Abstract
The consumption of human milk by a breastfeeding infant is associated with positive health outcomes, including lower risk of diarrheal disease, respiratory disease, otitis media, and in later life, less risk of chronic disease. These benefits may be mediated by antibodies, glycoproteins, glycolipids, oligosaccharides, and leukocytes. More recently, human milk extracellular vesicles (hMEVs) have been identified. HMEVs contain functional cargos, i.e., miRNAs and proteins, that may transmit information from the mother to promote infant growth and development. Maternal health conditions can influence hMEV composition. This review summarizes hMEV biogenesis and functional contents, reviews the functional evidence of hMEVs in the maternal–infant health relationship, and discusses challenges and opportunities in hMEV research.
1. Introduction
Extracellular vesicles (EVs; also known as exosomes) are cell-derived lipid bilayer submicron particles secreted from all types of mammalian cells into extracellular space. The primary function of EVs is to transport cellular components of the parent cells, including proteins, lipids, and nucleic acids, to recipient cells. They may elicit diverse complex biological processes within recipient cells, thereby influencing human physiology and pathology [1,2]. The discovery of vesicular transport machinery that governs vesicle trafficking from one cell and transfers cargos and elicits signaling in a recipient cell was so groundbreaking that it earned James Rothman, Randy Schekman, and Thomas Südhof the 2013 Nobel Prize in Physiology or Medicine [3]. EVs have been investigated to understand cell-to-cell communication and phenomena within the cellular microenvironment in various fields, including cancer biology [4,5], cardiology [6], coagulation [7,8], immunology [9], immunometabolism [10], neurology [11], and stem cell biology [12]. EVs released from specific cells have been studied for therapeutic purposes, including mesenchymal stem cell-derived EVs for regenerative medicine [13] and SARS-CoV-2 infection [14], and red blood cell-derived EVs for a drug delivery system [15]. EV molecular profiling has been investigated in clinically relevant biofluids, e.g., plasma [16], urine [17], cerebrospinal fluid [18], amniotic fluid [19], and saliva [20] as candidate biomarkers of disease diagnosis or prognosis.
Human milk, a complex and dynamic biofluid, contains nutrients that support infant growth as well as bioactive components that protect infants against various diseases [21,22,23,24]. Clinical and epidemiologic studies confirm the beneficial effects of feeding human milk over infant formula in preventing early and long-term diseases, e.g., necrotizing enterocolitis, neonatal sepsis, respiratory and gastrointestinal tract infections, allergic diseases, obesity, diabetes mellitus, and malignancies [21,22,23,24]. Knowledge regarding mechanisms by which human milk components deliver positive health outcomes to children and young adults is growing. The recognized human milk bioactive components include proteins (immunoglobulins, lactoferrin), growth factors, cytokines, adipokines, non-digestible oligosaccharides (2′-fucosyllactose (2′FL), lacto-N-tetraose (LNT), lacto-N-neotetraose (LNnT), sialyllactoses (3SL, 6SL)), leukocytes, and stem cells [25,26,27,28]. In 2007, Admyr et al. [29] reported that human milk contains EVs harboring major histocompatibility complex (MHC) class I/II, which can be immunosuppressive. Human milk extracellular vesicles (hMEVs) are now considered a functional component of human milk, and further elucidation of this biological system could provide a unique opportunity to study maternal-to-child biochemical communication with intergeneration health consequences.
Searching the PubMed database for (“human milk” OR breastmilk) AND (exosomes OR “extracellular vesicle”) yields 100 articles since 2007 with the majority published over the last five years (Figure 1). This increasing appreciation of the potential roles of hMEVs also suggests there are many unknown functions of hMEVs to be explored further. This review summarizes the known components of the hMEV biological system, including cell sources, vesicular biogenesis, subpopulations, and molecular composition. How these components interact with maternal conditions, and their potential biological influence on neonatal and infant growth and health, is of particular interest. Opportunities and challenges of future hMEV research include potential clinical applications of hMEV-based biomarkers to predict maternal–child health outcomes and hMEV-based therapy.
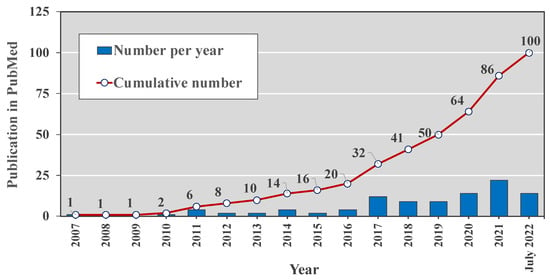
Figure 1.
The number of peer-reviewed publications in the PubMed database during 2007–2022 with search terms (“human milk” OR “breastmilk”) AND (“exosomes” OR “extracellular vesicle”).
2. Biology of hMEVs
2.1. Biogenesis and Subpopulations
Extracellular vesicle (EV) is a generic term covering three vesicle subpopulations: exosomes, microvesicles, and apoptotic bodies. While these EV subpopulations share the same plasma membrane and cytosolic components of the parent cells, they are different in intracellular origin, biogenesis, and release mechanisms, which results in various vesicular sizes and compositions [30,31].
Exosomes (approximately 40–150 nm) originate from the inward budding of endosomal membrane into intraluminal vesicles (ILVs) from which are generated multivesicular bodies (MVBs), which are transported to and fuse with the plasma membrane to be released as exosomes into the extracellular space [32,33] (Figure 2). The generation of multivesicular bodies is mediated by at least two distinct pathways and involves sorting of various molecules into intraluminal vesicles. The first pathway utilizes the Endosomal Sorting Complex Required for Transport (ESCRT). This machinery contains up to 30 proteins which can be divided into four protein complexes: ESCRT-0, -I, -II, -III, and the associated ATPase Vps4 complex [34,35,36,37]. ESCRT-0 recognizes and sorts the ubiquitinated cargo proteins into the lipid domain [38,39]. ESCRT-I and -II invaginate the late endosomal membrane to form buds with sorted cargos [40,41]. ESCRT-III de-ubiquitinates the protein cargo [42,43]. After recruiting the Vps4 complex to fully assemble the ESCRT-III-Vps4 scission machinery, this complex catalyzes vesicle abscission to produce the ILVs that form MVBs [44,45,46]. Vps4 also plays roles in ESCRT disassembly and recycling of subunits for further rounds of vesicle formation [47,48]. The second pathway of MVB formation is ESCRT-independent [32,33,49]. In this mechanism, MVB is generated from raft-based microdomains of endosomal compartments where the neutral sphingomylinase 2 (nSMase2) converts sphingolipids into ceramide [50]. This sphingolipid-to-ceramide conversion induces coalescence of the microdomains into larger structures which then promotes domain-induced budding and formations of ILVs and MVBs [32,49]. Inhibition of nSMase2 has been shown to reduce the release of exosomes in several types of cells, but not all, suggesting that the role of ceramide in exosome release varies among cell types [33,49,50,51,52,53,54].
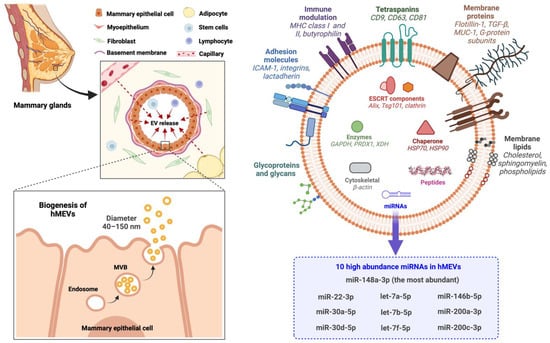
Figure 2.
HMEV biogenesis and compositions. EV, extracellular vesicles; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hMEVs, human milk extracellular vesicles; HSP, heat shock protein; ICAM-1, intercellular adhesion molecule 1; MHC, major histocompatibility complex; miRNAs, microRNAs; MUC-1, mucin-1; MVB, multivesicular bodies; PRDX1, peroxiredoxin 1; TGF-β, tumor necrosis factor-β; Tsg101, tumor susceptibility gene 101; XDH, xanthine dehydrogenase.
Unlike exosomes, microvesicles (usually 100–1000 nm in size) originate from the direct outward blebbing and pinching of the plasma membrane in a constitutive manner or upon stimulation [32,55]. Cytoplasmic protrusions are due to rearrangement of plasma membrane asymmetry induced by the Ca+2-dependent enzymes flippase (aminophospholipid translocase) and floppase (ABC transporter). Flippase translocates phosphatidylserine and other phospholipids from the inner leaflet to the outer leaflet; conversely, floppase translocates the phospholipids from the outer leaflet to the inner leaflet [55,56,57,58,59]. Cargo sorting and microvesicle shedding are tightly regulated by several small GTPases, including ARF6, Rab, Rac1, and RhoA or by modification of the lateral pressure of phospholipids via phosphatidylserine binding protein on the inner leaflet, or sphingomyelin/cholesterol binding proteins on the outer leaflet [55,60,61,62].
Apoptotic bodies, typically 1000–5000 nm, are released from dying apoptotic cells by the outward blebbing of the plasma membrane. This process is regulated by caspase-cleaved substrates such as Rho-associated kinase 1, plexin B2, and pannexin 1, which phosphorylate and activate the myosin light-chain that regulates blebbing [63,64]. Apoptotic bodies are usually packed with DNA fragments, proteins, and parts of cellular organelles. Apoptotic bodies are often underappreciated in the field of EV research. Apoptotic bodies may play roles as messengers from dying cells to regulate various cellular processes, such as cell clearance and homeostasis, pathogen dissemination, and immune responses [64].
The complexity of EVs does not allow them to be fully characterized by any single method; several biophysical and biochemical methods are required. To promote reproducibility of EV studies and enhance ability to interpret findings across studies, the International Society of Extracellular Vesicles has recommended the minimal information needed to define a preparation as extracellular vesicles (MISEV) in 2014 [30], with an update in 2018 [31]. Nanoscale-vesicle morphology and particle size distribution in tandem with enrichment of multiple exosomal biomarkers and depletion of common protein contaminants are required to claim the presence of exosomes in isolates [30,31]. Therefore, consistent with the consensus recommendation of the International Society of Extracellular Vesicles, the term extracellular vesicles or EVs are used instead of exosomes throughout this review with only a few exceptions [30,31]. The intent is to avoid the confusion in previous literature due to labeling several types of EV preparations as exosomes.
2.2. Cell Sources
Current evidence suggests that hMEVs are a mixed population of vesicles released from the local breast tissues and distant organ compartments [29,65,66]. HMEVs are mainly produced and secreted from mammary gland epithelial cells during lactation [29,65], some of which are attached to the surface of human milk fat globules (HMFGs). Cells in human milk, e.g., lymphocytes, macrophages, and stem cells, may also contribute to the EVs presented in human milk; in addition, EVs from other organ systems that transmigrate to milk through the systemic circulation can also contribute to the pool of EVs in human milk [26,27,29,66]. While the relative contribution of local and distant organ systems would define the characteristics and functions of hMEVs, the exact proportion has not been determined due to technical limitations. Advances in single EV technologies in which isolation and characterization of individual single-particle EVs [67,68,69,70] would facilitate the definition of source-specific hMEVs as part of the mixture of EVs in the human milk biological system.
2.3. Molecular Components
HMEVs are composed of proteins, nucleic acids, and lipids derived from plasma membrane and cytoplasm of the cells of origin (Figure 2). However, the functional cargos are not components of parent cells passively incorporated into hMEVs, but rather depend on active sorting mechanisms [71]. By high-throughput analyses, up to 920 proteins [72], 1523 miRNAs [73], and 395 lipids [74,75] have been identified in hMEVs. The expression and amount of hMEV molecular components vary depending on the maternal physiological and pathological states [76,77,78,79,80,81,82]. Nonetheless, some of the functional molecules are highly abundant and frequently identified in hMEV studies. These high abundance proteins and non-coding RNAs (ncRNAs) of hMEVs and their putative functions are summarized in Table 1.

Table 1.
Selected hMEV molecules and their potential functions.
The proteins tetraspanins (e.g., CD9, CD63, CD81), Tsg101, and Alix play crucial roles in the exosome biogenesis and are usually measured as the biomarkers of exosome enrichment during hMEV isolation and characterization. Lactadherin (or Milk Fat Globule-EGF-factor VIII; MFGE8), butyrophilin, and xanthine dehydrogenase/oxidase, major proteins of milk fat globules, are highly abundant in hMEVs and often serve as specific milk EV markers [90,91]. Butyrophilin, together with major histocompatibility complex (MHC) and transforming growth factor-β (TGF-β), may work in concert to support the immunomodulatory properties of hMEVs [29,94,96]. Furthermore, Butyrophilin and TGF-β in hMEVs may be responsible for the induction and differentiation of CD4+CD25+FoxP3+ T regulatory cells from peripheral blood mononuclear cells [29,85,96]. Mucin-1 expressed on hMEVs could bind DC-SIGN on monocyte-derived dendritic cells to inhibit HIV-1 viral transfer to CD4+T cells [100]. Adhesion molecules, i.e., intercellular adhesion molecule-1 (ICAM-1) and integrins, are on the hMEV surface and play roles in vesicular trafficking and recipient cell targeting [83,86]. Lactadherin, a milk glycoprotein that protects against symptomatic rotavirus infection [92], is also expressed on hMEVs [29,90,91]. Lactadherin may be responsible for the anti-rotavirus effect of hMEVs in vitro [132].
MicroRNAs (miRNAs) are small ncRNA molecules (~22 nucleotides) involved in epigenetic regulation and pre-transcriptional gene repression. Most miRNAs in hMEVs are derived from mammalian epithelial cells [66] and play critical roles in epigenetic programming of intestinal homeostasis, immunomodulation, metabolic regulation, and neurodevelopment during the postnatal period [73,105,133,134,135]. Recently, Ting et al. [103] conducted a systematic review on miRNAs in human milk and reported miR-148a-3p, miR-30a/d-5p, let-7a/b/f-5p, miR-22-3p, miR-146b-5p, and miR-200a/c-3p to be the top 10 highly abundant miRNAs in all human milk fractions including hMEVs. Note that miR-148a-3p is the most abundant miRNA in hMEVs, and is involved in multiple cellular processes, including regulating DNMT1-dependent DNA methylation [105,106,107], suppressing tumor growth and metastasis [106,107,108,109,110,111], mitigating NF-κB mediated intestinal inflammation [112], modulating angiogenesis [113,114], and exerting neuroprotective effect [115,116,117,118,119]. Thus, hMEV miRNAs exhibit a wide array of functions in both normal biology and disease, and warrant further study.
The EV membrane contains the proteins discussed above embedded in a lipid bilayer originating from the plasma membrane of parent cells; therefore, the major lipids are cholesterol, sphingomyelin, phospholipids, and ceramides [74]. In addition, lipidomic analysis identified 395 lipids in hMEVs isolated from term and preterm breastmilk [75]. The 50 lipids at greatest concentration are associated with the ERK/MAPK signaling pathway and intestinal cell regulation. Moreover, the molecular species of phosphatidylethanolamine PE(18:1/18:1), phosphatidylcholine PC(18:0/18:2), and PC(18:1/16:0), and phosphatidylserine PS(18:0/18:1) and PS(18:0/22:6) are enriched in hMEVs derived from both preterm and term milk; these species could support neurodevelopment and long-term health outcomes [75].
Human milk oligosaccharides (hMOS), the third most abundant component of human milk, inhibit binding of pathogens to the intestinal mucosa, stimulate growth of mutualist microbes in the gut (prebiotic), and modulate signaling and inflammation in the intestinal mucosa [28]. Using liquid chromatography-mass spectrometry, He et al. discovered that hMOS are also encapsulated by hMEVs from both colostrum and mature milk. For colostrum hMEVs, 2’-fucosyllactose (2’FL), lacto-N-tetraose/neotetraose (LNT/LNnT), lacto-N-difucohexaose (LDFH) were predominant; for mature milk hMEVs, 2’FL, lacto-N-fucopentaose I (LNFP I), and LNT/LNnT predominated [136]. 2’FL exhibits immunomodulatory and anti-infective properties [28,136,137,138,139,140,141]. In adherent invasive E. coli-infected mice, hMEV-encapsulated hMOS could attenuate intestinal inflammation comparable to or perhaps superior to that of free solutions of 2’FL [136]. Such research into the full potential of hMOS as part of hMEV functional cargos is a promising area of research.
A breakthrough study led by Flynn et al. revealed that some non-coding RNAs are modified by N-glycans and expressed on the outer surface of mammalian cells, i.e., cell-surface glycoRNAs [142]. Cell-surface glycoRNAs can interact with sialic-acid-binding immunoglobulin-like lectins (Siglecs) [142,143]. Thus, glycosylation of RNAs could help regulate cellular physiology and innate immunity. As EV plasma membranes originate from the parent cells, glycoRNAs could well be present on the surface of EVs as well, including hMEVs. GlycoRNA biogenesis remains opaque [144], and EV-surface glycoRNAs seems to be a rich research topic for understanding human milk as a biological system.
2.4. Bioavailability and Tissue Distribution
From a physiologic standpoint, a primary issue of hMEVs is their stability upon ingestion and during the gastrointestinal digestion of milk. HMEVs survive under simulated gastric/pancreatic digestion for at least one hour [145,146]. Using simulated gastric/pancreatic digestion in vitro, Liao et al. [146] demonstrated that the SDS-PAGE protein profiles were similar between undigested and digested hMEVs, and analysis by protein array indicates that Alix, ICAM-1, and flotillin-1 were intact in the digested hMEVs. Fluorophore-labeled digested hMEVs could be internalized by human intestinal epithelial crypt-like cells (HIEC) to the same degree as undigested vesicles, with approximately 10% of the internalized hMEVs located at the nucleus [146]. These findings suggest that the lipid bilayer of hMEVs enclose and protect their molecular cargos against degradation within the gastrointestinal tract.
A related physiologic question is the bioavailability and tissue distribution of hMEVs following oral consumption. In vitro, human vascular endothelial cells internalize bovine milk-derived EVs; this is a crucial step for the delivery of dietary EVs and their functional cargos to systemic circulation and peripheral tissues [147]. The rate of EV uptake by human vascular endothelial cells was decreased approximately 50% by removal of bovine milk-derived EV surface proteins by proteinase K or by the presence of D-galactose, a competitor of cell surface carbohydrate binding; these data suggest a significant role of glycoproteins in milk EV transmigration. [147]. In vivo, fluorophore-labeled bovine milk-derived EVs, administered to C57BL/6 mice retro-orbitally, transmigrate across vessels for delivery to tissues, mainly in the liver and spleen, with trace amounts detected in the stomach, intestines, and lungs [147]. Relative to intravenous administration in mice, oral gavage of fluorophore-labeled bovine milk-derived EVs showed an apparent bioavailability of 4% and 6% at 3 h and 24 h, respectively [148]. At 24 h, most intravenously administered bovine milk-derived EVs accumulated in liver, spleen, and brain of Balb/c mice, while tissue distribution of orally administered bovine milk-derived EVs (higher to lower) expanded to liver, spleen, kidneys, heart, lungs, and brain [148]. In addition, the distinct miRNA cargos of the orally administered bovine milk-derived EVs demonstrated their unique tissue distribution patterns [148]. Transfecting synthetic fluorophore-labeled miRNAs into bovine milk-derived EVs followed by oral administration resulted in tissue distribution patterns at 24 h (higher to lower) of miRNA-320a: liver, spleen, and kidneys; and of miR-34a and miR-155-5p: brain and spleen [148]. These data highlight the potential bioavailability and tissue selectivity of bovine milk-derived EVs and their miRNA cargos. Future studies are warranted to elucidate tissue distribution patterns following hMEV administration. Direct investigation of the underlying mechanisms should yield information of high utility in understanding the potential impact of hMEVs on both local and systemic physiology.
2.5. Biological Functions
HMEVs are heterogenous due to different cell sources and altered compositions during maternal health and disease. The net effect on target cells results from concerted actions of the sum of functional hMEV cargos delivered rather than from a single hMEV component [149]. Furthermore, a dose-response relationship will favor activities of highly abundant cargos (as shown in Table 1) relative to low abundance molecular components of hMEVs. Despite the dearth of data on the relationship between whole vesicle bioactivities and each hMEV functional moiety, current understanding supports several strong putative biological impacts: HMEVs promote gut maturation and homeostasis [133,149,150,151,152,153]. HMEVs can attenuate mucosal inflammation and perform other forms of immunomodulation [29,149,154]. HMEVs exhibit antiviral effects against HIV-1, rotavirus, respiratory syncytial virus (RSV), human cytomegalovirus (CMV), Zika virus, and Usutu virus [100,132,155,156]. Reported hMEV functions are summarized in Table 2.

Table 2.
HMEV functions.
Direct effects of hMEVs on neurodevelopment remain to be demonstrated. However, promising associations were observed between miR-148a-3p (the most abundant hMEV-derived miRNA) and neuroprotection in Alzheimer’s Disease [115,116], ischemic stroke [117], and temporal lobe epilepsy [118]. In both term and preterm infants, human milk feeding is associated with improved neurodevelopmental outcomes [157,158]. This has been partially attributed to hMOS, including 2’FL, 3’-sialyllactose (3’SL) and 6’-sialyllactose (6’SL), for their contribution to synaptic formation, neuro-transmission, and memory improvement [159,160,161,162,163,164,165]. HMEV-derived miR-148a-3p may work in concert with hMOS and other human milk components on brain development during infancy.
3. Maternal Conditions Influence hMEV Composition and Child Health Outcomes
Maternal health conditions impact the content of nutrients and bioactive components of human milk [166]. Whereas hMEVs transfer functional molecules from mothers to growing infants, studying alterations of hMEV functional cargos affected by maternal factors could serve as a key to understand intergenerational health consequences [76,77,79,82].
3.1. Maternal Stress
Maternal stress/psychological distress is associated with negative child health outcomes, including poor nutritional status [167], reduced linear growth [168], altered neurodevelopment [169,170], and increased risk of childhood asthma and atopic diseases [171,172]. In a cohort of 80 mothers, Bozack et al. [82] evaluated the association between maternal lifetime stress, including negative life events during pregnancy, and hMEV-derived miRNAs (hMEV-miRs). Among 205 hMEV-miRs, increased expression of six miRNAs, including miR-99b-3p, miR-96-5p, miR-550a-5p, miR-616-5p, miR-155-5p, and miR-604, were significantly associated with the measures of maternal stress [82]. These differentially expressed hMEV-miRs may be involved in epigenetic regulation of fatty acid metabolism, steroid biosynthesis, and the Hippo signaling pathway that regulates organ growth [82]. Dysregulation of the Hippo pathway is associated with metabolic diseases, e.g., obesity, diabetes, fatty liver, and cardiovascular disorders [82,173], and atopic diseases, including asthma [174,175]. Measuring direct biological impacts of maternal stress-induced hMEV-miR changes on early-life programming and long-term health outcomes in breastfed infants could lead to better understanding of one of the mechanisms of the long recognized but poorly understood connection between maternal stress and infant outcome.
3.2. Maternal Overweight/Obesity
Maternal overweight and obesity have impacts on human milk macronutrients and bioactive molecules with the potential to increase the long-term risk of child obesity and impaired neurodevelopment [176,177,178,179,180]. Overweight/obese mothers had lower hMEV-derived miR-148a and miR-30b at 1-month of lactation (30 normal weight vs. 30 overweight/obese). After controlling for gestational age, gender, and birth weight, both miR-148a and miR-30b intake were significantly associated with infant anthropometric measures [79]; for each fold decline in hMEV-derived miR-148a, the infant body weight and fat mass were increased by 0.6 kg and 0.3 kg, respectively. The significance of the relationship between hMEV-derived miR-148a and infant anthropometric measures diminished at 3 to 6 months of lactation [79]. MiR-148a is the precursor of miR-148a-3p, the most abundant miRNA in hMEVs, which has known neuroprotective and neuro-proliferative effects [103,115,116,117,118]. Accordingly, the reduction of hMEV-derived miR-148a in maternal overweight/obesity provides some mechanistic insight into increased risk of childhood obesity and unfavorable neurodevelopmental outcomes in obese mothers.
3.3. Maternal Diabetes
Type I diabetes is an autoimmune disease whose onset is typically in childhood. Children who were breastfed had half the risk of type 1 diabetes as those fed infant formula [181]. Mirza et al. [77] demonstrated that hMEVs of mothers with type 1 diabetes were enriched with immune-modulating miRNAs relative to healthy mothers (n = 26 in each group). Of the 631 identified miRNAs, 9 hMEV-miRs were significantly altered (6 upregulated and 3 downregulated) in mothers with type 1 diabetes [77]. These 9 hMEV-miRs are involved in cell cycle regulation and immune response processes, and included PI3K/AKT mediated proinflammatory cytokine production. Two miRNA mimics of miR-4497 and miR-3178, which are significantly upregulated in hMEVs from type 1 diabetic mothers, enhance the release of TNF-α pro-inflammatory cytokine in vitro from transfected THP1 monocytes [77]. However, hMEV-miRs from mothers with type I diabetes did not increase the risk of type I diabetes or other inflammatory diseases in their offspring.
Gestational diabetes mellitus (GDM) affected the miRNA cargos of hMEVs [78]. In a cohort of 32 GDM and 62 non-GDM, the levels of HMEV-miRs involved with metabolism (e.g., miR-148a, miR-30b, let-7a, and let-7d) were measured in milk along with infant growth and body composition in the first six months of life [78]. Levels of miR-148a, miR-30b, let-7a, and let-7d were lower in GDM human milk. MiR-148a were negatively associated, while levels of miR-30b were positively associated, to infant weight and fat mass at 1 month of age [78]. Thus, gestational diabetes mellitus mothers produced aberrant hMEV-miR levels, which were associated with abnormal metabolic outcomes in their nursing offspring.
3.4. Premature Delivery
Preterm delivery is associated with differences in functional cargos of hMEVs. Mourtzi et al. [80] compared hMEVs and their lncRNA levels in mothers of term (≥37 weeks of gestation) vs. preterm (<37 weeks of gestation) birth (n = 10 each group). Of the 31 hMEV-derived lncRNAs measured, 4 lncRNAs differed significantly in milks of the preterm group; LRRC75A-AS1 was higher, and CTC-444N24.11, CRNDE and LINC00657 (NORAD; non-coding RNA activated at DNA damage) were lower. LRRC75A-AS1 or SNHG29, involved in accelerating cellular senescence and triggering pro-inflammatory cytokine production, was also higher in preterm birth placentas [182]. The function of CTC-444N24.11 is unknown. CRNDE is a metabolic regulator that promotes aerobic glycolysis [183], while LINC00657 helps maintain genomic stability under stress [184,185]. Alterations of these lncRNAs in hMEVs are consistent with adaptive responses of the preterm infants against hypoxic conditions [80]. In addition, LINC00657 is one of the most abundant lncRNAs in hMEVs, and its decrease may serve as a biomarker of perinatal hypoxic stress [80].
The peptides present in hMEVs from mothers with term and preterm birth were compared by proteomics; 719 peptides were identified [81]. Differential expression and bioinformatic analyses revealed 70 peptides whose levels differed between groups, with 47 being higher in the term mothers, and 23 being lower. The biologic activities of these peptides mainly involve cell proliferation and development, biological adhesion, immune responses, and metabolic process [81]. These biologically active peptides included lactotransferrin (LTF)-derived peptide residues 79–96 (DGGFIYEAGLAPYKLRPV, transferrin-like 1 domain), and lactadherin (MFGE8)-derived peptide residues 24–47 (LDICSKNPCHNGGLCEEISQEVR, EGF-like domain) [81]. The LTF (residues 79–96) peptide probably has anti-inflammatory and antimicrobial properties, while the MFGE8 (24–47) peptide may exhibit cell growth promoting effects [81]. The preterm hMEVs induced greater cell proliferation and wound healing on FHC human intestinal cells compared to term hMEVs [81]. In a necrotizing enterocolitis (NEC) animal model, the oral administration of the preterm hMEVs protected the villous integrity from injury and restored enterocyte proliferation relative to untreated NEC-like mice [81]. This structure–function relationship suggests that mothers with preterm birth secrete hMEVs containing factors that protect against the consequences of the immature gut in their infants.
3.5. Maternal Allergic Sensitivity and Lifestyle
Levels of immunologic components of human milk, including hMEVs, are associated with maternal allergies and environmental factors [76,186,187,188]. Torregrosa Paredes et al. [76] characterized the influence of maternal allergic sensitivity and anthroposophic lifestyle on hMEV-derived protein levels and their relationship with the child sensitivity at 2 years of age. Two hMEV subpopulations were evaluated: HLA-DR-enriched hMEVs and CD63-enriched hMEVs. In the HLA-DR-enriched hMEV subpopulation, significantly lower levels of mucin-1 were detected in mothers with anthroposophic lifestyle compared to non-anthroposophic mothers [76]. In the CD63-enriched hMEV subpopulation, lower levels of mucin-1 were detected in sensitized mothers, while higher levels of HLA-ABC were associated with mothers whose children developed sensitization (allergen-specific IgE levels ≥0.35 kUA/L) [76]. This study highlights the complex interaction of maternal underlying conditions and environmental factors on hMEV composition and child health outcomes.
Table 3 summarizes current knowledge on hMEV molecular and functional changes affected by maternal health and disease states. These sets of significant proteins and miRNAs also hold promise as predictive biomarkers of child health outcomes.

Table 3.
Influence of maternal conditions on hMEV composition and potential health outcomes in children.
4. Challenges and Clinical Implications
HMEVs, like EVs in other biofluids and from stem cells, have promising potential clinical applications. However, several characteristics of hMEVs are distinct from other EVs. HMEVs are expressed as part of human breastmilk, harbor selective molecular packages of parent cells found primarily in the mammary gland and elicit epigenetic regulation and cell signaling in infant tissues. Thus, hMEVs represent another mechanism whereby human milk acts as a medium for communication from the mother to the infant, and current data suggest that this may be one of the primary effectors of this vertical communication. This review focuses on the EVs in human milk, but EVs undoubtedly have analogous functions in other mammals.
HMEVs are a complex biological system of human milk. They originate in parent cells in the local breast tissues and, to a lesser degree, distant organ systems. After the infants ingests the milk, their intestinal mucosa may be a primary target, but appreciable amounts of hMEVs are also absorbed into the infant circulation. The membrane contains adhesion molecules that can guide the vesicle to specific types of cells, both in the gut lining, and the distant organ systems after some of the hMEVs pass into the blood circulation [83,86,147,189,190,191]. The cargo of the hMEVs include both biologically active proteins and miRNAs that are capable of programing the recipient cells to induce physiological responses in breastfed infants. Understanding more about this system of mother to infant communication could serve two major clinical applications: predictive biomarkers and therapeutic agents (Figure 3).
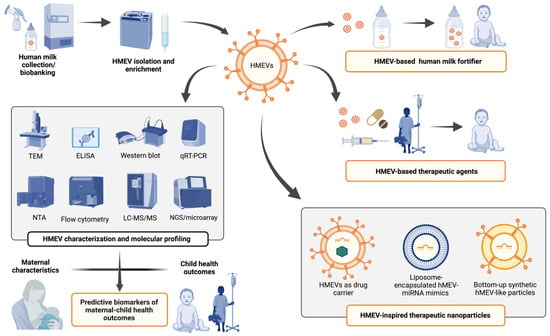
Figure 3.
Potential clinical applications of hMEVs as predictive biomarkers of maternal–child health outcomes and as a source or inspiration for novel nutraceuticals/therapeutics. Abbreviations: TEM, transmission electron microscopy, ELISA, enzyme-linked immunosorbent assay; qRT-PCR, quantitative real-time polymerase chain reaction; NTA, nanoparticle tracking analysis; LC-MS/MS, liquid chromatography-tandem mass spectrometry; NGS, next-generation sequencing; EV, extracellular vesicles; HMEV, human milk derived extracellular vesicles.
A common problem in searching for biomarkers is reproducibility across populations [192,193]. Several strategies help address this challenge. First, a large sample size during the discovery phase of biomarker research (e.g., 100s–1000s) combined with validation using a large-scale independent cohort or multi-center study would enhance the probability of finding valid and robust population-based biomarkers [192,193,194]. Second, a mechanistic biomarker that is directly involved in physio-pathological processes is a strong candidate [192,195]. The mechanistic marker would be improved further if it included multiplex biomarkers; measuring several markers in combination is likely to be more specific than a solitary descriptive biomarker [17,196,197]. While a population-based biomarker strategy is often constrained by budget, mechanistic/multiplex biomarkers may be more compatible with hMEVs functional proteins and miRNAs. The ideal is to incorporate both strategies in the form of hMEV-based (mechanistic/multiplex) biomarker studies conducted as an extension of population-based longitudinal birth cohorts, e.g., Boston (ClinicalTrials.gov Identifier NCT03228875), Influenza IMPRINT [198], MatCH [199], NEHO [200], NICE [201], and PREVAIL [202]. This would provide the strongest opportunity to discover hMEV-based biomarkers for predicting maternal–child health outcomes.
To develop hMEVs for therapeutic purposes, a major challenge is the quantity and quality of hMEVs available for research. In general, a single-step EV isolation method, i.e., high-speed ultracentrifugation without a prior step of low-speed centrifugation, polymer precipitation, ultrafiltration, or size-exclusion chromatography, provides high-to-intermediate yields, but such isolates may be contaminated with lipoproteins, or free and aggregated proteins [31]. A combination of isolation methods such as differential ultracentrifugation coupled with polymer precipitation or size-exclusion chromatography achieve a higher specificity with less contaminates, but usually with a lower recovery [31,203,204]. There is no single optimum/gold-standard method for EV isolation [31]. The isolation method must be chosen to consider compatibility and suitability to downstream applications. For example, a single-step method may be superior for large-scale hMEV biomarker studies, while combined methods may be more suitable to prepare hMEVs for therapeutic testing. A comparison of different methods for pre-processing and isolation of hMEVs [205] concluded: (i) milk fat should be removed prior to long-term milk storage; (ii) different combinations of isolation methods (differential ultracentrifugation plus serial filtration vs. precipitation (i.e., ExoQuick) plus serial filtration) provide comparable yields; (iii) combined methods were effective for hMEV isolation even with a small starting volume of 1.5 mL human milk [205].
As one of the human milk bioactive components, hMEVs are expected to be safe for oral consumption, and without toxicity, supporting its use as a nutraceutical/therapeutic agent. Perhaps one potential use of isolated hMEVs would be human milk fortification [206,207] to enhance the beneficial effects of maternal breastmilk or donor milk or to improve infant formula. For preterm infants, especially whose with very low birth weight, this could help reduce their high risk of morbidity and mortality [207,208]. Another application for isolated hMEVs would be as a therapeutic agent to mitigate intestinal inflammation and facilitate tissue repair in preterm infants with NEC [81,112]. HMEVs also harbor functional molecules with anti-tumor and anti-viral effects, e.g., lactadherin which can inhibit rotavirus [92] and miR-148a-3p which inhibits gastric and pancreatic cancers [109,110]. The therapeutic potential of bovine milk-derived EVs, including those EVs loaded with miR-148a-3p, have been investigated in colon cancer [209,210]. Future preclinical and clinical studies should evaluate the safety and efficacy of hMEV-based therapy against NEC, rotavirus infection, and adult gastrointestinal cancers.
A major hurdle for use of hMEV for therapeutics is the amount of human milk available for isolating hMEVs. Although human milk banks have large quantities of donor milk, it is mostly reserved for neonates in immediate need. Moreover, the stability of hMEVs to the processing and storage conditions of donor human milk requires investigation [211,212].
The synthesis of hMEV-inspired therapeutic nanoparticles is an alternative strategy for harnessing therapeutic potential of hMEVs. For example, synthetic EVs could be nanoparticle platforms for drug and gene delivery [15,213]. Targeted oral delivery by bovine milk EVs and hMEV-based carriers have been actively investigated to overcome the solubility limits and toxicity of the chemotherapeutic drug paclitaxel [214,215]. EVs also improve the oral bioavailability of small molecules, such as celastrol [216], anthocyanidins [217,218], and curcumin [219,220,221]. The stability and targeted delivery of small interfering RNAs (siRNAs) is also improved by using EV carriers [222,223,224]. SiRNA-loaded milk EVs are safe and effective in vivo [224,225].
The use of synthetic mimetics of hMEV-derived functional molecules can overcome the limited availability of human milk, errors during EV isolation and characterization, and also avoid any undesirable effects by unknown or unwanted EV components [226,227]. HMEV-inspired therapeutic liposomes incorporating desirable protein ligands and miRNAs (Table 2) would be amenable to standardization and large-scale clinical application. Quantitative engineering using stepwise quantitative loading demonstrated that the bottom-up synthetic fibroblast-liked EV mimics had precisely controlled lipid (43% cholesterol, 16% sphingomyelin, 38% phospholipids, 2% phosphatidic acid, 1% diacylglycerol), protein (CD9, CD63, CD81), and miRNA (miR-21, miR-124, miR-125, miR-126, miR-130, miR-132) components. These synthetic EV mimetics promoted wound healing and neovascularization in 2D and 3D in vitro models [227], akin to the known therapeutic potential of fibroblast-derived EVs on diabetic wound healing [228]. This synthetic bottom-up synthesis promises to harness the full potential, designability, and therapeutic effects of hMEV-like EV mimics in pediatric and adult populations in the future.
5. Conclusions
As a unique biological system of human milk, hMEVs deliver functional cargos of proteins, nucleic acids, and lipids from mothers to breastfeeding infants. These cargos induce epigenetic and physiological responses in growing infants, including accelerating gut maturation, attenuating mucosal inflammation, modulating the immune system, and preventing viral infection. The variation in hMEVs among mothers of diverse metabolic states provide special opportunities in searching for predictive biomarkers of child health outcomes, particularly for those that also may serve as mechanistic markers. Some of the biomarkers may also be relevant to broader applications in adult medicine. Therapeutic hMEVs, both natural isolates and inspired synthetics, hold great promise as novel biologics for human disease, and warrant expansion of our research efforts.
Author Contributions
Conceptualization, S.C. and D.S.N.; methodology, S.C.; data curation, S.C.; writing—original draft preparation, S.C.; writing—review and editing, A.L.M. and D.S.N.; visualization, S.C.; supervision, A.L.M. and D.S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
All figures created with BioRender.com (last accessed on 29 June 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stahl, P.D. Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S. Vesicular transport earns a Nobel. Trends Cell Biol. 2014, 24, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Möller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Panachan, J.; Rojsirikulchai, N.; Pongsakul, N.; Khowawisetsut, L.; Pongphitcha, P.; Siriboonpiputtana, T.; Chareonsirisuthigul, T.; Phornsarayuth, P.; Klinkulab, N.; Jinawath, N.; et al. Extracellular Vesicle-Based Method for Detecting MYCN Amplification Status of Pediatric Neuroblastoma. Cancers 2022, 14, 2627. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Greco, M.F.; Padró, T.; Badimon, L. Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis. Cells 2022, 11, 1845. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Repa, A.; Lisman, T.; Yerlikaya-Schatten, G.; Hau, C.; Pabinger, I.; Ay, C.; Nieuwland, R.; Thaler, J. Extracellular vesicles from amniotic fluid, milk, saliva, and urine expose complexes of tissue factor and activated factor VII. J. Thromb. Haemost. 2022. [Google Scholar] [CrossRef]
- Hu, Y.; Hell, L.; Kendlbacher, R.A.; Hajji, N.; Hau, C.; van Dam, A.; Berckmans, R.J.; Wisgrill, L.; Ay, C.; Pabinger, I.; et al. Human milk triggers coagulation via tissue factor-exposing extracellular vesicles. Blood Adv. 2020, 4, 6274–6282. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Pi, J.; Xu, J.-F. Emerging Role of Exosomes in Tuberculosis: From Immunity Regulations to Vaccine and Immunotherapy. Front. Immunol. 2021, 12, 628973. [Google Scholar] [CrossRef]
- de Candia, P.; De Rosa, V.; Gigantino, V.; Botti, G.; Ceriello, A.; Matarese, G. Immunometabolism of human autoimmune diseases: From metabolites to extracellular vesicles. FEBS Lett. 2017, 591, 3119–3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.H.; Cerione, R.A.; Antonyak, M.A. Extracellular vesicles and their roles in stem cell biology. Stem Cells 2020, 38, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Kongsomros, S.; Pongsakul, N.; Panachan, J.; Khowawisetsut, L.; Pattanapanyasat, K.; Hongeng, S.; Thitithanyanont, A. Anti-SARS-CoV-2 effect of extracellular vesicles released from mesenchymal stem cells. J. Extracell. Vesicles 2022, 11, e12201. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Netsirisawan, P.; Hongeng, S.; Chutipongtanate, S. Red Blood Cell Extracellular Vesicle-Based Drug Delivery: Challenges and Opportunities. Front. Med. 2021, 8, 761362. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lai, Y.; Ying, S.; Zhan, J.; Shen, Y. Plasma exosome-derived microRNAs expression profiling and bioinformatics analysis under cross-talk between increased low-density lipoprotein cholesterol level and ATP-sensitive potassium channels variant rs1799858. J. Transl. Med. 2020, 18, 459. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Greis, K.D. Multiplex Biomarker Screening Assay for Urinary Extracellular Vesicles Study: A Targeted Label-Free Proteomic Approach. Sci. Rep. 2018, 8, 15039. [Google Scholar] [CrossRef]
- Li, Y.; Gu, J.; Mao, Y.; Wang, X.; Li, Z.; Xu, X.; Chen, H.; Gui, Y. Cerebrospinal Fluid Extracellular Vesicles with Distinct Properties in Autoimmune Encephalitis and Herpes Simplex Encephalitis. Mol. Neurobiol. 2022, 59, 2441–2455. [Google Scholar] [CrossRef]
- Balbi, C.; Piccoli, M.; Barile, L.; Papait, A.; Armirotti, A.; Principi, E.; Reverberi, D.; Pascucci, L.; Becherini, P.; Varesio, L.; et al. First Characterization of Human Amniotic Fluid Stem Cell Extracellular Vesicles as a Powerful Paracrine Tool Endowed with Regenerative Potential. Stem Cells Transl. Med. 2017, 6, 1340–1355. [Google Scholar] [CrossRef]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget 2017, 8, 82459–82474. [Google Scholar] [CrossRef] [Green Version]
- AAP. Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horta, B.L. Breastfeeding: Investing in the Future. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2019, 14, S11–S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaprach, P.; Pongsakul, N.; Apiwattanakul, N.; Muanprasat, C.; Supapannachart, S.; Nuntnarumit, P.; Chutipongtanate, S. Evaluation of Fetal Intestinal Cell Growth and Antimicrobial Biofunctionalities of Donor Human Milk after Preparative Processes. Breastfeed. Med. 2018, 13, 215–220. [Google Scholar] [CrossRef]
- Chiangjong, W.; Panachan, J.; Vanichapol, T.; Pongsakul, N.; Pongphitcha, P.; Siriboonpiputtana, T.; Lerksuthirat, T.; Nuntnarumit, P.; Supapannachart, S.; Srisomsap, C.; et al. HMP-S7 Is a Novel Anti-Leukemic Peptide Discovered from Human Milk. Biomedicines 2021, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassiotou, F.; Hartmann, P.E. At the dawn of a new discovery: The potential of breast milk stem cells. Adv. Nutr. 2014, 5, 770–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizzari, G.; Morniroli, D.; Ceroni, F.; Verduci, E.; Consales, A.; Colombo, L.; Cerasani, J.; Mosca, F.; Giannì, M.L. Human Milk, More Than Simple Nourishment. Children 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S.; Morrow, A.L.; Newburg, D.S. Human Milk Oligosaccharides: Potential Applications in COVID-19. Biomedicines 2022, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyer, F.; Baur, A. Biogenesis and Functions of Exosomes and Extracellular Vesicles. Methods Mol. Biol. 2016, 1448, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henne, W.M.; Stenmark, H.; Emr, S.D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol. 2013, 5, a016766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vietri, M.; Radulovic, M.; Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Calistri, A.; Reale, A.; Palù, G.; Parolin, C. Why Cells and Viruses Cannot Survive without an ESCRT. Cells 2021, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y. The ESCRT Machinery: Remodeling, Repairing, and Sealing Membranes. Membranes 2022, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Raiborg, C.; Bache, K.G.; Gillooly, D.J.; Madshus, I.H.; Stang, E.; Stenmark, H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002, 4, 394–398. [Google Scholar] [CrossRef]
- Mayers, J.R.; Fyfe, I.; Schuh, A.L.; Chapman, E.R.; Edwardson, J.M.; Audhya, A. ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously. J. Biol. Chem. 2011, 286, 9636–9645. [Google Scholar] [CrossRef] [Green Version]
- Katzmann, D.J.; Babst, M.; Emr, S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001, 106, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Babst, M.; Katzmann, D.J.; Snyder, W.B.; Wendland, B.; Emr, S.D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 2002, 3, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Agromayor, M.; Martin-Serrano, J. Interaction of AMSH with ESCRT-III and deubiquitination of endosomal cargo. J. Biol. Chem. 2006, 281, 23083–23091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyuuma, M.; Kikuchi, K.; Kojima, K.; Sugawara, Y.; Sato, M.; Mano, N.; Goto, J.; Takeshita, T.; Yamamoto, A.; Sugamura, K.; et al. AMSH, an ESCRT-III associated enzyme, deubiquitinates cargo on MVB/late endosomes. Cell Struct. Funct. 2007, 31, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollert, T.; Wunder, C.; Lippincott-Schwartz, J.; Hurley, J.H. Membrane scission by the ESCRT-III complex. Nature 2009, 458, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Adell, M.A.Y.; Vogel, G.F.; Pakdel, M.; Müller, M.; Lindner, H.; Hess, M.W.; Teis, D. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J. Cell Biol. 2014, 205, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, J.; Pavlin, M.R.; Yan, S.; Righini, M.; Lee, I.-H.; Carlson, L.-A.; Bahrami, A.H.; Goldman, D.H.; Ren, X.; Hummer, G.; et al. ATP-dependent force generation and membrane scission by ESCRT-III and Vps4. Science 2018, 362, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Davies, B.A.; Azmi, I.F.; Payne, J.; Shestakova, A.; Horazdovsky, B.F.; Babst, M.; Katzmann, D.J. Coordination of substrate binding and ATP hydrolysis in Vps4-mediated ESCRT-III disassembly. Mol. Biol. Cell 2010, 21, 3396–3408. [Google Scholar] [CrossRef] [Green Version]
- Caillat, C.; Macheboeuf, P.; Wu, Y.; McCarthy, A.A.; Boeri-Erba, E.; Effantin, G.; Göttlinger, H.G.; Weissenhorn, W.; Renesto, P. Asymmetric ring structure of Vps4 required for ESCRT-III disassembly. Nat. Commun. 2015, 6, 8781. [Google Scholar] [CrossRef] [Green Version]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.K.; Young, R.F.; Ashraf, H.; Canty, J.M.J. Inhibiting Extracellular Vesicle Release from Human Cardiosphere Derived Cells with Lentiviral Knockdown of nSMase2 Differentially Effects Proliferation and Apoptosis in Cardiomyocytes, Fibroblasts and Endothelial Cells In Vitro. PLoS ONE 2016, 11, e0165926. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef] [Green Version]
- Phuyal, S.; Hessvik, N.P.; Skotland, T.; Sandvig, K.; Llorente, A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014, 281, 2214–2227. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, K.R.; Kantari-Mimoun, C.; Yin, M.; Pederzoli-Ribeil, M.; Angelot-Delettre, F.; Ceroi, A.; Grauffel, C.; Benhamou, M.; Reuter, N.; Saas, P.; et al. Proteinase 3 Is a Phosphatidylserine-binding Protein That Affects the Production and Function of Microvesicles. J. Biol. Chem. 2016, 291, 10476–10489. [Google Scholar] [CrossRef] [Green Version]
- Skočaj, M.; Yu, Y.; Grundner, M.; Resnik, N.; Bedina Zavec, A.; Leonardi, A.; Križaj, I.; Guella, G.; Maček, P.; Kreft, M.E.; et al. Characterisation of plasmalemmal shedding of vesicles induced by the cholesterol/sphingomyelin binding protein, ostreolysin A-mCherry. Biochim. Biophys. Acta 2016, 1858, 2882–2893. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [Green Version]
- Antonyak, M.A.; Wilson, K.F.; Cerione, R.A. R(h)oads to microvesicles. Small GTPases 2012, 3, 219–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedgwick, A.E.; Clancy, J.W.; Olivia Balmert, M.; D’Souza-Schorey, C. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Sci. Rep. 2015, 5, 14748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T.K.; Fonseka, P.; Tixeira, R.; Pathan, M.; Ang, C.-S.; Ozkocak, D.C.; Mathivanan, S.; Poon, I.K.H. Pannexin-1 channel regulates nuclear content packaging into apoptotic bodies and their size. Proteomics 2021, 21, e2000097. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.K.; Ozkocak, D.C.; Poon, I.K.H. Unleashing the therapeutic potential of apoptotic bodies. Biochem. Soc. Trans. 2020, 48, 2079–2088. [Google Scholar] [CrossRef]
- Gallier, S.; Vocking, K.; Post, J.A.; Van De Heijning, B.; Acton, D.; Van Der Beek, E.M.; Van Baalen, T. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids Surf. B Biointerfaces 2015, 136, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef]
- Smith, Z.J.; Lee, C.; Rojalin, T.; Carney, R.P.; Hazari, S.; Knudson, A.; Lam, K.; Saari, H.; Ibañez, E.L.; Viitala, T.; et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J. Extracell. Vesicles 2015, 4, 28533. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Ludwig, S.; Muller, L.; Hong, C.S.; Kirkwood, J.M.; Ferrone, S.; Whiteside, T.L. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J. Extracell. Vesicles 2018, 7, 1435138. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Chen, C. Toward characterizing extracellular vesicles at a single-particle level. J. Biomed. Sci. 2019, 26, 9. [Google Scholar] [CrossRef]
- Hilton, S.H.; White, I.M. Advances in the analysis of single extracellular vesicles: A critical review. Sens. Actuators Rep. 2021, 3, 100052. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Song, D.; Cao, X.; Wu, R.; Liu, B.; Ye, W.; Wu, J.; Yue, X. Comparative proteomic analysis of milk-derived exosomes in human and bovine colostrum and mature milk samples by iTRAQ-coupled LC-MS/MS. Food Res. Int. 2017, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kupsco, A.; Prada, D.; Valvi, D.; Hu, L.; Petersen, M.S.; Coull, B.; Grandjean, P.; Weihe, P.; Baccarelli, A.A. Human milk extracellular vesicle miRNA expression and associations with maternal characteristics in a population-based cohort from the Faroe Islands. Sci. Rep. 2021, 11, 5840. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Chen, X.; Qian, Y.; Wang, X.; Zhou, Y.; Yan, X.; Yu, B.; Yao, S.; Yu, Z.; Zhu, J.; et al. Lipidomic Profiling of Human Milk Derived Exosomes and Their Emerging Roles in the Prevention of Necrotizing Enterocolitis. Mol. Nutr. Food Res. 2021, 65, e2000845. [Google Scholar] [CrossRef]
- Torregrosa Paredes, P.; Gutzeit, C.; Johansson, S.; Admyre, C.; Stenius, F.; Alm, J.; Scheynius, A.; Gabrielsson, S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy 2014, 69, 463–471. [Google Scholar] [CrossRef]
- Mirza, A.H.; Kaur, S.; Nielsen, L.B.; Størling, J.; Yarani, R.; Roursgaard, M.; Mathiesen, E.R.; Damm, P.; Svare, J.; Mortensen, H.B.; et al. Breast Milk-Derived Extracellular Vesicles Enriched in Exosomes From Mothers With Type 1 Diabetes Contain Aberrant Levels of microRNAs. Front. Immunol. 2019, 10, 2543. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.B.; Fields, D.A.; Pezant, N.P.; Kharoud, H.K.; Gulati, S.; Jacobs, K.; Gale, C.A.; Kharbanda, E.O.; Nagel, E.M.; Demerath, E.W.; et al. Gestational Diabetes Mellitus Is Associated with Altered Abundance of Exosomal MicroRNAs in Human Milk. Clin. Ther. 2022, 44, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.B.; Chernausek, S.D.; Garman, L.D.; Pezant, N.P.; Plows, J.F.; Kharoud, H.K.; Demerath, E.W.; Fields, D.A. Human Milk Exosomal MicroRNA: Associations with Maternal Overweight/Obesity and Infant Body Composition at 1 Month of Life. Nutrients 2021, 13, 1091. [Google Scholar] [CrossRef]
- Mourtzi, N.; Siahanidou, T.; Tsifintaris, M.; Karamichali, E.; Tasiopoulou, A.; Sertedaki, A.; Pesmatzoglou, M.; Kapetanaki, A.; Liosis, G.; Baltatzis, G.; et al. lncRNA NORAD is consistently detected in breastmilk exosomes and its expression is downregulated in mothers of preterm infants. Int. J. Mol. Med. 2021, 48, 5049. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, X.; Zhang, L.; Cai, J.; Zhou, Y.; Liu, H.; Hu, Y.; Chen, W.; Xu, S.; Liu, P.; et al. Identification and Peptidomic Profiling of Exosomes in Preterm Human Milk: Insights Into Necrotizing Enterocolitis Prevention. Mol. Nutr. Food Res. 2019, 63, e1801247. [Google Scholar] [CrossRef] [PubMed]
- Bozack, A.K.; Colicino, E.; Rodosthenous, R.; Bloomquist, T.R.; Baccarelli, A.A.; Wright, R.O.; Wright, R.J.; Lee, A.G. Associations between maternal lifetime stressors and negative events in pregnancy and breast milk-derived extracellular vesicle microRNAs in the programming of intergenerational stress mechanisms (PRISM) pregnancy cohort. Epigenetics 2021, 16, 389–404. [Google Scholar] [CrossRef] [PubMed]
- van Herwijnen, M.J.C.; Zonneveld, M.I.; Goerdayal, S.; Nolte-’t Hoen, E.N.M.; Garssen, J.; Stahl, B.; Maarten Altelaar, A.F.; Redegeld, F.A.; Wauben, M.H.M. Comprehensive Proteomic Analysis of Human Milk-derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol. Cell. Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Gürsoy-Özdemir, Y.; Kaya, M.; Eslami Abriz, A.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef]
- Fujiwara, M.; Tsunoda, R.; Shigeta, S.; Yokota, T.; Baba, M. Human follicular dendritic cells remain uninfected and capture human immunodeficiency virus type 1 through CD54-CD11a interaction. J. Virol. 1999, 73, 3603–3607. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef]
- Vaswani, K.M.; Peiris, H.; Qin Koh, Y.; Hill, R.J.; Harb, T.; Arachchige, B.J.; Logan, J.; Reed, S.; Davies, P.S.W.; Mitchell, M.D. A complete proteomic profile of human and bovine milk exosomes by liquid chromatography mass spectrometry. Expert Rev. Proteom. 2021, 18, 719–735. [Google Scholar] [CrossRef]
- Sedykh, S.; Kuleshova, A.; Nevinsky, G. Milk Exosomes: Perspective Agents for Anticancer Drug Delivery. Int. J. Mol. Sci. 2020, 21, 6646. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; Guerrero, M.L.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998, 351, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Sugano, G.; Bernard-Pierrot, I.; Laé, M.; Battail, C.; Allory, Y.; Stransky, N.; Krumeich, S.; Lepage, M.-L.; Maille, P.; Donnadieu, M.-H.; et al. Milk fat globule--epidermal growth factor--factor VIII (MFGE8)/lactadherin promotes bladder tumor development. Oncogene 2011, 30, 642–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, F.; Morelli, A.E. Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin. Immunopathol. 2018, 40, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.L.; Weldon, A.K.; Dobbie, L.; Smith, A.J.H.; Mather, I.H. Expression of butyrophilin (Btn1a1) in lactating mammary gland is essential for the regulated secretion of milk-lipid droplets. Proc. Natl. Acad. Sci. USA 2004, 101, 10084–10089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, H.A.; Viney, J.L. Immune modulation by butyrophilins. Nat. Rev. Immunol. 2014, 14, 559–569. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Komarova, E.Y.; Suezov, R.V.; Nikotina, A.D.; Aksenov, N.D.; Garaeva, L.A.; Shtam, T.A.; Zhakhov, A.V.; Martynova, M.G.; Bystrova, O.A.; Istomina, M.S.; et al. Hsp70-containing extracellular vesicles are capable of activating of adaptive immunity in models of mouse melanoma and colon carcinoma. Sci. Rep. 2021, 11, 21314. [Google Scholar] [CrossRef]
- Wang, C.-H.; Zhang, C.; Xing, X.-H. Xanthine dehydrogenase: An old enzyme with new knowledge and prospects. Bioengineered 2016, 7, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Näslund, T.I.; Paquin-Proulx, D.; Paredes, P.T.; Vallhov, H.; Sandberg, J.K.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 2014, 28, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Ueno, K.; Koga, T.; Kato, K.; Golenbock, D.T.; Gendler, S.J.; Kai, H.; Kim, K.C. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am. J. Respir. Cell Mol. Biol. 2008, 38, 263–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, P.; McAuley, J. The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Tingö, L.; Ahlberg, E.; Johansson, L.; Pedersen, S.A.; Chawla, K.; Sætrom, P.; Cione, E.; Simpson, M.R. Non-Coding RNAs in Human Breast Milk: A Systematic Review. Front. Immunol. 2021, 12, 725323. [Google Scholar] [CrossRef] [PubMed]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk Cells Contain Numerous miRNAs that May Change with Milk Removal and Regulate Multiple Physiological Processes. Int. J. Mol. Sci. 2016, 17, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, W.; Zhu, S.; Yuan, M.; Cui, H.; Wang, L.; Luo, X.; Li, J.; Zhou, H.; Tang, Y.; Shen, N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 2010, 184, 6773–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liang, Z.; Xu, X.; Li, J.; Zhu, Y.; Meng, S.; Li, S.; Wang, S.; Xie, B.; Ji, A.; et al. miR-148a-3p represses proliferation and EMT by establishing regulatory circuits between ERBB3/AKT2/c-myc and DNMT1 in bladder cancer. Cell Death Dis. 2016, 7, e2503. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, Y.; Dang, H.; Wu, X. MicroRNA-148a-3p inhibits the proliferation of cervical cancer cells by regulating the expression levels of DNMT1 and UTF1. Oncol. Lett. 2021, 22, 617. [Google Scholar] [CrossRef]
- Okumura, S.; Hirano, Y.; Komatsu, Y. Stable duplex-linked antisense targeting miR-148a inhibits breast cancer cell proliferation. Sci. Rep. 2021, 11, 11467. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hong, L.; Yang, Z.; Tu, Y.; Xin, W.; Zha, M.; Tu, S.; Sun, G.; Li, Y.; Xiao, W. MicroRNA-148a-3p suppresses epithelial-to-mesenchymal transition and stemness properties via Wnt1-mediated Wnt/β-catenin pathway in pancreatic cancer. J. Cell. Mol. Med. 2020, 24, 13020–13035. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Guo, L. MicroRNA-148a-3p inhibits cancer progression and is a novel screening biomarker for gastric cancer. J. Clin. Lab. Anal. 2020, 34, e23454. [Google Scholar] [CrossRef]
- Paczkowska, J.; Janiszewska, J.; Bein, J.; Schneider, M.; Bednarek, K.; Ustaszewski, A.; Hartmann, S.; Hansmann, M.-L.; Giefing, M. The Tumor Suppressive mir-148a Is Epigenetically Inactivated in Classical Hodgkin Lymphoma. Cells 2020, 9, 2292. [Google Scholar] [CrossRef]
- Guo, M.-M.; Zhang, K.; Zhang, J.-H. Human Breast Milk-Derived Exosomal miR-148a-3p Protects Against Necrotizing Enterocolitis by Regulating p53 and Sirtuin 1. Inflammation 2022, 45, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Shrestha, A.; Liu, C.; Wu, P.; Cheng, B. MicroRNA 148a-3p promotes Thrombospondin-4 expression and enhances angiogenesis during tendinopathy development by inhibiting Krüppel-like factor 6. Biochem. Biophys. Res. Commun. 2018, 502, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, Y.; Wang, K.; Li, J.; Chu, T.; Shen, H. MicroRNA-148a-3p alleviates high glucose-induced diabetic retinopathy by targeting TGFB2 and FGF2. Acta Diabetol. 2020, 57, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, W.; Ge, H.; Li, K. Aberrant expression of miR-148a-3p in Alzheimer’s disease and its protective role against amyloid-β induced neurotoxicity. Neurosci. Lett. 2021, 756, 135953. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Jiang, H.; Ashraf, G.M.; Liu, J.; Wang, L.; Zhao, K.; Liu, M.; Li, Z.; Liu, R. Implications of miR-148a-3p/p35/PTEN signaling in tau hyperphosphorylation and autoregulatory feedforward of Akt/CREB in Alzheimer’s disease. Mol. Ther. Nucleic Acids 2022, 27, 256–275. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Liu, X.; Ouyang, Y. Silencing lncRNA HOTAIR improves the recovery of neurological function in ischemic stroke via the miR-148a-3p/KLF6 axis. Brain Res. Bull. 2021, 176, 43–53. [Google Scholar] [CrossRef]
- Yu, Y.; Du, L.; Zhang, J. Febrile Seizure-Related miR-148a-3p Exerts Neuroprotection by Promoting the Proliferation of Hippocampal Neurons in Children with Temporal Lobe Epilepsy. Dev. Neurosci. 2021, 43, 312–320. [Google Scholar] [CrossRef]
- Hu, C.-W.; Tseng, C.-W.; Chien, C.-W.; Huang, H.-C.; Ku, W.-C.; Lee, S.-J.; Chen, Y.-J.; Juan, H.-F. Quantitative proteomics reveals diverse roles of miR-148a from gastric cancer progression to neurological development. J. Proteome Res. 2013, 12, 3993–4004. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hepworth, A.R.; Lefèvre, C.; Hartmann, P.E.; Geddes, D.T.; Hassiotou, F. Human Milk MicroRNA and Total RNA Differ Depending on Milk Fractionation. J. Cell. Biochem. 2015, 116, 2397–2407. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.-Y.; Mei, J.-W.; Xiang, S.-S.; Li, H.-F.; Ma, Q.; Song, X.-L.; Wang, Z.; Zhang, Y.-C.; Liu, Y.-C.; Jin, Y.-P.; et al. MicroRNA-30a-5p inhibits gallbladder cancer cell proliferation, migration and metastasis by targeting E2F7. Cell Death Dis. 2018, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zong, K.; Wang, X.; Dou, D.; Lv, P.; Zhang, Z.; Li, H. miR-30d suppresses proliferation and invasiveness of pancreatic cancer by targeting the SOX4/PI3K-AKT axis and predicts poor outcome. Cell Death Dis. 2021, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.L.; Jiang, X.; Rom, S. let-7 microRNAs: Their Role in Cerebral and Cardiovascular Diseases, Inflammation, Cancer, and Their Regulation. Biomedicines 2021, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.-T.; Liu, Z.; Feng, J.; Zhao, W.; Hao, T.; Ding, W.-Y.; Chu, J.-P.; Gao, L.-J. MiR-22-3p Regulates Cell Proliferation and Inhibits Cell Apoptosis through Targeting the eIF4EBP3 Gene in Human Cervical Squamous Carcinoma Cells. Int. J. Med. Sci. 2018, 15, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Senese, R.; Cioffi, F.; Petito, G.; de Lange, P.; Russo, A.; Goglia, F.; Lanni, A.; Potenza, N. miR-22-3p is involved in gluconeogenic pathway modulated by 3,5-diiodo-L-thyronine (T2). Sci. Rep. 2019, 9, 16645. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Elbaum Shiff, Y.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef]
- Correia, N.C.; Fragoso, R.; Carvalho, T.; Enguita, F.J.; Barata, J.T. MiR-146b negatively regulates migration and delays progression of T-cell acute lymphoblastic leukemia. Sci. Rep. 2016, 6, 31894. [Google Scholar] [CrossRef]
- Mitsumura, T.; Ito, Y.; Chiba, T.; Matsushima, T.; Kurimoto, R.; Tanaka, Y.; Kato, T.; Uchida, K.; Ito, T.; Yamamoto, K.; et al. Ablation of miR-146b in mice causes hematopoietic malignancy. Blood Adv. 2018, 2, 3483–3491. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef]
- Wu, Q.; Ye, X.; Xiong, Y.; Zhu, H.; Miao, J.; Zhang, W.; Wan, J. The Protective Role of microRNA-200c in Alzheimer’s Disease Pathologies Is Induced by Beta Amyloid-Triggered Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2016, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, J.; Wang, Q.; Jiang, H.; Zeng, L.; Li, Z.; Liu, R. MicroRNA-200a-3p Mediates Neuroprotection in Alzheimer-Related Deficits and Attenuates Amyloid-Beta Overproduction and Tau Hyperphosphorylation via Coregulating BACE1 and PRKACB. Front. Pharmacol. 2019, 10, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Civra, A.; Francese, R.; Donalisio, M.; Tonetto, P.; Coscia, A.; Sottemano, S.; Balestrini, R.; Faccio, A.; Cavallarin, L.; Moro, G.E.; et al. Human Colostrum and Derived Extracellular Vesicles Prevent Infection by Human Rotavirus and Respiratory Syncytial Virus in Vitro. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2021, 37, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Elbaum Shiff, Y.; Golan-Gerstl, R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. J. Transl. Med. 2019, 17, 325. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, M.; Tong, M.; Yang, L.; Pang, L.; Chen, L.; Xu, G.; Chi, X.; Hong, Q.; Ni, Y.; et al. miR-148a is Associated with Obesity and Modulates Adipocyte Differentiation of Mesenchymal Stem Cells through Wnt Signaling. Sci. Rep. 2015, 5, 9930. [Google Scholar] [CrossRef] [Green Version]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 2019, 7, 245–262. [Google Scholar] [CrossRef]
- He, Y.; He, Z.; Leone, S.; Liu, S. Milk Exosomes Transfer Oligosaccharides into Macrophages to Modulate Immunity and Attenuate Adherent-Invasive E. coli (AIEC) Infection. Nutrients 2021, 13, 3198. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Leone, S.; Newburg, D.S. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunol. 2014, 7, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to Those Who Are Breastfed, Infants Fed a Formula Containing 2’-Fucosyllactose Have Lower Inflammatory Cytokines in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2’-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.-T.; Nanthakumar, N.N.; Newburg, D.S. The Human Milk Oligosaccharide 2’-Fucosyllactose Quenches Campylobacter jejuni-Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 2016, 146, 1980–1990. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; Overbeek, S.A.; Kostadinova, A.I.; Stahl, B.; Garssen, J.; Van’t Land, B.; Willemsen, L.E.M. Exposure of Intestinal Epithelial Cells to 2’-Fucosyllactose and CpG Enhances Galectin Release and Instructs Dendritic Cells to Drive Th1 and Regulatory-Type Immune Development. Biomolecules 2020, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.A.; Pedram, K.; Malaker, S.A.; Batista, P.J.; Smith, B.A.H.; Johnson, A.G.; George, B.M.; Majzoub, K.; Villalta, P.W.; Carette, J.E.; et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 2021, 184, 3109–3124.e22. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.; Schnaar, R.L. Siglec Ligands. Cells 2021, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Chevet, E.; De Matteis, M.A.; Eskelinen, E.-L.; Farhan, H. RNA, a new member in the glycan-club that gets exposed at the cell surface. Traffic 2021, 22, 362–363. [Google Scholar] [CrossRef]
- Kahn, S.; Liao, Y.; Du, X.; Xu, W.; Li, J.; Lönnerdal, B. Exosomal MicroRNAs in Milk from Mothers Delivering Preterm Infants Survive in Vitro Digestion and Are Taken Up by Human Intestinal Cells. Mol. Nutr. Food Res. 2018, 62, e1701050. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Du, X.; Li, J.; Lönnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61, 1700082. [Google Scholar] [CrossRef]
- Kusuma, R.J.; Manca, S.; Friemel, T.; Sukreet, S.; Nguyen, C.; Zempleni, J. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am. J. Physiol. Cell Physiol. 2016, 310, C800–C807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zonneveld, M.I.; van Herwijnen, M.J.C.; Fernandez-Gutierrez, M.M.; Giovanazzi, A.; de Groot, A.M.; Kleinjan, M.; van Capel, T.M.M.; Sijts, A.J.A.M.; Taams, L.S.; Garssen, J.; et al. Human milk extracellular vesicles target nodes in interconnected signalling pathways that enhance oral epithelial barrier function and dampen immune responses. J. Extracell. Vesicles 2021, 10, e12071. [Google Scholar] [CrossRef]
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Brawner, K.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Zhang, Y.; Yan, D.-Y.; Wang, Y.; Xu, X.; Zhao, Y.-C.; Xiao, T.-T. Protective Effects of Human Milk-Derived Exosomes on Intestinal Stem Cells Damaged by Oxidative Stress. Cell Transplant. 2020, 29, 963689720912690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Zhang, R.; Qian, T.; Peng, X.; He, W.; Zheng, S.; Cao, Y.; Pierro, A.; Shen, C. A comparison of exosomes derived from different periods breast milk on protecting against intestinal organoid injury. Pediatr. Surg. Int. 2019, 35, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Lee, C.; Chusilp, S.; Bhalla, M.; Li, B.; Pitino, M.; Seo, S.; O’Connor, D.L.; Pierro, A. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int. 2020, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Elbaum-Shiff, Y.; Koroukhov, N.; Shilo, I.; Musseri, M.; Golan-Gerstl, R. Cow and Human Milk-Derived Exosomes Ameliorate Colitis in DSS Murine Model. Nutrients 2020, 12, 2589. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Cirrincione, S.; Rittà, M.; Lamberti, C.; Civra, A.; Francese, R.; Tonetto, P.; Sottemano, S.; Manfredi, M.; Lorenzato, A.; et al. Extracellular Vesicles in Human Preterm Colostrum Inhibit Infection by Human Cytomegalovirus In Vitro. Microorganisms 2020, 8, 1087. [Google Scholar] [CrossRef] [PubMed]
- Francese, R.; Civra, A.; Donalisio, M.; Volpi, N.; Capitani, F.; Sottemano, S.; Tonetto, P.; Coscia, A.; Maiocco, G.; Moro, G.E.; et al. Anti-Zika virus and anti-Usutu virus activity of human milk and its components. PLoS Negl. Trop. Dis. 2020, 14, e0008713. [Google Scholar] [CrossRef]
- Chetta, K.E.; Schulz, E.V.; Wagner, C.L. Outcomes improved with human milk intake in preterm and full-term infants. Semin. Perinatol. 2021, 45, 151384. [Google Scholar] [CrossRef]
- Lockyer, F.; McCann, S.; Moore, S.E. Breast Milk Micronutrients and Infant Neurodevelopmental Outcomes: A Systematic Review. Nutrients 2021, 13, 3848. [Google Scholar] [CrossRef]
- Wang, B.; McVeagh, P.; Petocz, P.; Brand-Miller, J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 2003, 78, 1024–1029. [Google Scholar] [CrossRef]
- Mudd, A.T.; Fleming, S.A.; Labhart, B.; Chichlowski, M.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary Sialyllactose Influences Sialic Acid Concentrations in the Prefrontal Cortex and Magnetic Resonance Imaging Measures in Corpus Callosum of Young Pigs. Nutrients 2017, 9, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveros, E.; Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Buck, R.; Rueda, R.; Martín, M.J. Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats. Nutrients 2018, 10, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.; Zhu, Z.; Li, T.; Baluyot, K.; Howell, B.R.; Hazlett, H.C.; Elison, J.T.; Hauser, J.; Sprenger, N.; Wu, D.; et al. Human milk 3’-Sialyllactose is positively associated with language development during infancy. Am. J. Clin. Nutr. 2021, 114, 588–597. [Google Scholar] [CrossRef]
- Pisa, E.; Martire, A.; Chiodi, V.; Traversa, A.; Caputo, V.; Hauser, J.; Macrì, S. Exposure to 3’Sialyllactose-Poor Milk during Lactation Impairs Cognitive Capabilities in Adulthood. Nutrients 2021, 13, 4191. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Human and Bovine Milk Oligosaccharides Elicit Improved Recognition Memory Concurrent With Alterations in Regional Brain Volumes and Hippocampal mRNA Expression. Front. Neurosci. 2020, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Dietary Oligofructose Alone or in Combination with 2’-Fucosyllactose Differentially Improves Recognition Memory and Hippocampal mRNA Expression. Nutrients 2020, 12, 2131. [Google Scholar] [CrossRef] [PubMed]
- de Weerth, C.; Aatsinki, A.-K.; Azad, M.B.; Bartol, F.F.; Bode, L.; Collado, M.C.; Dettmer, A.M.; Field, C.J.; Guilfoyle, M.; Hinde, K.; et al. Human milk: From complex tailored nutrition to bioactive impact on child cognition and behavior. Crit. Rev. Food Sci. Nutr. 2022, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Rondó, P.H.C.; Rezende, G.; Lemos, J.O.; Pereira, J.A. Maternal stress and distress and child nutritional status. Eur. J. Clin. Nutr. 2013, 67, 348–352. [Google Scholar] [CrossRef] [Green Version]
- Susiloretni, K.A.; Smith, E.R.; Suparmi; Marsum; Agustina, R.; Shankar, A.H. The psychological distress of parents is associated with reduced linear growth of children: Evidence from a nationwide population survey. PLoS ONE 2021, 16, e0246725. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.-C.; Jacobs, M.; Pradhan, S.; Kapse, K.; Zhao, L.; Niforatos-Andescavage, N.; Vezina, G.; du Plessis, A.J.; Limperopoulos, C. Association of Prenatal Maternal Psychological Distress With Fetal Brain Growth, Metabolism, and Cortical Maturation. JAMA Netw. Open 2020, 3, e1919940. [Google Scholar] [CrossRef]
- Pierce, L.J.; Thompson, B.L.; Gharib, A.; Schlueter, L.; Reilly, E.; Valdes, V.; Roberts, S.; Conroy, K.; Levitt, P.; Nelson, C.A. Association of Perceived Maternal Stress During the Perinatal Period With Electroencephalography Patterns in 2-Month-Old Infants. JAMA Pediatr. 2019, 173, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Mathilda Chiu, Y.-H.; Rosa, M.J.; Jara, C.; Wright, R.O.; Coull, B.A.; Wright, R.J. Prenatal and postnatal stress and asthma in children: Temporal- and sex-specific associations. J. Allergy Clin. Immunol. 2016, 138, 740–747.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, I.R.V.; Sly, P.D.; Schmidt, L.A.; van Lieshout, R.J.; Bienenstock, J.; Holt, P.G.; Arck, P.C. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J. Allergy Clin. Immunol. 2014, 134, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, A.; Lupse, B.; Maedler, K. Hippo Signaling: Key Emerging Pathway in Cellular and Whole-Body Metabolism. Trends Endocrinol. Metab. 2018, 29, 492–509. [Google Scholar] [CrossRef]
- Fodor, L.E.; Gézsi, A.; Ungvári, L.; Semsei, A.F.; Gál, Z.; Nagy, A.; Gálffy, G.; Tamási, L.; Kiss, A.; Antal, P.; et al. Investigation of the Possible Role of the Hippo/YAP1 Pathway in Asthma and Allergy. Allergy. Asthma Immunol. Res. 2017, 9, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Zheng, R.; Li, F.; Xin, J.-Y.; Chen, S.-L.; Zhu, X.-J.; Gu, X.; Dai, M.-D.; Yang, Y.-F.; Chu, H.-Y.; et al. Genetic variants in Hippo pathway genes are associated with house dust mite-induced allergic rhinitis in a Chinese population. Clin. Transl. Allergy 2021, 11, e12077. [Google Scholar] [CrossRef] [PubMed]
- Young, B.E.; Johnson, S.L.; Krebs, N.F. Biological determinants linking infant weight gain and child obesity: Current knowledge and future directions. Adv. Nutr. 2012, 3, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; George, B.; Williams, M.; Whitaker, K.; Allison, D.B.; Teague, A.; Demerath, E.W. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr. Obes. 2017, 12 (Suppl. S1), 78–85. [Google Scholar] [CrossRef] [Green Version]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, A.B.S. The impact of maternal obesity on human milk macronutrient composition: A systematic review and meta-analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef] [Green Version]
- Zamanillo, R.; Sánchez, J.; Serra, F.; Palou, A. Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients 2019, 11, 2589. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.; Kalish, B.T. The impact of maternal obesity on childhood neurodevelopment. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2021, 41, 928–939. [Google Scholar] [CrossRef]
- Lund-Blix, N.A.; Dydensborg Sander, S.; Størdal, K.; Nybo Andersen, A.-M.; Rønningen, K.S.; Joner, G.; Skrivarhaug, T.; Njølstad, P.R.; Husby, S.; Stene, L.C. Infant Feeding and Risk of Type 1 Diabetes in Two Large Scandinavian Birth Cohorts. Diabetes Care 2017, 40, 920–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Hu, H.; Chen, Q.; Zhang, Y.; Chen, W.; Huang, Q.; Chen, X.; Li, J.; Zhong, M. Long non-coding RNA SNHG29 regulates cell senescence via p53/p21 signaling in spontaneous preterm birth. Placenta 2021, 103, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.C.; Graham, L.D.; Molloy, P.L. CRNDE, a long non-coding RNA responsive to insulin/IGF signaling, regulates genes involved in central metabolism. Biochim. Biophys. Acta 2014, 1843, 372–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kopp, F.; Chang, T.-C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Elguindy, M.M.; Mendell, J.T. NORAD-induced Pumilio phase separation is required for genome stability. Nature 2021, 595, 303–308. [Google Scholar] [CrossRef]
- Berdi, M.; de Lauzon-Guillain, B.; Forhan, A.; Castelli, F.A.; Fenaille, F.; Charles, M.-A.; Heude, B.; Junot, C.; Adel-Patient, K. Immune components of early breastmilk: Association with maternal factors and with reported food allergy in childhood. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2019, 30, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demers-Mathieu, V.; Mathijssen, G.; Dapra, C.; Do, D.M.; Medo, E. Active free secretory component and secretory IgA in human milk: Do maternal vaccination, allergy, infection, mode of delivery, nutrition and active lifestyle change their concentrations? Pediatr. Res. 2021, 89, 795–802. [Google Scholar] [CrossRef]
- Seppo, A.E.; Choudhury, R.; Pizzarello, C.; Palli, R.; Fridy, S.; Rajani, P.S.; Stern, J.; Martina, C.; Yonemitsu, C.; Bode, L.; et al. Traditional Farming Lifestyle in Old Older Mennonites Modulates Human Milk Composition. Front. Immunol. 2021, 12, 741513. [Google Scholar] [CrossRef]
- Sadri, M.; Shu, J.; Kachman, S.D.; Cui, J.; Zempleni, J. Milk exosomes and miRNA cross the placenta and promote embryo survival in mice. Reproduction 2020, 160, 501–509. [Google Scholar] [CrossRef]
- Ngu, A.; Wang, S.; Wang, H.; Khanam, A.; Zempleni, J. Milk exosomes in nutrition and drug delivery. Am. J. Physiol. Cell Physiol. 2022, 322, C865–C874. [Google Scholar] [CrossRef]
- Zhou, F.; Ebea, P.; Mutai, E.; Wang, H.; Sukreet, S.; Navazesh, S.; Dogan, H.; Li, W.; Cui, J.; Ji, P.; et al. Small Extracellular Vesicles in Milk Cross the Blood-Brain Barrier in Murine Cerebral Cortex Endothelial Cells and Promote Dendritic Complexity in the Hippocampus and Brain Function in C57BL/6J Mice. Front. Nutr. 2022, 9, 838543. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S. Breaking the ice: Urine proteomics of medullary sponge kidney disease. Kidney Int. 2017, 91, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S.; Chatchen, S.; Svasti, J. Plasma prefractionation methods for proteomic analysis and perspectives in clinical applications. Proteom. Clin. Appl. 2017, 11, 1600135. [Google Scholar] [CrossRef] [PubMed]
- Taché, V.; Baer, R.J.; Currier, R.J.; Li, C.-S.; Towner, D.; Waetjen, L.E.; Jelliffe-Pawlowski, L.L. Population-based biomarker screening and the development of severe preeclampsia in California. Am. J. Obstet. Gynecol. 2014, 211, 377.e1–377.e8. [Google Scholar] [CrossRef] [Green Version]
- Narayan, V.; Thompson, E.W.; Demissei, B.; Ho, J.E.; Januzzi, J.L.J.; Ky, B. Mechanistic Biomarkers Informative of Both Cancer and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lindstrom, T.M.; Cheung, R.K.; Sokolove, J. Mechanistic biomarkers for clinical decision making in rheumatic diseases. Nat. Rev. Rheumatol. 2013, 9, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Schoenhoff, F.S.; Savage, W.J.; Zhang, P.; Van Eyk, J.E. Multiplex assays for biomarker research and clinical application: Translational science coming of age. Proteom. Clin. Appl. 2010, 4, 271–284. [Google Scholar] [CrossRef]
- Butler, D. Long-term studies will track indelible marks of first flu. Nature 2019, 569, 464–465. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Mishra, G.D.; Moss, K.M.; Yang, I.A.; Lycett, K.; Knibbs, L.D. Maternal and Childhood Ambient Air Pollution Exposure and Mental Health Symptoms and Psychomotor Development in Children: An Australian Population-Based Longitudinal Study. Environ. Int. 2022, 158, 107003. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, S.; Drago, G.; Colombo, P.; Alesci, A.; Augello, P.; Bisbano, A.; Bucolo, A.; Dattoli, P.; De Sole, R.; La Runa, V.; et al. Three contaminated sites in southern Italy. The Neonatal Environment and Health Outcomes cohort: Protocol for a longitudinal birth cohort study. BMJ Open 2019, 9, e029471. [Google Scholar] [CrossRef] [Green Version]
- Barman, M.; Murray, F.; Bernardi, A.I.; Broberg, K.; Bölte, S.; Hesselmar, B.; Jacobsson, B.; Jonsson, K.; Kippler, M.; Rabe, H.; et al. Nutritional impact on Immunological maturation during Childhood in relation to the Environment (NICE): A prospective birth cohort in northern Sweden. BMJ Open 2018, 8, e022013. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Staat, M.A.; DeFranco, E.A.; McNeal, M.M.; Cline, A.R.; Conrey, S.C.; Schlaudecker, E.P.; Piasecki, A.M.; Burke, R.M.; Niu, L.; et al. Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis in US Mothers and Children Aged 0-2: PREVAIL Cohort Study. JMIR Res. Protoc. 2021, 10, e22222. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.-K.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E.; et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef]
- Koh, Y.Q.; Almughlliq, F.B.; Vaswani, K.; Peiris, H.N.; Mitchell, M.D. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front. Biosci. 2018, 23, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Wijenayake, S.; Eisha, S.; Tawhidi, Z.; Pitino, M.A.; Steele, M.A.; Fleming, A.S.; McGowan, P.O. Comparison of methods for pre-processing, exosome isolation, and RNA extraction in unpasteurized bovine and human milk. PLoS ONE 2021, 16, e0257633. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Boquien, C.-Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef]
- Parker, M.G.; Stellwagen, L.M.; Noble, L.; Kim, J.H.; Poindexter, B.B.; Puopolo, K.M. Promoting Human Milk and Breastfeeding for the Very Low Birth Weight Infant. Pediatrics 2021, 148, 054272. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. North Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [Green Version]
- Babaker, M.A.; Aljoud, F.A.; Alkhilaiwi, F.; Algarni, A.; Ahmed, A.; Khan, M.I.; Saadeldin, I.M.; Alzahrani, F.A. The Therapeutic Potential of Milk Extracellular Vesicles on Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 6812. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Acebo, L.; de Las Hazas, M.-C.L.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for miRNA-Based Therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef]
- Zonneveld, M.I.; Brisson, A.R.; van Herwijnen, M.J.C.; Tan, S.; van de Lest, C.H.A.; Redegeld, F.A.; Garssen, J.; Wauben, M.H.M.; Nolte-’t Hoen, E.N.M. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell. Vesicles 2014, 3, 24215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyczynska, U.; Bartlomiejczyk, M.A.; Stanczak, M.M.; Sztromwasser, P.; Wesolowska, A.; Barbarska, O.; Pawlikowska, E.; Fendler, W. Impact of processing method on donated human breast milk microRNA content. PLoS ONE 2020, 15, e0236126. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Kandimalla, R.; Aqil, F.; Alhakeem, S.S.; Jeyabalan, J.; Tyagi, N.; Agrawal, A.; Yan, J.; Spencer, W.; Bondada, S.; Gupta, R.C. Targeted Oral Delivery of Paclitaxel Using Colostrum-Derived Exosomes. Cancers 2021, 13, 3700. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Kausar, H.; Agrawal, A.K.; Jeyabalan, J.; Kyakulaga, A.-H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Munagala, R.; Parker, L.; Gupta, R.C. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017, 8, 4100–4107. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Mudd, A.M.; Kyakulaga, A.H.; Singh, I.P.; Vadhanam, M.V.; Gupta, R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017, 393, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vashisht, M.; Rani, P.; Onteru, S.K.; Singh, D. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl. Biochem. Biotechnol. 2017, 183, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Gupta, R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Vallejo, F.; Cattivelli, A.; Del Pozo-Acebo, L.; Del Saz, A.; López de Las Hazas, M.C.; Dávalos, A.; Espín, J.C. Milk-Derived Exosomes as Nanocarriers to Deliver Curcumin and Resveratrol in Breast Tissue and Enhance Their Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 2860. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, S.; Rani, P.; Onteru, S.K.; Singh, D. Small Interfering RNA in Milk Exosomes Is Resistant to Digestion and Crosses the Intestinal Barrier In Vitro. J. Agric. Food Chem. 2017, 65, 9506–9513. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Xu, H.; Zuo, L.; Li, C.; Qiao, G.; Guo, M.; Zheng, L.; Leitgeb, M.; Lin, X. Exosomes-coated bcl-2 siRNA inhibits the growth of digestive system tumors both in vitro and in vivo. Int. J. Biol. Macromol. 2020, 161, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Wilcher, S.A.; Gupta, R.C. Milk exosomes—Natural nanoparticles for siRNA delivery. Cancer Lett. 2019, 449, 186–195. [Google Scholar] [CrossRef]
- Matsuda, A.; Moirangthem, A.; Angom, R.S.; Ishiguro, K.; Driscoll, J.; Yan, I.K.; Mukhopadhyay, D.; Patel, T. Safety of bovine milk derived extracellular vesicles used for delivery of RNA therapeutics in zebrafish and mice. J. Appl. Toxicol. 2020, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; de Vrij, J.; van der Vlist, E.J.; Geragousian, B.; van Bloois, L.; Mastrobattista, E.; Schiffelers, R.M.; Wauben, M.H.M.; Broekman, M.L.D.; Nolte-’t Hoen, E.N.M. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J. Control. release Off. J. Control. Release Soc. 2015, 200, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Staufer, O.; Dietrich, F.; Rimal, R.; Schröter, M.; Fabritz, S.; Boehm, H.; Singh, S.; Möller, M.; Platzman, I.; Spatz, J.P. Bottom-up assembly of biomedical relevant fully synthetic extracellular vesicles. Sci. Adv. 2021, 7, eabg6666. [Google Scholar] [CrossRef]
- Geiger, A.; Walker, A.; Nissen, E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem. Biophys. Res. Commun. 2015, 467, 303–309. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).