The Critical Role of Hypoxia in the Re-Differentiation of Human Articular Chondrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Articular Chondrocytes
2.2. Primary Isolation and Culture of Human Chondrocytes
2.3. Estimating the De-Differentiation Rate of Human Articular Chondrocytes in Monolayer Culture
2.4. Manufacturing of Non-Adhesive Microwells
2.5. Formation of Human Cartilage Spheroids
2.6. Live/dead Viability Assay
2.7. Morphometric Evaluation
2.8. Glycosaminoglycans Staining
2.9. Gene Expression Analysis
2.10. Statistical Analysis
3. Results
3.1. Anthropometric Features of Cartilage Donors
3.2. De-Differentiation of Articular Chondrocytes in Traditional 2D Cell Culture
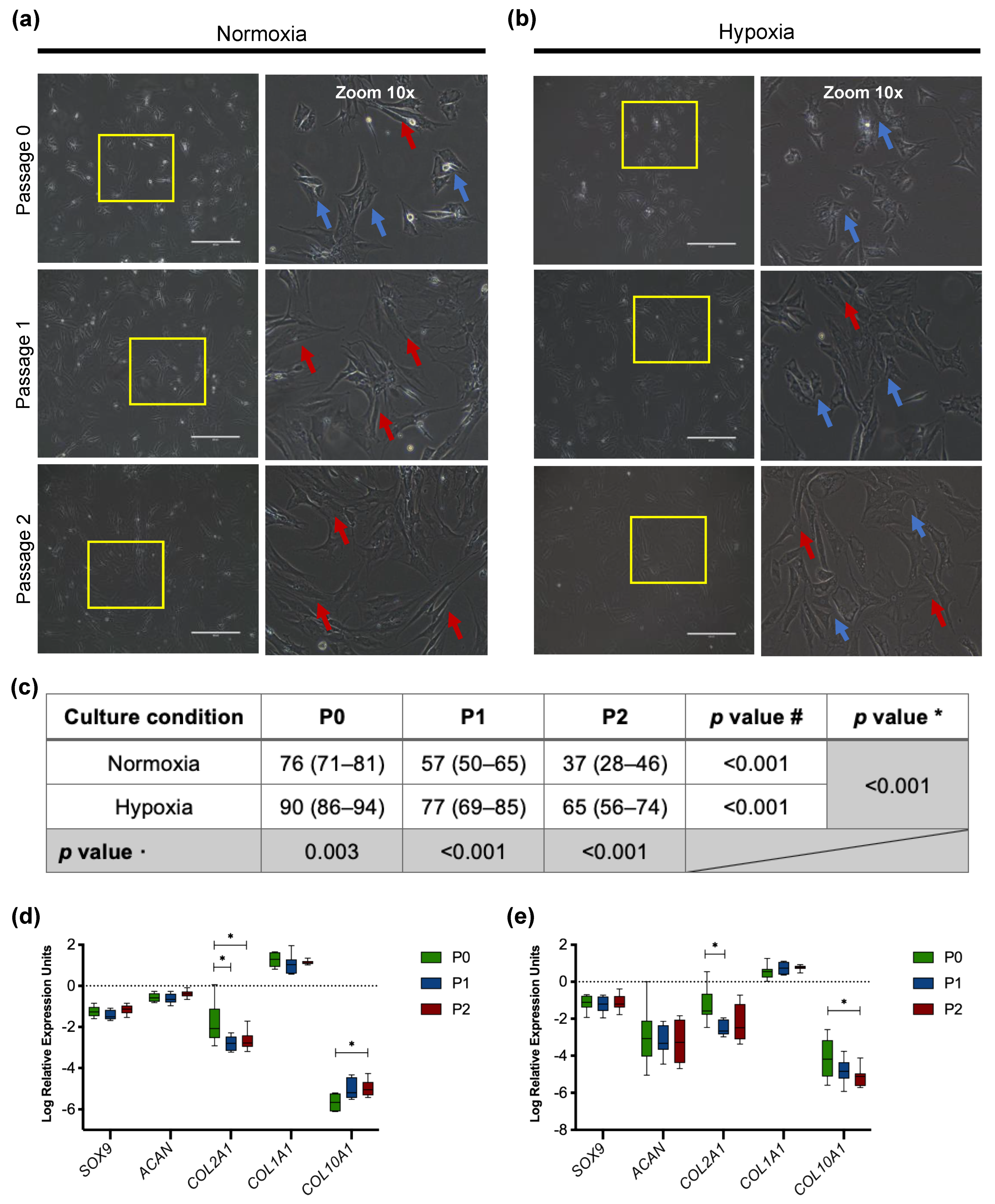
3.2.1. Morphology of Passaged Articular Chondrocytes
3.2.2. Downregulation of Chondrogenic Genes in Cultured Chondrocytes
3.3. Developing Scaffold-Free Spheroids from Human De-Differentiated Chondrocytes
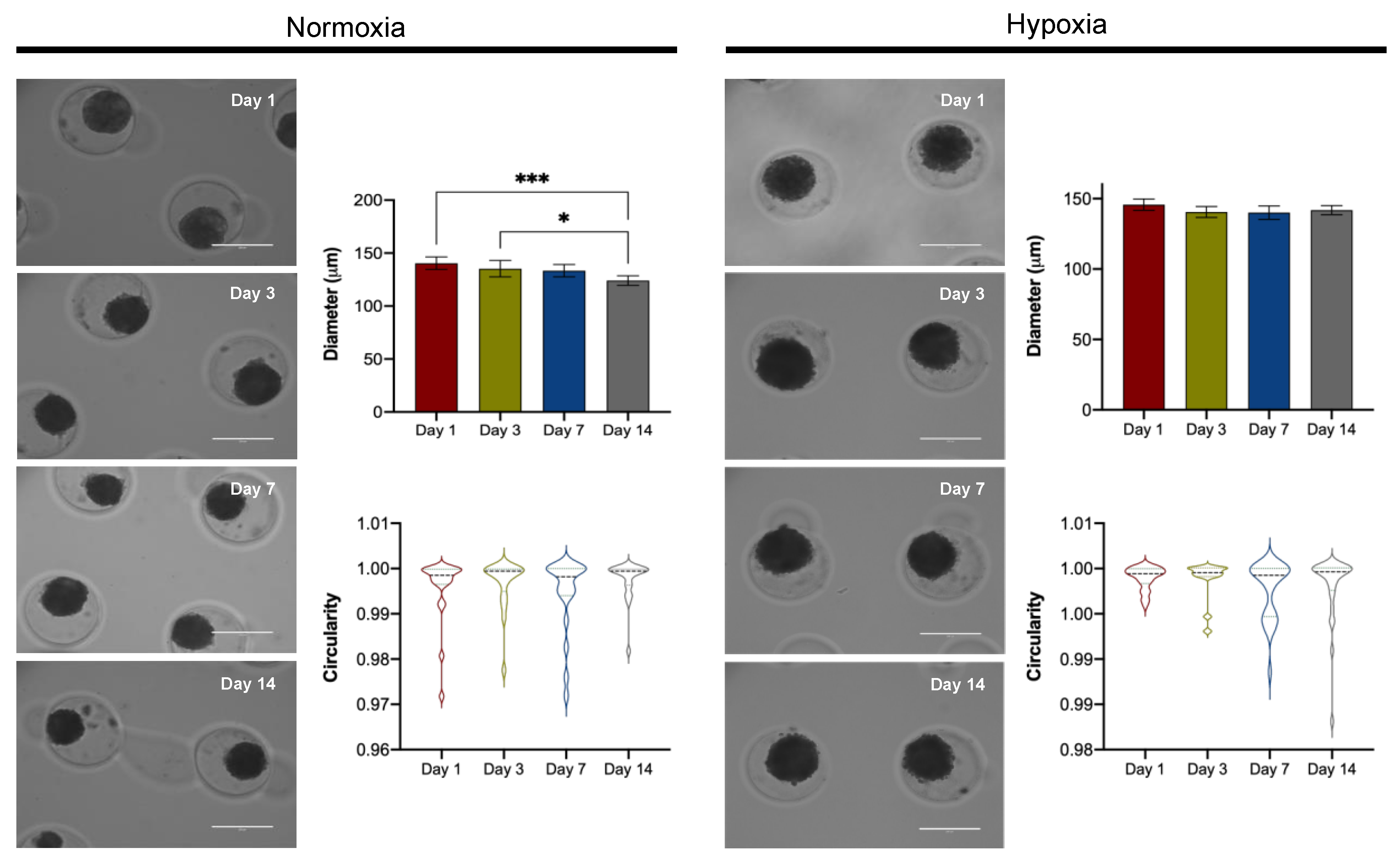
3.3.1. Spheroid Morphology
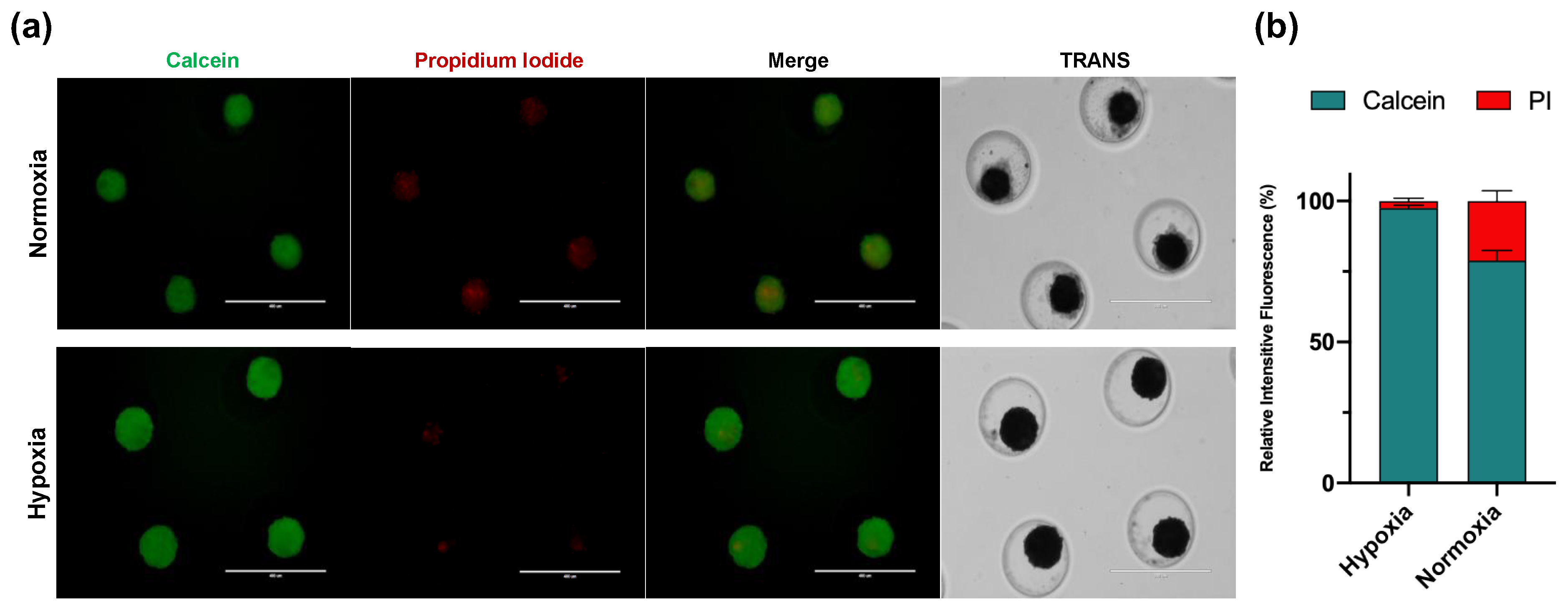
3.3.2. Viability
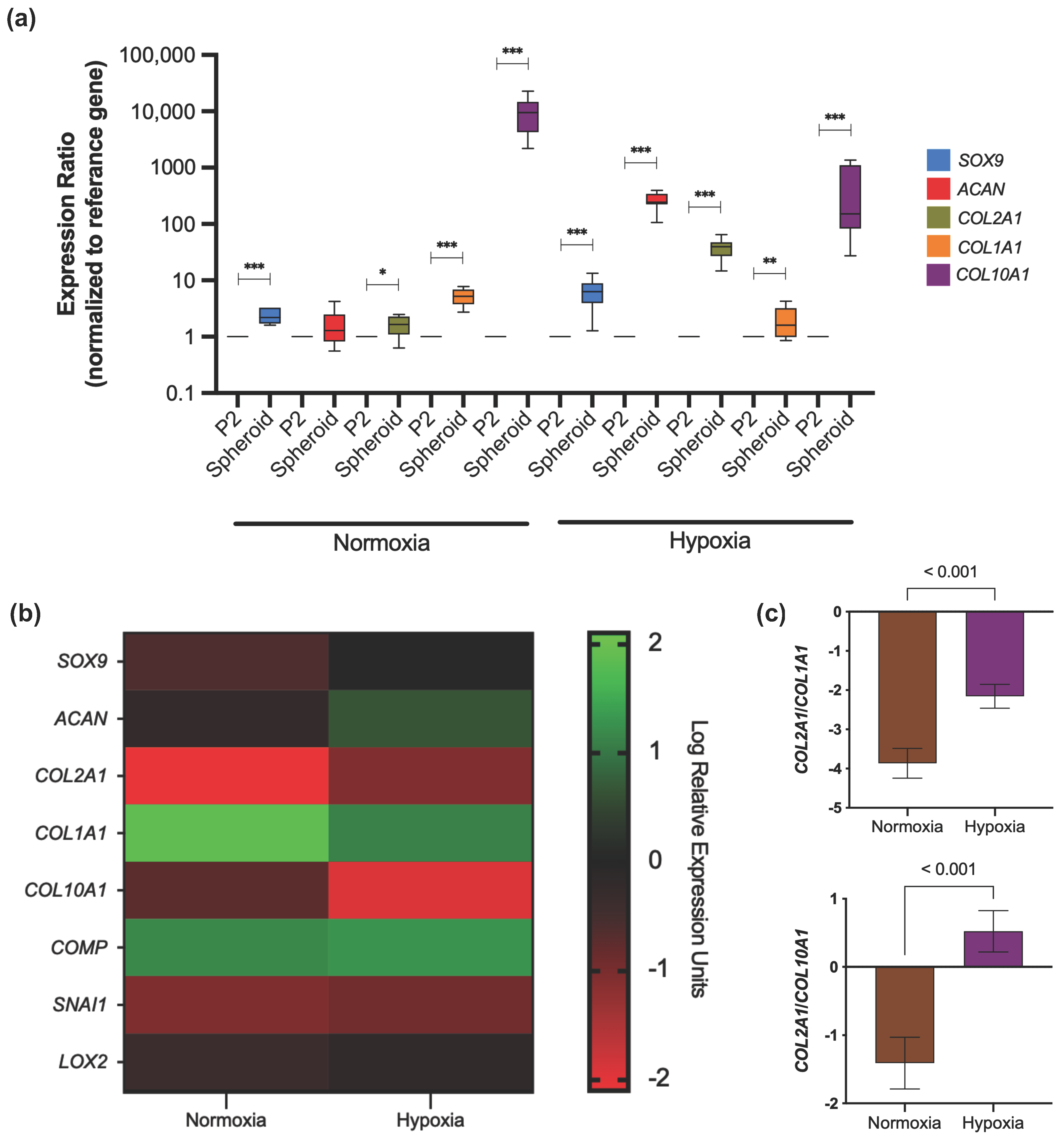
3.3.3. Extracellular Matrix Production
3.3.4. Gene Expression Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abhishek, A.; Doherty, M. Diagnosis and clinical presentation of osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 45–66. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Mennan, C.; Garcia, J.; McCarthy, H.; Owen, S.; Perry, J.; Wright, K.; Banerjee, R.; Richardson, J.B.; Roberts, S. Human Articular Chondrocytes Retain Their Phenotype in Sustained Hypoxia While Normoxia Promotes Their Immunomodulatory Potential. Cartilage 2019, 10, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Choi, Y.J.; Kim, H.S.; Park, S.H.; Choi, B.H.; Kim, J.H.; Song, B.R.; Min, B.H. Development of three-dimensional articular cartilage construct using silica nano-patterned substrate. PLoS ONE 2019, 14, e0208291. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, J.; Zhang, X.; Li, H.; Jiang, L.; Qin, J. Hypoxia combined with spheroid culture improves cartilage specific function in chondrocytes. Integr. Biol. 2015, 7, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ecke, A.; Lutter, A.H.; Scholka, J.; Hansch, A.; Becker, R.; Anderer, U. Tissue Specific Differentiation of Human Chondrocytes Depends on Cell Microenvironment and Serum Selection. Cells 2019, 8, 934. [Google Scholar] [CrossRef]
- Scalzone, A.; Ferreira, A.M.; Tonda-Turo, C.; Ciardelli, G.; Dalgarno, K.; Gentile, P. The interplay between chondrocyte spheroids and mesenchymal stem cells boosts cartilage regeneration within a 3D natural-based hydrogel. Sci. Rep. 2019, 9, 14630. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Sato, M.; Kim, H.W.; Mochida, J. Transplantatation of scaffold-free spheroids composed of synovium-derived cells and chondrocytes for the treatment of cartilage defects of the knee. Eur. Cell Mater. 2011, 22, 275–290, discussion 290. [Google Scholar] [CrossRef]
- Cooke, M.E.; Allon, A.A.; Cheng, T.; Kuo, A.C.; Kim, H.T.; Vail, T.P.; Marcucio, R.S.; Schneider, R.A.; Lotz, J.C.; Alliston, T. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr. Cartil. 2011, 19, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Hoffman, T.; Wu, A.; Kohn, J. An Innovative Laboratory Procedure to Expand Chondrocytes with Reduced Dedifferentiation. Cartilage 2018, 9, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Urlic, I.; Ivkovic, A. Cell Sources for Cartilage Repair-Biological and Clinical Perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef] [PubMed]
- Zubillaga, V.; Alonso-Varona, A.; Fernandes, S.C.M.; Salaberria, A.M.; Palomares, T. Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 1004. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Leduc, T.; Desance, M.; Hervieu, M.; Legendre, F.; Ollitrault, D.; de Vienne, C.; Herlicoviez, M.; Galera, P.; Demoor, M. Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges. Int. J. Mol. Sci. 2017, 18, 1933. [Google Scholar] [CrossRef] [PubMed]
- Adesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, Y.; Xu, X.; Xiong, J.; Ouyang, K.; Duan, L.; Wang, D. Cell-to-Cell Culture Inhibits Dedifferentiation of Chondrocytes and Induces Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells. Biomed. Res. Int. 2019, 2019, 5871698. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, V.P.; Tan, K.L.; Vasavada, S.; Bova, W.; Barnard, J.; Muschler, G.F. Characterization of heterogeneous primary human cartilage-derived cell population using non-invasive live-cell phase-contrast time-lapse imaging. Cytotherapy 2021, 23, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Wongin, S.; Waikakul, S.; Chotiyarnwong, P.; Siriwatwechakul, W.; Kino-Oka, M.; Kim, M.H.; Viravaidya-Pasuwat, K. Maintenance of human chondrogenic phenotype on a dendrimer-immobilized surface for an application of cell sheet engineering. BMC Biotechnol. 2018, 18, 14. [Google Scholar] [CrossRef]
- De Moor, L.; Beyls, E.; Declercq, H. Scaffold Free Microtissue Formation for Enhanced Cartilage Repair. Ann. Biomed. Eng. 2020, 48, 298–311. [Google Scholar] [CrossRef]
- Salgado, C.; Jordan, O.; Allemann, E. Osteoarthritis In Vitro Models: Applications and Implications in Development of Intra-Articular Drug Delivery Systems. Pharmaceutics 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.A.; Nordin, N.; Hussin, P.; Mehat, M.Z.; Tan, S.W.; Fakurazi, S. Optimization of Protocol for Isolation of Chondrocytes from Human Articular Cartilage. Cartilage 2021, 13, 872S–884S. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.G.; Liu, G.; Coimbra, I.B.; Piera-Velazquez, S.; Crowl, R.M.; Jimenez, S.A. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis. Rheum. 2002, 46, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Tew, S.R.; Murdoch, A.D.; Rauchenberg, R.P.; Hardingham, T.E. Cellular methods in cartilage research: Primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods 2008, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, S.; Hombauer, M.; Truppe, M.; Vecsei, V.; Schlegel, W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Jt. Surg Br. 2004, 86, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, A.V.; Vakhrushev, I.V.; Basok, Y.B.; Grigor’ev, A.M.; Kirsanova, L.A.; Lupatov, A.Y.; Sevastianov, V.I.; Yarygin, K.N. Chondrogeneic Potential of MSC from Different Sources in Spheroid Culture. Bull. Exp. Biol. Med. 2021, 170, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Leijten, J.C.; Wu, L.; Kip, M.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthr. Cartil. 2013, 21, 599–603. [Google Scholar] [CrossRef]
- Aurich, M.; Hofmann, G.O.; Gras, F.; Rolauffs, B. Human osteochondritis dissecans fragment-derived chondrocyte characteristics ex vivo, after monolayer expansion-induced de-differentiation, and after re-differentiation in alginate bead culture. BMC Musculoskelet. Disord. 2018, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, J.; Lv, S.; Zhao, Y.; Sun, D.; Zheng, Y.; Li, X.; Zhang, L.; Chi, G.; Li, Y. Overexpression of long non-coding RNA AP001505.9 inhibits human hyaline chondrocyte dedifferentiation. Aging (Albany N. Y.) 2021, 13, 11433–11454. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Bevilacqua, C.; Cappella, M.; Manzotti, S.; Greco, F. Engineered articular cartilage: Influence of the scaffold on cell phenotype and proliferation. J. Mater. Sci. Mater. Med. 2003, 14, 713–716. [Google Scholar] [CrossRef]
- Sulaiman, S.; Chowdhury, S.R.; Fauzi, M.B.; Rani, R.A.; Yahaya, N.H.M.; Tabata, Y.; Hiraoka, Y.; Binti Haji Idrus, R.; Min Hwei, N. 3D Culture of MSCs on a Gelatin Microsphere in a Dynamic Culture System Enhances Chondrogenesis. Int. J. Mol. Sci. 2020, 21, 2688. [Google Scholar] [CrossRef] [PubMed]
- Klinder, A.; Kussauer, S.; Hiemer, B.; Wree, A.; Bader, R.; Jonitz-Heincke, A. Influence of Conditioned Media on the Re-Differentiation Capacity of Human Chondrocytes in 3D Spheroid Cultures. J. Clin. Med. 2020, 9, 2798. [Google Scholar] [CrossRef]
- Foldager, C.B.; Nielsen, A.B.; Munir, S.; Ulrich-Vinther, M.; Soballe, K.; Bunger, C.; Lind, M. Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop. 2011, 82, 234–240. [Google Scholar] [CrossRef]
- Caron, M.M.; Emans, P.J.; Coolsen, M.M.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.J.; Lee, A.; Anderson, J.; Parreno, J.; Theodoropoulos, J.; Backstein, D.; Kandel, R. Redifferentiated Chondrocytes in Fibrin Gel for the Repair of Articular Cartilage Lesions. Am. J. Sports Med. 2019, 47, 2348–2359. [Google Scholar] [CrossRef]
- Markway, B.D.; Cho, H.; Johnstone, B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res. Ther. 2013, 15, R92. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, W.; Usui, M.; Sano, K.; Kawano, A.; Okinaga, T.; Nakashima, K.; Nakazawa, K.; Nishihara, T. 3D spheroid culture models for chondrocytes using polyethylene glycol-coated microfabricated chip. Biomed. Res. 2020, 41, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chavez, R.D.; Serra, R. Scaffoldless tissue-engineered cartilage for studying transforming growth factor beta-mediated cartilage formation. Biotechnol. Prog. 2020, 36, e2897. [Google Scholar] [CrossRef] [PubMed]
- Dehne, T.; Karlsson, C.; Ringe, J.; Sittinger, M.; Lindahl, A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res. Ther. 2009, 11, R133. [Google Scholar] [CrossRef] [PubMed]
- Tallheden, T.; Bengtsson, C.; Brantsing, C.; Sjogren-Jansson, E.; Carlsson, L.; Peterson, L.; Brittberg, M.; Lindahl, A. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res. Ther. 2005, 7, R560–R568. [Google Scholar] [CrossRef]
- Ma, K.; Zhu, B.; Wang, Z.; Cai, P.; He, M.; Ye, D.; Yan, G.; Zheng, L.; Yang, L.; Zhao, J. Articular chondrocyte-derived extracellular vesicles promote cartilage differentiation of human umbilical cord mesenchymal stem cells by activation of autophagy. J. Nanobiotechnol. 2020, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lei, G.; Lin, Z.; Yang, Y.; Lin, H.; Tuan, R.S. Engineering hyaline cartilage from mesenchymal stem cells with low hypertrophy potential via modulation of culture conditions and Wnt/β-catenin pathway. Biomaterials 2019, 192, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef]
- Thielen, N.G.M.; Neefjes, M.; Vitters, E.L.; van Beuningen, H.M.; Blom, A.B.; Koenders, M.I.; van Lent, P.; van de Loo, F.A.J.; Blaney Davidson, E.N.; van Caam, A.P.M.; et al. Identification of Transcription Factors Responsible for a Transforming Growth Factor-beta-Driven Hypertrophy-like Phenotype in Human Osteoarthritic Chondrocytes. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.T.; Vinardell, T.; Kelly, D.J. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.G.; Buckley, C.T.; Thorpe, S.D.; Kelly, D.J. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J. Biomech. 2010, 43, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, V.V.; Dahlin, R.L.; Wright, S.; Kasper, F.K.; Mikos, A.G. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 2013, 34, 4266–4273. [Google Scholar] [CrossRef]
- Omelyanenko, N.P.; Karalkin, P.A.; Bulanova, E.A.; Koudan, E.V.; Parfenov, V.A.; Rodionov, S.A.; Knyazeva, A.D.; Kasyanov, V.A.; Babichenko, I.I.; Chkadua, T.Z.; et al. Extracellular Matrix Determines Biomechanical Properties of Chondrospheres during Their Maturation In Vitro. Cartilage 2020, 11, 521–531. [Google Scholar] [CrossRef]

| Gene Symbol | Primer Qiagen Id or Costume Sequence | qRT-PCR Condition |
|---|---|---|
| SOX9 | PPH02125A | 60 °C–60 seg |
| ACAN | PPH06097E | 60 °C–60 seg |
| COMP | PPH07086B | 60 °C–60 seg |
| SNAI1 | PPH02459B | 60 °C–60 seg |
| LOXL2 | PPH10275A | 60 °C–60 seg |
| RPL27 | PPH00443B | 60 °C–60 seg |
| COL1A1 | PPH01299F | 60 °C–60 seg |
| COL2A1 | Forward ATGAGGGCGCGGTAGAGA Reverse CCCTGACACCGAAGGACAG | 62 °C–47 seg |
| COL10A1 | Forward CCCAGCACGCAGAATCCAT Reverse CCTGTGGGCATTTGGTATCG | 58 °C–60 seg |
| Characteristics | Total (n = 15) | Normoxia (n = 5) | Hypoxia (n = 10) | p-Value |
|---|---|---|---|---|
| Age, years | 43 ± 15 | 36 ± 11 | 47 ± 15 | 0.16 |
| BMI | 26.6 ± 4.1 | 25.1 ± 4.8 | 27.4 ± 3.8 | 0.33 |
| Gender ** | ||||
| Male | 11 (73%) | 4 (80%) | 7 (70%) | 0.59 |
| Procedure ** | ||||
| TKR | 9 (60%) | 3 (60%) | 6 (60%) | 0.71 |
| KA | 6 (40%) | 2 (40%) | 4 (40%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Armenta, C.; Suarez-Ahedo, C.; Olivos-Meza, A.; Camacho-Rea, M.C.; Martínez-Gómez, L.E.; Jimenez-Gutierrez, G.E.; Martínez-Nava, G.A.; Gomez-Quiroz, L.E.; Pineda, C.; López-Reyes, A. The Critical Role of Hypoxia in the Re-Differentiation of Human Articular Chondrocytes. Cells 2022, 11, 2553. https://doi.org/10.3390/cells11162553
Martinez-Armenta C, Suarez-Ahedo C, Olivos-Meza A, Camacho-Rea MC, Martínez-Gómez LE, Jimenez-Gutierrez GE, Martínez-Nava GA, Gomez-Quiroz LE, Pineda C, López-Reyes A. The Critical Role of Hypoxia in the Re-Differentiation of Human Articular Chondrocytes. Cells. 2022; 11(16):2553. https://doi.org/10.3390/cells11162553
Chicago/Turabian StyleMartinez-Armenta, Carlos, Carlos Suarez-Ahedo, Anell Olivos-Meza, María C. Camacho-Rea, Laura E. Martínez-Gómez, Guadalupe Elizabeth Jimenez-Gutierrez, Gabriela A. Martínez-Nava, Luis E. Gomez-Quiroz, Carlos Pineda, and Alberto López-Reyes. 2022. "The Critical Role of Hypoxia in the Re-Differentiation of Human Articular Chondrocytes" Cells 11, no. 16: 2553. https://doi.org/10.3390/cells11162553
APA StyleMartinez-Armenta, C., Suarez-Ahedo, C., Olivos-Meza, A., Camacho-Rea, M. C., Martínez-Gómez, L. E., Jimenez-Gutierrez, G. E., Martínez-Nava, G. A., Gomez-Quiroz, L. E., Pineda, C., & López-Reyes, A. (2022). The Critical Role of Hypoxia in the Re-Differentiation of Human Articular Chondrocytes. Cells, 11(16), 2553. https://doi.org/10.3390/cells11162553







