IL-10 Producing B Cells Protect against LPS-Induced Murine Preterm Birth by Promoting PD1- and ICOS-Expressing T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments and Sample Collection
2.2. Cell Staining and Flow Cytometry
2.3. Cytokine and Chemokine Detection in Sera and Peritoneal Lavage
2.4. Measurement of Progesterone Concentrations
2.5. Data Analysis and Statistics
3. Results
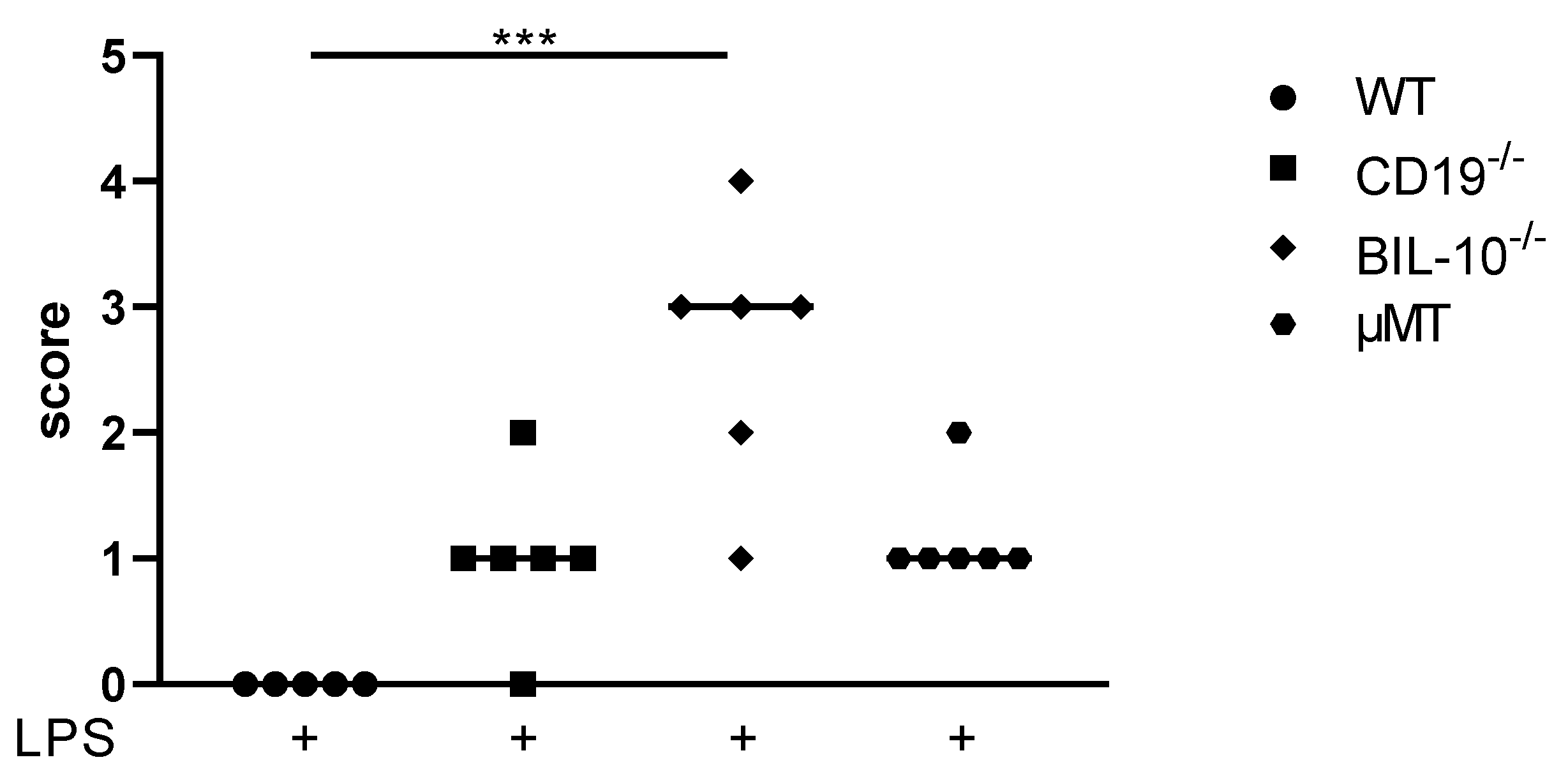
3.1. Loss of B Cell-Specific IL-10 Influences Maternal Well-Being after LPS-Induced Preterm Birth
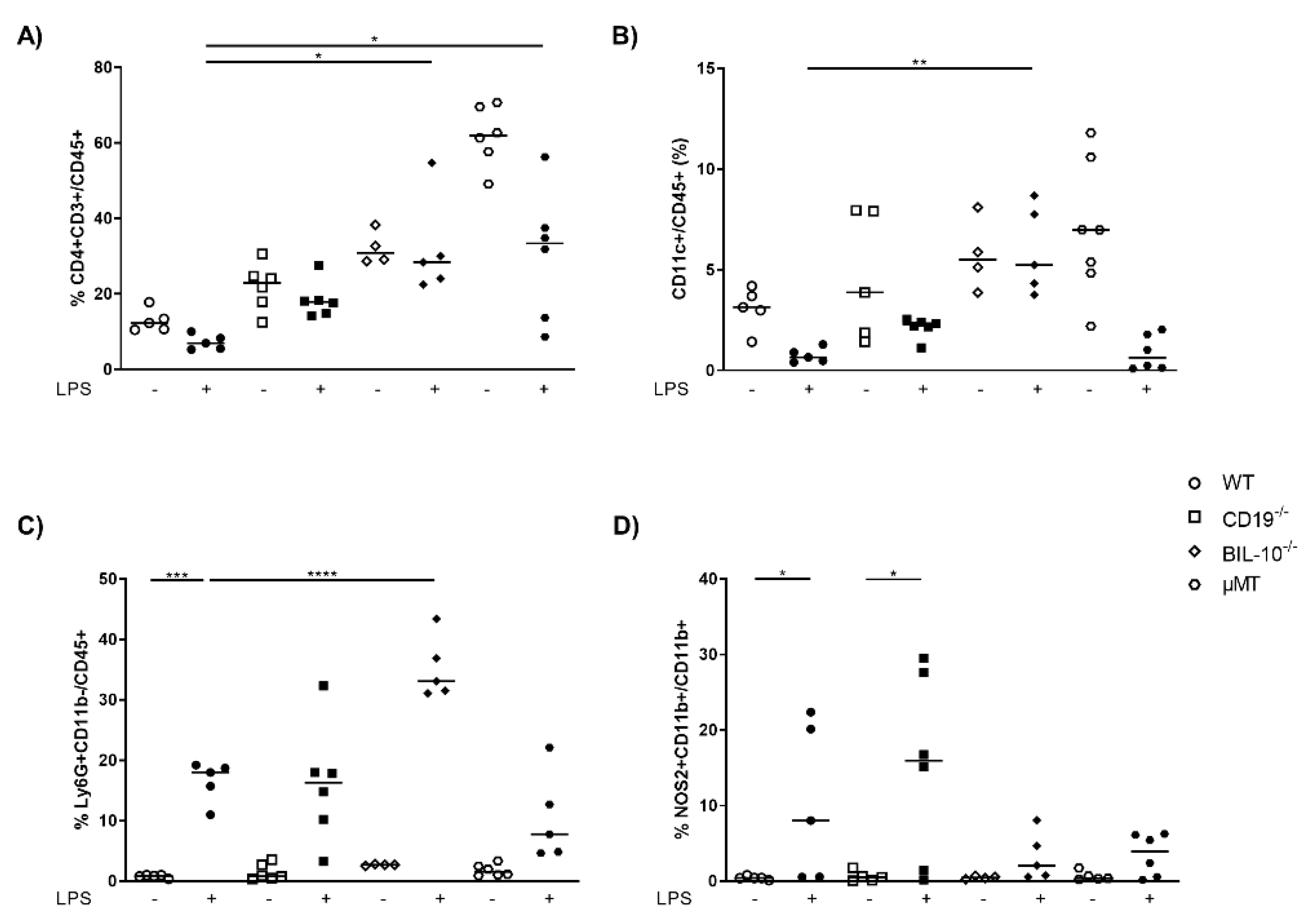
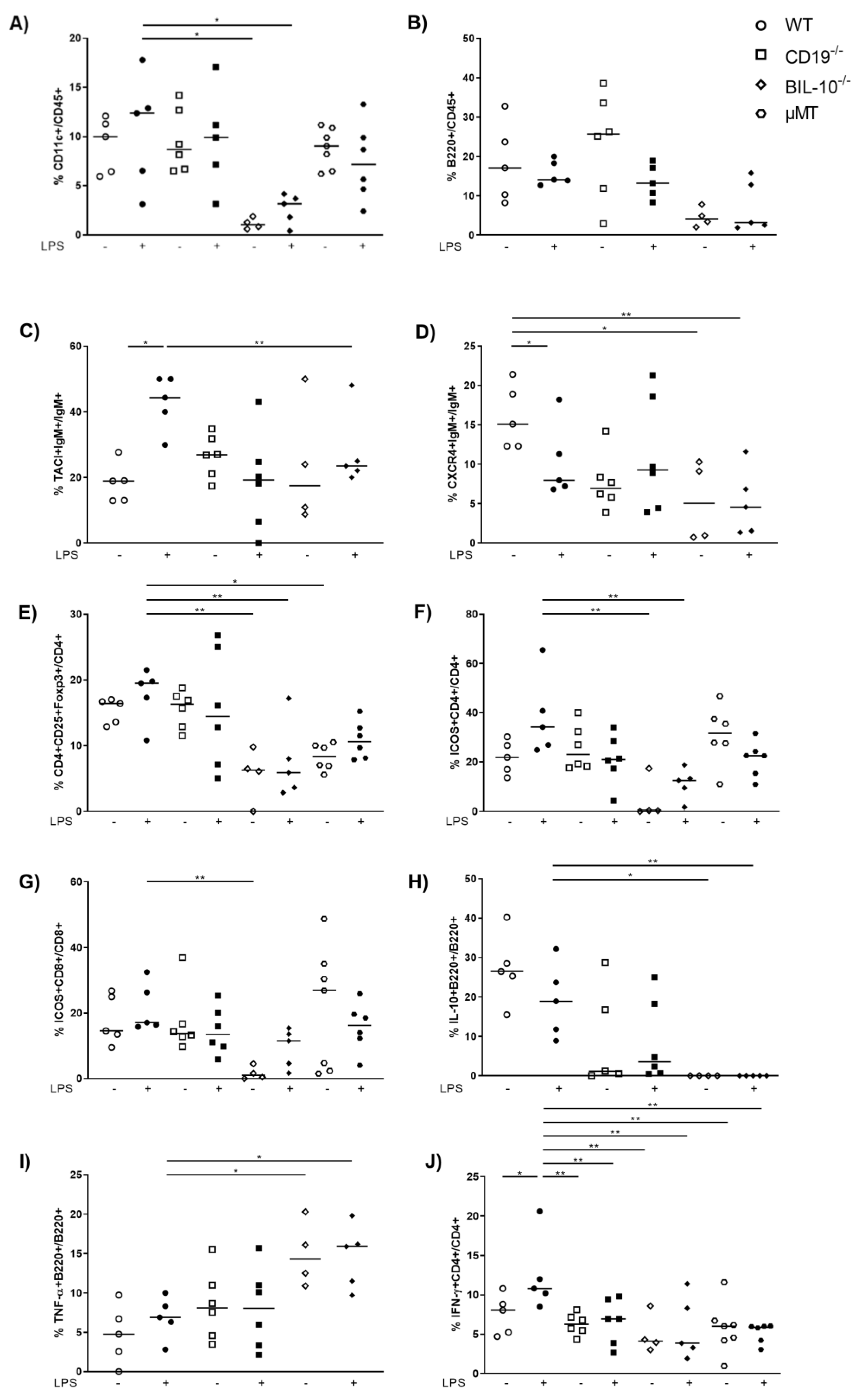
3.2. B Cell-Specific IL-10 Deficiency Impacts on the Cellular Composition and Cytokine Concentrations in Peritoneal Cavity after LPS Challenge at gd16
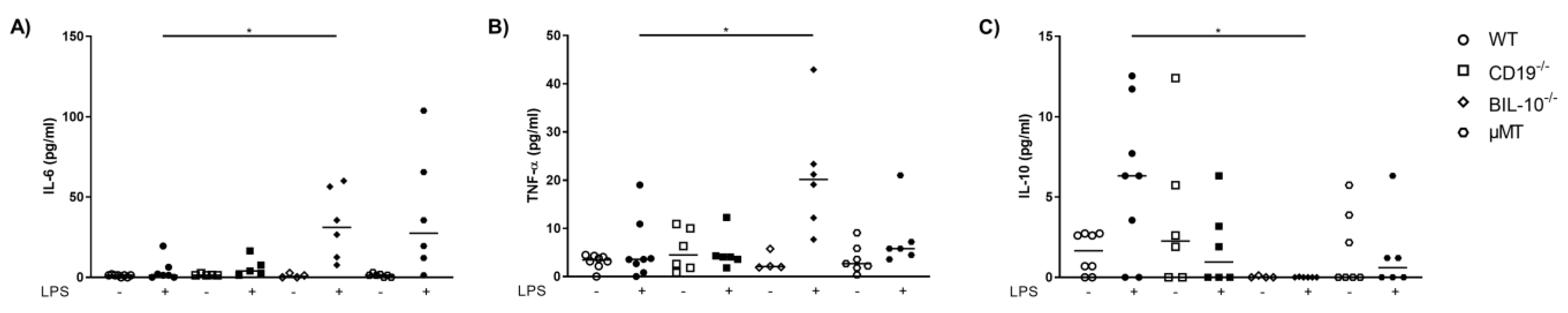
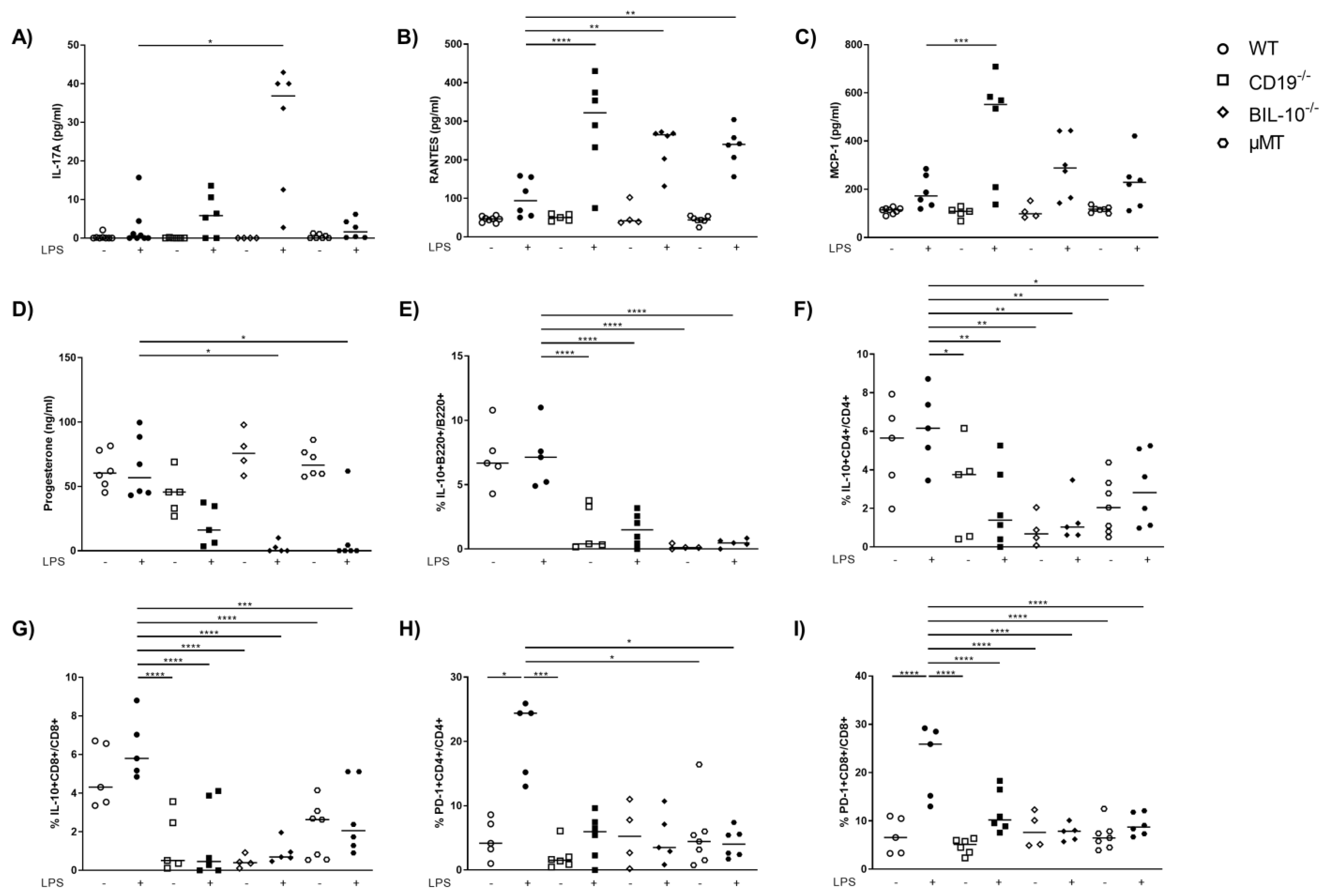
3.3. CD19 Deficiency or B Cell Specific IL-10 Knockout Influence the Level of Cytokines and Chemokines Observed in Blood from Pregnant Animals That Received LPS
3.4. B Cell-Specific IL-10 Deficiency Influences the Frequency of Uterine Cell Populations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113 (Suppl. S3), 17–42. [Google Scholar] [CrossRef]
- Busse, M.; Campe, K.-N.J.; Redlich, A.; Oettel, A.; Hartig, R.; Costa, S.-D.; Zenclussen, A. Regulatory B Cells Are Decreased and Impaired in Their Function in Peripheral Maternal Blood in Pre-term Birth. Front. Immunol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Busse, M.; Redlich, A.; Hartig, R.; Costa, S.-D.; Rathert, H.; Fest, S.; Zenclussen, A.C. Imbalance between inflammatory and regulatory cord blood B cells following pre-term birth. J. Reprod. Immunol. 2021, 145, 103319. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B Cells, Antibodies, and More. Clin. J. Am. Soc. Nephrol. 2016, 11, 137–154. [Google Scholar] [CrossRef]
- Berthelot, J.-M.; Jamin, C.; Amrouche, K.; Le Goff, B.; Maugars, Y.; Youinou, P. Regulatory B cells play a key role in immune system balance. Joint. Bone Spine 2013, 80, 18–22. [Google Scholar] [CrossRef]
- Shang, J.; Zha, H.; Sun, Y. Phenotypes, Functions, and Clinical Relevance of Regulatory B Cells in Cancer. Front. Immunol. 2020, 11, 582657. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Haque, N. Reproductive immunomodulatory functions of B cells in pregnancy. Int. Rev. Immunol. 2020, 39, 53–66. [Google Scholar] [CrossRef]
- Canellada, A.; Färber, A.; Zenclussen, A.; Gentile, T.; Dokmetjian, J.; Keil, A.; Blois, S.; Miranda, S.; Berod, L.; Gutiérrez, G.; et al. Interleukin Regulation of Asymmetric Antibody Synthesized by Isolated Placental B Cells. Am. J. Reprod. Immunol. 2002, 48, 275–282. [Google Scholar] [CrossRef]
- Kalinderi, K.; Delkos, D.; Kalinderis, M.; Athanasiadis, A.; Kalogiannidis, I. Urinary tract infection during pregnancy: Current concepts on a common multifaceted problem. J. Obstet. Gynaecol. 2018, 38, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Puopolo, K.M.; Hansen, N.I.; Sánchez, P.J.; Bell, E.F.; Carlo, W.A.; Cotten, C.M.; D’Angio, C.T.; Kazzi, S.N.J.; Poindexter, B.B.; et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the Need for Novel Prevention Strategies. JAMA Pediatr. 2020, 174, e200593. [Google Scholar] [CrossRef] [PubMed]
- Andreakos, E.; Sacre, S.; Smith, C.; Lundberg, A.; Kiriakidis, S.; Stonehouse, T.; Monaco, C.; Feldmann, M.; Foxwell, B.M. Distinct pathways of LPS-induced NF-κB activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood 2004, 103, 2229–2237. [Google Scholar] [CrossRef]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.-I.; Mühlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide Stimulates the MyD88-Independent Pathway and Results in Activation of IFN-Regulatory Factor 3 and the Expression of a Subset of Lipopolysaccharide-Inducible Genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Su, I.-H.; Miyake, K.; Nagai, Y.; Akashi, S.; Mecklenbräuker, I.; Rajewsky, K.; Kimoto, M.; Tarakhovsky, A. The Toll-like Receptor Protein Rp105 Regulates Lipopolysaccharide Signaling in B Cells. J. Exp. Med. 2000, 192, 23–30. [Google Scholar] [CrossRef]
- Nagai, Y.; Yanagibashi, T.; Watanabe, Y.; Ikutani, M.; Kariyone, A.; Ohta, S.; Hirai, Y.; Kimoto, M.; Miyake, K.; Takatsu, K. The RP105/MD-1 complex is indispensable for TLR4/MD-2-dependent proliferation and IgM-secreting plasma cell differentiation of marginal zone B cells. Int. Immunol. 2012, 24, 389–400. [Google Scholar] [CrossRef]
- Yazawa, N.; Fujimoto, M.; Sato, S.; Miyake, K.; Asano, N.; Nagai, Y.; Takeuchi, O.; Takeda, K.; Okochi, H.; Akira, S.; et al. CD19 regulates innate immunity by the toll-like receptor RP105 signaling in B lymphocytes. Blood 2003, 102, 1374–1380. [Google Scholar] [CrossRef]
- Busse, M.; Langwisch, S.; Tedford, K.; Fischer, K.-D.; Zenclussen, A.C. Maternal B cell signaling orchestrates fetal development in mice. Development 2022, 149, dev199783. [Google Scholar] [CrossRef] [PubMed]
- Roers, A.; Siewe, L.; Strittmatter, E.; Deckert, M.; Schlüter, D.; Stenzel, W.; Gruber, A.D.; Krieg, T.; Rajewsky, K.; Muller, W. T Cell–specific Inactivation of the Interleukin 10 Gene in Mice Results in Enhanced T Cell Responses but Normal Innate Responses to Lipopolysaccharide or Skin Irritation. J. Exp. Med. 2004, 200, 1289–1297. [Google Scholar] [CrossRef]
- Rickert, R.C.; Rajewsky, K.; Roes, J. Impairment of T-cell-dependent B-cell responses and B-l cell development in CD19-deficient mice. Nature 1995, 376, 352–355. [Google Scholar] [CrossRef]
- Kitamura, D.; Roes, J.; Kuhn, R.; Rajewsky, K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 1991, 350, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Ehrentraut, S.; Scharm, M.; Wang, H.; Hartig, R.; Morse, H.C., 3rd; Zenclussen, A.C. Plasma Cell Alloantigen 1 and IL-10 Secretion Define Two Distinct Peritoneal B1a B Cell Subsets With Opposite Functions, PC1high Cells Being Protective and PC1low Cells Harmful for the Growing Fetus. Front. Immunol. 2018, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Genuino, R.M.; Dimova, T.; You, Y.; Aldo, P.; Hayball, J.D.; Mor, G.; Diener, K.R. Trophoblasts promote induction of a regulatory phenotype in B cells that can protect against detrimental T cell–mediated inflammation. Am. J. Reprod. Immunol. 2019, 82, e13187. [Google Scholar] [CrossRef] [PubMed]
- Fettke, F.; Schumacher, A.; Canellada, A.; Toledo, N.; Bekeredjian-Ding, I.; Bondt, A.; Wuhrer, M.; Costa, S.-D.; Zenclussen, A.C. Maternal and Fetal Mechanisms of B Cell Regulation during Pregnancy: Human Chorionic Gonadotropin Stimulates B Cells to Produce IL-10 While Alpha-Fetoprotein Drives Them into Apoptosis. Front. Immunol. 2016, 7, 495. [Google Scholar] [CrossRef]
- Jensen, F.; Muzzio, D.; Soldati, R.; Fest, S.; Zenclussen, A.C. Regulatory B10 Cells Restore Pregnancy Tolerance in a Mouse Model1. Biol. Reprod. 2013, 89, 90. [Google Scholar] [CrossRef]
- Rolle, L.; Tehran, M.M.; Morell, A.; Raeva, Y.; Schumacher, A.; Hartig, R.; Costa, S.-D.; Jensen, F.; Zenclussen, A.C. Cutting Edge: IL-10-Producing Regulatory B Cells in Early Human Pregnancy. Am. J. Reprod. Immunol. 2013, 70, 448–453. [Google Scholar] [CrossRef]
- Cyster, J.G.; Allen, C.D. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef]
- Busse, M.; Campe, K.-N.J.; Nowak, D.; Schumacher, A.; Plenagl, S.; Langwisch, S.; Tiegs, G.; Reinhold, A.; Zenclussen, A.C. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci. Rep. 2019, 9, 9335. [Google Scholar] [CrossRef]
- Robertson, S.A.; Skinner, R.J.; Care, A. Essential Role for IL-10 in Resistance to Lipopolysaccharide-Induced Preterm Labor in Mice. J. Immunol. 2006, 177, 4888–4896. [Google Scholar] [CrossRef]
- Busse, M.; Scharm, M.; Oettel, A.; Redlich, A.; Costa, S.-D.; Zenclussen, A.C. Enhanced S100B expression in T and B lymphocytes in spontaneous preterm birth and preeclampsia. J. Perinat. Med. 2022, 50, 157–166. [Google Scholar] [CrossRef]
- Ao, M.; Miyauchi, M.; Furusho, H.; Inubushi, T.; Kitagawa, M.; Nagasaki, A.; Sakamoto, S.; Kozai, K.; Takata, T. Dental Infection of Porphyromonas gingivalis Induces Preterm Birth in Mice. PLoS ONE 2015, 10, e0137249. [Google Scholar] [CrossRef]
- Van Boeckel, S.R.; Hrabalkova, L.; Baker, T.L.; MacPherson, H.; Frew, L.; Boyle, A.K.; McHugh, B.J.; Wilson, K.; Norman, J.; Dorin, J.R.; et al. Cathelicidins and the Onset of Labour. Sci. Rep. 2019, 9, 7356. [Google Scholar] [CrossRef]
- Crawford, A.; Angelosanto, J.M.; Nadwodny, K.L.; Blackburn, S.D.; Wherry, E.J. A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection. PLOS Pathog. 2011, 7, e1002098. [Google Scholar] [CrossRef]
- Von Luettichau, I.; Nelson, P.J.; Pattison, J.M.; Van De Rijn, M.; Huie, P.; Warnke, R.; Wiedermann, C.J.; Stahl, R.A.; Sibley, R.K.; Krensky, A.M. RANTES chemokine expression in diseased and normal human tissues. Cytokine 1996, 8, 89–98. [Google Scholar] [CrossRef]
- Amabebe, E.; Reynolds, S.; He, X.; Wood, R.; Stern, V.; Anumba, D.O.C. Infection/inflammation-associated preterm delivery within 14 days of presentation with symptoms of preterm labour: A multivariate predictive model. PLoS ONE 2019, 14, e0222455. [Google Scholar] [CrossRef]
- Hentschke, M.; Krauspenhar, B.; Guwzinski, A.; Caruso, F.; Silveira, I.; Antonello, I.; Gadonski, G.; Poli-De-Figueiredo, C.; Da Costa, B.P. PP040. Expression of RANTES (CCL5) in maternal plasma, fetal plasma and placenta in pre-eclampsia and normotensive controls. Pregnancy Hypertens 2012, 2, 263. [Google Scholar] [CrossRef]
- Diamond, A.K.; Sweet, L.M.; Oppenheimer, K.H.; Bradley, D.F.; Phillippe, M. Modulation of Monocyte Chemotactic Protein-1 Expression During Lipopolysaccharide-Induced Preterm Delivery in the Pregnant Mouse. Reprod. Sci. 2007, 14, 548–559. [Google Scholar] [CrossRef]
- Shynlova, O.; Nedd-Roderique, T.; Li, Y.; Dorogin, A.; Lye, S.J. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J. Cell. Mol. Med. 2013, 17, 90–102. [Google Scholar] [CrossRef]
- Thomson, A.J.; Telfer, J.F.; Young, A.; Campbell, S.; Stewart, C.J.; Cameron, I.T.; Greer, I.A.; Norman, J.E. Leukocytes infiltrate the myometrium during human parturition: Further evidence that labour is an inflammatory process. Hum. Reprod. 1999, 14, 229–236. [Google Scholar] [CrossRef]
- Osman, I.; Young, A.; Ledingham, M.A.; Thomson, A.J.; Jordan, F.; Greer, I.A.; Norman, J.E. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 2003, 9, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Abrahams, V.M. Neutrophils in preterm birth: Friend or foe? Placenta 2020, 102, 17–20. [Google Scholar] [CrossRef]
- Norton, R.S. Anti-Infective Peptides to Enhance the Host Innate Response: Design, Development and Delivery. Protein Pept. Lett. 2018, 25, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Juhas, U.; Ryba-Stanisławowska, M.; Szargiej, P.; Myśliwska, J. Different pathways of macrophage activation and polarization. Postepy. Hig. Med. Dosw. 2015, 69, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Jaiswal, M.K.; Ilievski, V.; Beaman, K.D.; Jilling, T.; Hirsch, E. Platelet-Activating Factor: A Role in Preterm Delivery and an Essential Interaction with Toll-Like Receptor Signaling in Mice. Biol. Reprod. 2014, 91, 119. [Google Scholar] [CrossRef] [PubMed]
- Aikio, O.; Vuopala, K.; Pokela, M.-L.; Hallman, M. Diminished Inducible Nitric Oxide Synthase Expression in Fulminant Early-Onset Neonatal Pneumonia. Pediatrics 2000, 105, 1013–1019. [Google Scholar] [CrossRef]
- Azizieh, F.; Raghupathy, R. IL-10 and pregnancy complications. Clin. Exp. Obstet. Gynecol. 2017, 44, 252–258. [Google Scholar] [CrossRef]
- Kaislasuo, J.; Simpson, S.; Petersen, J.F.; Peng, G.; Aldo, P.; Lokkegaard, E.; Paidas, M.; Pal, L.; Guller, S.; Mor, G. IL-10 to TNFα ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am. J. Reprod. Immunol. 2020, 83, e13195. [Google Scholar] [CrossRef]
- Huang, B.; Faucette, A.N.; Pawlitz, M.; Pei, B.; Goyert, J.; Zhou, J.; El-Hage, N.G.; Deng, J.; Lin, J.; Yao, F.; et al. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat. Med. 2017, 23, 128–135. [Google Scholar] [CrossRef]
- Wafula, P.O.; Teles, A.; Schumacher, A.; Pohl, K.; Yagita, H.; Volk, H.-D.; Zenclussen, A.C. PD-1 but not CTLA-4 Blockage Abrogates the Protective Effect of Regulatory T Cells in a Pregnancy Murine Model. Am. J. Reprod. Immunol. 2009, 62, 283–292. [Google Scholar] [CrossRef]
- Taglauer, E.S.; Yankee, T.; Petroff, M.G. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J. Reprod. Immunol. 2009, 80, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Tang, C.; Gong, Y.; Liu, Y.; Rao, J.; Chen, S.; Qu, W.; Wu, D.; Lei, L.; Chen, L. PD-1/PD-L1 regulates Treg differentiation in pregnancy-induced hypertension. Braz. J. Med. Biol. Res. 2018, 51, e7334. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Y.; Wang, S.-C.; Lin, Y.-K.; Li, D.-J.; DU, M.-R. Tim-3 and PD-1 regulate CD8+ T cell function to maintain early pregnancy in mice. J. Reprod. Dev. 2017, 63, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, L.; Hu, X.; Ji, J.; Mor, G.; Liao, A. The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum. Reprod. 2019, 34, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.; Soto, C.D.A.; Tometten, M.; Klapp, B.F.; Margni, R.A.; Arck, P.C. Lineage, Maturity, and Phenotype of Uterine Murine Dendritic Cells Throughout Gestation Indicate a Protective Role in Maintaining Pregnancy. Biol. Reprod. 2004, 70, 1018–1023. [Google Scholar] [CrossRef]
- Bizargity, P.; Bonney, E.A. Dendritic cells: A family portrait at mid-gestation. Immunology 2009, 126, 565–578. [Google Scholar] [CrossRef]
- Shima, T.; Nakashima, A.; Yasuda, I.; Ushijima, A.; Inada, K.; Tsuda, S.; Yoshino, O.; Tomura, M.; Saito, S. Uterine CD11c+ cells induce the development of paternal antigen-specific Tregs via seminal plasma priming. J. Reprod. Immunol. 2020, 141, 103165. [Google Scholar] [CrossRef]
- Langat, D.L.; Wheaton, D.A.; Platt, J.S.; Sifers, T.; Hunt, J.S. Signaling Pathways for B Cell-Activating Factor (BAFF) and a Proliferation-Inducing Ligand (APRIL) in Human Placenta. Am. J. Pathol. 2008, 172, 1303–1311. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhang, Y.-M.; Zhang, X.-M.; Tao, J. Effect of TACI Signaling on Humoral Immunity and Autoimmune Diseases. J. Immunol. Res. 2015, 2015, 247426. [Google Scholar] [CrossRef]
- Meng, L.; Almeida, L.N.; Clauder, A.-K.; Lindemann, T.; Luther, J.; Link, C.; Hofmann, K.; Kulkarni, U.; Wong, D.M.; David, J.-P.; et al. Bone Marrow Plasma Cells Modulate Local Myeloid-Lineage Differentiation via IL-10. Front. Immunol. 2019, 10, 1183. [Google Scholar] [CrossRef]
- Dickinson, G.S.; Akkoyunlu, M.; Bram, R.J.; Alugupalli, K.R. BAFF receptor and TACI in B-1b cell maintenance and antibacterial responses. Ann. N. Y. Acad. Sci. 2015, 1362, 57–67. [Google Scholar] [CrossRef]
- Morbach, H.; Schickel, J.-N.; Cunningham-Rundles, C.; Conley, M.E.; Reisli, I.; Franco, J.; Meffre, E. CD19 controls Toll-like receptor 9 responses in human B cells. J. Allergy Clin. Immunol. 2016, 137, 889–898.e6. [Google Scholar] [CrossRef]
- Hua, C.; Audo, R.; Yeremenko, N.; Baeten, D.; Hahne, M.; Combe, B.; Morel, J.; Daïen, C. A proliferation inducing ligand (APRIL) promotes IL-10 production and regulatory functions of human B cells. J. Autoimmun. 2016, 73, 64–72. [Google Scholar] [CrossRef]
- Nie, Y.; Waite, J.; Brewer, F.; Sunshine, M.J.; Littman, D.R.; Zou, Y.-R. The Role of CXCR4 in Maintaining Peripheral B Cell Compartments and Humoral Immunity. J. Exp. Med. 2004, 200, 1145–1156. [Google Scholar] [CrossRef]
- Moon, H.; Lee, J.-G.; Shin, S.H.; Kim, T.J. LPS-Induced Migration of Peritoneal B-1 Cells is Associated with Upregulation of CXCR4 and Increased Migratory Sensitivity to CXCL12. J. Korean Med. Sci. 2012, 27, 27–35. [Google Scholar] [CrossRef]
- Löhning, M.; Hutloff, A.; Kallinich, T.; Mages, H.W.; Bonhagen, K.; Radbruch, A.; Hamelmann, E.; Kroczek, R.A. Expression of ICOS In Vivo Defines CD4+ Effector T Cells with High Inflammatory Potential and a Strong Bias for Secretion of Interleukin 10. J. Exp. Med. 2003, 197, 181–193. [Google Scholar] [CrossRef]
- Riella, L.V.; Dada, S.; Chabtini, L.; Smith, B.; Huang, L.; Dakle, P.; Mfarrej, B.; D’Addio, F.; Adams, L.-T.; Kochupurakkal, N.; et al. B7h (ICOS-L) Maintains Tolerance at the Fetomaternal Interface. Am. J. Pathol. 2013, 182, 2204–2213. [Google Scholar] [CrossRef]
- Kälble, F.; Mai, C.; Wagner, M.; Schober, L.; Schaier, M.; Zeier, M.; Spratte, J.; Fluhr, H.; Steinborn, A. Aberrant ICOS+-T cell differentiation in women with spontaneous preterm labor. Am. J. Reprod. Immunol. 2016, 76, 415–425. [Google Scholar] [CrossRef]
- Wagner, M.I.; Jöst, M.; Spratte, J.; Schaier, M.; Mahnke, K.; Meuer, S.; Zeier, M.; Steinborn, A. Differentiation of ICOS+ and ICOS− recent thymic emigrant regulatory T cells (RTE Tregs) during normal pregnancy, pre-eclampsia and HELLP syndrome. Clin. Exp. Immunol. 2016, 183, 129–142. [Google Scholar] [CrossRef]
- Monteiro, C.; Kasahara, T.; Sacramento, P.M.; Dias, A.; Leite, S.; Silva, V.G.; Gupta, S.; Agrawal, A.; Bento, C.A.M. Human pregnancy levels of estrogen and progesterone contribute to humoral immunity by activating TFH/B cell axis. Eur. J. Immunol. 2021, 51, 167–179. [Google Scholar] [CrossRef]
- Monteiro, C.; Kasahara, T.M.; Castro, J.R.; Sacramento, P.M.; Hygino, J.; Centurião, N.; Cassano, T.; Lopes, L.M.F.; Leite, S.; Silva, V.G.; et al. Pregnancy favors the expansion of circulating functional follicular helper T Cells. J. Reprod. Immunol. 2017, 121, 1–10. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Z.; Zhang, S.; Ren, J.; Ma, X.; Qin, C.; Tian, F.; Zhang, Y.; Lin, Y. Characterization of T follicular helper cells in allogeneic normal pregnancy and PDL1 blockage-induced abortion. Sci. Rep. 2016, 6, 36560. [Google Scholar] [CrossRef] [Green Version]
| Chemokine | WT PBS | WT LPS | CD19−/− PBS | CD19−/− LPS | BIL-10−/− PBS | BIL-10−/− LPS | µMT PBS | µMT LPS | p |
|---|---|---|---|---|---|---|---|---|---|
| IP-10 | 13.9 | 32.6 | 13.6 | 45.4 | 19.0 | 28.3 | 7.7 | 15.6 | <0.0001 |
| LIX | 334.0 | 587.0 | 733.5 | 841.5 | 205.5 | 341.3 | 662.7 | 440.7 | 0.0176 |
| MDC | 86.8 | 150.2 | 121.8 | 136.5 | 108.7 | 106.0 | 88.2 | 93.3 | 0.0386 |
| MIP-1α | 28.6 | 72.2 | 39.9 | 97.6 | 41.1 | 85.7 | 31.7 | 59.4 | <0.0001 |
| MIP-1β | 26.2 | 124.9 | 17.0 | 100.3 | 36.5 | 76.0 | 9.0 | 38.6 | 0.0002 |
| Eotaxin | 179.6 | 350.6 | 200.3 | 278.3 | 199.1 | 237.4 | 144.8 | 202.7 | 0.0057 |
| KC | 130.5 | 195.7 | 134.8 | 314.9 | 142.9 | 185.9 | 112.8 | 141.3 | <0.0001 |
| MCP-1 | 111.6 | 190.2 | 103.7 | 457.0 | 108.1 | 294.9 | 116.2 | 228.8 | <0.0001 |
| MIP-3α | 82.2 | 100.2 | 79.5 | 86.8 | 72.6 | 88.0 | 63.8 | 68.9 | 0.0268 |
| RANTES | 46.1 | 101.2 | 50.6 | 292.6 | 55.7 | 234.3 | 44.5 | 234.0 | <0.0001 |
| TARC | 42.7 | 102.2 | 52.0 | 94.8 | 49.1 | 74.3 | 39.2 | 44.3 | 0.0180 |
| MIG | 38.3 | 115.3 | 55.5 | 117.1 | 58.3 | 99.9 | 40.2 | 69.6 | <0.0001 |
| Cell Population | WT PBS | WT LPS | CD19−/− PBS | CD19−/− LPS | BIL-10−/− PBS | BIL-10−/− LPS | µMT PBS | µMT LPS | p |
|---|---|---|---|---|---|---|---|---|---|
| B220+ | 61.0 | 57.3 | 42.7 | 42.9 | 54.5 | 40.6 | 0.2972 | ||
| CD4+CD25+Foxp3+ | 8.4 | 8.4 | 7.0 | 9.7 | 3.7 | 4.9 | 4.7 | 4.2 | 0.0019 |
| ICOS+CD4+ | 8.6 | 5.6 | 5.2 | 5.1 | 1.6 | 2.0 | 3.3 | 2.6 | <0.0001 |
| ICOS+CD8+ | 2.0 | 2.3 | 2.7 | 2.5 | 0.8 | 0.6 | 1.4 | 1.5 | 0.0461 |
| PD-1+CD4+ | 4.9 | 3.4 | 5.2 | 3.1 | 1.3 | 0.9 | 2.5 | 1.8 | 0.0036 |
| PD-1+CD8+ | 2.0 | 3.1 | 2.6 | 3.7 | 1.0 | 1.0 | 2.7 | 2.4 | 0.0352 |
| IL-10+CD4+ | 5.2 | 5.0 | 1.9 | 1.9 | 1.0 | 0.9 | 2.3 | 2.5 | 0.0036 |
| IL-10+CD4+ Treg | 6.5 | 7.0 | 3.5 | 3.4 | 1.2 | 2.1 | 3.8 | 3.4 | 0.0555 |
| IL-10+CD8+ | 5.6 | 4.9 | 2.7 | 2.3 | 0.6 | 1.3 | 2.6 | 2.4 | 0.0055 |
| Cell Population | WT PBS | WT LPS | CD19−/− PBS | CD19−/− LPS | BIL-10−/− PBS | BIL-10−/− LPS | µMT PBS | µMT LPS | p |
|---|---|---|---|---|---|---|---|---|---|
| B220+ | 65.0 | 62.0 | 46.3 | 56.1 | 46.5 | 61.9 | 0.0041 | ||
| CD4+CD25+Foxp3+ | 8.3 | 10.9 | 6.2 | 9.3 | 2.4 | 2.8 | 4.4 | 6.9 | 0.0002 |
| ICOS+CD4+ | 12.9 | 17.1 | 9.8 | 9.8 | 0.7 | 3.0 | 7.6 | 7.4 | <0.0001 |
| ICOS+CD8+ | 14.1 | 14.1 | 7.2 | 12.2 | 0.6 | 1.1 | 9.8 | 8.5 | 0.0062 |
| PD-1+CD4+ | 12.9 | 17.1 | 9.8 | 9.8 | 1.59 | 2.8 | 7.6 | 7.4 | <0.0001 |
| PD-1+CD8+ | 9.8 | 9.0 | 9.8 | 9.7 | 1.3 | 1.9 | 7.0 | 9.1 | 0.1422 |
| IL-10+CD4+ | 1.9 | 4.82 | 1.8 | 1.3 | 0.3 | 0.7 | 1.9 | 2.2 | 0.0967 |
| IL-10+CD4+ Treg | 2.2 | 2.5 | 3.5 | 2.5 | 0.3 | 0.9 | 2.6 | 2.0 | 0.1539 |
| IL-10+CD8+ | 3.5 | 3.3 | 2.7 | 1.6 | 0.4 | 0.7 | 2.4 | 2.7 | 0.0037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busse, M.; Zenclussen, A.C. IL-10 Producing B Cells Protect against LPS-Induced Murine Preterm Birth by Promoting PD1- and ICOS-Expressing T Cells. Cells 2022, 11, 2690. https://doi.org/10.3390/cells11172690
Busse M, Zenclussen AC. IL-10 Producing B Cells Protect against LPS-Induced Murine Preterm Birth by Promoting PD1- and ICOS-Expressing T Cells. Cells. 2022; 11(17):2690. https://doi.org/10.3390/cells11172690
Chicago/Turabian StyleBusse, Mandy, and Ana Claudia Zenclussen. 2022. "IL-10 Producing B Cells Protect against LPS-Induced Murine Preterm Birth by Promoting PD1- and ICOS-Expressing T Cells" Cells 11, no. 17: 2690. https://doi.org/10.3390/cells11172690
APA StyleBusse, M., & Zenclussen, A. C. (2022). IL-10 Producing B Cells Protect against LPS-Induced Murine Preterm Birth by Promoting PD1- and ICOS-Expressing T Cells. Cells, 11(17), 2690. https://doi.org/10.3390/cells11172690






