Discovery of Novel HSP27 Inhibitors as Prospective Anti-Cancer Agents Utilizing Computer-Assisted Therapeutic Discovery Approaches
Abstract
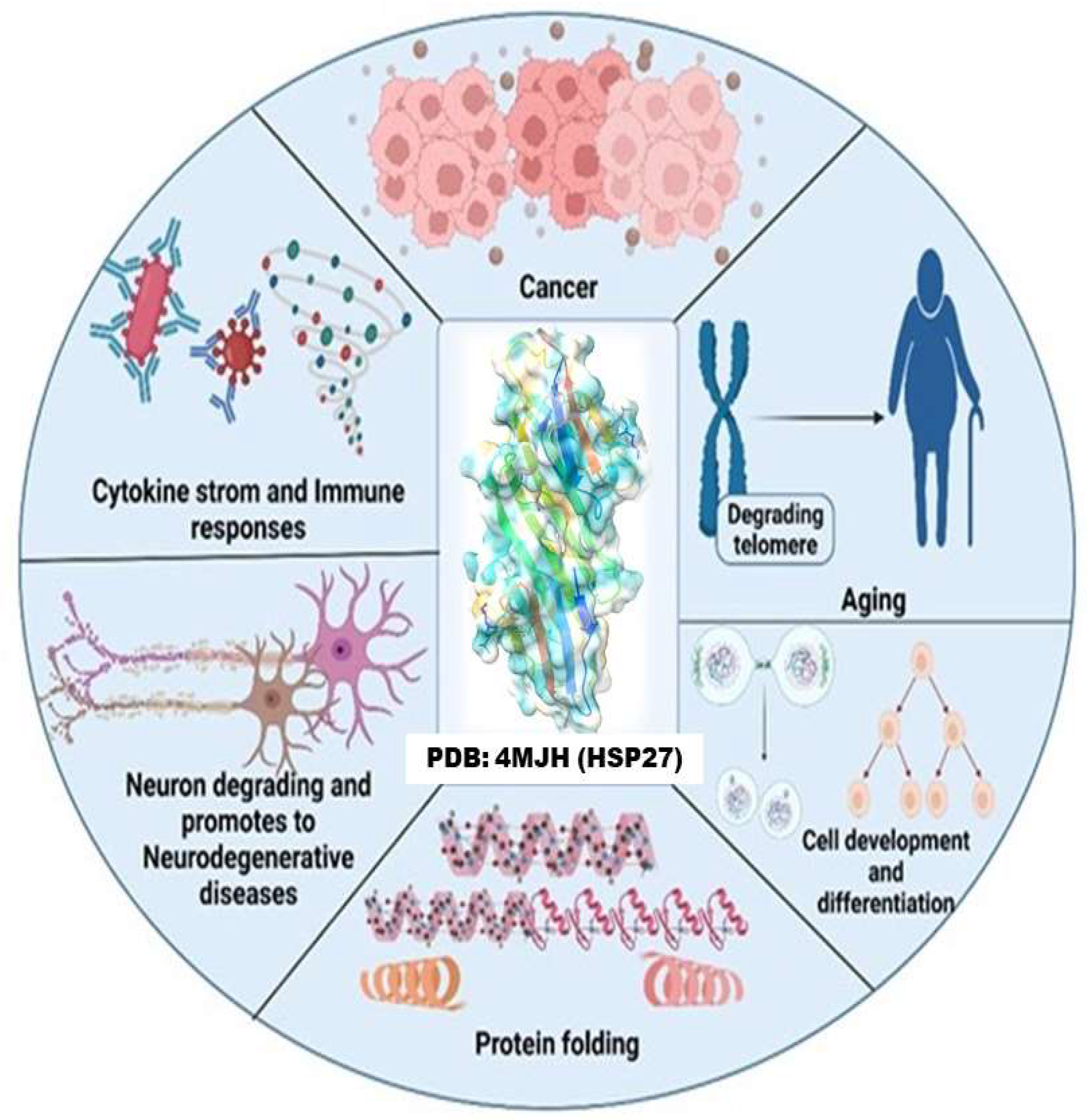
:1. Introduction
2. Materials and Methods
2.1. Compound Datasets
2.2. Screening of Compound Library for Drug Likeness
2.3. Selection and Preparation of Protein Target
2.4. Preparation of Compounds for Molecular Docking against HSP27
2.5. Molecular Docking of Compounds against HSP27
2.6. ADMET Prediction In Silico
2.7. Molecular Dynamics Simulations
3. Results
3.1. Drug Likeness Screening
3.2. Docking-Derived Binding Affinity
3.3. ADMET Prediction
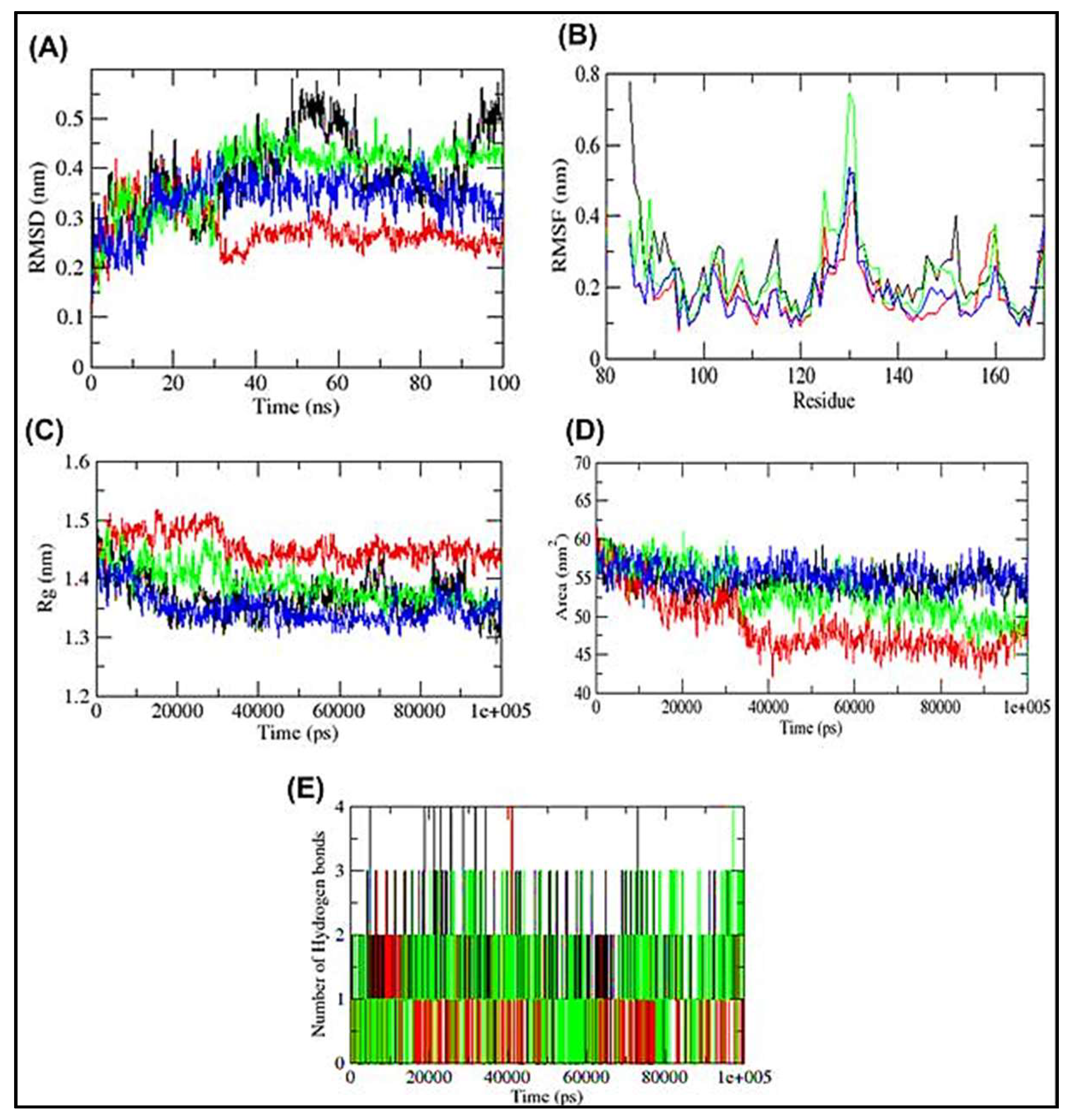
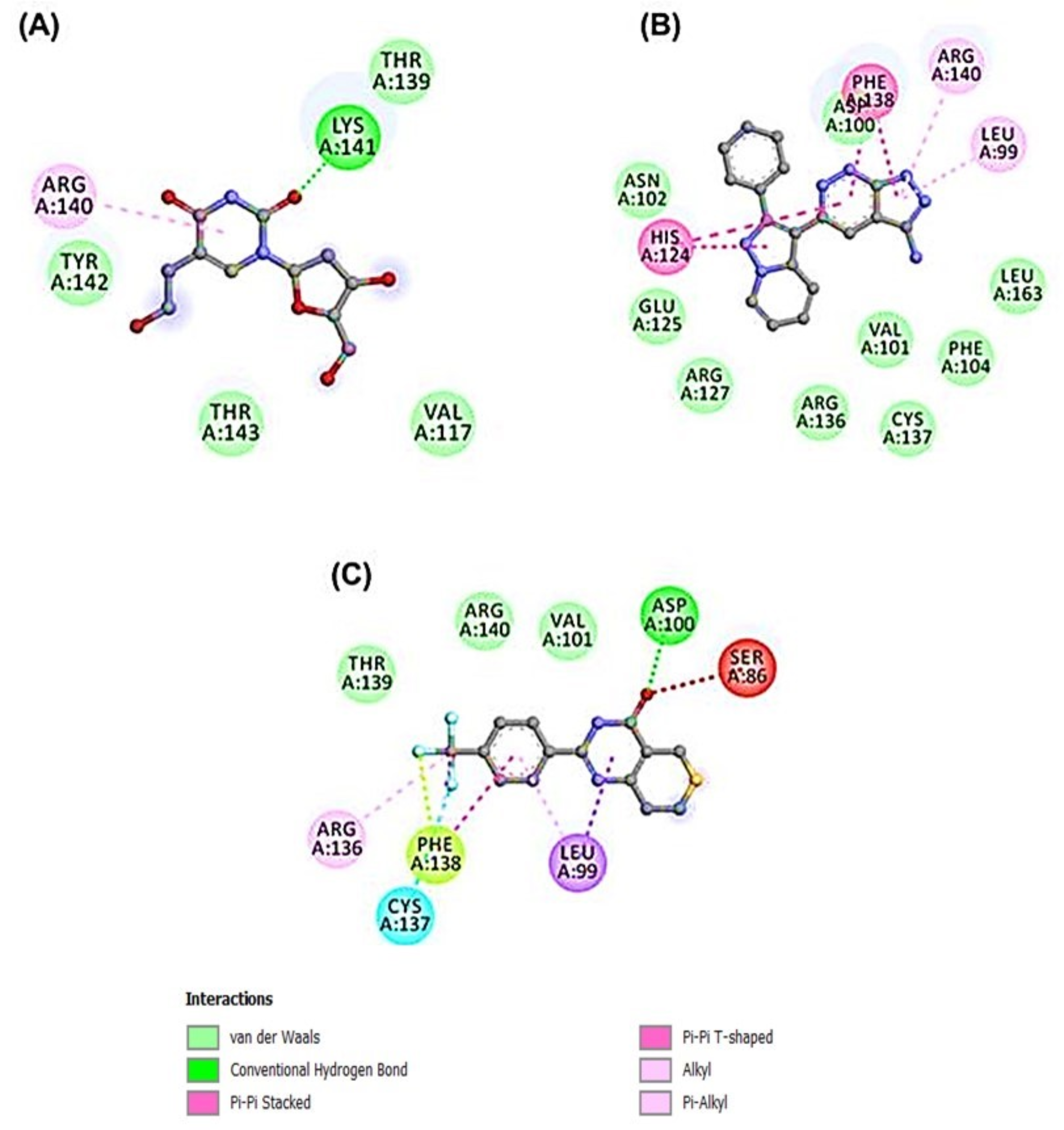
3.4. Molecular Dynamics Simulation of HSP27-Drug Complexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alderson, T.R.; Roche, J.; Gastall, H.Y.; Dias, D.M.; Pritišanac, I.; Ying, J.; Bax, A.; Benesch, J.L.P.; Baldwin, A.J. Local unfolding of the HSP27 monomer regulates chaperone activity. Nat. Commun. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuramitsu, Y. Is HSP27 a Key Molecule or a Biomarker of Cancers? J. Gastrointest. Dig. Syst. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.-Y.; Choi, S.-K.; Seo, S.H.; Jo, H.; Shin, J.-H.; Na, Y.; Lee, Y.-S.; Kwon, Y. Specific Roles of HSP27 S15 Phosphorylation Augmenting the Nuclear Function of HER2 to Promote Trastuzumab Resistance. Cancers 2020, 12, 1540. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Kam, H.; Kim, K.-Y.; Park, S.I.; Lee, Y.-S. Targeting Heat Shock Protein 27 in Cancer: A Druggable Target for Cancer Treatment? Cancers 2019, 11, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, S.; Zhao, H.; Dart, A.; Wang, Y.; Ruge, F.; Gao, Y.; Wei, C.; Wu, Y.; Jiang, W.G. Heat shock protein 27 is a potential indicator for response to YangZheng XiaoJi and chemotherapy agents in cancer cells. Int. J. Oncol. 2016, 49, 1839–1847. [Google Scholar] [CrossRef] [Green Version]
- Katsogiannou, M.; Andrieu, C.; Rocchi, P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front. Genet. 2014, 5, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Liu, D.; Kong, X.; Dong, M. Clinicopathological Significance of Heat Shock Protein (HSP) 27 Expression in Gastric Cancer: A Updated Meta-Analysis. Evidence-Based Complement. Altern. Med. 2020, 2020, 7018562. [Google Scholar] [CrossRef]
- Nappi, L.; Aguda, A.H.; Al Nakouzi, N.; Lelj-Garolla, B.; Beraldi, E.; Lallous, N.; Thi, M.; Moore, S.; Fazli, L.; Battsogt, D.; et al. Ivermectin inhibits HSP27 and potentiates efficacy of oncogene targeting in tumor models. J. Clin. Investig. 2019, 130, 699–714. [Google Scholar] [CrossRef]
- Shimura, H.; Miura-Shimura, Y.; Kosik, K.S. Binding of Tau to Heat Shock Protein 27 Leads to Decreased Concentration of Hyperphosphorylated Tau and Enhanced Cell Survival. J. Biol. Chem. 2004, 279, 17957–17962. [Google Scholar] [CrossRef] [Green Version]
- Miller-Graziano, C.L.; De, A.; Laudanski, K.; Herrmann, T.; Bandyopadhyay, S. HSP27: An anti-infl ammatory and immunomodulatory stress protein. Novartis Found. Symp. 2008, 291, 196–211. [Google Scholar] [PubMed]
- Hell-Pourmojib, M.; Neuner, P.; Knobler, R.; Trautinger, F.; Fischer, H.; Rezaie, S.; Kindås-Mügge, I. Differential Expression of a Novel Gene in Response to hsp27 and Cell Differentiation in Human Keratinocytes. J. Investig. Dermatol. 2002, 119, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Ng, Y.-C. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim. Biophys. Acta (BBA) -Mol. Basis Dis. 2006, 1762, 103–109. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Shen, F.; Qi, P.; Zhai, Z.; Wang, Z. miR-541-3p enhances the radiosensitivity of prostate cancer cells by inhibiting HSP27 expression and downregulating β-catenin. Cell Death Discov. 2021, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Bodzek, P.; Damasiewicz-Bodzek, A.; Janosz, I.; Witek, L.; Olejek, A. Heat shock protein 27 (HSP27) in patients with ovarian cancer. Ginekol. Polska 2021, 92, 837–843. [Google Scholar] [CrossRef]
- Rajesh, Y.; Banerjee, A.; Pal, I.; Biswas, A.; das, S.; Dey, K.K.; Kapoor, N.; Ghosh, A.K.; Mitra, P.; Mandal, M. Delineation of crosstalk between HSP27 and MMP-2/MMP-9: A synergistic therapeutic avenue for glioblastoma management. Biochim. Biophys. Acta (BBA) -Gen. Subj. 2019, 1863, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Xu, Y.; Ge, H.; Li, G.; Wu, J. The clinicopathological and prognostic value of HSP27 in hepatocellular carcinoma: A systematic review and meta-analysis. OncoTargets Ther. 2018, ume 11, 1293–1303. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tao, X.; Jin, G.; Jin, H.; Wang, N.; Hu, F.; Luo, Q.; Shu, H.; Zhao, F.; Yao, M.; et al. A Targetable Molecular Chaperone Hsp27 Confers Aggressiveness in Hepatocellular Carcinoma. Theranostics 2016, 6, 558–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; Hong, S.; Kim, J.; Kim, M.; Chang, Y.H.; Hong, Y.J.; Lee, J.K.; Park, I. Induction of HSP27 and HSP70 by constitutive overexpression of Redd1 confers resistance of lung cancer cells to ionizing radiation. Oncol. Rep. 2019, 41, 3119–3126. [Google Scholar] [CrossRef]
- Xu; Yu, Z.; Zhi, J.; Zhong, X.; Peng, X.; Xu, A. Clinical significance of HSP27 expression in colorectal cancer. Mol. Med. Rep. 2010, 3, 953–958. [Google Scholar] [CrossRef]
- Guo, Y.; Ziesch, A.; Hocke, S.; Kampmann, E.; Ochs, S.; De Toni, E.N.; Göke, B.; Gallmeier, E. Overexpression of heat shock protein 27 (HSP27) increases gemcitabine sensitivity in pancreatic cancer cells through S-phase arrest and apoptosis. J. Cell. Mol. Med. 2014, 19, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Weeks, S.D.; Muranova, L.; Heirbaut, M.; Beelen, S.; Strelkov, S.V.; Gusev, N.B. Characterization of human small heat shock protein HSPB1 α-crystallin domain localized mutants associated with hereditary motor neuron diseases. Sci. Rep. 2018, 8, 688. [Google Scholar] [CrossRef] [Green Version]
- Mostoufi, A.; Eucefifar, H.; Beygi, F.; Fereidoonnezhad, M. In Silico Screening Studies on Methanesulfonamide Derivatives as Dual Hsp27 and Tubulin Inhibitors Using QSAR and Molecular Docking. J. Sci. 2018, 29, 221–240. [Google Scholar] [CrossRef]
- Kamada, M.; So, A.; Muramaki, M.; Rocchi, P.; Beraldi, E.; Gleave, M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol. Cancer Ther. 2007, 6, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, G.K.A.; Ecroyd, H.; Liu, C.; Cox, D.; Cascio, D.; Sawaya, M.R.; Collier, M.P.; Stroud, J.; Carver, J.A.; Baldwin, A.J.; et al. The structured core domain of αB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc. Natl. Acad. Sci. USA 2014, 111, E1562–E1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroganov, O.V.; Novikov, F.N.; Zeifman, A.A.; Stroylov, V.S.; Chilov, G.G. TSAR, a new graph-theoretical approach to computational modeling of protein side-chain flexibility: Modeling of ionization properties of proteins. Proteins Struct. Funct. Bioinform. 2011, 79, 2693–2710. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Fossa, P.; Cichero, E. In silico evaluation of human small heat shock protein HSP27: Homology modeling, mutation analyses and docking studies. Bioorg. Med. Chem. 2015, 23, 3215–3220. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; De Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2018, 35, 1067–1069. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; Van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.Y.; Uzairu, A. In silico QSAR and molecular docking studies of sulfur containing shikonin oxime derivatives as anti-cancer agent for colon cancer. Radiol. Infect. Dis. 2019, 6, 108–121. [Google Scholar] [CrossRef]
- Heinrich, J.C.; Donakonda, S.; Haupt, V.J.; Lennig, P.; Zhang, Y.; Schroeder, M. New HSP27 inhibitors efficiently suppress drug resistance development in cancer cells. Oncotarget 2016, 7, 68156–68169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokhande, K.B.; Nagar, S.; Swamy, K.V. Molecular interaction studies of Deguelin and its derivatives with Cyclin D1 and Cyclin E in cancer cell signaling pathway: The computational approach. Sci. Rep. 2019, 9, 1778. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Bala, R.; Sindhu, R.K.; Zehravi, M.; Madaan, R.; Ramproshad, S.; Mondal, B.; Dey, A.; Rahman, H.; Cavalu, S. Bioactive Based Nanocarriers for the Treatment of Viral Infections and SARS-CoV-2. Nanomaterials 2022, 12, 1530. [Google Scholar] [CrossRef]
- Zehravi, M.; Kabir, J.; Akter, R.; Malik, S.; Ashraf, G.M.; Tagde, P.; Ramproshad, S.; Mondal, B.; Rahman, H.; Mohan, A.G.; et al. A Prospective Viewpoint on Neurological Diseases and Their Biomarkers. Molecules 2022, 27, 3516. [Google Scholar] [CrossRef]
- You, I.-S.; Sharma, S.; Fadriquela, A.; Bajgai, J.; Thi, T.T.; Rahman, H.; Sung, J.; Kwon, H.-U.; Lee, S.-Y.; Kim, C.-S.; et al. Antioxidant Properties of Hydrogen Gas Attenuates Oxidative Stress in Airway Epithelial Cells. Molecules 2021, 26, 6375. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bajgai, J.; Antonio, J.M.; Fadriquela, A.; Trinh, T.T.; Rahman, H.; Vira, K.; Sofian, A.-N.; Kim, C.-S.; Lee, K.-J. Anti-Hyperglycemic Effect of Magnesium-Enhanced Alkaline-Reduced Water on High Glucose-Induced Oxidative Stress in Renal Tubular Epithelial Cells. Processes 2022, 10, 919. [Google Scholar] [CrossRef]


| S/N | Molecules | PID | MW | PAINS Alert | Brenk Alert | Lead Likeness Violations |
|---|---|---|---|---|---|---|
| 1 | A-966492 | 16666333 | 324.35 | 0 | 0 | 0 |
| 2 | A2AR antagonist 1 | 53466958 | 309.3 | 0 | 0 | 0 |
| 3 | AG-14361 | 9840076 | 320.39 | 0 | 0 | 0 |
| 4 | APY29 | 42627755 | 332.36 | 0 | 0 | 0 |
| 5 | ASP-9521 | 25210792 | 330.42 | 0 | 0 | 0 |
| 6 | AZD1480 | 16659841 | 348.77 | 0 | 0 | 0 |
| 7 | AZ32 | 134814488 | 328.37 | 0 | 0 | 0 |
| 8 | Broxyquinoline | 2453 | 302.95 | 0 | 0 | 0 |
| 9 | CCT128930 | 17751819 | 341.84 | 0 | 0 | 0 |
| 10 | DB07268 | 16058637 | 321.33 | 0 | 0 | 0 |
| 11 | Eupatilin | 5273755 | 344.32 | 0 | 0 | 0 |
| 12 | E7820 | 196970 | 336.37 | 0 | 0 | 0 |
| 13 | Fluorescein | 16850 | 332.31 | 0 | 0 | 0 |
| 14 | Flubendazole | 35802 | 313.28 | 0 | 0 | 0 |
| 15 | FR 180204 | 11493598 | 327.34 | 0 | 0 | 0 |
| 16 | GNE-0877 | 69093374 | 339.32 | 0 | 0 | 0 |
| 17 | Hydroquinidine | 91503 | 326.43 | 0 | 0 | 0 |
| 18 | (+)-Isocorynoline | 10143 | 341.4 | 0 | 0 | 0 |
| 19 | Kenpaullone | 3820 | 327.18 | 0 | 0 | 0 |
| 20 | KW-2449 | 11427553 | 332.4 | 0 | 0 | 0 |
| 21 | Lificiguat (YC-1) | 5712 | 304.34 | 0 | 0 | 0 |
| 22 | LIT-927 | 137287575 | 328.75 | 0 | 0 | 0 |
| 23 | Longdaysin | 49830252 | 335.33 | 0 | 0 | 0 |
| 24 | ML167 | 44968231 | 335.36 | 0 | 0 | 0 |
| 25 | MSC2530818 | 118879529 | 340.81 | 0 | 0 | 0 |
| 26 | Niraparib (MK-4827) | 24958200 | 320.39 | 0 | 0 | 0 |
| 27 | Nocodazole | 4122 | 301.32 | 0 | 0 | 0 |
| 28 | Olanzapine | 135398745 | 312.43 | 0 | 0 | 0 |
| 29 | Omeprazole | 4594 | 345.42 | 0 | 0 | 0 |
| 30 | Oxfendazole | 40854 | 315.35 | 0 | 0 | 0 |
| 31 | Pimobendan | 4823 | 334.37 | 0 | 0 | 0 |
| 32 | PNU 282987 | 9795278 | 300.2 | 0 | 0 | 0 |
| 33 | PI-103 | 9884685 | 348.36 | 0 | 0 | 0 |
| 34 | PP2 | 4878 | 301.77 | 0 | 0 | 0 |
| 35 | PP121 | 24905142 | 319.36 | 0 | 0 | 0 |
| 36 | R112 | 9904854 | 312.3 | 0 | 0 | 0 |
| 37 | Ruxolitinib (INCB018424) | 25126798 | 306.37 | 0 | 0 | 0 |
| 38 | SCH58261 | 176408 | 345.36 | 0 | 0 | 0 |
| 39 | SGC2085 | 121231417 | 312.41 | 0 | 0 | 0 |
| 40 | S-Ruxolitinib (INCB018424) | 50878566 | 306.37 | 0 | 0 | 0 |
| 41 | Tenatoprazole | 636411 | 346.4 | 0 | 0 | 0 |
| 42 | Torkinib (PP242) | 135565635 | 308.34 | 0 | 0 | 0 |
| 43 | TG100-115 | 10427712 | 346.34 | 0 | 0 | 0 |
| 44 | Valdecoxib | 119607 | 314.36 | 0 | 0 | 0 |
| 45 | XL413 (BMS-863233) | 135564632 | 325.17 | 0 | 0 | 0 |
| 46 | XAV-939 | 135418940 | 312.31 | 0 | 0 | 0 |
| 47 | ZM241385 | 176407 | 337.34 | 0 | 0 | 0 |
| S/N | Ligand | Binding Energy (kcal/mol) |
|---|---|---|
| 1. | A-966492 | −7.2 |
| 2. | A2AR antagonist 1 | −5.4 |
| 3. | AG-14361 | −6.4 |
| 4. | APY29 | −7.7 |
| 5. | ASP-9521 | −5.6 |
| 6. | AZD1480 | −6.2 |
| 7. | AZ32 | −6.8 |
| 8. | Brivudine (BVDU) | −6.0 |
| 9. | Broxyquinoline | −5.3 |
| 10. | CCT128930 | −6.0 |
| 11. | DB07268 | −6.9 |
| 12. | Eupatilin | −6.4 |
| 13. | E7820 | −7.1 |
| 14. | Fluorescein | −8.2 |
| 15. | Flubendazole | −7.3 |
| 16. | FR 180204 | −8.1 |
| 17. | GNE-0877 | −6.2 |
| 18. | Hydroquinidine | −6.1 |
| 19. | (+)-Isocorynoline | −6.4 |
| 20. | J2 | −5.8 |
| 21. | Kenpaullone | −7.5 |
| 22. | KW-2449 | −6.9 |
| 23. | Lificiguat (YC-1) | −6.5 |
| 24. | LIT-927 | −6.6 |
| 25. | Longdaysin | −6.5 |
| 26. | ML167 | −7.3 |
| 27. | MSC2530818 | −7.2 |
| 28. | Niraparib (MK-4827) | −6.7 |
| 29. | Nocodazole | −6.3 |
| 30. | Olanzapine | −5.9 |
| 31. | Omeprazole | −6.0 |
| 32. | Oxfendazole | −6.5 |
| 33. | Pimobendan | −7.2 |
| 34. | PNU 282987 | −5.8 |
| 35. | PI-103 | −6.7 |
| 36. | PP2 | −6.4 |
| 37. | PP121 | −6.7 |
| 38. | R112 | −6.8 |
| 39. | Ruxolitinib (INCB018424) | −6.9 |
| 40. | SCH58261 | −6.4 |
| 41. | SGC2085 | −6.7 |
| 42. | S-Ruxolitinib (INCB018424) | −7.2 |
| 43. | Tenatoprazole | −5.8 |
| 44. | Torkinib (PP242) | −6.9 |
| 45. | TG100-115 | −7.6 |
| 46. | Valdecoxib | −6.8 |
| 47. | XL413 (BMS-863233) | −6.9 |
| 48. | XAV-939 | −7.3 |
| 49. | ZM241385 | −6.7 |
| ADMET PROFILES | Kenpaullone | Pimobendan | Fluorescein | Flubendazole | E7820 | TG100-115 | FR180204 |
|---|---|---|---|---|---|---|---|
| Ames mutagenesis | + | + | - | - | + | - | - |
| Blood Brain Barrier | + | + | - | + | + | + | + |
| Caco-2 | + | - | - | + | - | - | + |
| CYP1A2 inhibition | + | + | - | + | + | + | + |
| CYP2C19 inhibition | - | + | - | - | + | + | + |
| CYP2C9 inhibition | - | - | + | - | - | + | - |
| CYP2C9 substrate | - | - | - | - | + | - | - |
| CYP2D6 inhibition | + | - | - | - | - | - | - |
| CYP2D6 substrate | - | - | - | - | - | - | - |
| CYP3A4 inhibition | + | + | + | - | + | - | - |
| CYP3A4 substrate | + | + | + | + | + | - | + |
| Human either-a-go-go inhibition | - | + | - | + | - | - | - |
| OCT2 inhibitior | - | - | - | - | - | - | - |
| Human Intestinal Absorption | + | + | + | + | + | + | + |
| Human oral bioavailability | + | + | + | - | + | + | + |
| P-glycoprotein inhibitior | - | - | - | - | - | - | - |
| P-glycoprotein substrate | - | - | - | - | - | - | + |
| Subcellular localization | Mitochondria | Mitochondria | Mitochondria | Mitochondria | Lysosomes | Mitochondria | Mitochondria |
| ADMET PROFILES | A-966492 | APY29 | ML167 | S-Ruxolitinib | MSC2530818 | XAV-939 | BVDU |
| Ames mutagenesis | - | - | - | - | - | - | - |
| Blood Brain Barrier | + | + | + | + | + | + | + |
| Caco-2 | - | - | - | - | + | + | - |
| CYP1A2 inhibition | + | + | + | + | - | + | - |
| CYP2C19 inhibition | + | - | + | - | + | - | - |
| CYP2C9 inhibition | + | - | + | - | + | - | - |
| CYP2C9 substrate | - | - | - | - | + | - | - |
| CYP2D6 inhibition | - | - | - | - | - | - | - |
| CYP2D6 substrate | - | - | - | - | - | - | - |
| CYP3A4 inhibition | - | - | - | - | - | - | - |
| CYP3A4 substrate | + | + | + | + | + | - | - |
| Human either-a-go-go inhibition | - | + | + | + | - | - | - |
| OCT2 inhibitor | + | + | - | + | - | + | - |
| Human Intestinal Absorption | + | + | + | + | + | + | - |
| Human oral bioavailability | + | + | - | + | - | - | + |
| P-glycoprotein inhibitor | + | - | + | - | - | - | - |
| P-glycoprotein substrate | + | + | - | + | - | - | - |
| Subcellular localization | Mitochondria | Mitochondria | Mitochondria | Mitochondria | Mitochondria | Mitochondria | Nucleus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, H.I.; Ajayi, A.T.; Mukerjee, N.; Aborode, A.T.; Hasan, M.M.; Maitra, S.; Bello, R.O.; Alabere, H.O.; Sanusi, A.A.; Awolaja, O.O.; et al. Discovery of Novel HSP27 Inhibitors as Prospective Anti-Cancer Agents Utilizing Computer-Assisted Therapeutic Discovery Approaches. Cells 2022, 11, 2412. https://doi.org/10.3390/cells11152412
Umar HI, Ajayi AT, Mukerjee N, Aborode AT, Hasan MM, Maitra S, Bello RO, Alabere HO, Sanusi AA, Awolaja OO, et al. Discovery of Novel HSP27 Inhibitors as Prospective Anti-Cancer Agents Utilizing Computer-Assisted Therapeutic Discovery Approaches. Cells. 2022; 11(15):2412. https://doi.org/10.3390/cells11152412
Chicago/Turabian StyleUmar, Haruna Isiyaku, Adeola Temitayo Ajayi, Nobendu Mukerjee, Abdullahi Tunde Aborode, Mohammad Mehedi Hasan, Swastika Maitra, Ridwan O. Bello, Hafsat O. Alabere, Afees A. Sanusi, Olamide O. Awolaja, and et al. 2022. "Discovery of Novel HSP27 Inhibitors as Prospective Anti-Cancer Agents Utilizing Computer-Assisted Therapeutic Discovery Approaches" Cells 11, no. 15: 2412. https://doi.org/10.3390/cells11152412
APA StyleUmar, H. I., Ajayi, A. T., Mukerjee, N., Aborode, A. T., Hasan, M. M., Maitra, S., Bello, R. O., Alabere, H. O., Sanusi, A. A., Awolaja, O. O., Alshehri, M. M., Chukwuemeka, P. O., Aljarba, N. H., Alkahtani, S., Malik, S., Alexiou, A., Ghosh, A., & Rahman, M. H. (2022). Discovery of Novel HSP27 Inhibitors as Prospective Anti-Cancer Agents Utilizing Computer-Assisted Therapeutic Discovery Approaches. Cells, 11(15), 2412. https://doi.org/10.3390/cells11152412








