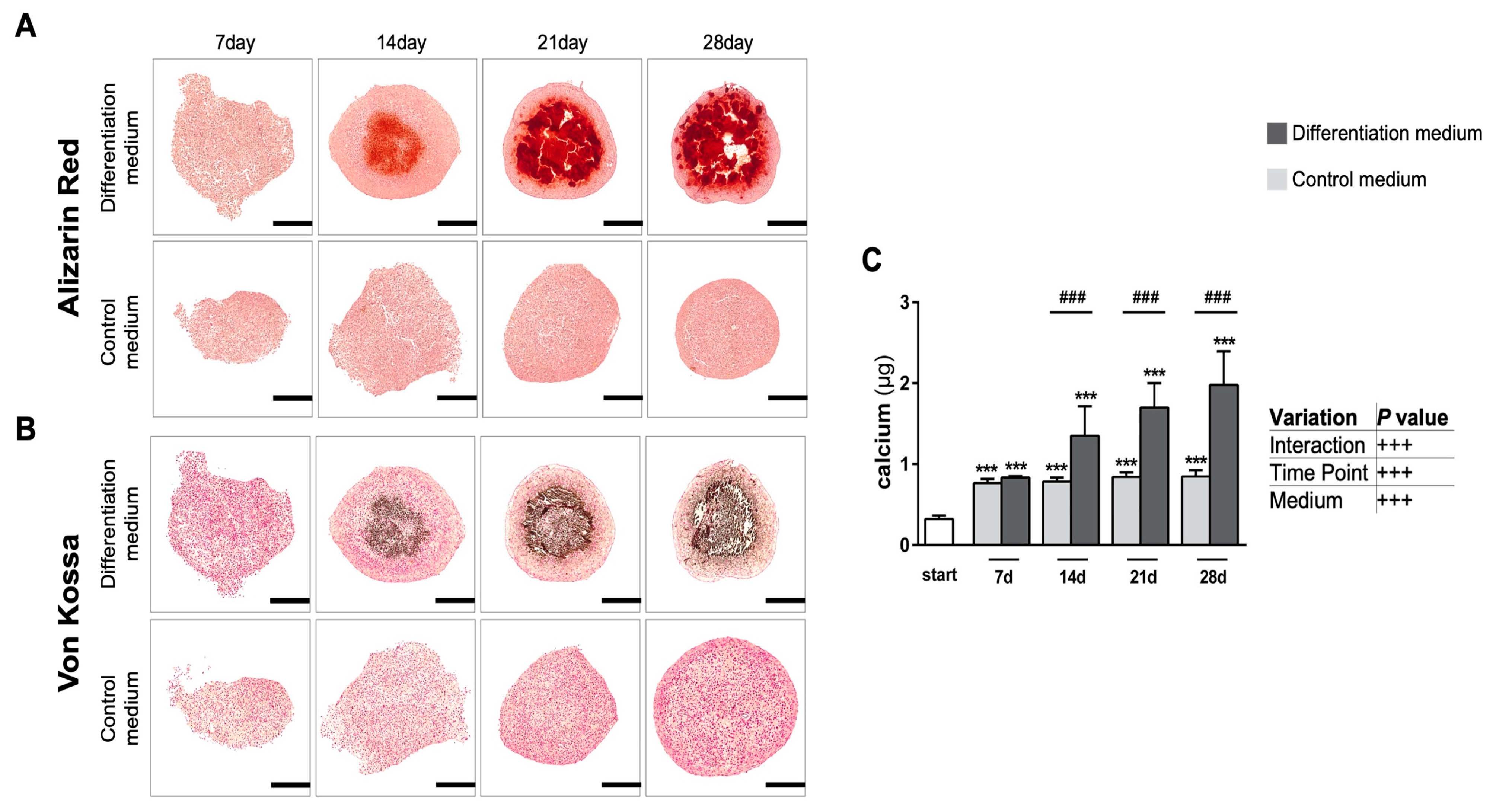

3.1.3. Formation of Native Hydroxyapatite-like Biomineralization in Spheroid Cultures

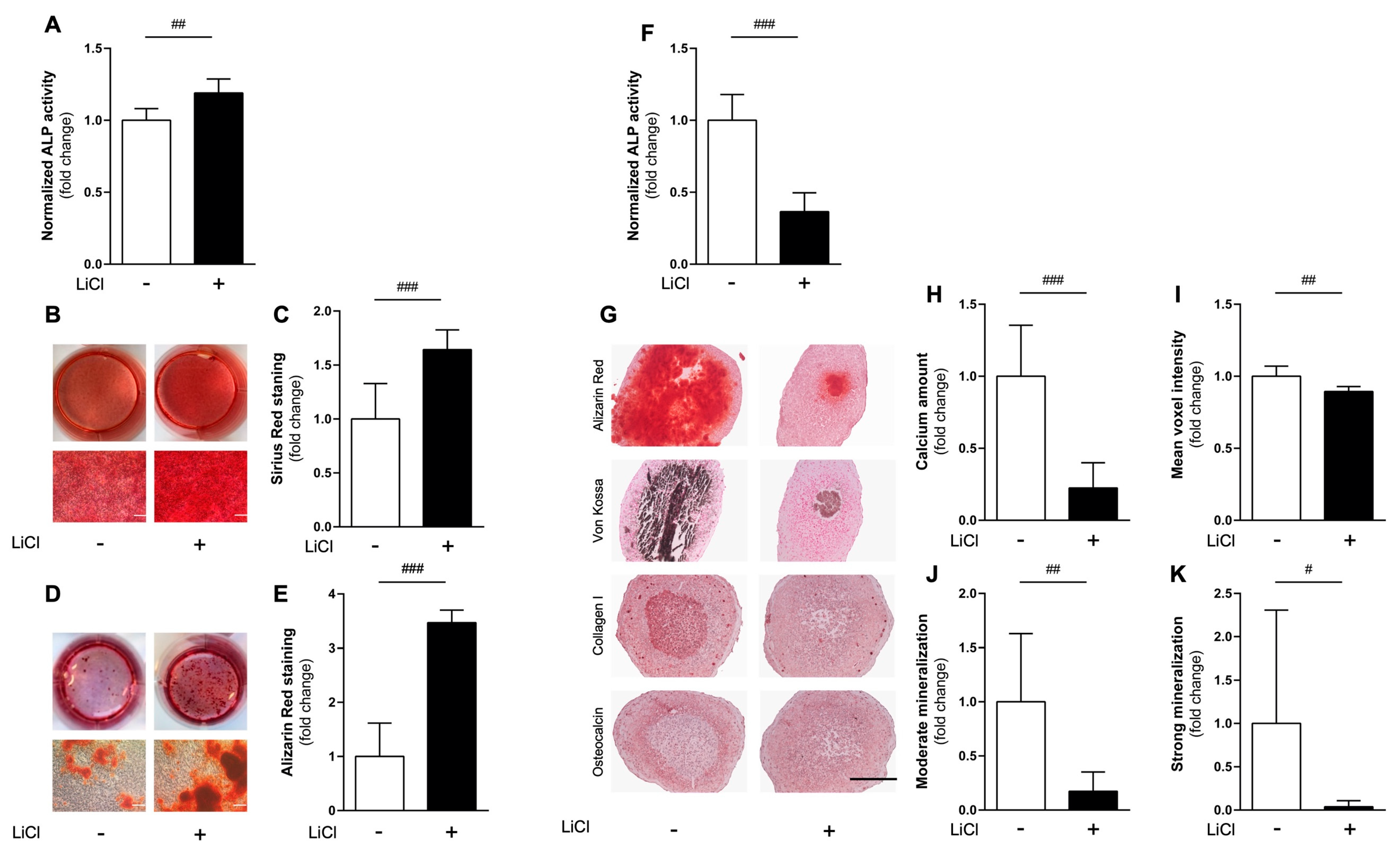
3.2. Comparing Effects of Pharmacological cWnt Agonist Lithium in 2D vs. 3D
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell Dev. Biol. 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Karasik, D.; Rivadeneira, F.; Johnson, M.L. The genetics of bone mass and susceptibility to bone diseases. Nat. Rev. Rheumatol. 2016, 12, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Michou, L.; Kolta, S.; Debiais, F.; Cortet, B.; Guggenbuhl, P. High bone mass in adults. Jt. Bone Spine 2018, 85, 693–699. [Google Scholar] [CrossRef]
- Andersen, T.L.; Abdelgawad, M.E.; Kristensen, H.B.; Hauge, E.M.; Rolighed, L.; Bollerslev, J.; Kjærsgaard-Andersen, P.; Delaisse, J.M. Understanding coupling between bone resorption and formation: Are reversal cells the missing link? Am. J. Pathol. 2013, 183, 235–246. [Google Scholar] [CrossRef]
- Reid, I.R. A broader strategy for osteoporosis interventions. Nat. Rev. Endocrinol. 2020, 16, 333–339. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Abrahamsen, B.; Al-Daghri, N.M.; Brandi, M.L.; Cannata-Andia, J.; Cortet, B.; Dimai, H.P.; Ferrari, S.; et al. Identification and management of patients at increased risk of osteoporotic fracture: Outcomes of an ESCEO expert consensus meeting. Osteoporos. Int. 2017, 28, 2023–2034. [Google Scholar] [CrossRef]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Brown, K.M.; Tracy, D.K. Lithium: The pharmacodynamic actions of the amazing ion. Adv. Psychopharmacol. 2013, 3, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Clément-Lacroix, P.; Ai, M.; Morvan, F.; Roman-Roman, S.; Vayssière, B.; Belleville, C.; Estrera, K.; Warman, M.L.; Baron, R.; Rawadi, G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 17406–17411. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Petersen, O.W.; Wang, F.; Larabell, C.A.; Briand, P.; Damsky, C.; Bissell, M.J. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997, 137, 231–245. [Google Scholar] [CrossRef]
- VanDussen, K.L.; Marinshaw, J.M.; Shaikh, N.; Miyoshi, H.; Moon, C.; Tarr, P.I.; Ciorba, M.A.; Stappenbeck, T.S. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 2015, 64, 911–920. [Google Scholar] [CrossRef]
- Sloan, S.A.; Andersen, J.; Pașca, A.M.; Birey, F.; Pașca, S.P. Generation and assembly of human brain region-specific three-dimensional cultures. Nat. Protoc. 2018, 13, 2062–2085. [Google Scholar] [CrossRef]
- Peng, W.C.; Logan, C.Y.; Fish, M.; Anbarchian, T.; Aguisanda, F.; Álvarez-Varela, A.; Wu, P.; Jin, Y.; Zhu, J.; Li, B.; et al. Inflammatory Cytokine TNFα Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell 2018, 175, 1607–1619.e15. [Google Scholar] [CrossRef]
- Kale, S.; Biermann, S.; Edwards, C.; Tarnowski, C.; Morris, M.; Long, M.W. Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat. Biotechnol. 2000, 18, 954–958. [Google Scholar] [CrossRef]
- Kim, J.; Adachi, T. Cell Condensation Triggers the Differentiation of Osteoblast Precursor Cells to Osteocyte-Like Cells. Front. Bioeng. Biotechnol. 2019, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kigami, H.; Adachi, T. Comparative gene expression analysis for pre-osteoblast MC3T3-E1 cells under non-adhesive culture toward osteocyte differentiation. J. Biosci. Bioeng. 2021, 132, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Jähn, K.; Richards, R.G.; Archer, C.W.; Stoddart, M.J. Pellet culture model for human primary osteoblasts. Eur. Cell Mater. 2010, 20, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Restle, L.; Costa-Silva, D.; Lourenço, E.S.; Bachinski, R.F.; Batista, A.C.; Linhares, A.B.R.; Alves, G.G. A 3D Osteoblast In Vitro Model for the Evaluation of Biomedical Materials. Adv. Mater. Sci. Eng. 2015, 2015, 268930. [Google Scholar] [CrossRef]
- Gaitán-Salvatella, I.; López-Villegas, E.O.; González-Alva, P.; Susate-Olmos, F.; Álvarez-Pérez, M.A. Case Report: Formation of 3D Osteoblast Spheroid Under Magnetic Levitation for Bone Tissue Engineering. Front. Mol. Biosci. 2021, 8, 672518. [Google Scholar] [CrossRef]
- Souza, W.; Piperni, S.G.; Laviola, P.; Rossi, A.L.; Rossi, M.I.D.; Archanjo, B.S.; Leite, P.E.; Fernandes, M.H.; Rocha, L.A.; Granjeiro, J.M.; et al. The two faces of titanium dioxide nanoparticles bio-camouflage in 3D bone spheroids. Sci. Rep. 2019, 9, 9309. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Dar, M.S.; Gesty-Palmer, D.; El-Shewy, H.M.; Robinson, K.M.; Haycraft, C.J.; Barth, J.L. Transcriptomic characterization of signaling pathways associated with osteoblastic differentiation of MC-3T3E1 cells. PLoS ONE 2019, 14, e0204197. [Google Scholar] [CrossRef]
- Gibon, E.; Batke, B.; Jawad, M.U.; Fritton, K.; Rao, A.; Yao, Z.; Biswal, S.; Gambhir, S.S.; Goodman, S.B. MC3T3-E1 osteoprogenitor cells systemically migrate to a bone defect and enhance bone healing. Tissue Eng. Part A 2012, 18, 968–973. [Google Scholar] [CrossRef]
- Sudo, H.; Kodama, H.A.; Amagai, Y.; Yamamoto, S.; Kasai, S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Biol. 1983, 96, 191–198. [Google Scholar] [CrossRef]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cell Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K.; Miyake, T. Divide, accumulate, differentiate: Cell condensation in skeletal development revisited. Int. J. Dev. Biol. 1995, 39, 881–893. [Google Scholar] [PubMed]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Köllmer, M.; Buhrman, J.S.; Zhang, Y.; Gemeinhart, R.A. Markers Are Shared Between Adipogenic and Osteogenic Differentiated Mesenchymal Stem Cells. J. Dev. Biol. Tissue Eng. 2013, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A review of old concepts with new standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, M.; Shinar, D.; Rodan, G.A. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J. Bone Miner. Res. 1990, 5, 831–842. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 1995, 15, 1858–1869. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Harris, S.E.; Rosser, J.; Dallas, M.R.; Dallas, S.L.; Camacho, N.P.; Boyan, B.; Boskey, A. Von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif. Tissue Int. 2003, 72, 537–547. [Google Scholar] [CrossRef]
- Van der Linden, J.C.; Waarsing, J.H.; Weinans, H. The use of micro-CT to study bone architecture dynamics noninvasively. Drug Discov. Today Technol. 2006, 3, 213–219. [Google Scholar] [CrossRef]
- Fragoulis, A.; Schenkel, J.; Herzog, M.; Schellenberg, T.; Jahr, H.; Pufe, T.; Trautwein, C.; Kensler, T.W.; Streetz, K.L.; Wruck, C.J. Nrf2 Ameliorates DDC-Induced Sclerosing Cholangitis and Biliary Fibrosis and Improves the Regenerative Capacity of the Liver. Toxicol. Sci. 2019, 169, 485–498. [Google Scholar] [CrossRef]
- Fragoulis, A.; Biller, K.; Fragoulis, S.; Lex, D.; Uhlig, S.; Reiss, L.K. Reference Gene Selection for Gene Expression Analyses in Mouse Models of Acute Lung Injury. Int. J. Mol. Sci. 2021, 22, 7853. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Tullberg-Reinert, H.; Jundt, G. In situ measurement of collagen synthesis by human bone cells with a Sirius Red-based colorimetric microassay: Effects of transforming growth factor β2 and ascorbic acid 2-phosphate. Histochem. Cell Biol. 1999, 112, 271–276. [Google Scholar] [CrossRef]
- Hwang, P.W.; Horton, J.A. Variable osteogenic performance of MC3T3-E1 subclones impacts their utility as models of osteoblast biology. Sci. Rep. 2019, 9, 8299. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Beckmann, R.; Fragoulis, A.; Conrads, C.; Pavanram, P.; Nebelung, S.; Wolf, M.; Wruck, C.J.; Jahr, H.; Pufe, T. Nrf2/ARE Signaling Directly Regulates SOX9 to Potentially Alter Age-Dependent Cartilage Degeneration. Antioxidants 2022, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Hellingman, C.A.; Verwiel, E.T.; Slagt, I.; Koevoet, W.; Poublon, R.M.; Nolst-Trenité, G.J.; Baatenburg de Jong, R.J.; Jahr, H.; van Osch, G.J. Differences in cartilage-forming capacity of expanded human chondrocytes from ear and nose and their gene expression profiles. Cell Transpl. 2011, 20, 925–940. [Google Scholar] [CrossRef]
- Gremse, F.; Stärk, M.; Ehling, J.; Menzel, J.R.; Lammers, T.; Kiessling, F. Imalytics Preclinical: Interactive Analysis of Biomedical Volume Data. Theranostics 2016, 6, 328–341. [Google Scholar] [CrossRef]
- Li, L.; Peng, X.; Qin, Y.; Wang, R.; Tang, J.; Cui, X.; Wang, T.; Liu, W.; Pan, H.; Li, B. Acceleration of bone regeneration by activating Wnt/β-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci. Rep. 2017, 7, 45204. [Google Scholar] [CrossRef]
- Bicer, M.; Cottrell, G.S.; Widera, D. Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells. Stem Cell Res. Ther. 2021, 12, 31. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional. Front. Pharm. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, U.; Bar-Lev, M.; Bellows, C.G.; Aubin, J.E. Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone 1988, 9, 155–163. [Google Scholar] [CrossRef]
- El-Serafi, A.T.; Wilson, D.I.; Roach, H.I.; Oreffo, R.O. Developmental plasticity of human foetal femur-derived cells in pellet culture: Self assembly of an osteoid shell around a cartilaginous core. Eur. Cells Mater. 2011, 21, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SlAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Dunwoodie, S.L. The role of hypoxia in development of the Mammalian embryo. Dev. Cell. 2009, 17, 755–773. [Google Scholar] [CrossRef]
- Amarilio, R.; Viukov, S.V.; Sharir, A.; Eshkar-Oren, I.; Johnson, R.S.; Zelzer, E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 2007, 134, 3917–3928. [Google Scholar] [CrossRef]
- Provot, S.; Zinyk, D.; Gunes, Y.; Kathri, R.; Le, Q.; Kronenberg, H.M.; Johnson, R.S.; Longaker, M.T.; Giaccia, A.J.; Schipani, E. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 2007, 177, 451–464. [Google Scholar] [CrossRef]
- Hall, B.K.; Miyake, T. All for one and one for all: Condensations and the initiation of skeletal development. Bioessays 2000, 22, 138–147. [Google Scholar] [CrossRef]
- Owen, T.A.; Aronow, M.; Shalhoub, V.; Barone, L.M.; Wilming, L.; Tassinari, M.S.; Kennedy, M.B.; Pockwinse, S.; Lian, J.B.; Stein, G.S. Progressive development of the rat osteoblast phenotype in vitro: Reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J. Cell. Physiol. 1990, 143, 420–430. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B.; Owen, T.A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. Faseb J. 1990, 4, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A. Induction and patterning of intramembranous bone. Front. Biosci. (Landmark Ed.) 2011, 16, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Deegan, A.J.; Aydin, H.M.; Hu, B.; Konduru, S.; Kuiper, J.H.; Yang, Y. A facile in vitro model to study rapid mineralization in bone tissues. Biomed. Eng. Online 2014, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Curcio, E.; Salerno, S.; Barbieri, G.; De Bartolo, L.; Drioli, E.; Bader, A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials 2007, 28, 5487–5497. [Google Scholar] [CrossRef]
- Palumbo, C.; Ferretti, M.; De Pol, A. Apoptosis during intramembranous ossification. J. Anat. 2003, 203, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1649. [Google Scholar] [CrossRef]
- Abzhanov, A.; Rodda, S.J.; McMahon, A.P.; Tabin, C.J. Regulation of skeletogenic differentiation in cranial dermal bone. Development 2007, 134, 3133–3144. [Google Scholar] [CrossRef]
- Jabalee, J.; Hillier, S.; Franz-Odendaal, T.A. An investigation of cellular dynamics during the development of intramembranous bones: The scleral ossicles. J. Anat. 2013, 223, 311–320. [Google Scholar] [CrossRef]
- Roberts, S.J.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Uncovering the periosteum for skeletal regeneration: The stem cell that lies beneath. Bone 2015, 70, 10–18. [Google Scholar] [CrossRef]
- Allen, M.R.; Hock, J.M.; Burr, D.B. Periosteum: Biology, regulation, and response to osteoporosis therapies. Bone 2004, 35, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Alcorta-Sevillano, N.; Macías, I.; Infante, A.; Rodríguez, C.I. Deciphering the Relevance of Bone ECM Signaling. Cells 2020, 9, 2630. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.K. Role of osteopontin in modulation of hydroxyapatite formation. Calcif. Tissue Int. 2013, 93, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Spevak, L.; Paschalis, E.; Doty, S.B.; McKee, M.D. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif. Tissue Int. 2002, 71, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; Azari, F.; Sørensen, E.S.; Kaartinen, M.T.; McKee, M.D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 2007, 282, 15872–15883. [Google Scholar] [CrossRef] [PubMed]
- Roach, H.I. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol. Int. 1994, 18, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Holm, E.; Gleberzon, J.S.; Liao, Y.; Sørensen, E.S.; Beier, F.; Hunter, G.K.; Goldberg, H.A. Osteopontin mediates mineralization and not osteogenic cell development in vitro. Biochem. J. 2014, 464, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Carlsen, B.; Rudkin, G.; Berry, M.; Ishida, K.; Yamaguchi, D.T.; Miller, T.A. Osteopontin is a negative regulator of proliferation and differentiation in MC3T3-E1 pre-osteoblastic cells. Bone 2004, 34, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharm. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E.; Liu, F.; Malaval, L.; Gupta, A.K. Osteoblast and chondroblast differentiation. Bone 1995, 17, 77s–83s. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Poundarik, A.A.; Boskey, A.; Gundberg, C.; Vashishth, D. Biomolecular regulation, composition and nanoarchitecture of bone mineral. Sci. Rep. 2018, 8, 1191. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.V.; Carr, S.A. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry 1982, 21, 2538–2547. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.H.; van der Jagt, O.P.; Punt, B.J.; Verhaar, J.A.; van Leeuwen, J.P.; Weinans, H.; Jahr, H. Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: An in vitro study. BMC Musculoskelet. Disord. 2010, 11, 188. [Google Scholar] [CrossRef]
- Jansen, J.H.; Eijken, M.; Jahr, H.; Chiba, H.; Verhaar, J.A.; van Leeuwen, J.P.; Weinans, H. Stretch-induced inhibition of Wnt/beta-catenin signaling in mineralizing osteoblasts. J. Orthop. Res. 2010, 28, 390–396. [Google Scholar] [CrossRef]
- Zur Nieden, N.I.; Kempka, G.; Ahr, H.J. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation 2003, 71, 18–27. [Google Scholar] [CrossRef]
- Addison, W.N.; Nelea, V.; Chicatun, F.; Chien, Y.C.; Tran-Khanh, N.; Buschmann, M.D.; Nazhat, S.N.; Kaartinen, M.T.; Vali, H.; Tecklenburg, M.M.; et al. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: An ultrastructural, compositional and comparative analysis with mouse bone. Bone 2015, 71, 244–256. [Google Scholar] [CrossRef]
- Cowan, C.M.; Aghaloo, T.; Chou, Y.F.; Walder, B.; Zhang, X.; Soo, C.; Ting, K.; Wu, B. MicroCT evaluation of three-dimensional mineralization in response to BMP-2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 2007, 13, 501–512. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Rohde, C.; Nierenberg, A.A.; Østergaard, S.D. Association of Lithium Treatment With the Risk of Osteoporosis in Patients With Bipolar Disorder. JAMA Psychiatry 2022, 79, 454–463. [Google Scholar] [CrossRef]
- Warden, S.J.; Hassett, S.M.; Bond, J.L.; Rydberg, J.; Grogg, J.D.; Hilles, E.L.; Bogenschutz, E.D.; Smith, H.D.; Fuchs, R.K.; Bliziotes, M.M.; et al. Psychotropic drugs have contrasting skeletal effects that are independent of their effects on physical activity levels. Bone 2010, 46, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, L.; Hu, X.; Liao, S.; Pathak, J.L.; Liu, J. Lithium chloride enhances bone regeneration and implant osseointegration in osteoporotic conditions. J. Bone Miner. Metab. 2017, 35, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Hongisto, V.; Jernström, S.; Fey, V.; Mpindi, J.P.; Kleivi Sahlberg, K.; Kallioniemi, O.; Perälä, M. High-throughput 3D screening reveals differences in drug sensitivities between culture models of JIMT1 breast cancer cells. PLoS ONE 2013, 8, e77232. [Google Scholar] [CrossRef]
- Ryves, W.J.; Harwood, A.J. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem. Biophys. Res. Commun. 2001, 280, 720–725. [Google Scholar] [CrossRef]
- Van der Horst, G.; van der Werf, S.M.; Farih-Sips, H.; van Bezooijen, R.L.; Löwik, C.W.; Karperien, M. Downregulation of Wnt signaling by increased expression of Dickkopf-1 and -2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J. Bone Miner. Res. 2005, 20, 1867–1877. [Google Scholar] [CrossRef]
- Vaes, B.L.; Dechering, K.J.; van Someren, E.P.; Hendriks, J.M.; van de Ven, C.J.; Feijen, A.; Mummery, C.L.; Reinders, M.J.; Olijve, W.; van Zoelen, E.J.; et al. Microarray analysis reveals expression regulation of Wnt antagonists in differentiating osteoblasts. Bone 2005, 36, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Imamura, A.; Kajiya, H.; Fujisaki, S.; Maeshiba, M.; Yanagi, T.; Kojima, H.; Ohno, J. Three-dimensional spheroids of mesenchymal stem/stromal cells promote osteogenesis by activating stemness and Wnt/β-catenin. Biochem. Biophys. Res. Commun. 2020, 523, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Saleh, F.; Carstairs, A.; Etheridge, S.L.; Genever, P. Real-Time Analysis of Endogenous Wnt Signalling in 3D Mesenchymal Stromal Cells. Stem. Cells Int. 2016, 2016, 7132529. [Google Scholar] [CrossRef] [Green Version]
| Gene | Primer Sequence 5″ → 3″ | Annealing Temp [°C] | Amplicon Length | Accession No. |
|---|---|---|---|---|
| Alpl | F: CCA ACT CTT TTG TGC CAG AGA R: GGC TAC ATT GGT GTT GAG CTT TT | 60.0 | 110 mer | NM_001287172.1 |
| Bglap | F: GCC CAG ACC TAG CAG ACA C R: TGG GCT TGG CAT CTG TGA G | 59.0 | 97 mer | NM_007541 |
| Col1a1 | F: CTA CTA CCG GGC CGA TGA TG R: CGA TCC AGT ACT CTC CGC TC | 59.0 | 188 mer | NM_007742.3 |
| Hprt | F: TCA GTC AAC GGG GGA CAT AAA R: GGG GCT GTA CTG CTT AAC CAG | 61.0 | 142 mer | NM_013556.2 |
| Runx2 | F: GCC AGG CAG GTG CTT CAG AAC T R: CTG GGC GGG GTG TAG GTA AAG | 59.0 | 133 mer | NM_001146038.2 |
| Sdha | F: GGA ACA CTC CAA AAA CAG ACC T R: CCA CCA CTG CGT ATT GAG TAG AA | 60.0 | 106 mer | NM_023281.1 |
| Spp1 | F: GAG AAG CTT TAC AGC CTG CAC C R: ATT GGA ATT GCT TGG AAG AGT TTC T | 59.0 | 150 mer | NM_001204233.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koblenzer, M.; Weiler, M.; Fragoulis, A.; Rütten, S.; Pufe, T.; Jahr, H. Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model. Cells 2022, 11, 2702. https://doi.org/10.3390/cells11172702
Koblenzer M, Weiler M, Fragoulis A, Rütten S, Pufe T, Jahr H. Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model. Cells. 2022; 11(17):2702. https://doi.org/10.3390/cells11172702
Chicago/Turabian StyleKoblenzer, Maximilian, Marek Weiler, Athanassios Fragoulis, Stephan Rütten, Thomas Pufe, and Holger Jahr. 2022. "Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model" Cells 11, no. 17: 2702. https://doi.org/10.3390/cells11172702
APA StyleKoblenzer, M., Weiler, M., Fragoulis, A., Rütten, S., Pufe, T., & Jahr, H. (2022). Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model. Cells, 11(17), 2702. https://doi.org/10.3390/cells11172702







