Abstract
Slowness of movement initiation is a cardinal motor feature of Parkinson’s disease (PD) and is not fully reverted by current dopaminergic treatments. This trouble could be due to the dysfunction of executive processes and, in particular, of inhibitory control of response initiation, a function possibly associated with the noradrenergic (NA) system. The implication of NA in the network supporting proactive inhibition remains to be elucidated using pharmacological protocols. For that purpose, we administered 150 μg of clonidine to 15 healthy subjects and 12 parkinsonian patients in a double-blind, randomized, placebo-controlled design. Proactive inhibition was assessed by means of a Go/noGo task, while pre-stimulus brain activity was measured by event-related functional MRI. Acute reduction in noradrenergic transmission induced by clonidine enhanced difficulties initiating movements reflected by an increase in omission errors and modulated the activity of the anterior node of the proactive inhibitory network (dorsomedial prefrontal and anterior cingulate cortices) in PD patients. We conclude that NA contributes to movement initiation by acting on proactive inhibitory control via the α2-adrenoceptor. We suggest that targeting noradrenergic dysfunction may represent a new treatment approach in some of the movement initiation disorders seen in Parkinson’s disease.
1. Introduction
Although Parkinson’s disease (PD) has long been considered a motor and dopaminergic disease, numerous non-dopaminergic neurotransmitter systems are implicated in its motor features (Figure 1A) [1]. Current drugs that modulate the acetylcholinergic, glutamatergic, histaminergic, adenosinergic, GABAergic, serotonergic or noradrenergic (NA) systems might thus provide clinical benefits as add-on therapies to L-dopa by targeting symptoms that may be mediated by nondopaminergic systems [1,2,3,4]. However, the neurochemical bases of numerous motor subfunctions and non-motor functions that modulate movement control are still obscure, making it difficult to associate symptoms with potentially relevant pharmacological solutions.
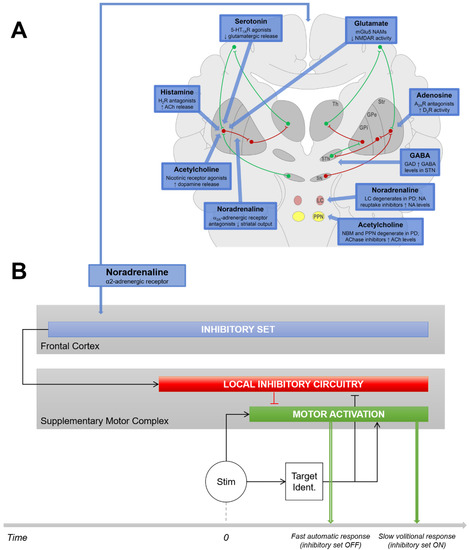
Figure 1.
Issues and Hypotheses. (A) Nondopaminergic neurotransmitter systems involved in the motor features of PD. Taken from [1] and reproduced with permission. (B) We hypothesize that the impairment in initiating movements in PD patients might be related to the NA system. Indeed, the NA system likely plays a substantial role in proactive response inhibition: a cortico–ganglio–thalamo–cortical function is intended to inhibit movement triggering mechanisms by anticipation to prevent erroneous responses when the context is uncertain. When proactive inhibition is ON, motor responses are delayed with respect to a condition that does not require inhibition (fast automatic responses) because it takes additional time to release inhibition after the stimulus has been identified. PD patients are known to have enhanced difficulties to trigger automatic responses when the context does not require action restraint. This might be due to the fact that PD patients are often locked into a mode of control, maintaining inappropriate proactive inhibition over willed movements (i.e., troubles to switch from controlled to automatic behavior). If this disorder is associated with the NA system, manipulating noradrenergic tonus by means of clonidine, an α2-AR agonist, should induce brain activation differences in the proactive inhibition network associated with the lengthening of reaction time in PD patients with respect to healthy controls. GPe, globus pallidus externa; GPi, globus pallidus interna; LC, locus coeruleus; SN, substantia nigra; Str, striatum; STN, subthalamic nucleus; Th, thalamus.
Within this context, akinesia, referred to as slowness in movement initiation [5,6], has long been considered a pure motor symptom of PD, related to dysfunctions of the circuit linking the motor cortices to specific sensorimotor territories within the basal ganglia nuclei [7,8]. However, this view fails to explain experimental observations, such as the fact that lesions of the motor thalamus do not result in akinesia [9], or that internal pallidum lesions do not improve it [10]. Moreover, slowness in movement initiation is not fully reverted by dopaminergic treatments [11,12,13]. It is therefore unlikely that only a dysfunction of the motor cortico–subcortical circuit fully accounts for slowness in movement initiation.
Impairment in initiating movements in PD might also be related to executive dysfunctions [14]; in particular, to abnormal proactive inhibitory control [12,15,16]. Proactive inhibition is a pivotal mechanism which gates movement initiation in anticipation of stimulation when the context is uncertain and requires action restraint (Figure 1B) [17,18,19,20]. Proactive inhibition has been considered the default state of the executive system [19,20,21]. In healthy subjects, it takes less than 300 ms to release proactive inhibition and allow response triggering after an appropriate stimulus has been identified [21]. It takes no more time to release this default state of inhibition after a cue has removed uncertainty about upcoming stimulation, i.e., to switch from controlled to automatic behavior [22,23]. This ability would be impaired in PD patients [14], who would have difficulties releasing the default proactive inhibitory mode of control, even when the situation does not require action restraint [15]. This would substantially delay the initiation of responses, especially when the release of proactive inhibition must be internally driven (Figure 1B).
The difficulty of PD patients to release proactive inhibition is not fully compensated by dopaminergic medication [12]. Multiple clues point to the possible role of the NA system. Notably, the NA system has been involved in executive functions that likely share common mechanisms with proactive inhibition [24,25,26,27,28,29,30,31]. More specifically, clonidine, a specific α2-adrenergic receptor (AR) agonist, was found to cancel the positive action of subthalamic nucleus-deep brain stimulation (STN-DBS) on akinesia suggesting, at least in part, a noradrenergic-dependent STN-DBS efficiency in movement initiation [16,32].
As disorders of proactive inhibitory control in PD may account for various clinical symptoms ranging from akinesia (impaired ability to release proactive inhibition [15]) to impulsivity (impaired ability to sustain proactive inhibition [33,34]), increased knowledge on how noradrenaline may affect the neural network underlying this executive function may fuel the development of more optimal pharmacological treatments. Accordingly, to test the hypothesis that NA dysfunction plays a role in the pathophysiology of akinesia in PD, we manipulated NA tonus by means of clonidine, and assessed the ability of parkinsonian patients to control movement initiation with respect to healthy subjects in a double-blind placebo-controlled functional MRI study.
2. Materials and Methods
2.1. Participants
Two groups participated in the study. Fifteen healthy control subjects (aged from 41 to 70 years, six males), with no history of neurologic or psychiatric disorder, were recruited from advertisement. Twelve idiopathic parkinsonian patients (aged from 45 to 70 years, eight males), with no history of neurological disorder other than PD and current psychiatry comorbidity, were also enrolled. All participants were right-handed with normal or corrected-to-normal vision and underwent a medical screening. Only subjects with a systolic blood pressure above 100 mmHg and a diastolic blood pressure above 70 mmHg were included in the study. Exclusion criteria for the two groups were: ferromagnetic implanted materials; claustrophobia; pregnancy; a history of cholinergic or noradrenergic medications; uncontrolled hypertension; glaucoma; and scoring above 130 on the Mattis Dementia Rating Scale. All patients were tested on regular PD medication. Demographic and clinical characteristics of participants are presented in Table 1.

Table 1.
Demographic and clinical characteristics of the participants.
2.2. Drug Design
A double-blind, placebo-controlled, cross-over design was used with nine healthy subjects and eight patients randomized to receive a single oral dose of a lactose placebo on a first session, followed by 150 µg of clonidine on a second session, as well as six controls and five patients randomized to receive the drug first, followed by the placebo. For each subject, testing sessions were separated by at least 5 days. Each participant was tested at approximately the same time of the day (afternoon). They were instructed to abstain from caffeine, nicotine and other psycho-active substances from 24 h before the start of the session. Since peak plasma concentration for clonidine is achieved approximately 1–3 h following oral dosing [35], functional MRI sessions started 90 min after administration and lasted 1 h. Clonidine has well-established anti-hypertensive properties; accordingly, blood pressure was monitored for subject safety. Measurements were taken every 30 min, starting from drug administration until the end of the functional MRI scans. None of the participants reported any side effects of the medication.
2.3. Experimental Design and Apparatus
Subjects were asked to react as fast as possible to visual Go stimuli by pressing a nonmagnetic handgrip with their right hand (Figure 2; see detailed procedure of stimulus design and presentation in [15]).
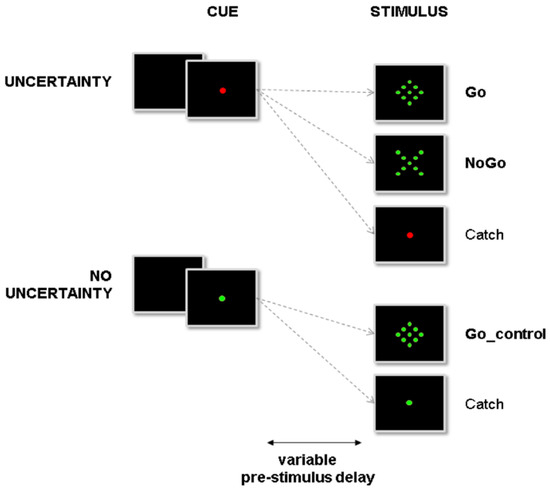
Figure 2.
Overview of the experimental setup. Subjects were instructed to react to the presentation of a go signal (diamond) by pressing a button. At the beginning of a trial, the central fixation point (cue) could turn either red or green, indicating, respectively, that NoGo stimuli (X) could or could not be presented. In the former condition (uncertainty condition), proactive inhibition was required during the pre-stimulus period to avoid erroneous automatic responses to NoGo stimuli. In the latter condition, proactive inhibition was not required during the pre-stimulus period. Subjects could react automatically to any upcoming target (no uncertainty condition).
In order to optimize the discriminative power of the fMRI contrast intended to reveal proactive control related activity, we used only the longest pre-stimulus delays (four to six seconds) [21]. The experiment was divided into two acquisition sessions—placebo/clonidine—with four runs each. Each run was composed of 20 Go trials, 20 NoGo trials, 20 Go_control trials and 20 catch trials (no stimulus), randomly presented, for a sum of 80 trials/condition of interest, giving a total of 320 trials for each session.
Time series from the handgrip were sampled at 1000 Hz (12 bits A/D converter). Force signals were filtered using a second-order Butterworth filter (dual pass 30 Hz lowpass cut-off frequency). Reaction times (RTs) were obtained after standard time series analyses [37]. Based on the distributions of baseline fluctuations and response peaks, movement initiation was defined as the time at which the grip force exceeded the baseline mean force signal + 35% to reach response peak force. RT was defined as the time elapsed between stimulus presentation and movement initiation. As it increases as a function of the time needed to release proactive inhibition, RT is an appropriate marker of response inhibition in this kind of task [15,19,38,39]. Inappropriate responses to NoGo signals or in absence of signal (commission errors) were analyzed to index difficulties in implementing proactive inhibition (impulsivity). Go trials without response (omission errors) were analyzed to index difficulties in releasing proactive inhibition (akinesia).
Images were acquired on a 1.5-T Siemens MRI scanner, equipped with a circular polarized head coil. For each participant, we acquired a high-resolution structural T1-weighted image (EPI sequence, resolution 1 × 1 × 1 mm) in sagittal orientation, covering the whole brain. For functional imaging, we used a T2*-weighted echoplanar sequence, covering the whole brain with 28 interleaved 3.44-mm-thick/0-mm-gap axial slices (repetition time = 2620 ms, echo time = 60 ms, flip angle = 90°, field of view = 220 cm, 64 × 64 matrix of 3.44 × 3.44 × 4.4 mm voxels).
2.4. Data Processing
Neuroimaging data were processed using the Statistical Parametric Mapping software (SPM8; http///www.fil.ion.ucl.ac.uk/spm/ first accessed on 1 September 2014), according to the general linear model. In order to account for magnetic saturation, the effects of the first five functional volumes of each run were removed. The other 240 images were corrected for differences in slice acquisition time. They were then realigned for the correction of head movements. Scans displaying more than 1.5% variation in global intensity, and scans showing more than 0.5 mm/time repetition in scan-to-scan motion, were considered as outliers and repaired using the ArtRepair SPM toolbox (http://spnl.stanford.edu/tools/ArtRepair/ArtRepair.html, first accessed on 1 September 2014). The DARTEL toolbox was used to perform spatial normalization on an MNI template. Data were smoothed spatially using an isotropic Gaussian filter (8mm full width at half maximum).
All events were convolved with a canonical hemodynamic response function after being time-locked to the onset of the red or green cue and modeled according to both their onset and duration. The analysis focused on the pre-stimulus period, while other events were considered as events of non-interest in the statistical analysis.
Based on the studies referenced in the introduction section, a mask encompassing all regions was found to induce potential proactive inhibition-related activity based on the aal atlas [40]. This mask includes the dorsal premotor cortex (PMd), the supplementary motor area (SMA), the dorsomedial prefrontal cortex, the inferior frontal gyrus (rIFG), the angular gyrus, the insula, the thalamus, the striatum and the STN. Data were high pass-filtered (cutoff frequency: 128 s) and summarized into one contrast per subject: the intensity of the pre-stimulus period signal was contrasted to the baseline signal intensity in each voxel.
2.5. Statistical Analysis
Behavioral data: Reaction time (RT) and various error rates were used as dependent variables. False alarms (responses without stimulation), abnormally short responses (RTs < 150 ms) and wrong responses (responses to NoGo stimuli) were pooled together and considered as commission errors. Missed targets (no response to Go trials) and abnormally delayed responses (RT > 1500 ms) were considered as Omission errors. The percentages of omission and commission errors were analyzed after ArcSin transforms. The experimental design was originally intended to run analyses of variance (ANOVA) with group factor and repeated measures. However, testing for data normality (Shapiro–Wilks test and Q–Q plots) and homogeneity of variances (Levene’s test) precluded applying ANOVAs. We therefore used two samples of t-tests or the non-parametric unpaired Wilcoxon test, depending on normality. RStudio 2021.09.0 was used to perform all analyses.
Event-related analysis of BOLD signal changes: In the statistical analysis, 10 event types were defined at the first level. This included two periods—pre-stimulus and post-stimulus—for five types of trial (Go_control, go, NoGo, catch_control, catch_NoGo). The events were time-locked to the onset of the cue, modeled according to their onset and their duration and convolved with a canonical hemodynamic response function. Data were high pass-filtered at 128 s and summarized into two contrasts per subject. PD patients are known to be locked into a mode of control, maintaining anticipated inhibition over willed movements even when the situation does not require proactive inhibition [15]. In other words, the green cue modality is a suitable condition for testing automatic response without inhibition in healthy subjects vs. inappropriate proactive inhibition in PD patients. To assess the interaction between drug and disease effects during the pre-stimulus period, we performed the [(green cue_(clonidine-placebo)_Patients)-(green cue_(clonidine-placebo)_Controls)] contrast. The statistical parametric group maps were generated with a random-effects model. The individual statistical maps were entered into a two-sample t-test: PD vs. controls. All maps were thresholded at p < 0.001, uncorrected for display purposes, and all results were reported after peak-level cluster-wise family wise error (FWE) correction with a significant threshold of p < 0.05. Finally, we also used a ROI-based analysis approach to focus on the locus coeruleus (LC), which constituted of two 10 mm spheres centered at ±4, −26 and −15 (MNI coordinates) in the pontine region of the brainstem [41,42].
3. Results
3.1. Behavioral Data
Reaction time (RT): We first tested the effect of treatment (placebo vs clonidine) for all group × task conditions. No significant effect was reported (all p > 0.6). We then tested the effect of task (uncertainty vs. no uncertainty) for both groups, independently of the treatment condition. The RT to Go trials were shorter in the no uncertainty than in the uncertainty condition for both groups (control group: 402 ± 74 vs. 468 ± 55 ms; t (53.3) = −3.87; p < 0.001) (PD group: 479 ± 86 vs. 533 ± 82 ms; W = 171; p < 0.05). Finally, we tested the effect of group for both conditions of task, independently of the treatment condition. The RT of PD patients were longer than the RT of control subjects, both in the no uncertainty (W = 168; p < 0.001) and in the uncertainty (W = 173; p < 0.001) conditions (Figure 3).
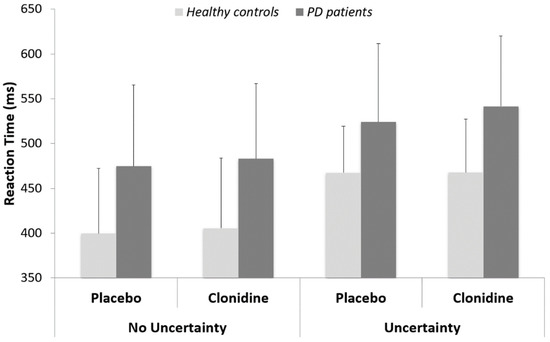
Figure 3.
Reaction times (means and standard deviations) for PD patients versus healthy matched controls.
Commission errors: No significant effect was found.
Omissions: A significant effect of treatment (W = 1763; p < 0.05) revealed more omissions in the clonidine (7 ± 10%) than in the placebo condition (4 ± 6%). There was a significant effect of group in the clonidine condition (W = 237; p < 0.05), where PD patients produced significantly more omission errors (12 ± 15%) than controls (3 ± 5%). The effect of group just approached conventional thresholds in the placebo condition (W = 272; p = 0.055), whereas PD patients produced more omission errors (6 ± 10%) than controls (3 ± 7%). No other significant effect was found (Figure 4).
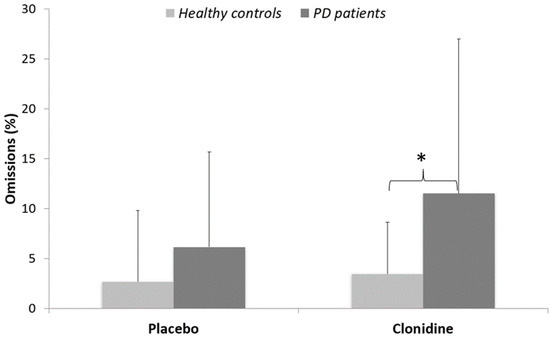
Figure 4.
Percentages of omissions (means and standard deviations) for PD patients versus healthy matched controls. * p < 0.05.
3.2. Imaging Data
By comparing PD patients to healthy controls in the no uncertainty condition, clonidine was found to increase in comparison to the placebo, with brain activation within the dorsal ACC extending to the superior medial frontal gyrus (Figure 5 and Table 2). The ROI analysis revealed a significant cluster with increased BOLD signal within the LC (including 25 voxels, p = 0.012 corrected at the cluster level; Z = 3.65). There was no decrease in brain activation with clonidine compared to the placebo.
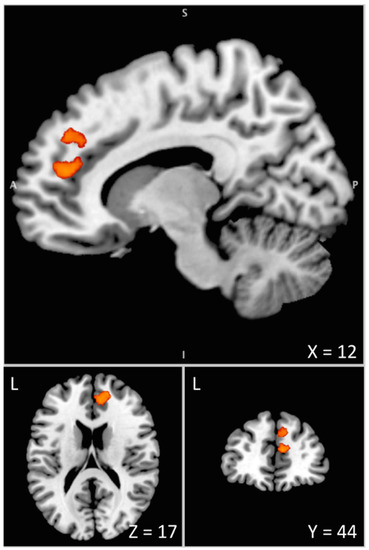
Figure 5.
Interaction between drug and disease effects during the pre-stimulus period assessed by means of the [(green cue_(clonidine-placebo)_Patients)-(green cue_(clonidine-placebo)_Controls)] contrast. The differential increase in BOLD signal under clonidine between PD patients and healthy controls is overlaid on the Colin 27 brain template in the MNI space visualized with the Mango software [43]. This overactivation of the anterior node of the proactive inhibition network (mPFC/ACC) is associated with the enhanced difficulty of PD patients to initiate movements when action restraint is not required (control condition with no uncertainty) under the effect of clonidine. L = Left. A = Anterior. S = Superior. X, Y, Z are coordinates in mm in the MNI space.

Table 2.
Location of increased BOLD signal in patients with PD versus healthy controls during the control condition (with no uncertainty) under clonidine versus placebo.
4. Discussion
The present study provides pharmacological functional MRI evidence that noradrenaline contributes to movement initiation via the modulation of the α2-adrenoceptor and inhibitory control. The difficulty for PD patients to initiate movements with respect to controls, classically observed by RT, was enhanced under clonidine as pinpointed by an increase in omission errors. Functional MRI, assessing the specific difficulty of patients to release proactive inhibition when the situation does not require action restraint, revealed BOLD signal changes under clonidine in the LC and the anterior node of the proactive inhibitory network.
4.1. Noradrenergic Modulation of Movement Initiation Control
The present behavioral data confirm that PD patients not only have difficulties initiating movements when the context requires action restraint, but also have difficulties initiating movements when the context does not require action restraint. This is consistent with previous studies suggesting that PD patients are locked into a mode of control that inhibits, in advance, movement-triggering mechanisms to prevent undesired automatic responses to stimuli. In other words, PD patients would keep refraining from reacting to any upcoming stimulus, even when the situation requires automatic responding [12,15,16].
Behaviorally, clonidine did not significantly impair RT, neither in PD patients nor in healthy subjects, consistent with a previous study [44]. However, clonidine increased the rate of omissions with respect to the placebo, and this effect was more pronounced in PD patients who produced significantly more omission errors under clonidine than healthy controls. The fact that more trials were missed (with no response at all) suggests that the effect of reducing the NA tonus did not gradually impede the time needed to release inhibition (which would have been observed through the increase in RT). It is tempting to speculate that clonidine has literally prevented the release of inhibition in a substantial number of trials for which movement initiation was not possible at all, reminiscent of freezing behavior.
4.2. Noradrenergic Modulation of the Proactive Inhibitory Network
In the present study, BOLD changes in the LC and in the anterior node of the proactive network (mPFC/ACC) were associated with the differential effect of clonidine observed between PD patients and healthy controls in the critical experimental condition, revealing the frequently reported deficits of patients in initiating movements (Figure 5).
Previous studies already evidenced the importance of LC neuromodulation in behavioral adjustments and sensorimotor performance optimization [31,45,46,47]. Some suggested a possible role of the NA system in the ability to inhibit inappropriate movement [48,49,50,51]. However, most of this research has focused on the inhibition of movement in reaction to the presentation of a stop signal [52]. Here, our data suggested that the LC-NA system might interact with higher brain regions before any stimulus is presented to set a level of responsiveness adapted to the context. This executive function consists of modulating the tonic process, which inhibits anticipatorily, and by default, movement triggering mechanisms, which is dysfunctional in PD patients [15]. The joint modulation of activity of LC and mPFC/ACC, a central node of the proactive inhibition network [18,21], is consistent with the view that that the LC likely plays more specific roles than just autonomic arousal, and likely has a specific influence on cortical target networks supporting various cognitive and executive functions [53]. Here, our results support the hypothesis that reduced central NA activity via clonidine stimulation of the α2-ARs substantially modulates the activity of the LC and the dmFC in PD patients whose cortical NA transmission is already compromised [54,55,56]. This cortical node is the source of the ‘neural brake’ mechanism that blocks specific ongoing motor activity [57,58,59]. It is known as the “veto” area as it plays a pivotal role in intentional inhibitory control and in the initiation of voluntary action [60,61].
Although BOLD fluctuations remain difficult to interpret with regard to the potential confounds between neural excitation and inhibition [62], our observations are in line with previous results from animal studies. For instance, it was reported that stimulation of postsynaptic α2-AR produces hypoactivity in open filed tests in rats [63,64,65], while local selective blockade of those receptors in the monkey prefrontal cortex leads to increase locomotor activity and impulsivity [66]. Specifically, the BOLD signal increase observed in the dmFC in the present study is in good agreement with animal studies reporting hyperactivity of the mPFC pyramidal neurons after lesion of the LC [67], while LC stimulation has been shown to inhibit neuronal firing in the same region [68].
4.3. Relevance to a Noradrenergic Approach to Current Medical Therapies in PD
Functional brain measures may be more sensitive than behavioral indexes to subtle pharmacological effects [69]. Our study measured an acute effect of clonidine, while several weeks of daily treatment with clonidine were generally needed to exert its maximal beneficial effects, such as a reduction in impulsivity in patients with Tourette syndrome or with ADHD [70]. In other terms, BOLD changes observed in this study with clonidine could precede behavioral effects induced by chronic administration. Targeting non-dopaminergic medications is a major issue in PD therapy in general and is more specifically critical for akinesia, which is not fully reverted by current dopaminergic treatments [11,12,71]. The present proof-of-concept study might open the way to future clinical trials.
It has become increasingly apparent that the neuropathological changes of PD extend well beyond the nigrostriatal system, pointing especially to the early involvement of the LC in the neurodegenerative process underlying the disease [72,73]. Of interest, the NA system is thought to be involved in the pathophysiology of gait disorders in PD, such as freezing of gait which can be viewed as a failure to initiate movement [74,75,76].
On the basis of the present results, it might be relevant to explore the effect of an α2-AR antagonist in PD patients. Indeed, given that clonidine, an α2-AR agonist, negatively affects proactive inhibitory control, it is tempting to speculate that an α2-AR antagonist might conversely improve specific executive functions and behaviors, such as impulse control disorders [51,77,78,79,80,81,82]. Previously, it has been shown that α2-AR antagonists improve tremor and rigidity in the reserpinized rat [83], have a potent effect on levodopa-induced dyskinesia in a PD monkey model [84,85,86], and can extend the anti-parkinsonian effect of levodopa in MPTP-treated monkeys [87,88]. In PD patients, although conflicting results have been reported, preliminary clinical trials have suggested that this therapeutic approach reduces dyskinesia when given in combination with levodopa [89,90].
There are still, however, numerous issues to be dealt with before considering new NA drugs as potential add-on therapies to L-dopa in PD. The literature is very confusing and sometimes controversial about the potential anti-parkinsonian impact of an α2-AR antagonist. This is likely due to differences in NA subtype selectivity, differences in functional specificity with regards to neural mechanisms and to the non-selective binding of most pharmacological agents. Indeed, there are three subtypes of α2-ARs, including α2A, α2B and α2C [91,92], each with a distinct distribution and function in the brain [93]. However, the individual role of each of these subtypes is still unclear. For instance, while α2A/C antagonism (such as fipamezole and idazoxan) can potentially reduce dyskinesia in patients [89,94], working memory deficits are partly compensated by α2A-ARs agonists, such as guanfacine or clonidine [95,96,97], but overexpression of α2C-ARs can impair the ability to perform spatial and nonspatial cognitive tasks [98]. Furthermore, there are often strong confounds when using pharmaceuticals that do not target unique receptors or transporters, such as clozapine which is an α2-ARs antagonist, but which also acts as an antagonist at the dopamine D2 receptor, and modulates serotonin and acetylcholine [99]. Thus, although it has been reported to reduce LID without worsening PD [100], the anti-adrenergic properties of clozapine have never really been highlighted [3]. Accordingly, in vivo imaging of all receptors could further elucidate the potential important roles of α2 ARs in PD patients, not only in movement control but also in the context of cognitive decline and non-motor symptoms. This is a current technical challenge for molecular imaging [101].
5. Conclusions
Although no convincing solution has been provided to date [2,4], the present results might have important implications in setting the ground for new add-on NA treatment approaches. Yet, further pharmacological investigations are warranted to support this hypothesis. Recent technological and methodological developments in molecular imaging might offer new opportunities to better understand the role of NA and adrenoceptors in neurodegeneration, a central issue in PD pathophysiology [101,102,103,104,105,106,107]. A condition for success is certainly to combine molecular imaging with clinical and behavioral analyses, yielding refined functional segregation [5,108]. Of particular interest is the new possibility to reliably investigate in α2 density in vivo in large human samples [109]. Understanding the NA pathogenic mechanisms that might contribute to disease progression and associated complications is indeed a prerequisite for developing novel neuroprotective or efficient disease-modifying therapies with individually tailored care.
Author Contributions
Conceptualization, M.C., J.R., B.B. and P.B.; methodology, B.B. and P.B.; software, M.C., E.M. and J.R.; validation, M.C., B.B. and P.B.; formal analysis, A.P., M.C. and E.M.; investigation, A.P., M.C. and B.B.; resources, P.B. and B.B.; data curation, B.B.; writing—original draft preparation, A.P., M.C. and B.B.; writing—review and editing, C.L., S.T., E.M., J.R., P.B. and B.B.; visualization, C.L.; supervision, B.B.; project administration, B.B.; funding acquisition, P.B. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the French National Agency of Research (grants numbers ANR-09-MNPS-039-01 to P.B. and ANR-16-CE37-0014-01 to B.B.) and by Fédération Française des Groupements de Parkinsoniens (FFGP-077030 to B.B.).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Ethical Committee in Biomedical Research (N° CPP 11/094).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors will be happy to support request for a formal data sharing agreement.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kalia, L.V.; Brotchie, J.M.; Fox, S.H. Novel Nondopaminergic Targets for Motor Features of Parkinson’s Disease: Review of Recent Trials: Nondopaminergic Targets for Motor Features of PD. Mov. Disord. 2013, 28, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Latapi, P.; Bhowmick, S.S.; Saranza, G.; Fox, S.H. Non-Dopaminergic Treatments for Motor Control in Parkinson’s Disease: An Update. CNS Drugs 2020, 34, 1025–1044. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H. Non-Dopaminergic Treatments for Motor Control in Parkinson’s Disease. Drugs 2013, 73, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, D.G.; Reyes, N.G.D.; Fox, S.H. Newly Approved and Investigational Drugs for Motor Symptom Control in Parkinson’s Disease. Drugs 2022, 82, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Spay, C.; Meyer, G.; Welter, M.-L.; Lau, B.; Boulinguez, P.; Ballanger, B. Functional Imaging Correlates of Akinesia in Parkinson’s Disease: Still Open Issues. NeuroImage Clin. 2018, 21, 101644. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Clinical Neurophysiology of Akinesia. Rev. Neurol. 1990, 146, 585–590. [Google Scholar]
- Alexander, G.E.; Crutcher, M.D. Functional Architecture of Basal Ganglia Circuits: Neural Substrates of Parallel Processing. Trends Neurosci. 1990, 13, 266–271. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D.; DeLong, M.R. Basal Ganglia-Thalamocortical Circuits: Parallel Substrates for Motor, Oculomotor, “Prefrontal” and “Limbic” Functions. Prog. Brain Res. 1990, 85, 119–146. [Google Scholar]
- Canavan, A.G.M.; Nixon, P.D.; Passingham, R.E. Motor Learning in Monkeys (Macaca Fascicularis) with Lesions in Motor Thalamus. Exp. Brain Res. 1989, 77, 113–126. [Google Scholar] [CrossRef]
- Marsden, C.D.; Obeso, J.A. The Functions of the Basal Ganglia and the Paradox of Stereotaxic Surgery in Parkinson’s Disease. Brain J. Neurol. 1994, 117, 877–897. [Google Scholar] [CrossRef]
- Ballanger, B.; Gil, R.; Audiffren, M.; Desmurget, M. Perceptual Factors Contribute to Akinesia in Parkinson’s Disease. Exp. Brain Res. 2007, 179, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Favre, E.; Ballanger, B.; Thobois, S.; Broussolle, E.; Boulinguez, P. Deep Brain Stimulation of the Subthalamic Nucleus, but Not Dopaminergic Medication, Improves Proactive Inhibitory Control of Movement Initiation in Parkinson’s Disease. Neurother. J. Am. Soc. Exp. Neurother. 2013, 10, 154–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, N.; Nandi, D.; Oram, R.; Stein, J.F.; Aziz, T.Z. Pedunculopontine Nucleus Electric Stimulation Alleviates Akinesia Independently of Dopaminergic Mechanisms. NeuroReport 2006, 17, 639–641. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Rothwell, J.C. Inhibitory Dysfunction Contributes to Some of the Motor and Non-Motor Symptoms of Movement Disorders and Psychiatric Disorders. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160198. [Google Scholar] [CrossRef] [PubMed]
- Criaud, M.; Poisson, A.; Thobois, S.; Metereau, E.; Redouté, J.; Ibarrola, D.; Baraduc, P.; Broussolle, E.; Strafella, A.P.; Ballanger, B.; et al. Slowness in Movement Initiation Is Associated with Proactive Inhibitory Network Dysfunction in Parkinson’s Disease. J. Park. Dis. 2016, 6, 433–440. [Google Scholar] [CrossRef]
- Albares, M.; Thobois, S.; Favre, E.; Broussolle, E.; Polo, G.; Domenech, P.; Boulinguez, P.; Ballanger, B. Interaction of Noradrenergic Pharmacological Manipulation and Subthalamic Stimulation on Movement Initiation Control in Parkinson’s Disease. Brain Stimulat. 2015, 8, 27–35. [Google Scholar] [CrossRef]
- Jaffard, M.; Benraiss, A.; Longcamp, M.; Velay, J.-L.; Boulinguez, P. Cueing Method Biases in Visual Detection Studies. Brain Res. 2007, 1179, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Jaffard, M.; Longcamp, M.; Velay, J.-L.; Anton, J.-L.; Roth, M.; Nazarian, B.; Boulinguez, P. Proactive Inhibitory Control of Movement Assessed by Event-Related FMRI. NeuroImage 2008, 42, 1196–1206. [Google Scholar] [CrossRef]
- Criaud, M.; Longcamp, M.; Anton, J.-L.; Nazarian, B.; Roth, M.; Sescousse, G.; Strafella, A.P.; Ballanger, B.; Boulinguez, P. Testing the Physiological Plausibility of Conflicting Psychological Models of Response Inhibition: A Forward Inference FMRI Study. Behav. Brain Res. 2017, 333, 192–202. [Google Scholar] [CrossRef]
- Criaud, M.; Anton, J.-L.; Nazarian, B.; Longcamp, M.; Metereau, E.; Boulinguez, P.; Ballanger, B. The Human Basal Ganglia Mediate the Interplay between Reactive and Proactive Control of Response through Both Motor Inhibition and Sensory Modulation. Brain Sci. 2021, 11, 560. [Google Scholar] [CrossRef]
- Criaud, M.; Wardak, C.; Ben Hamed, S.; Ballanger, B.; Boulinguez, P. Proactive Inhibitory Control of Response as the Default State of Executive Control. Front. Psychol. 2012, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Isoda, M.; Hikosaka, O. Switching from Automatic to Controlled Action by Monkey Medial Frontal Cortex. Nat. Neurosci. 2007, 10, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, O.; Isoda, M. Switching from Automatic to Controlled Behavior: Cortico-Basal Ganglia Mechanisms. Trends Cogn. Sci. 2010, 14, 154–161. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Robbins, T.W. Noradrenergic Modulation of Cognition: Therapeutic Implications. J. Psychopharmacol. 2013, 27, 694–718. [Google Scholar] [CrossRef]
- Bari, A.; Robbins, T.W. Noradrenergic versus Dopaminergic Modulation of Impulsivity, Attention and Monitoring Behaviour in Rats Performing the Stop-Signal Task: Possible Relevance to ADHD. Psychopharmacology 2013, 230, 89–111. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The Locus Coeruleus–Noradrenergic System: Modulation of Behavioral State and State-Dependent Cognitive Processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Bouret, S.; Sara, S.J. Network Reset: A Simplified Overarching Theory of Locus Coeruleus Noradrenaline Function. Trends Neurosci. 2005, 28, 574–582. [Google Scholar] [CrossRef]
- Ramos, B.P.; Arnsten, A.F.T. Adrenergic Pharmacology and Cognition: Focus on the Prefrontal Cortex. Pharmacol. Ther. 2007, 113, 523–536. [Google Scholar] [CrossRef]
- Tait, D.S.; Brown, V.J.; Farovik, A.; Theobald, D.E.; Dalley, J.W.; Robbins, T.W. Lesions of the Dorsal Noradrenergic Bundle Impair Attentional Set-Shifting in the Rat. Eur. J. Neurosci. 2007, 25, 3719–3724. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. Adaptive Gain and the Role of the Locus Coeruleus-Norepinephrine System in Optimal Performance. J. Comp. Neurol. 2005, 493, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Spay, C.; Albares, M.; Lio, G.; Thobois, S.; Broussolle, E.; Lau, B.; Ballanger, B.; Boulinguez, P. Clonidine Modulates the Activity of the Subthalamic-Supplementary Motor Loop: Evidence from a Pharmacological Study Combining Deep Brain Stimulation and Electroencephalography Recordings in Parkinsonian Patients. J. Neurochem. 2018, 146, 333–347. [Google Scholar] [CrossRef] [Green Version]
- Ballanger, B.; van Eimeren, T.; Moro, E.; Lozano, A.M.; Hamani, C.; Boulinguez, P.; Pellecchia, G.; Houle, S.; Poon, Y.Y.; Lang, A.E.; et al. Stimulation of the Subthalamic Nucleus and Impulsivity: Release Your Horses. Ann. Neurol. 2009, 66, 817–824. [Google Scholar] [CrossRef]
- Meyer, G.M.; Spay, C.; Beliakova, A.; Gaugain, G.; Pezzoli, G.; Cilia, R. Inhibitory Control Dysfunction in Parkinsonian Impulse Control Disorders. Brain 2020, 143, 3734–3747. [Google Scholar] [CrossRef] [PubMed]
- Anavekar, S.N.; Jarrott, B.; Toscano, M.; Louis, W.J. Pharmacokinetic and Pharmacodynamic Studies of Oral Clonidine in Normotensive Subjects. Eur. J. Clin. Pharmacol. 1982, 23, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic Review of Levodopa Dose Equivalency Reporting in Parkinson’s Disease: Systematic Review of LED Reporting in PD. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Boulinguez, P.; Blouin, J.; Nougier, V. The Gap Effect for Eye and Hand Movements in Double-Step Pointing. Exp. Brain Res. 2001, 138, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Criaud, M.; Boulinguez, P. Have We Been Asking the Right Questions When Assessing Response Inhibition in Go/No-Go Tasks with FMRI? A Meta-Analysis and Critical Review. Neurosci. Biobehav. Rev. 2013, 37, 11–23. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Aron, A.R. Unconsciously Triggered Response Inhibition Requires an Executive Setting. J. Exp. Psychol. Gen. 2014, 143, 56–61. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Keren, N.I.; Lozar, C.T.; Harris, K.C.; Morgan, P.S.; Eckert, M.A. In Vivo Mapping of the Human Locus Coeruleus. NeuroImage 2009, 47, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.J.; Kirilina, E.; Otaduy, M.C.G.; Ivanov, D.; Acosta-Cabronero, J.; Callaghan, M.F.; Lambert, C.; Cardenas-Blanco, A.; Pine, K.; Passamonti, L.; et al. Locus Coeruleus Imaging as a Biomarker for Noradrenergic Dysfunction in Neurodegenerative Diseases. Brain 2019, 142, 2558–2571. [Google Scholar] [CrossRef] [PubMed]
- Kochunov, P.; Lancaster, J.; Thompson, P.; Toga, A.W.; Brewer, P.; Hardies, J.; Fox, P. An Optimized Individual Target Brain in the Talairach Coordinate System. NeuroImage 2002, 17, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Riekkinen, M.; Kejonen, K.; Jäkälä, P.; Soininen, H.; Riekkinen, P. Reduction of Noradrenaline Impairs Attention and Dopamine Depletion Slows Responses in Parkinson’s Disease: Noradrenaline, Dopamine and Attention in Parkinson’s Disease. Eur. J. Neurosci. 1998, 10, 1429–1435. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Aston-Jones, G.; Cohen, J.D. Decision Making, the P3, and the Locus Coeruleus--Norepinephrine System. Psychol. Bull. 2005, 131, 510–532. [Google Scholar] [CrossRef]
- Bouret, S. Locus Coeruleus, Noradrenaline, and Behavior: Network Effect, Network Effects? Neuron 2019, 103, 554–556. [Google Scholar] [CrossRef]
- Chandler, D.J.; Jensen, P.; McCall, J.G.; Pickering, A.E.; Schwarz, L.A.; Totah, N.K. Redefining Noradrenergic Neuromodulation of Behavior: Impacts of a Modular Locus Coeruleus Architecture. J. Neurosci. 2019, 39, 8239–8249. [Google Scholar] [CrossRef]
- Bari, A.; Eagle, D.M.; Mar, A.C.; Robinson, E.S.J.; Robbins, T.W. Dissociable Effects of Noradrenaline, Dopamine, and Serotonin Uptake Blockade on Stop Task Performance in Rats. Psychopharmacology 2009, 205, 273–283. [Google Scholar] [CrossRef]
- Eagle, D.M.; Bari, A.; Robbins, T.W. The Neuropsychopharmacology of Action Inhibition: Cross-Species Translation of the Stop-Signal and Go/No-Go Tasks. Psychopharmacology 2008, 199, 439–456. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Hampshire, A.; Müller, U.; Rubia, K.; del Campo, N.; Craig, K.; Regenthal, R.; Suckling, J.; Roiser, J.P.; Grant, J.E.; et al. Atomoxetine Modulates Right Inferior Frontal Activation During Inhibitory Control: A Pharmacological Functional Magnetic Resonance Imaging Study. Biol. Psychiatry 2009, 65, 550–555. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Housden, C.R.; Regenthal, R.; Barker, R.A.; Müller, U.; Rowe, J.; Sahakian, B.J.; Robbins, T.W. Targeting Impulsivity in Parkinson’s Disease Using Atomoxetine. Brain 2014, 137, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R. From Reactive to Proactive and Selective Control: Developing a Richer Model for Stopping Inappropriate Responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jone, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus Coeruleus: A New Look at the Blue Spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Vazey, E.M.; Aston-Jones, G. The Emerging Role of Norepinephrine in Cognitive Dysfunctions of Parkinson’s Disease. Front. Behav. Neurosci. 2012, 6, 48. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Kopin, I.J.; Basile, M.J.; Mash, D.C. Catechols in Post-Mortem Brain of Patients with Parkinson Disease: Brain Catechols in Parkinson Disease. Eur. J. Neurol. 2011, 18, 703–710. [Google Scholar] [CrossRef]
- Nayyar, T.; Bubser, M.; Ferguson, M.C.; Neely, M.D.; Goodwin, J.S.; Montine, T.J.; Deutch, A.Y.; Ansah, T.A. Cortical Serotonin and Norepinephrine Denervation in Parkinsonism: Preferential Loss of the Beaded Serotonin Innervation. Eur. J. Neurosci. 2009, 30, 207–216. [Google Scholar] [CrossRef]
- Filevich, E.; Kühn, S.; Haggard, P. Intentional Inhibition in Human Action: The Power of ‘No’. Neurosci. Biobehav. Rev. 2012, 36, 1107–1118. [Google Scholar] [CrossRef]
- Brass, M.; Haggard, P. To Do or Not to Do: The Neural Signature of Self-Control. J. Neurosci. 2007, 27, 9141–9145. [Google Scholar] [CrossRef]
- Brass, M.; Haggard, P. The What, When, Whether Model of Intentional Action. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2008, 14, 319–325. [Google Scholar] [CrossRef]
- Cho, S.S.; Pellecchia, G.; Ko, J.H.; Ray, N.; Obeso, I.; Houle, S.; Strafella, A.P. Effect of Continuous Theta Burst Stimulation of the Right Dorsolateral Prefrontal Cortex on Cerebral Blood Flow Changes during Decision Making. Brain Stimulat. 2012, 5, 116–123. [Google Scholar] [CrossRef]
- Narayanan, N.S.; Horst, N.K.; Laubach, M. Reversible Inactivations of Rat Medial Prefrontal Cortex Impair the Ability to Wait for a Stimulus. Neuroscience 2006, 139, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K. What We Can Do and What We Cannot Do with FMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Herman, Z.S.; Brus, R.; Drybański, A.; Szkilnik, R.; Slomińska-Zurek, J. Influence of 6-Hydroxydopamine on the Behavioral Effects Induced by Apomorphine or Clonidine in Rats. Psychopharmacology 1976, 50, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Nassif, S.; Kempf, E.; Cardo, B.; Velley, L. Neurochemical Lesion of the Locus Coeruleus of the Rat Does Not Suppress the Sedative Effect of Clonidine. Eur. J. Pharmacol. 1983, 91, 69–76. [Google Scholar] [CrossRef]
- Spyraki, C.; Arbuthnott, G.W.; Fibiger, H.C. The Effect of DSP-4 on Some Positively Reinforced Operant Behaviors in the Rat. Pharmacol. Biochem. Behav. 1982, 16, 197–202. [Google Scholar] [CrossRef]
- Ma, C.-L.; Arnsten, A.F.T.; Li, B.-M. Locomotor Hyperactivity Induced by Blockade of Prefrontal Cortical A2-Adrenoceptors in Monkeys. Biol. Psychiatry 2005, 57, 192–195. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.J.; Liu, J.; Ali, U.; Gui, Z.H.; Hui, Y.P.; Wang, T.; Chen, L.; Li, Q. Noradrenergic Lesion of the Locus Coeruleus Increases the Firing Activity of the Medial Prefrontal Cortex Pyramidal Neurons and the Role of A2-Adrenoceptors in Normal and Medial Forebrain Bundle Lesioned Rats. Brain Res. 2010, 1324, 64–74. [Google Scholar] [CrossRef]
- Thierry, A.M.; Godbout, R.; Mantz, J.; Pirot, S.; Glowinski, J. Differential Influence of Dopaminergic and Noradrenergic Afferents on Their Target Cells in the Rat Prefrontal Cortex. Clin. Neuropharmacol. 1992, 15, 139A–140A. [Google Scholar] [CrossRef]
- Wilkinson, D.; Halligan, P. The Relevance of Behavioural Measures for Functional-Imaging Studies of Cognition. Nat. Rev. Neurosci. 2004, 5, 67–73. [Google Scholar] [CrossRef]
- Nair, V.; Mahadevan, S. Randomised Controlled Study-Efficacy of Clonidine versus Carbamazepine in Children with ADHD. J. Trop. Pediatr. 2009, 55, 116–121. [Google Scholar] [CrossRef]
- Narabayashi, H. The Neural Mechanisms and Progressive Nature of Symptoms of Parkinson’s Disease—Based on Clinical, Neurophysiological and Morphological Studies. J. Neural Transm. Park. Dis. Dement. Sect. 1995, 10, 63–75. [Google Scholar] [CrossRef]
- Zarow, C.; Lyness, S.A.; Mortimer, J.A.; Chui, H.C. Neuronal Loss Is Greater in the Locus Coeruleus Than Nucleus Basalis and Substantia Nigra in Alzheimer and Parkinson Diseases. Arch. Neurol. 2003, 60, 337. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Moreau, C.; Delval, A.; Defebvre, L.; Dujardin, K.; Duhamel, A.; Petyt, G.; Vuillaume, I.; Corvol, J.-C.; Brefel-Courbon, C.; Ory-Magne, F.; et al. Methylphenidate for Gait Hypokinesia and Freezing in Patients with Parkinson’s Disease Undergoing Subthalamic Stimulation: A Multicentre, Parallel, Randomised, Placebo-Controlled Trial. Lancet Neurol. 2012, 11, 589–596. [Google Scholar] [CrossRef]
- Taylor, N.L.; Wainstein, G.; Quek, D.; Lewis, S.J.G.; Shine, J.M.; Ehgoetz Martens, K.A. The Contribution of Noradrenergic Activity to Anxiety-Induced Freezing of Gait. Mov. Disord. 2022, 37, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.A.; Sato, T.; Muramatsu, S. Freezing of Gait in Parkinson’s Disease Is Associated with Reduced 6-[18F]Fluoro-l-m-Tyrosine Uptake in the Locus Coeruleus. Park. Dis. 2016, 2016, 5430920. [Google Scholar] [CrossRef] [PubMed]
- Marsh, L.; Biglan, K.; Gerstenhaber, M.; Williams, J.R. Atomoxetine for the Treatment of Executive Dysfunction in Parkinson’s Disease: A Pilot Open-Label Study. Mov. Disord. 2009, 24, 277–282. [Google Scholar] [CrossRef]
- Ye, Z.; Altena, E.; Nombela, C.; Housden, C.R.; Maxwell, H.; Rittman, T.; Huddleston, C.; Rae, C.L.; Regenthal, R.; Sahakian, B.J.; et al. Improving Response Inhibition in Parkinson’s Disease with Atomoxetine. Biol. Psychiatry 2015, 77, 740–748. [Google Scholar] [CrossRef]
- Yssel, J.D.; O’Neill, E.; Nolan, Y.M.; Connor, T.J.; Harkin, A. Treatment with the Noradrenaline Re-Uptake Inhibitor Atomoxetine Alone and in Combination with the A2-Adrenoceptor Antagonist Idazoxan Attenuates Loss of Dopamine and Associated Motor Deficits in the LPS Inflammatory Rat Model of Parkinson’s Disease. Brain. Behav. Immun. 2018, 69, 456–469. [Google Scholar] [CrossRef]
- Ye, Z.; Rae, C.L.; Nombela, C.; Ham, T.; Rittman, T.; Jones, P.S.; Rodríguez, P.V.; Coyle-Gilchrist, I.; Regenthal, R.; Altena, E.; et al. Predicting Beneficial Effects of Atomoxetine and Citalopram on Response Inhibition in Parkinson’s Disease with Clinical and Neuroimaging Measures: Predicting Treatment Response in PD. Hum. Brain Mapp. 2016, 37, 1026–1037. [Google Scholar] [CrossRef]
- Delaville, C.; Deurwaerdère, P.D.; Benazzouz, A. Noradrenaline and Parkinson’s Disease. Front. Syst. Neurosci. 2011, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; LeWitt, P.A.; Kaufmann, H. Norepinephrine Deficiency in Parkinson’s Disease: The Case for Noradrenergic Enhancement: Norepinephrine Deficiency in PD. Mov. Disord. 2014, 29, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, F.C. Pharmacological Characteristics of Tremor, Rigidity and Hypokinesia Induced by Reserpine in Rat. Neuropharmacology 1987, 26, 1431–1440. [Google Scholar] [CrossRef]
- Bezard, E.; Brefel, C.; Tison, F.; Peyro-SaintPaul, H.; Ladure, P.; Rascol, O.; Gross, C.E. Effect of the A2 Adrenoreceptor Antagonist, Idazoxan, on Motor Disabilities in MPTP-Treated Monkey. Prog. Neuropsychopharmacol. Biol. Psychiatry 1999, 23, 1237–1246. [Google Scholar] [CrossRef]
- Savola, J.-M.; Hill, M.; Engstrom, M.; Merivuori, H.; Wurster, S.; McGuire, S.G.; Fox, S.H.; Crossman, A.R.; Brotchie, J.M. Fipamezole (JP-1730) Is a Potent A2 Adrenergic Receptor Antagonist That Reduces Levodopa-Induced Dyskinesia in the MPTP-Lesioned Primate Model of Parkinson’s Disease. Mov. Disord. 2003, 18, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Grondin, R.; Hadj Tahar, A.; Doan, V.D.; Ladure, P.; Bédard, P.J. Noradrenoceptor Antagonism with Idazoxan Improves l-Dopa-Induced Dyskinesias in MPTP Monkeys. Naunyn. Schmiedebergs Arch. Pharmacol. 2000, 361, 181–186. [Google Scholar] [CrossRef]
- Domino, E.F.; Ni, L.; Colpaert, F.; Marien, M. Effects of (+/−)-Idazoxan Alone and in Combination with L-DOPA Methyl Ester in MPTP-Induced Hemiparkinsonian Monkeys. Receptors Channels 2003, 9, 335–338. [Google Scholar] [CrossRef]
- Henry, B.; Fox, S.H.; Peggs, D.; Crossman, A.R.; Brotchie, J.M. The A2-Adrenergic Receptor Antagonist Idazoxan Reduces Dyskinesia and Enhances Anti-Parkinsonian Actions of L-Dopa in the MPTP-Lesioned Primate Model of Parkinson’s Disease. Mov. Disord. 1999, 14, 744–753. [Google Scholar] [CrossRef]
- Rascol, O.; Arnulf, I.; Peyro-Saint Paul, H.; Brefel-Courbon, C.; Vidailhet, M.; Thalamas, C.; Bonnet, A.m.; Descombes, S.; Bejjani, B.; Fabre, N.; et al. Idazoxan, an Alpha-2 Antagonist, and L-DOPA-Induced Dyskinesias in Patients with Parkinson’s Disease. Mov. Disord. 2001, 16, 708–713. [Google Scholar] [CrossRef]
- Colosimo, C.; Craus, A. Noradrenergic Drugs for Levodopa-Induced Dyskinesia. Clin. Neuropharmacol. 2003, 26, 299–305. [Google Scholar] [CrossRef]
- Bylund, D.B. Heterogeneity of Alpha-2 Adrenergic Receptors. Pharmacol. Biochem. Behav. 1985, 22, 835–843. [Google Scholar] [CrossRef]
- Bylund, D.B. Pharmacological Characteristics of Alpha-2 Adrenergic Receptor Subtypes. Ann. N. Y. Acad. Sci. 1995, 763, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scheinin, M.; Lomasney, J.W.; Hayden-Hixson, D.M.; Schambra, U.B.; Caron, M.G.; Lefkowitz, R.J.; Fremeau, R.T. Distribution of Alpha 2-Adrenergic Receptor Subtype Gene Expression in Rat Brain. Brain Res. Mol. Brain Res. 1994, 21, 133–149. [Google Scholar] [CrossRef]
- Lewitt, P.A.; Hauser, R.A.; Lu, M.; Nicholas, A.P.; Weiner, W.; Coppard, N.; Leinonen, M.; Savola, J.-M. Randomized Clinical Trial of Fipamezole for Dyskinesia in Parkinson Disease (FJORD Study). Neurology 2012, 79, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, S.G.; Podell, D.M.; Arnsten, A.F. Noradrenergic Alpha-2 Receptor Agonists Reverse Working Memory Deficits Induced by the Anxiogenic Drug, FG7142, in Rats. Pharmacol. Biochem. Behav. 2000, 67, 397–403. [Google Scholar] [CrossRef]
- Li, B.M.; Mao, Z.M.; Wang, M.; Mei, Z.T. Alpha-2 Adrenergic Modulation of Prefrontal Cortical Neuronal Activity Related to Spatial Working Memory in Monkeys. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1999, 21, 601–610. [Google Scholar] [CrossRef]
- Jäkälä, P.; Riekkinen, M.; Sirviö, J.; Koivisto, E.; Kejonen, K.; Vanhanen, M.; Riekkinen, P. Guanfacine, but Not Clonidine, Improves Planning and Working Memory Performance in Humans. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1999, 20, 460–470. [Google Scholar] [CrossRef]
- Björklund, M.; Sirviö, J.; Sallinen, J.; Scheinin, M.; Kobilka, B.K.; Riekkinen, P. Alpha2C-Adrenoceptor Overexpression Disrupts Execution of Spatial and Non-Spatial Search Patterns. Neuroscience 1999, 88, 1187–1198. [Google Scholar] [CrossRef]
- Khokhar, J.Y.; Henricks, A.M.; Kirk, E.; Green, A.I. Unique Effects of Clozapine: A Pharmacological Perspective. Adv. Pharmacol. 2018, 82, 137–162. [Google Scholar] [CrossRef]
- Durif, F.; Debilly, B.; Galitzky, M.; Morand, D.; Viallet, F.; Borg, M.; Thobois, S.; Broussolle, E.; Rascol, O. Clozapine Improves Dyskinesias in Parkinson Disease: A Double-Blind, Placebo-Controlled Study. Neurology 2004, 62, 381–388. [Google Scholar] [CrossRef]
- Nahimi, A.; Kinnerup, M.B.; Sommerauer, M.; Gjedde, A.; Borghammer, P. Molecular Imaging of the Noradrenergic System in Idiopathic Parkinson’s Disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 141, pp. 251–274. [Google Scholar]
- Nahimi, A.; Sommerauer, M.; Kinnerup, M.B.; Østergaard, K.; Wintherdahl, M.; Jacobsen, J.; Schacht, A.; Johnsen, B.; Damholdt, M.F.; Borghammer, P.; et al. Noradrenergic Deficits in Parkinson Disease Imaged with 11 C-MeNER. J. Nucl. Med. 2018, 59, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Doppler, C.E.J.; Kinnerup, M.B.; Brune, C.; Farrher, E.; Betts, M.; Fedorova, T.D.; Schaldemose, J.L.; Knudsen, K.; Ismail, R.; Seger, A.D.; et al. Regional Locus Coeruleus Degeneration Is Uncoupled from Noradrenergic Terminal Loss in Parkinson’s Disease. Brain 2021, 144, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Kinnerup, M.B.; Sommerauer, M.; Damholdt, M.F.; Schaldemose, J.L.; Ismail, R.; Terkelsen, A.J.; Stær, K.; Hansen, A.; Fedorova, T.D.; Knudsen, K.; et al. Preserved Noradrenergic Function in Parkinson’s Disease Patients with Rest Tremor. Neurobiol. Dis. 2021, 152, 105295. [Google Scholar] [CrossRef]
- Andersen, K.B.; Hansen, A.K.; Sommerauer, M.; Fedorova, T.D.; Knudsen, K.; Vang, K.; Van Den Berge, N.; Kinnerup, M.; Nahimi, A.; Pavese, N.; et al. Altered Sensorimotor Cortex Noradrenergic Function in Idiopathic REM Sleep Behaviour Disorder – A PET Study. Parkinsonism Relat. Disord. 2020, 75, 63–69. [Google Scholar] [CrossRef]
- Sommerauer, M.; Fedorova, T.D.; Hansen, A.K.; Knudsen, K.; Otto, M.; Jeppesen, J.; Frederiksen, Y.; Blicher, J.U.; Geday, J.; Nahimi, A.; et al. Evaluation of the Noradrenergic System in Parkinson’s Disease: An 11C-MeNER PET and Neuromelanin MRI Study. Brain 2018, 141, 496–504. [Google Scholar] [CrossRef]
- Sommerauer, M.; Hansen, A.K.; Parbo, P.; Fedorova, T.D.; Knudsen, K.; Frederiksen, Y.; Nahimi, A.; Barbe, M.T.; Brooks, D.J.; Borghammer, P. Decreased Noradrenaline Transporter Density in the Motor Cortex of Parkinson’s Disease Patients: Cortical Noradrenaline Transporter. Mov. Disord. 2018, 33, 1006–1010. [Google Scholar] [CrossRef]
- Meyer, G.M.; Spay, C.; Laurencin, C.; Ballanger, B.; Sescousse, G.; Boulinguez, P. Functional Imaging Studies of Impulse Control Disorders in Parkinson’s Disease Need a Stronger Neurocognitive Footing. Neurosci. Biobehav. Rev. 2019, 98, 164–176. [Google Scholar] [CrossRef]
- Laurencin, C.; Lancelot, S.; Gobert, F.; Redouté, J.; Mérida, I.; Iecker, T.; Liger, F.; Irace, Z.; Greusard, E.; Lamberet, L.; et al. Modeling [11C]Yohimbine PET Human Brain Kinetics with Test-Retest Reliability, Competition Sensitivity Studies and Search for a Suitable Reference Region. NeuroImage 2021, 240, 118328. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).