Fibrotic Scar in CNS Injuries: From the Cellular Origins of Fibroblasts to the Molecular Processes of Fibrotic Scar Formation
Abstract
1. Introduction
2. Fibrotic Scar Components
3. Timing of Fibrotic Scar Formation in the CNS
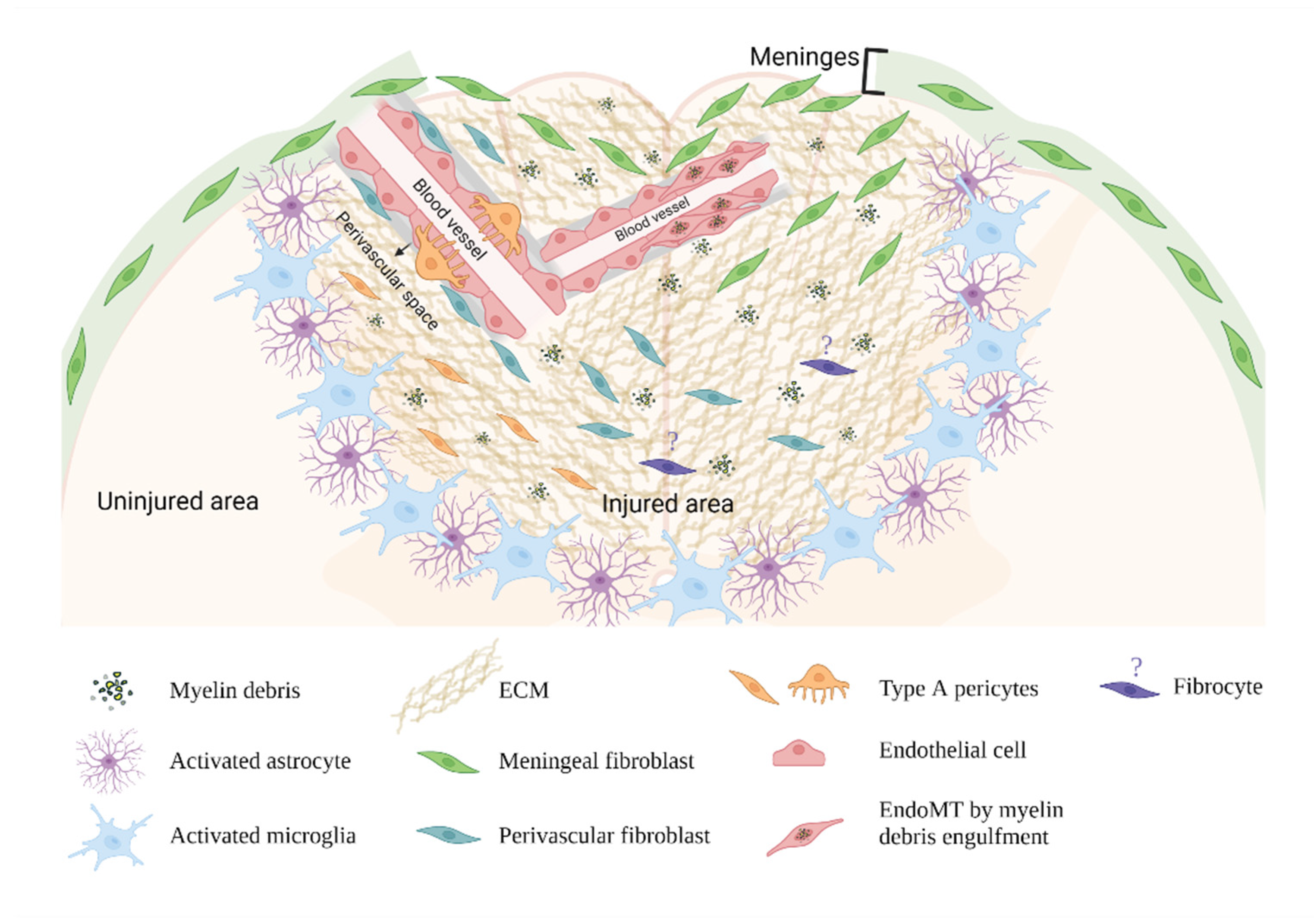
4. The Cellular Origins of Fibroblasts in the Fibrotic Scar
4.1. Meningeal Fibroblasts
4.2. Perivascular Fibroblasts
4.3. Pericytes
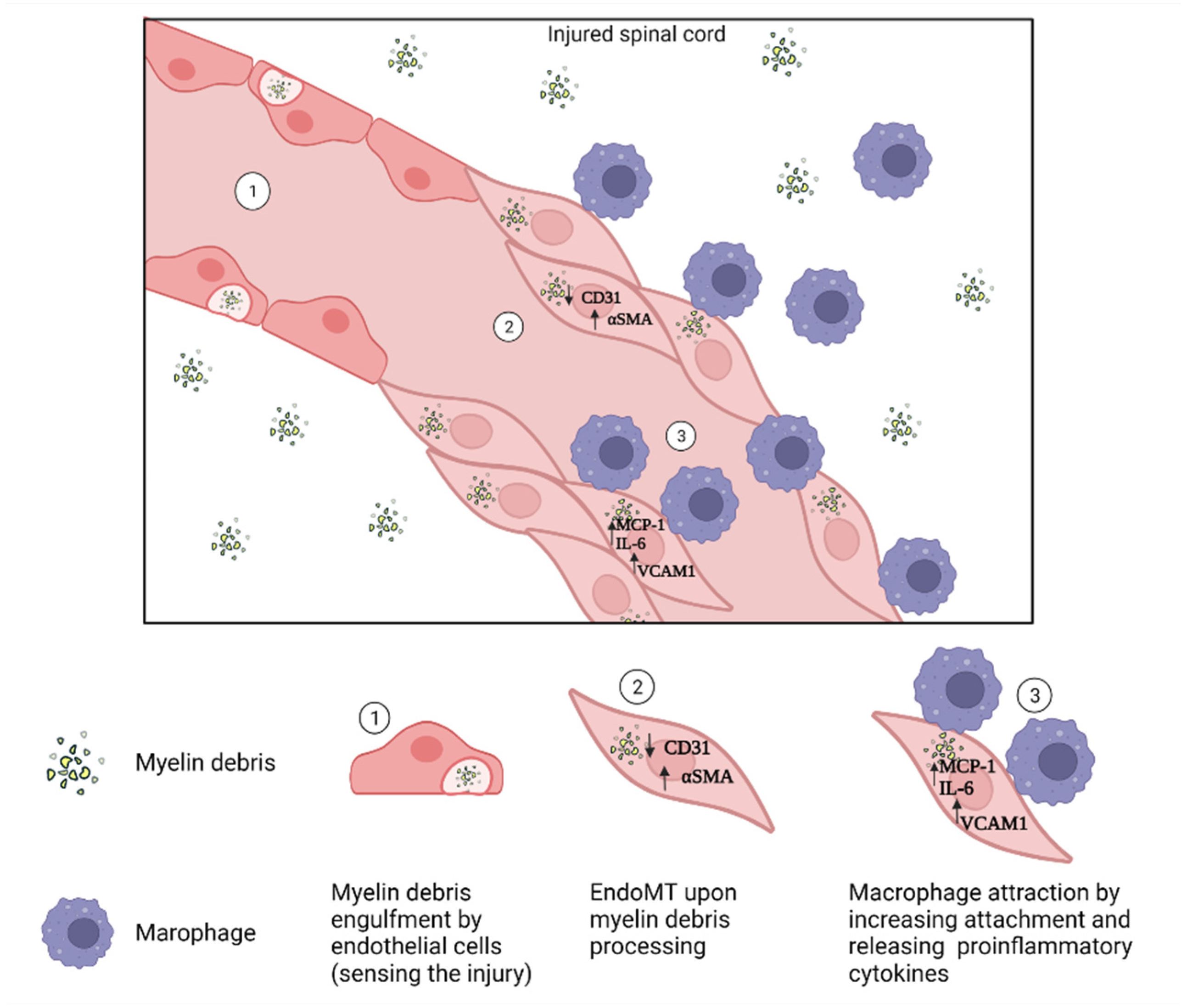
4.4. Endothelial Cells
4.5. Circulating Blood Fibrocytes
4.6. Approaches for Studying the Origins of Fibroblasts in the Fibrotic Scar
5. The Mechanism of Fibrotic Scar Formation
5.1. Fibroblast Activation by Profibrotic Cytokines
5.1.1. TGF-β
5.1.2. PDGFs
5.2. Direct Activation of Fibroblasts
5.3. Mechanical Activation (Self-Activation)
6. Potential Role of Fibroblasts in Neuroinflammation
7. Fibrotic Scar in CNS Tissue Remodeling and Regeneration
8. Therapeutic Targets for Fibrotic Scar
8.1. Targeting Macrophages
8.2. Targeting Fibroblasts and Fibroblast-like Cells
8.3. Targeting Profibrotic Cytokines
8.4. Targeting Collagen
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122 Pt 18, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Gyorfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Konigshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Roll, F.J.; Boyles, J.; Bissell, D.M. Hepatic lipocytes: The principal collagen-producing cells of normal rat liver. Proc. Natl. Acad. Sci. USA 1985, 82, 8681–8685. [Google Scholar] [CrossRef]
- Ma, P.F.; Gao, C.C.; Yi, J.; Zhao, J.L.; Liang, S.Q.; Zhao, Y.; Ye, Y.C.; Bai, J.; Zheng, Q.J.; Dou, K.F.; et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J. Hepatol 2017, 67, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; van Baarlen, J.; Storm, G.; Prakash, J. The interplay of the Notch signaling in hepatic stellate cells and macrophages determines the fate of liver fibrogenesis. Sci. Rep. 2015, 5, 18272. [Google Scholar] [CrossRef]
- Akcora, B.O.; Dathathri, E.; Ortiz-Perez, A.; Gabriel, A.V.; Storm, G.; Prakash, J.; Bansal, R. TG101348, a selective JAK2 antagonist, ameliorates hepatic fibrogenesis in vivo. FASEB J. 2019, 33, 9466–9475. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef]
- Cho, N.; Razipour, S.E.; McCain, M.L. Featured Article: TGF-beta1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts. Exp. Biol. Med. 2018, 243, 601–612. [Google Scholar] [CrossRef]
- Saxena, A.; Bujak, M.; Frunza, O.; Dobaczewski, M.; Gonzalez-Quesada, C.; Lu, B.; Gerard, C.; Frangogiannis, N.G. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res. 2014, 103, 217–227. [Google Scholar] [CrossRef]
- Hu, S.; Yang, M.; Huang, S.; Zhong, S.; Zhang, Q.; Ding, H.; Xiong, X.; Hu, Z.; Yang, Y. Different Roles of Resident and Non-resident Macrophages in Cardiac Fibrosis. Front. Cardiovasc Med. 2022, 9, 818188. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Geng, M.; Huang, Y.; Han, G.; Jing, R.; Lin, C.; Zhang, X.; Zhang, M.; Fan, G.; Wang, F.; et al. Upregulation of Wilms’ Tumor 1 in epicardial cells increases cardiac fibrosis in dystrophic mice. Cell Death Differ. 2022. [Google Scholar] [CrossRef] [PubMed]
- Falke, L.L.; Gholizadeh, S.; Goldschmeding, R.; Kok, R.J.; Nguyen, T.Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol 2015, 11, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.C.; Huang, X.R.; Meng, X.; Lan, H.Y. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J. Am. Soc. Nephrol 2010, 21, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xia, Y.; Li, F.; Tang, Y.; Nie, J.; Liu, Y.; Zhou, Z.; Zhang, H.; Hou, F.F. Kindlin-2 mediates activation of TGF-beta/Smad signaling and renal fibrosis. J. Am. Soc. Nephrol. 2013, 24, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Gwon, M.G.; An, H.J.; Kim, J.Y.; Kim, W.H.; Gu, H.; Kim, H.J.; Leem, J.; Jung, H.J.; Park, K.K. Anti-fibrotic effects of synthetic TGF-beta1 and Smad oligodeoxynucleotide on kidney fibrosis in vivo and in vitro through inhibition of both epithelial dedifferentiation and endothelial-mesenchymal transitions. FASEB J. 2020, 34, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.L.; Glass Ii, W.F. Renal interstitial fibrosis: A critical evaluation of the origin of myofibroblasts. Contrib Nephrol. 2011, 169, 73–93. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef]
- Lehmann, M.; Buhl, L.; Alsafadi, H.N.; Klee, S.; Hermann, S.; Mutze, K.; Ota, C.; Lindner, M.; Behr, J.; Hilgendorff, A.; et al. Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir Res. 2018, 19, 175. [Google Scholar] [CrossRef]
- Kurosaki, T.; Kanda, H.; Hashizume, J.; Sato, K.; Harasawa, H.; Nakamura, T.; Sasaki, H.; Kodama, Y. Delivery of pDNA to the Lung by Lipopolyplexes Using N-Lauroylsarcosine and Effect on the Pulmonary Fibrosis. Pharmaceutics 2021, 13, 1983. [Google Scholar] [CrossRef]
- Roach, K.M.; Castells, E.; Dixon, K.; Mason, S.; Elliott, G.; Marshall, H.; Poblocka, M.A.; Macip, S.; Richardson, M.; Khalfaoui, L.; et al. Evaluation of Pirfenidone and Nintedanib in a Human Lung Model of Fibrogenesis. Front. Pharmacol. 2021, 12, 679388. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Primers 2015, 1, 15002. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Takagi, K.; Hara, M.; Fukasawa, C.; Sugiura, T.; Nishimagi, E.; Harigai, M.; Kamatani, N. Angiotensin II in the lesional skin of systemic sclerosis patients contributes to tissue fibrosis via angiotensin II type 1 receptors. Arthritis Rheum. 2004, 50, 216–226. [Google Scholar] [CrossRef]
- Hasegawa, M.; Matsushita, Y.; Horikawa, M.; Higashi, K.; Tomigahara, Y.; Kaneko, H.; Shirasaki, F.; Fujimoto, M.; Takehara, K.; Sato, S. A novel inhibitor of Smad-dependent transcriptional activation suppresses tissue fibrosis in mouse models of systemic sclerosis. Arthritis Rheum 2009, 60, 3465–3475. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Beckett, J.D.; Bagirzadeh, R.; Creamer, T.J.; Shah, A.A.; McMahan, Z.; Paik, J.J.; Sampedro, M.M.; MacFarlane, E.G.; Beer, M.A.; et al. Epigenetic activation and memory at a TGFB2 enhancer in systemic sclerosis. Sci. Transl. Med. 2019, 11, eaaw0790. [Google Scholar] [CrossRef]
- Kawano, H.; Kimura-Kuroda, J.; Komuta, Y.; Yoshioka, N.; Li, H.P.; Kawamura, K.; Li, Y.; Raisman, G. Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res. 2012, 349, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Wanner, I.B.; Anderson, M.A.; Song, B.; Levine, J.; Fernandez, A.; Gray-Thompson, Z.; Ao, Y.; Sofroniew, M.V. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 2013, 33, 12870–12886. [Google Scholar] [CrossRef] [PubMed]
- Levine, J. The reactions and role of NG2 glia in spinal cord injury. Brain Res. 2016, 1638, 199–208. [Google Scholar] [CrossRef]
- Hackett, A.R.; Lee, D.H.; Dawood, A.; Rodriguez, M.; Funk, L.; Tsoulfas, P.; Lee, J.K. STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiol. Dis. 2016, 89, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Hesp, Z.C.; Yoseph, R.Y.; Suzuki, R.; Jukkola, P.; Wilson, C.; Nishiyama, A.; McTigue, D.M. Proliferating NG2-Cell-Dependent Angiogenesis and Scar Formation Alter Axon Growth and Functional Recovery After Spinal Cord Injury in Mice. J. Neurosci. 2018, 38, 1366–1382. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J. Neurosci. 1994, 14, 4716–4730. [Google Scholar] [CrossRef] [PubMed]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallieres, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.E.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; He, T.; Wen, R.; Li, Y.; Chen, T.; Huang, S.; Wang, Y.; Tang, Y.; Shen, F.; et al. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics 2021, 11, 1232–1248. [Google Scholar] [CrossRef] [PubMed]
- Milich, L.M.; Ryan, C.B.; Lee, J.K. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 2019, 137, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Soderblom, C.; Luo, X.; Blumenthal, E.; Bray, E.; Lyapichev, K.; Ramos, J.; Krishnan, V.; Lai-Hsu, C.; Park, K.K.; Tsoulfas, P.; et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 2013, 33, 13882–13887. [Google Scholar] [CrossRef] [PubMed]
- Bundesen, L.Q.; Scheel, T.A.; Bregman, B.S.; Kromer, L.F. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J. Neurosci. 2003, 23, 7789–7800. [Google Scholar] [CrossRef] [PubMed]
- Fehlberg, C.R.; Lee, J.K. Fibrosis in the central nervous system: From the meninges to the vasculature. Cell Tissue Res. 2022, 387, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dorrier, C.E.; Jones, H.E.; Pintaric, L.; Siegenthaler, J.A.; Daneman, R. Emerging roles for CNS fibroblasts in health, injury and disease. Nat. Rev. Neurosci. 2022, 23, 23–34. [Google Scholar] [CrossRef]
- Manrique-Castano, D.; ElAli, A. Neurovascular Reactivity in Tissue Scarring Following Cerebral Ischemia. In Cerebral Ischemia; Pluta, R., Ed.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.; Menounos, S.; Choi, J.P.; Hansbro, P.M.; Diwan, A.D.; Das, A. Blood-Spinal Cord Barrier: Its Role in Spinal Disorders and Emerging Therapeutic Strategies. NeuroSci 2022, 3, 1–27. [Google Scholar] [CrossRef]
- Goritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisen, J. A pericyte origin of spinal cord scar tissue. Science 2011, 333, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Soderblom, C.; Krishnan, V.; Ashbaugh, J.; Bethea, J.R.; Lee, J.K. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol. Dis. 2015, 74, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.O.; Kalkitsas, J.; Kelahmetoglu, Y.; Estrada, C.P.; Tatarishvili, J.; Holl, D.; Jansson, L.; Banitalebi, S.; Amiry-Moghaddam, M.; Ernst, A.; et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 2021, 12, 5501. [Google Scholar] [CrossRef] [PubMed]
- Dorrier, C.E.; Aran, D.; Haenelt, E.A.; Sheehy, R.N.; Hoi, K.K.; Pintaric, L.; Chen, Y.; Lizama, C.O.; Cautivo, K.M.; Weiner, G.A.; et al. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 2021, 24, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Miyamoto, O.; Shibuya, S.; Zhang, X.; Yamamoto, T.; Itano, T. Expression and role of type I collagen in a rat spinal cord contusion injury model. Neurosci. Res. 2007, 58, 371–377. [Google Scholar] [CrossRef]
- Klapka, N.; Muller, H.W. Collagen matrix in spinal cord injury. J. Neurotrauma 2006, 23, 422–435. [Google Scholar] [CrossRef]
- Hermanns, S.; Klapka, N.; Muller, H.W. The collagenous lesion scar--an obstacle for axonal regeneration in brain and spinal cord injury. Restor Neurol. Neurosci. 2001, 19, 139–148. [Google Scholar] [PubMed]
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017, 23, 818–828. [Google Scholar] [CrossRef]
- Cooper, J.G.; Jeong, S.J.; McGuire, T.L.; Sharma, S.; Wang, W.; Bhattacharyya, S.; Varga, J.; Kessler, J.A. Fibronectin EDA forms the chronic fibrotic scar after contusive spinal cord injury. Neurobiol. Dis. 2018, 116, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Camand, E.; Morel, M.P.; Faissner, A.; Sotelo, C.; Dusart, I. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur. J. Neurosci. 2004, 20, 1161–1176. [Google Scholar] [CrossRef]
- Tate, C.C.; Tate, M.C.; LaPlaca, M.C. Fibronectin and laminin increase in the mouse brain after controlled cortical impact injury. J. Neurotrauma 2007, 24, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, X.; Yang, J.; Kress, B.T.; Tong, J.; Liu, H.; Takano, T.; Zhao, Y.; Nedergaard, M. Improved axonal regeneration after spinal cord injury in mice with conditional deletion of ephrin B2 under the GFAP promoter. Neuroscience 2013, 241, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Davies, J.E.; Davies, S.J. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J. Neurosci. Res. 2003, 71, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, R.J.; Giger, R.J.; Ruitenberg, M.J.; Holtmaat, A.J.; De Wit, J.; De Winter, F.; Verhaagen, J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol. Cell Neurosci. 1999, 13, 143–166. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Zhu, Y.; Soderblom, C.; Trojanowsky, M.; Lee, D.H.; Lee, J.K. Fibronectin Matrix Assembly after Spinal Cord Injury. J. Neurotrauma 2015, 32, 1158–1167. [Google Scholar] [CrossRef]
- Komuta, Y.; Teng, X.; Yanagisawa, H.; Sango, K.; Kawamura, K.; Kawano, H. Expression of Transforming Growth Factor-Beta Receptors in Meningeal Fibroblasts of the Injured Mouse Brain. Cell Mol. Neurobiol. 2010, 30, 101–111. [Google Scholar] [CrossRef]
- Wang, X.; Cao, K.; Sun, X.; Chen, Y.; Duan, Z.; Sun, L.; Guo, L.; Bai, P.; Sun, D.; Fan, J.; et al. Macrophages in spinal cord injury: Phenotypic and functional change from exposure to myelin debris. Glia 2015, 63, 635–651. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.K.; MacPherson, A.M.; Grewal, H.; Strnad, F.; Jones, J.W.; Yu, J.; Pierzchalski, K.; Kane, M.A.; Herson, P.S.; Siegenthaler, J.A. Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke. BMC Neurosci. 2016, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Vanlandewijck, M.; He, L.; Mae, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.L.; Pease, D.C. Cicatrix formation in rat cerebral cortex as revealed by electron microscopy. Am. J. Pathol. 1959, 35, 1017–1041. [Google Scholar]
- Carbonell, A.L.; Boya, J. Ultrastructural study on meningeal regeneration and meningo-glial relationships after cerebral stab wound in the adult rat. Brain Res. 1988, 439, 337–344. [Google Scholar] [CrossRef]
- Shearer, M.C.; Fawcett, J.W. The astrocyte/meningeal cell interface—A barrier to successful nerve regeneration? Cell Tissue Res. 2001, 305, 267–273. [Google Scholar] [CrossRef]
- Fernandez, E.; Pallini, R. Connective tissue scarring in experimental spinal cord lesions: Significance of dural continuity and role of epidural tissues. Acta Neurochir. 1985, 76, 145–148. [Google Scholar] [CrossRef]
- Yahn, S.L.; Li, J.; Goo, I.; Gao, H.; Brambilla, R.; Lee, J.K. Fibrotic scar after experimental autoimmune encephalomyelitis inhibits oligodendrocyte differentiation. Neurobiol. Dis. 2020, 134, 104674. [Google Scholar] [CrossRef]
- Bonney, S.K.; Sullivan, L.T.; Cherry, T.J.; Daneman, R.; Shih, A.Y. Distinct features of brain perivascular fibroblasts and mural cells revealed by in vivo two-photon imaging. bioRxiv 2021. [Google Scholar] [CrossRef]
- DeSisto, J.; O’Rourke, R.; Jones, H.E.; Pawlikowski, B.; Malek, A.D.; Bonney, S.; Guimiot, F.; Jones, K.L.; Siegenthaler, J.A. Single-Cell Transcriptomic Analyses of the Developing Meninges Reveal Meningeal Fibroblast Diversity and Function. Dev. Cell 2020, 54, 43–59.e44. [Google Scholar] [CrossRef]
- Riew, T.R.; Choi, J.H.; Kim, H.L.; Jin, X.; Lee, M.Y. PDGFR-beta-Positive Perivascular Adventitial Cells Expressing Nestin Contribute to Fibrotic Scar Formation in the Striatum of 3-NP Intoxicated Rats. Front. Mol. Neurosci. 2018, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.J.; Sun, N.; Lee, H.; Godlewski, B.; Mathys, H.; Galani, K.; Zhou, B.; Jiang, X.; Ng, A.P.; Mantero, J.; et al. Single-cell dissection of the human brain vasculature. Nature 2022, 603, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef]
- Zehendner, C.M.; Sebastiani, A.; Hugonnet, A.; Bischoff, F.; Luhmann, H.J.; Thal, S.C. Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex. Sci. Rep. 2015, 5, 13497. [Google Scholar] [CrossRef]
- Klement, W.; Blaquiere, M.; Zub, E.; de Bock, F.; Boux, F.; Barbier, E.; Audinat, E.; Lerner-Natoli, M.; Marchi, N. A pericyte-glia scarring develops at the leaky capillaries in the hippocampus during seizure activity. Epilepsia 2019, 60, 1399–1411. [Google Scholar] [CrossRef]
- Fernández-Klett, F.; Potas, J.R.; Hilpert, D.; Blazej, K.; Radke, J.; Huck, J.; Engel, O.; Stenzel, W.; Genové, G.; Priller, J. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb Blood Flow Metab. 2013, 33, 428–439. [Google Scholar] [CrossRef]
- Sundberg, C.; Ivarsson, M.; Gerdin, B.; Rubin, K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab. Investig. 1996, 74, 452–466. [Google Scholar] [PubMed]
- Lin, S.L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008, 173, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Lin, S.L.; Kobayashi, A.; Hudson, T.E.; Nowlin, B.T.; Bonventre, J.V.; Valerius, M.T.; McMahon, A.P.; Duffield, J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 2010, 176, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chang, F.C.; Wu, C.F.; Chou, Y.H.; Hsu, H.L.; Chiang, W.C.; Shen, J.; Chen, Y.M.; Wu, K.D.; Tsai, T.J.; et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011, 80, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Mintz, A.; Delbono, O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am. J. Physiol. Cell Physiol. 2013, 305, C1098–C1113. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef]
- Preston, A.N.; Cervasio, D.A.; Laughlin, S.T. Visualizing the brain’s astrocytes. Methods Enzymol. 2019, 622, 129–151. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, M.; Ying, Y.; Wu, Q.; Huang, Z.; Ni, W.; Wang, X.; Xu, H.; Bennett, S.; Xiao, J.; et al. Versatile subtypes of pericytes and their roles in spinal cord injury repair, bone development and repair. Bone Res. 2022, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, E.; Sutton, A.B.; Allen, T.D.; Schor, A.M. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J. Cell Sci. 1992, 103 Pt 2, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; van Zonneveld, A.J.; ten Dijke, P. Transforming growth factor beta-induced endothelial-to-mesenchymal transition: A switch to cardiac fibrosis? Trends Cardiovasc Med. 2008, 18, 293–298. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Li, Z.; Jimenez, S.A. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am. J. Pathol. 2011, 179, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Souilhol, C.; Harmsen, M.C.; Evans, P.C.; Krenning, G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc Res. 2018, 114, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Frid, M.G.; Kale, V.A.; Stenmark, K.R. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: In vitro analysis. Circ. Res. 2002, 90, 1189–1196. [Google Scholar] [CrossRef]
- Krenning, G.; Moonen, J.A.J.; van Luyn, M.J.A.; Harmsen, M.C. Vascular smooth muscle cells for use in vascular tissue engineering obtained by endothelial-to-mesenchymal transdifferentiation (EnMT) on collagen matrices. Biomaterials 2008, 29, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Li, Y.; Zhang, H.; Li, Z.; Zhang, Y.; Zhang, L.; Ju, J.; Liu, X.; Chen, X.; et al. Endothelial to mesenchymal transition contributes to arsenic-trioxide-induced cardiac fibrosis. Sci. Rep. 2016, 6, 33787. [Google Scholar] [CrossRef] [PubMed]
- Moonen, J.R.; Krenning, G.; Brinker, M.G.; Koerts, J.A.; van Luyn, M.J.; Harmsen, M.C. Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc Res. 2010, 86, 506–515. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Horie, M.; Mihira, H.; Saito, A. Molecular Analysis of Endothelial-mesenchymal Transition Induced by Transforming Growth Factor-beta Signaling. J. Vis. Exp. 2018, 138, 57577. [Google Scholar] [CrossRef]
- Potenta, S.; Zeisberg, E.; Kalluri, R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer 2008, 99, 1375–1379. [Google Scholar] [CrossRef]
- Man, S.; Sanchez Duffhues, G.; Ten Dijke, P.; Baker, D. The therapeutic potential of targeting the endothelial-to-mesenchymal transition. Angiogenesis 2019, 22, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Watabe, T. Emerging roles of inflammation-mediated endothelial-mesenchymal transition in health and disease. Inflamm Regen 2022, 42, 9. [Google Scholar] [CrossRef]
- Logan, A.; Berry, M.; Gonzalez, A.M.; Frautschy, S.A.; Sporn, M.B.; Baird, A. Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur. J. Neurosci. 1994, 6, 355–363. [Google Scholar] [CrossRef]
- Yoshioka, N.; Kimura-Kuroda, J.; Saito, T.; Kawamura, K.; Hisanaga, S.; Kawano, H. Small molecule inhibitor of type I transforming growth factor-beta receptor kinase ameliorates the inhibitory milieu in injured brain and promotes regeneration of nigrostriatal dopaminergic axons. J. Neurosci Res. 2011, 89, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Potenta, S.E.; Sugimoto, H.; Zeisberg, M.; Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2008, 19, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bertram, J.F. Review: Endothelial-myofibroblast transition, a new player in diabetic renal fibrosis. Nephrology 2010, 15, 507–512. [Google Scholar] [CrossRef]
- Rieder, F.; Kessler, S.P.; West, G.A.; Bhilocha, S.; de la Motte, C.; Sadler, T.M.; Gopalan, B.; Stylianou, E.; Fiocchi, C. Inflammation-induced endothelial-to-mesenchymal transition: A novel mechanism of intestinal fibrosis. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zheng, Y.; Sun, L.; Badea, S.R.; Jin, Y.; Liu, Y.; Rolfe, A.J.; Sun, H.; Wang, X.; Cheng, Z.; et al. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nat. Neurosci. 2019, 22, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Munji, R.N.; Soung, A.L.; Weiner, G.A.; Sohet, F.; Semple, B.D.; Trivedi, A.; Gimlin, K.; Kotoda, M.; Korai, M.; Aydin, S.; et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain barrier dysfunction module. Nat. Neurosci. 2019, 22, 1892–1902. [Google Scholar] [CrossRef]
- Derada Troletti, C.; Fontijn, R.D.; Gowing, E.; Charabati, M.; van Het Hof, B.; Didouh, I.; van der Pol, S.M.A.; Geerts, D.; Prat, A.; van Horssen, J.; et al. Inflammation-induced endothelial to mesenchymal transition promotes brain endothelial cell dysfunction and occurs during multiple sclerosis pathophysiology. Cell Death Dis. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, L.; Wang, Y.; Xu, R.; Peng, S.; Zhou, L.; Deng, Z. Ischemia-reperfusion injury of brain induces endothelial-mesenchymal transition and vascular fibrosis via activating let-7i/TGF-betaR1 double-negative feedback loop. FASEB J. 2020, 34, 7178–7191. [Google Scholar] [CrossRef]
- Bellini, A.; Mattoli, S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab. Investig. 2007, 87, 858–870. [Google Scholar] [CrossRef]
- Bucala, R.; Spiegel, L.A.; Chesney, J.; Hogan, M.; Cerami, A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1994, 1, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Donnelly, S.C.; Peng, T.; Bucala, R.; Metz, C.N. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J. Immunol. 2001, 166, 7556–7562. [Google Scholar] [CrossRef] [PubMed]
- Mori, L.; Bellini, A.; Stacey, M.A.; Schmidt, M.; Mattoli, S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp. Cell Res. 2005, 304, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Medbury, H.J.; Tarran, S.L.; Guiffre, A.K.; Williams, M.M.; Lam, T.H.; Vicaretti, M.; Fletcher, J.P. Monocytes contribute to the atherosclerotic cap by transformation into fibrocytes. Int. Angiol. 2008, 27, 114–123. [Google Scholar] [CrossRef]
- Kisseleva, T.; Uchinami, H.; Feirt, N.; Quintana-Bustamante, O.; Segovia, J.C.; Schwabe, R.F.; Brenner, D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006, 45, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Haudek, S.B.; Xia, Y.; Huebener, P.; Lee, J.M.; Carlson, S.; Crawford, J.R.; Pilling, D.; Gomer, R.H.; Trial, J.; Frangogiannis, N.G.; et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 18284–18289. [Google Scholar] [CrossRef] [PubMed]
- Reese, C.; Lee, R.; Bonner, M.; Perry, B.; Heywood, J.; Silver, R.M.; Tourkina, E.; Visconti, R.P.; Hoffman, S. Fibrocytes in the fibrotic lung: Altered phenotype detected by flow cytometry. Front. Pharmacol. 2014, 5, 141. [Google Scholar] [CrossRef][Green Version]
- Aldrich, A.; Kielian, T. Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am. J. Pathol. 2011, 179, 2952–2962. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Yang, R.; Zhang, Q.; Chen, L.; Huang, D.; Zeng, J.; Yang, C.; Zhang, T. TGF-beta Secretion by M2 Macrophages Induces Glial Scar Formation by Activating Astrocytes In Vitro. J. Mol. Neurosci. 2019, 69, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.A.; Chen, Q.; Kramer, M.S.; Hesson, D.P.; Argentieri, R.L.; Peng, X.; Gulati, M.; Homer, R.J.; Russell, T.; van Rooijen, N.; et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int. J. Biochem. Cell Biol. 2011, 43, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Agudelo, J.S.; Camara, N.O. Macrophages During the Fibrotic Process: M2 as Friend and Foe. Front. Immunol 2015, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Glim, J.E.; Niessen, F.B.; Everts, V.; van Egmond, M.; Beelen, R.H. Platelet derived growth factor-CC secreted by M2 macrophages induces alpha-smooth muscle actin expression by dermal and gingival fibroblasts. Immunobiology 2013, 218, 924–929. [Google Scholar] [CrossRef]
- Ignotz, R.A.; Massague, J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar] [CrossRef]
- Vaughan, M.B.; Howard, E.W.; Tomasek, J.J. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp. Cell Res. 2000, 257, 180–189. [Google Scholar] [CrossRef]
- Kovacs, E.J.; DiPietro, L.A. Fibrogenic cytokines and connective tissue production. FASEB J. 1994, 8, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Li, N.; Teng, W.; Wang, M.; Zhang, Y.; Xiao, Z. TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci. Rep. 2016, 6, 32231. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Klempt, N.D.; Sirimanne, E.; Gunn, A.J.; Klempt, M.; Singh, K.; Williams, C.; Gluckman, P.D. Hypoxia-ischemia induces transforming growth factor beta 1 mRNA in the infant rat brain. Brain Res. Mol. Brain Res. 1992, 13, 93–101. [Google Scholar] [CrossRef]
- Lindholm, D.; Castren, E.; Kiefer, R.; Zafra, F.; Thoenen, H. Transforming growth factor-beta 1 in the rat brain: Increase after injury and inhibition of astrocyte proliferation. J. Cell Biol. 1992, 117, 395–400. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Liu, W.; Wu, J.; Shao, Y.; Zhang, X. The role of thrombospondin-1 and transforming growth factor-beta after spinal cord injury in the rat. J. Clin. Neurosci. 2009, 16, 818–821. [Google Scholar] [CrossRef]
- Patel, R.K.; Prasad, N.; Kuwar, R.; Haldar, D.; Abdul-Muneer, P.M. Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury. Brain Behav. Immun. 2017, 64, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Buss, A.; Pech, K.; Kakulas, B.A.; Martin, D.; Schoenen, J.; Noth, J.; Brook, G.A. TGF-beta1 and TGF-beta2 expression after traumatic human spinal cord injury. Spinal Cord 2008, 46, 364–371. [Google Scholar] [CrossRef]
- Logan, A.; Green, J.; Hunter, A.; Jackson, R.; Berry, M. Inhibition of glial scarring in the injured rat brain by a recombinant human monoclonal antibody to transforming growth factor-beta2. Eur. J. Neurosci. 1999, 11, 2367–2374. [Google Scholar] [CrossRef]
- Lehrmann, E.; Kiefer, R.; Christensen, T.; Toyka, K.V.; Zimmer, J.; Diemer, N.H.; Hartung, H.P.; Finsen, B. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia 1998, 24, 437–448. [Google Scholar] [CrossRef]
- Kiefer, R.; Schweitzer, T.; Jung, S.; Toyka, K.V.; Hartung, H.P. Sequential expression of transforming growth factor-beta1 by T-cells, macrophages, and microglia in rat spinal cord during autoimmune inflammation. J. Neuropathol. Exp. Neurol. 1998, 57, 385–395. [Google Scholar] [CrossRef] [PubMed]
- De Groot, C.J.; Montagne, L.; Barten, A.D.; Sminia, P.; Van Der Valk, P. Expression of transforming growth factor (TGF)-beta1, -beta2, and -beta3 isoforms and TGF-beta type I and type II receptors in multiple sclerosis lesions and human adult astrocyte cultures. J. Neuropathol. Exp. Neurol. 1999, 58, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Yamagishi, T.; Hokari, S.; Nakamura, H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: Roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat. Rec. 2000, 258, 119–127. [Google Scholar] [CrossRef]
- Diez, M.; Musri, M.M.; Ferrer, E.; Barbera, J.A.; Peinado, V.I. Endothelial progenitor cells undergo an endothelial-to-mesenchymal transition-like process mediated by TGFbetaRI. Cardiovasc Res. 2010, 88, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Bradham, W.S.; Gleaves, L.A.; De Taeye, B.; Murphy, S.B.; Covington, J.W.; Vaughan, D.E. Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: Involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation 2010, 122, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H.; et al. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef]
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.J.; Ten Dijke, P. TGF-beta-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol. 2020, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.L.; Puckett, W.R.; Hiester, E.D.; Quencer, R.M.; Marcillo, A.E.; Post, M.J.; Bunge, R.P. MR-pathologic comparisons of wallerian degeneration in spinal cord injury. AJNR Am. J. Neuroradiol. 1995, 16, 125–133. [Google Scholar]
- Ek, C.J.; Habgood, M.D.; Dennis, R.; Dziegielewska, K.M.; Mallard, C.; Wheaton, B.; Saunders, N.R. Pathological changes in the white matter after spinal contusion injury in the rat. PLoS ONE 2012, 7, e43484. [Google Scholar] [CrossRef]
- Lampron, A.; Larochelle, A.; Laflamme, N.; Prefontaine, P.; Plante, M.M.; Sanchez, M.G.; Yong, V.W.; Stys, P.K.; Tremblay, M.E.; Rivest, S. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J. Exp. Med. 2015, 212, 481–495. [Google Scholar] [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.I.; Roth, G.J.; Hilberg, F.; Muller-Quernheim, J.; Prasse, A.; Zissel, G.; Schnapp, A.; Park, J.E. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur. Respir. J. 2007, 29, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Takamura, N.; Renaud, L.; da Silveira, W.A.; Feghali-Bostwick, C. PDGF Promotes Dermal Fibroblast Activation via a Novel Mechanism Mediated by Signaling Through MCHR1. Front. Immunol. 2021, 12, 745308. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Moon, A.; Kim, H.R. Both platelet-derived growth factor receptor (PDGFR)-alpha and PDGFR-beta promote murine fibroblast cell migration. Biochem Biophys Res. Commun. 2001, 282, 697–700. [Google Scholar] [CrossRef]
- Bonner, J.C.; Badgett, A.; Osornio-Vargas, A.R.; Hoffman, M.; Brody, A.R. PDGF-stimulated fibroblast proliferation is enhanced synergistically by receptor-recognized alpha 2-macroglobulin. J. Cell Physiol. 1990, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, F.; Olinga, P.; Poelstra, K.; Beljaars, L. Targeted Therapies in Liver Fibrosis: Combining the Best Parts of Platelet-Derived Growth Factor BB and Interferon Gamma. Front. Med. 2015, 2, 72. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, M.; Gesualdo, L.; Sabbah, G.M.; Abboud, H.E. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J. Clin. Investig. 1989, 84, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, M.; Milani, S.; Herbst, H.; DeFranco, R.; Grappone, C.; Gentilini, A.; Caligiuri, A.; Pellegrini, G.; Ngo, D.V.; Romanelli, R.G.; et al. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am. J. Pathol. 1996, 148, 785–800. [Google Scholar] [PubMed]
- Beljaars, L.; Weert, B.; Geerts, A.; Meijer, D.K.; Poelstra, K. The preferential homing of a platelet derived growth factor receptor-recognizing macromolecule to fibroblast-like cells in fibrotic tissue. Biochem Pharmacol. 2003, 66, 1307–1317. [Google Scholar] [CrossRef]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef]
- Betsholtz, C. Biology of platelet-derived growth factors in development. Birth Defects Res. C Embryo Today 2003, 69, 272–285. [Google Scholar] [CrossRef]
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Zhang, A.; Wang, S.; Hu, C.; Chen, W.; Shen, Y.; Tan, R.; Sun, Y.; Xu, Q. Targeting the PDGF-B/PDGFR-beta Interface with Destruxin A5 to Selectively Block PDGF-BB/PDGFR-betabeta Signaling and Attenuate Liver Fibrosis. eBioMedicine 2016, 7, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Ma, L.; Fan, B.; Wu, J.; Yang, Z.; Wang, L. Role of PDGFR-beta/PI3K/AKT signaling pathway in PDGF-BB induced myocardial fibrosis in rats. Am. J. Transl Res. 2014, 6, 714–723. [Google Scholar] [PubMed]
- Kishi, M.; Aono, Y.; Sato, S.; Koyama, K.; Azuma, M.; Abe, S.; Kawano, H.; Kishi, J.; Toyoda, Y.; Okazaki, H.; et al. Blockade of platelet-derived growth factor receptor-beta, not receptor-alpha ameliorates bleomycin-induced pulmonary fibrosis in mice. PLoS ONE 2018, 13, e0209786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.K.; Cheng, Y.; Liang Cheng, M.; Yu, L.; Mu, M.; Li, H.; Liu, Y.; Zhang, B.; Yao, Y.; Guo, H.; et al. Focal Adhesion Kinase Regulates Fibroblast Migration via Integrin beta-1 and Plays a Central Role in Fibrosis. Sci. Rep. 2016, 6, 19276. [Google Scholar] [CrossRef]
- Musiime, M.; Chang, J.; Hansen, U.; Kadler, K.E.; Zeltz, C.; Gullberg, D. Collagen Assembly at the Cell Surface: Dogmas Revisited. Cells 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, J.; Jing, Z.; Jin, D. Platelet-derived growth factor (PDGF)-AA: A self-imposed cytokine in the proliferation of human fetal osteoblasts. Cytokine 2000, 12, 1271–1274. [Google Scholar] [CrossRef]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; Van Steeg, H.; Dolle, M.E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Bowen-Pope, D.F.; Raines, E.W. Platelet-derived growth factor: Its potential roles in wound healing, atherosclerosis, neoplasia, and growth and development. Ciba Found. Symp. 1985, 116, 98–112. [Google Scholar] [CrossRef]
- Ivey, M.J.; Kuwabara, J.T.; Riggsbee, K.L.; Tallquist, M.D. Platelet-derived growth factor receptor-alpha is essential for cardiac fibroblast survival. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H330–H344. [Google Scholar] [CrossRef] [PubMed]
- Zymek, P.; Bujak, M.; Chatila, K.; Cieslak, A.; Thakker, G.; Entman, M.L.; Frangogiannis, N.G. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J. Am. Coll Cardiol 2006, 48, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, S.; Liu, Y.; Hu, X.; Li, Y.; Xiao, Z.; Chen, Y.; Tian, D.; Xu, X.; Cheng, L.; et al. SU16f inhibits fibrotic scar formation and facilitates axon regeneration and locomotor function recovery after spinal cord injury by blocking the PDGFRbeta pathway. J. Neuroinflammation 2022, 19, 95. [Google Scholar] [CrossRef] [PubMed]
- Funk, L.H.; Hackett, A.R.; Bunge, M.B.; Lee, J.K. Tumor necrosis factor superfamily member APRIL contributes to fibrotic scar formation after spinal cord injury. J. Neuroinflammation 2016, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lavine, K.J.; Epelman, S.; Evans, S.A.; Weinheimer, C.J.; Barger, P.M.; Mann, D.L. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J. Am. Heart Assoc. 2015, 4, e001993. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Kelley, K.; Melichian, D.S.; Tamaki, Z.; Fang, F.; Su, Y.; Feng, G.; Pope, R.M.; Budinger, G.R.; Mutlu, G.M.; et al. Toll-like receptor 4 signaling augments transforming growth factor-beta responses: A novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am. J. Pathol. 2013, 182, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.; Querejeta, R.; Gonzalez, A.; Larman, M.; Diez, J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: Potential role of lysyl oxidase. Hypertension 2012, 60, 677–683. [Google Scholar] [CrossRef]
- Rahman, N.; O'Neill, E.; Irnaten, M.; Wallace, D.; O'Brien, C. Corneal Stiffness and Collagen Cross-Linking Proteins in Glaucoma: Potential for Novel Therapeutic Strategy. J. Ocul. Pharmacol. Ther. 2020, 36, 582–594. [Google Scholar] [CrossRef]
- Niu, L.; Jia, Y.; Wu, M.; Liu, H.; Feng, Y.; Hu, Y.; Zhang, X.; Gao, D.; Xu, F.; Huang, G. Matrix stiffness controls cardiac fibroblast activation through regulating YAP via AT1 R. J. Cell Physiol. 2020, 235, 8345–8357. [Google Scholar] [CrossRef]
- Herum, K.M.; Choppe, J.; Kumar, A.; Engler, A.J.; McCulloch, A.D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell 2017, 28, 1871–1882. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.G.; Sicard, D.; Sharma, S.; Van Gulden, S.; McGuire, T.L.; Cajiao, M.P.; Tschumperlin, D.J.; Kessler, J.A. Spinal Cord Injury Results in Chronic Mechanical Stiffening. J. Neurotrauma 2020, 37, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Eckart, A.; Haidari, S.; Tirniceriu, A.; Lorenz, M.; von Bruhl, M.L.; Gartner, F.; Khandoga, A.G.; Legate, K.R.; Pless, R.; et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat. Immunol 2013, 14, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bajenoff, M.; Egen, J.G.; Koo, L.Y.; Laugier, J.P.; Brau, F.; Glaichenhaus, N.; Germain, R.N. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006, 25, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Balabanov, R.; Beaumont, T.; Dore-Duffy, P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J. Neurosci. Res. 1999, 55, 578–587. [Google Scholar] [CrossRef]
- Mazur, A.; Holthoff, E.; Vadali, S.; Kelly, T.; Post, S.R. Cleavage of Type I Collagen by Fibroblast Activation Protein-alpha Enhances Class A Scavenger Receptor Mediated Macrophage Adhesion. PLoS ONE 2016, 11, e0150287. [Google Scholar] [CrossRef]
- Verbeek, M.M.; Westphal, J.R.; Ruiter, D.J.; de Waal, R.M. T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. J. Immunol. 1995, 154, 5876–5884. [Google Scholar]
- Shechter, R.; Schwartz, M. CNS sterile injury: Just another wound healing? Trends Mol. Med. 2013, 19, 135–143. [Google Scholar] [CrossRef]
- Dias, D.O.; Goritz, C. Fibrotic scarring following lesions to the central nervous system. Matrix Biol. 2018, 68–69, 561–570. [Google Scholar] [CrossRef]
- Fernandez-Klett, F.; Priller, J. The fibrotic scar in neurological disorders. Brain Pathol. 2014, 24, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.O.; Kim, H.; Holl, D.; Werne Solnestam, B.; Lundeberg, J.; Carlen, M.; Goritz, C.; Frisen, J. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell 2018, 173, 153–165.e122. [Google Scholar] [CrossRef] [PubMed]
- Stichel, C.C.; Muller, H.W. The CNS lesion scar: New vistas on an old regeneration barrier. Cell Tissue Res. 1998, 294, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stichel, C.C.; Hermanns, S.; Luhmann, H.J.; Lausberg, F.; Niermann, H.; D’Urso, D.; Servos, G.; Hartwig, H.G.; Muller, H.W. Inhibition of collagen IV deposition promotes regeneration of injured CNS axons. Eur. J. Neurosci. 1999, 11, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Stichel, C.C.; Niermann, H.; D'Urso, D.; Lausberg, F.; Hermanns, S.; Muller, H.W. Basal membrane-depleted scar in lesioned CNS: Characteristics and relationships with regenerating axons. Neuroscience 1999, 93, 321–333. [Google Scholar] [CrossRef]
- Klapka, N.; Hermanns, S.; Straten, G.; Masanneck, C.; Duis, S.; Hamers, F.P.; Muller, D.; Zuschratter, W.; Muller, H.W. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur. J. Neurosci. 2005, 22, 3047–3058. [Google Scholar] [CrossRef]
- Esmaeili, M.; Berry, M.; Logan, A.; Ahmed, Z. Decorin treatment of spinal cord injury. Neural. Regen Res. 2014, 9, 1653–1656. [Google Scholar] [CrossRef]
- Hellal, F.; Hurtado, A.; Ruschel, J.; Flynn, K.C.; Laskowski, C.J.; Umlauf, M.; Kapitein, L.C.; Strikis, D.; Lemmon, V.; Bixby, J.; et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 2011, 331, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, R.J.; Anderson, P.N.; Verhaagen, J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur. J. Neurosci. 2001, 13, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Moon, L.D.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Iwanami, A.; Nakamura, M.; Kishino, A.; Kikuchi, K.; Shibata, S.; Okano, H.J.; Ikegami, T.; Moriya, A.; Konishi, O.; et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 2006, 12, 1380–1389. [Google Scholar] [CrossRef]
- Moon, L.D.; Asher, R.A.; Rhodes, K.E.; Fawcett, J.W. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat. Neurosci. 2001, 4, 465–466. [Google Scholar] [CrossRef]
- Joosten, E.A.; Dijkstra, S.; Brook, G.A.; Veldman, H.; Bar, P.R. Collagen IV deposits do not prevent regrowing axons from penetrating the lesion site in spinal cord injury. J. Neurosci. Res. 2000, 62, 686–691. [Google Scholar] [CrossRef]
- Risling, M.; Fried, K.; Linda, H.; Carlstedt, T.; Cullheim, S. Regrowth of motor axons following spinal cord lesions: Distribution of laminin and collagen in the CNS scar tissue. Brain Res. Bull. 1993, 30, 405–414. [Google Scholar] [CrossRef]
- Yao, L.; Daly, W.; Newland, B.; Yao, S.; Wang, W.; Chen, B.K.; Madigan, N.; Windebank, A.; Pandit, A. Improved axonal regeneration of transected spinal cord mediated by multichannel collagen conduits functionalized with neurotrophin-3 gene. Gene Ther. 2013, 20, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kanchiku, T.; Imajo, Y.; Yoshida, Y.; Nishida, N.; Gondo, T.; Yoshii, S.; Taguchi, T. Artificial collagen-filament scaffold promotes axon regeneration and long tract reconstruction in a rat model of spinal cord transection. Med. Mol. Morphol. 2015, 48, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Menezes, K.; de Menezes, J.R.; Nascimento, M.A.; Santos Rde, S.; Coelho-Sampaio, T. Polylaminin, a polymeric form of laminin, promotes regeneration after spinal cord injury. FASEB J. 2010, 24, 4513–4522. [Google Scholar] [CrossRef] [PubMed]
- King, V.R.; Hewazy, D.; Alovskaya, A.; Phillips, J.B.; Brown, R.A.; Priestley, J.V. The neuroprotective effects of fibronectin mats and fibronectin peptides following spinal cord injury in the rat. Neuroscience 2010, 168, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.M.; Zhao, L.R.; Westerman, M.; Lovick, D.; Furcht, L.T.; McCarthy, J.B.; Low, W.C. Enhancement of nigral graft survival in rat brain with the systemic administration of synthetic fibronectin peptide V. Neuroscience 2000, 100, 521–530. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Kawaguchi, R.; Zhang, Y.; Wang, Q.; Monavarfeshani, A.; Yang, Z.; Chen, B.; Shi, Z.; Meng, H.; et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 2020, 587, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Cooper, J.G.; Ifergan, I.; McGuire, T.L.; Xu, D.; Hunter, Z.; Sharma, S.; McCarthy, D.; Miller, S.D.; Kessler, J.A. Intravenous immune-modifying nanoparticles as a therapy for spinal cord injury in mice. Neurobiol. Dis. 2017, 108, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Kobayakawa, K.; Saito, T.; Hara, M.; Kijima, K.; Ohkawa, Y.; Harada, A.; Okazaki, K.; Ishihara, K.; Yoshida, S.; et al. Periostin Promotes Scar Formation through the Interaction between Pericytes and Infiltrating Monocytes/Macrophages after Spinal Cord Injury. Am. J. Pathol. 2017, 187, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, C.; Zhang, Y.P.; Shields, L.B.; Han, Y.; Burke, D.A.; Xu, X.M.; Shields, C.B. Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J. Neurotrauma 2006, 23, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Anderson, R.; Pham, T.; Bhatia, N.; Steward, O.; Gupta, R. Role of early surgical decompression of the intradural space after cervical spinal cord injury in an animal model. J. Bone Joint Surg. Am. 2010, 92, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C. Spinal cord injury: Is monitoring from the injury site the future? Crit Care 2016, 20, 308. [Google Scholar] [CrossRef]
- Streijger, F.; Kim, K.T.; So, K.; Manouchehri, N.; Shortt, K.; Okon, E.B.; Morrison, C.; Fong, A.; Gupta, R.; Allard Brown, A.; et al. Duraplasty in Traumatic Thoracic Spinal Cord Injury: Impact on Spinal Cord Hemodynamics, Tissue Metabolism, Histology, and Behavioral Recovery Using a Porcine Model. J. Neurotrauma 2021, 38, 2937–2955. [Google Scholar] [CrossRef] [PubMed]
- Phang, I.; Werndle, M.C.; Saadoun, S.; Varsos, G.; Czosnyka, M.; Zoumprouli, A.; Papadopoulos, M.C. Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure, and vascular pressure reactivity index in patients with traumatic spinal cord injury: Injured spinal cord pressure evaluation study. J. Neurotrauma 2015, 32, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Kohta, M.; Kohmura, E.; Yamashita, T. Inhibition of TGF-beta1 promotes functional recovery after spinal cord injury. Neurosci Res. 2009, 65, 393–401. [Google Scholar] [CrossRef]
- Manaenko, A.; Lekic, T.; Barnhart, M.; Hartman, R.; Zhang, J.H. Inhibition of transforming growth factor-beta attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage. Stroke 2014, 45, 828–834. [Google Scholar] [CrossRef]
- Pan, D.; Yang, F.; Zhu, S.; Li, Y.; Ning, G.; Feng, S. Inhibition of TGF-beta repairs spinal cord injury by attenuating EphrinB2 expressing through inducing miR-484 from fibroblast. Cell Death Discov. 2021, 7, 319. [Google Scholar] [CrossRef]
- Moon, L.D.; Fawcett, J.W. Reduction in CNS scar formation without concomitant increase in axon regeneration following treatment of adult rat brain with a combination of antibodies to TGFbeta1 and beta2. Eur. J. Neurosci. 2001, 14, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Shi, W.; Yi, S.J.; Chen, H.; Groffen, J.; Heisterkamp, N. TGFbeta signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Hales, N.J.; Beattie, J.F. Novel inhibitors of prolyl 4-hydroxylase. 5. The intriguing structure-activity relationships seen with 2,2'-bipyridine and its 5,5'-dicarboxylic acid derivatives. J. Med. Chem. 1993, 36, 3853–3858. [Google Scholar] [CrossRef]
- Duncan, M.R.; Frazier, K.S.; Abramson, S.; Williams, S.; Klapper, H.; Huang, X.; Grotendorst, G.R. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: Down-regulation by cAMP. FASEB J. 1999, 13, 1774–1786. [Google Scholar] [CrossRef]
- Vogelaar, C.F.; Konig, B.; Krafft, S.; Estrada, V.; Brazda, N.; Ziegler, B.; Faissner, A.; Muller, H.W. Pharmacological Suppression of CNS Scarring by Deferoxamine Reduces Lesion Volume and Increases Regeneration in an In Vitro Model for Astroglial-Fibrotic Scarring and in Rat Spinal Cord Injury In Vivo. PLoS ONE 2015, 10, e0134371. [Google Scholar] [CrossRef]
- Kadler, K.E.; Hill, A.; Canty-Laird, E.G. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin Cell Biol. 2008, 20, 495–501. [Google Scholar] [CrossRef]
- Chung, H.J.; Steplewski, A.; Chung, K.Y.; Uitto, J.; Fertala, A. Collagen fibril formation. A new target to limit fibrosis. J. Biol. Chem. 2008, 283, 25879–25886. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.J.; La Marca, F.; Park, P. The role of heat shock proteins in spinal cord injury. Neurosurg. Focus 2008, 25, E4. [Google Scholar] [CrossRef]
- Tasab, M.; Batten, M.R.; Bulleid, N.J. Hsp47: A molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J. 2000, 19, 2204–2211. [Google Scholar] [CrossRef]
- Nagai, N.; Hosokawa, M.; Itohara, S.; Adachi, E.; Matsushita, T.; Hosokawa, N.; Nagata, K. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 2000, 150, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Naitoh, M.; Hosokawa, N.; Kubota, H.; Tanaka, T.; Shirane, H.; Sawada, M.; Nishimura, Y.; Nagata, K. Upregulation of HSP47 and collagen type III in the dermal fibrotic disease, keloid. Biochem. Biophys Res. Commun. 2001, 280, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Sunamoto, M.; Kuze, K.; Tsuji, H.; Ohishi, N.; Yagi, K.; Nagata, K.; Kita, T.; Doi, T. Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress collagen accumulation in experimental glomerulonephritis. Lab. Investig. 1998, 78, 967–972. [Google Scholar] [PubMed]
- Liu, Y.; Liu, J.; Quimbo, A.; Xia, F.; Yao, J.; Clamme, J.P.; Zabludoff, S.; Zhang, J.; Ying, W. Anti-HSP47 siRNA lipid nanoparticle ND-L02-s0201 reverses interstitial pulmonary fibrosis in preclinical rat models. ERJ Open Res. 2021, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, S.; Hu, X.; Li, Y.; You, X.; Tian, D.; Cheng, L.; Zheng, M.; Jing, J. Fibrotic Scar After Spinal Cord Injury: Crosstalk With Other Cells, Cellular Origin, Function, and Mechanism. Front. Cell Neurosci. 2021, 15, 720938. [Google Scholar] [CrossRef] [PubMed]
| Fibrotic Scar Components | Specific Integrant | Reference |
|---|---|---|
| Fibroblast/Fibroblast-like cells | ||
| -- | [36,44,45,46,47] | |
| ECM | Collagen I, IV | [48,49,50,51] |
| Fibronectin | [36,52,53,54] | |
| Laminins | [36,54] | |
| Others | EphB2 | [37,55] |
| Phosphacan | [56] | |
| NG2 | [56] | |
| Tenascin | [56] | |
| Semaphorin III | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayazi, M.; Zivkovic, S.; Hammel, G.; Stefanovic, B.; Ren, Y. Fibrotic Scar in CNS Injuries: From the Cellular Origins of Fibroblasts to the Molecular Processes of Fibrotic Scar Formation. Cells 2022, 11, 2371. https://doi.org/10.3390/cells11152371
Ayazi M, Zivkovic S, Hammel G, Stefanovic B, Ren Y. Fibrotic Scar in CNS Injuries: From the Cellular Origins of Fibroblasts to the Molecular Processes of Fibrotic Scar Formation. Cells. 2022; 11(15):2371. https://doi.org/10.3390/cells11152371
Chicago/Turabian StyleAyazi, Maryam, Sandra Zivkovic, Grace Hammel, Branko Stefanovic, and Yi Ren. 2022. "Fibrotic Scar in CNS Injuries: From the Cellular Origins of Fibroblasts to the Molecular Processes of Fibrotic Scar Formation" Cells 11, no. 15: 2371. https://doi.org/10.3390/cells11152371
APA StyleAyazi, M., Zivkovic, S., Hammel, G., Stefanovic, B., & Ren, Y. (2022). Fibrotic Scar in CNS Injuries: From the Cellular Origins of Fibroblasts to the Molecular Processes of Fibrotic Scar Formation. Cells, 11(15), 2371. https://doi.org/10.3390/cells11152371






