Liver Regeneration by Hematopoietic Stem Cells: Have We Reached the End of the Road?
Abstract
1. Introduction
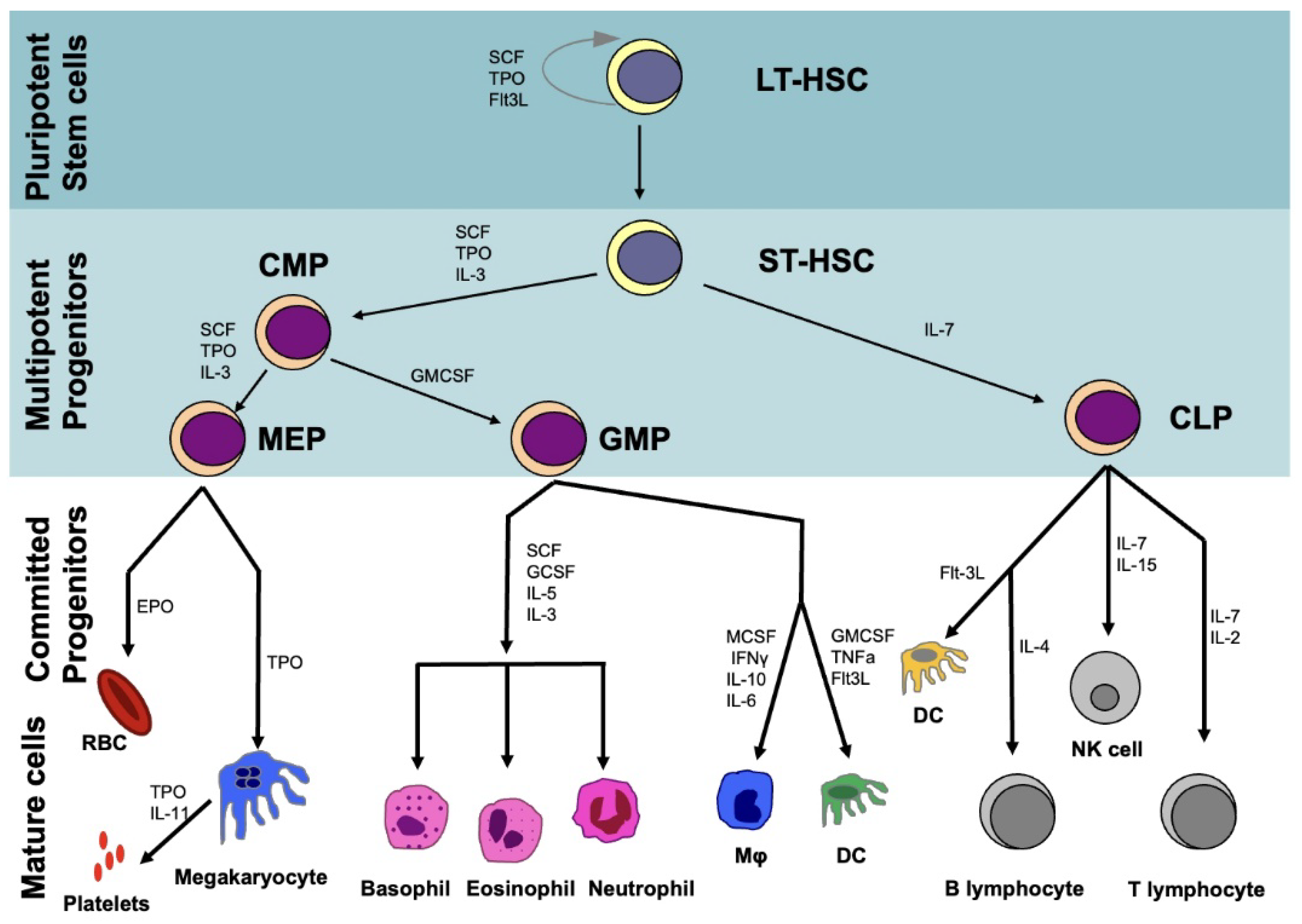
2. Hematopoietic System
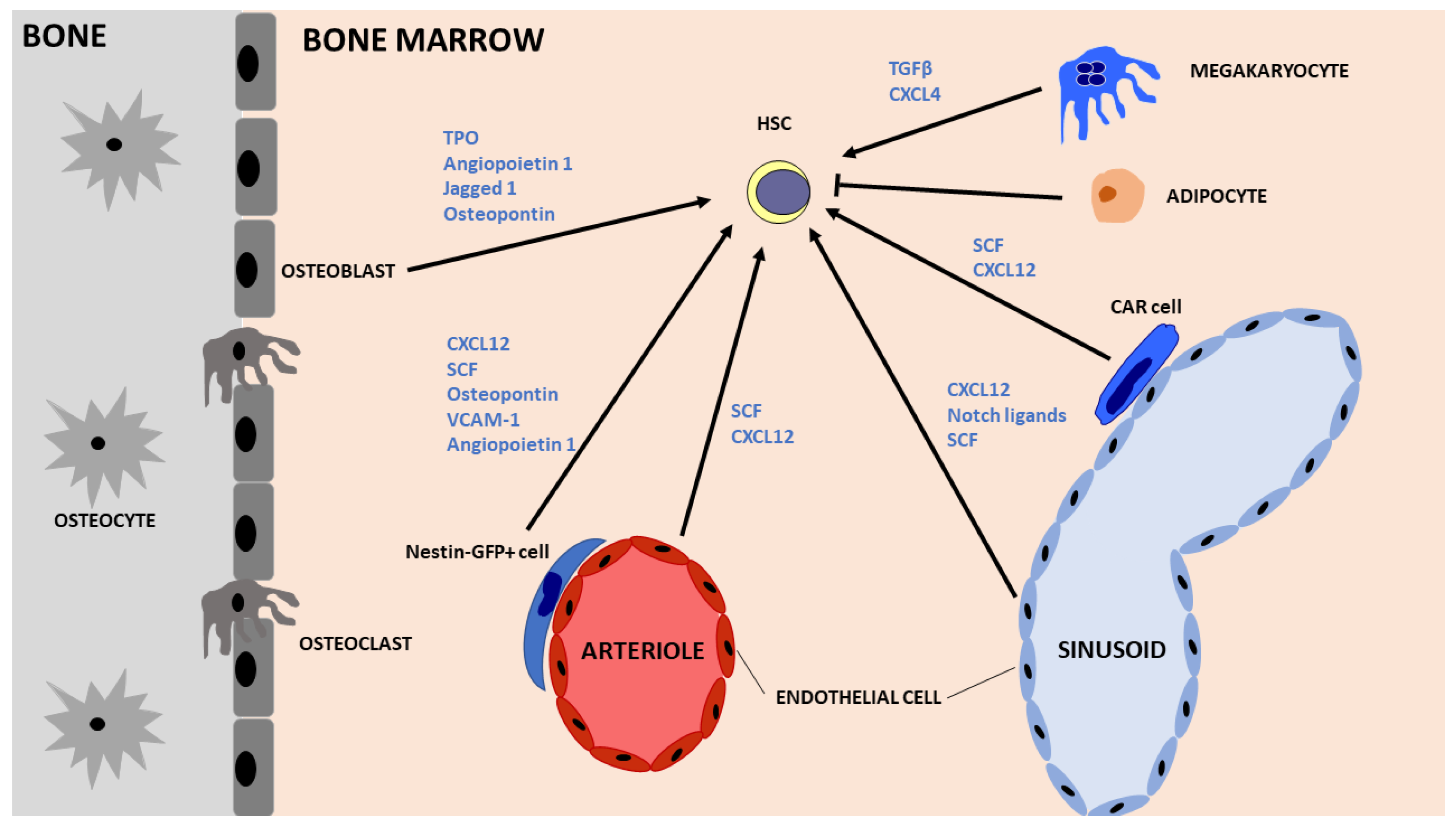
2.1. Hematopoietic Stem Cells (HSCs)
2.2. Embryonic Hematopoiesis
3. Fetal Liver Crosstalk with HSCs
4. Inherent Liver Regeneration
5. HSC-Mediated Liver Regeneration
6. Clinical Trials of HSC Transplantation or Mobilisation in Patients with Liver Disease
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trefts, E.; Gannon, M.; Wasserman, D.H. The Liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Furuya, K.; Zheng, Y.-W.; Oda, T. Novel Alternative Transplantation Therapy for Orthotopic Liver Transplantation in Liver Failure: A Systematic Review. World J. Transplant. 2020, 10, 64–78. [Google Scholar] [CrossRef]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human Hepatocyte Transplantation for Liver Disease: Current Status and Future Perspectives. Pediatr. Res. 2018, 83, 232–240. [Google Scholar] [CrossRef]
- Giancotti, A.; D’Ambrosio, V.; Corno, S.; Pajno, C.; Carpino, G.; Amato, G.; Vena, F.; Mondo, A.; Spiniello, L.; Monti, M.; et al. Current Protocols and Clinical Efficacy of Human Fetal Liver Cell Therapy in Patients with Liver Disease: A Literature Review. Cytotherapy 2022, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Nikokiraki, C.; Psaraki, A.; Roubelakis, M.G. The Potential Clinical Use of Stem/Progenitor Cells and Organoids in Liver Diseases. Cells 2022, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Hackl, V.; Esser, H.; Meszaros, A.T.; Fodor, M.; Öfner, D.; Troppmair, J.; Schneeberger, S.; Hautz, T. Cell-Based Regeneration and Treatment of Liver Diseases. Int. J. Mol. Sci. 2021, 22, 10276. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Trohatou, O.; Roubelakis, M.G. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell. Reprogramming 2017, 19, 217–224. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, S.; Shi, X.; Cao, H.; Li, L. A Pooled Analysis of Mesenchymal Stem Cell-Based Therapy for Liver Disease. Stem Cell Res. Ther. 2018, 9, 72. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Wu, X.; Xu, X.; Niu, J. The Assessment of Mesenchymal Stem Cells Therapy in Acute on Chronic Liver Failure and Chronic Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Stem Cell Res. Ther. 2022, 13, 204. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Afshari, A.; Shamdani, S.; Uzan, G.; Naserian, S.; Azarpira, N. Different Approaches for Transformation of Mesenchymal Stem Cells into Hepatocyte-like Cells. Stem Cell Res. Ther. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yuan, L.; An, Z.; Shi, D.; Xin, J.; Jiang, J.; Ren, K.; Chen, J.; Guo, B.; Zhou, X.; et al. DLL4 Restores Damaged Liver by Enhancing HBMSC Differentiation into Cholangiocytes. Stem Cell Res. 2020, 47, 101900. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Takeuchi, S.; Watanabe, T.; Yoshida, T.; Nojiri, S.; Ogawa, M.; Terai, S. Mesenchymal Stem Cell Therapies for Liver Cirrhosis: MSCs as “Conducting Cells” for Improvement of Liver Fibrosis and Regeneration. Inflamm. Regen. 2019, 39, 18. [Google Scholar] [CrossRef] [PubMed]
- Psaraki, A.; Ntari, L.; Karakostas, C.; Korrou-Karava, D.; Roubelakis, M.G. Extracellular Vesicles Derived from Mesenchymal Stem/Stromal Cells: The Regenerative Impact in Liver Diseases. Hepatology 2022, 75, 1590–1603. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Hannan, N.R.F.; Segeritz, C.-P.; Touboul, T.; Vallier, L. Production of Hepatocyte-like Cells from Human Pluripotent Stem Cells. Nat. Protoc. 2013, 8, 430–437. [Google Scholar] [CrossRef]
- Basma, H.; Soto–Gutiérrez, A.; Yannam, G.R.; Liu, L.; Ito, R.; Yamamoto, T.; Ellis, E.; Carson, S.D.; Sato, S.; Chen, Y.; et al. Differentiation and Transplantation of Human Embryonic Stem Cell–Derived Hepatocytes. Gastroenterology 2009, 136, 990–999.e4. [Google Scholar] [CrossRef]
- Tolosa, L.; Caron, J.; Hannoun, Z.; Antoni, M.; López, S.; Burks, D.; Castell, J.V.; Weber, A.; Gomez-Lechon, M.-J.; Dubart-Kupperschmitt, A. Transplantation of HESC-Derived Hepatocytes Protects Mice from Liver Injury. Stem Cell Res. Ther. 2015, 6, 246. [Google Scholar] [CrossRef]
- Tasnim, F.; Xing, J.; Huang, X.; Mo, S.; Wei, X.; Tan, M.-H.; Yu, H. Generation of Mature Kupffer Cells from Human Induced Pluripotent Stem Cells. Biomaterials 2019, 192, 377–391. [Google Scholar] [CrossRef]
- Povero, D.; Pinatel, E.M.; Leszczynska, A.; Goyal, N.P.; Nishio, T.; Kim, J.; Kneiber, D.; de Araujo Horcel, L.; Eguchi, A.; Ordonez, P.M.; et al. Human Induced Pluripotent Stem Cell–Derived Extracellular Vesicles Reduce Hepatic Stellate Cell Activation and Liver Fibrosis. JCI Insight 2019, 4, e125652. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Fu, X.; Xu, Y. The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Front. Immunol. 2017, 8, 645. [Google Scholar] [CrossRef]
- Yoshihara, M.; Hayashizaki, Y.; Murakawa, Y. Genomic Instability of IPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev. Rep. 2017, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef]
- Lan, L.; Liu, R.; Qin, L.-Y.; Cheng, P.; Liu, B.-W.; Zhang, B.-Y.; Ding, S.-Z.; Li, X.-L. Transplantation of Bone Marrow-Derived Endothelial Progenitor Cells and Hepatocyte Stem Cells from Liver Fibrosis Rats Ameliorates Liver Fibrosis. World J. Gastroenterol. 2018, 24, 237–247. [Google Scholar] [CrossRef]
- Kaur, S.; Tripathi, D.; Dongre, K.; Garg, V.; Rooge, S.; Mukopadhyay, A.; Sakhuja, P.; Sarin, S.K. Increased Number and Function of Endothelial Progenitor Cells Stimulate Angiogenesis by Resident Liver Sinusoidal Endothelial Cells (SECs) in Cirrhosis through Paracrine Factors. J. Hepatol. 2012, 57, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, E.; Kin, M.; Torimura, T.; Nakamura, T.; Kumemura, H.; Hanada, S.; Hisamoto, T.; Yoshida, T.; Kawaguchi, T.; Baba, S.; et al. Endothelial Progenitor Cell Transplantation Improves the Survival Following Liver Injury in Mice. Gastroenterology 2006, 130, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Z.-D.; Wu, N.; Cong, X.; Fei, R.; Chen, H.-S.; Wei, L. Transplanted Endothelial Progenitor Cells Ameliorate Carbon Tetrachloride-Induced Liver Cirrhosis in Rats. Liver Transplant. 2009, 15, 1092–1100. [Google Scholar] [CrossRef]
- D’Avola, D.; Fernández-Ruiz, V.; Carmona-Torre, F.; Méndez, M.; Pérez-Calvo, J.; Prósper, F.; Andreu, E.; Herrero, J.I.; Iñarrairaegui, M.; Fuertes, C.; et al. Phase 1–2 Pilot Clinical Trial in Patients with Decompensated Liver Cirrhosis Treated with Bone Marrow–Derived Endothelial Progenitor Cells. Transl. Res. 2017, 188, 80–91.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, L.; Ma, X.; Ma, W.; Wang, C.; Lu, Y.; Chen, Y.; An, L.; An, W.; Yang, Y. Monitoring of Intrasplenic Hepatocyte Transplantation for Acute-on-Chronic Liver Failure: A Prospective Five-Year Follow-Up Study. Transplant. Proc. 2014, 46, 192–198. [Google Scholar] [CrossRef]
- Bilir, B.; Guinette, D.; Karrer, F.; Kumpe, D.; Krysl, J.; Stephens, J.; Mcgavran, L.; Ostrowska, A.; Durham, J. Hepatocyte Transplantation in Acute Liver Failure. Liver Transplant. 2000, 6, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pareja, E.; Gomez-Lechon, M.J.; Cortes, M.; Bonora-Centelles, A.; Castell, J.V.; Mir, J. Human Hepatocyte Transplantation in Patients with Hepatic Failure Awaiting a Graft. Eur. Surg. Res. 2013, 50, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Overturf, K.; Al-Dhalimy, M.; Tanguay, R.; Brantly, M.; Ou, C.-N.; Finegold, M.; Grompe, M. Hepatocytes Corrected by Gene Therapy are Selected in Vivo in a Murine Model of Hereditary Tyrosinaemia Type I. Nat. Genet. 1996, 12, 266–273. [Google Scholar] [CrossRef]
- Ding, J.; Yannam, G.R.; Roy-Chowdhury, N.; Hidvegi, T.; Basma, H.; Rennard, S.I.; Wong, R.J.; Avsar, Y.; Guha, C.; Perlmutter, D.H.; et al. Spontaneous Hepatic Repopulation in Transgenic Mice Expressing Mutant Human A1-Antitrypsin by Wild-Type Donor Hepatocytes. J. Clin. Investig. 2011, 121, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Chaijitraruch, N.; Fitzpatrick, E.; Bansal, S.; Filippi, C.; Lehec, S.C.; Heaton, N.D.; Kane, P.; Verma, A.; Hughes, R.D.; et al. Alginate Microencapsulated Human Hepatocytes for the Treatment of Acute Liver Failure in Children. J. Hepatol. 2020, 72, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, C.M.; Syed, I.H.; Qamar, A.; Taher-Uz, Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation 1994, 58, 951–952. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Habeeb, A.; Parveen, N.; Naseem, B.; Babu, R.P.; Capoor, A.K.; Habibullah, C.M. Peritoneal Transplantation of Human Fetal Hepatocytes for the Treatment of Acute Fatty Liver of Pregnancy: A Case Report. Trop. Gastroenterol. 2005, 25, 141–143. [Google Scholar]
- Patterson, A.M.; Pelus, L.M. G-CSF in Stem Cell Mobilization: New Insights, New Questions. Ann. Blood 2016, 2, 10. [Google Scholar] [CrossRef]
- Sawai, C.M.; Babovic, S.; Upadhaya, S.; Knapp, D.J.H.F.; Lavin, Y.; Lau, C.M.; Goloborodko, A.; Feng, J.; Fujisaki, J.; Ding, L.; et al. Hematopoietic Stem Cells are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity 2016, 45, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zheng, Z.; Cheng, T. New Paradigms on Hematopoietic Stem Cell Differentiation. Protein Cell 2020, 11, 34–44. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Igarashi, K.J.; Nakauchi, H. Haematopoietic Stem Cell Self-Renewal In Vivo and Ex Vivo. Nat. Rev. Genet. 2020, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gurudutta, G. Epigenetic Regulation of Hematopoietic Stem Cells. Int. J. Stem Cells 2016, 9, 36–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pouzolles, M.; Oburoglu, L.; Taylor, N.; Zimmermann, V.S. Hematopoietic Stem Cell Lineage Specification. Curr. Opin. Hematol. 2016, 23, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Hu, W.; Park, C.Y. The Role of MicroRNAs in Hematopoietic Stem Cell and Leukemic Stem Cell Function. Ther. Adv. Hematol. 2011, 2, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.; Mauch, P.; Vergilio, J.-A.; Sackstein, R.; Down, J.D. Distribution of Hematopoietic Stem Cells in the Bone Marrow According to Regional Hypoxia. Proc. Natl. Acad. Sci. USA 2007, 104, 5431–5436. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Pivarnik, G.; Winkel, B.; Canty, K.J.; Harley, B.; Mahoney, J.E.; Park, S.-Y.; Lu, J.; Protopopov, A.; Silberstein, L.E. Quantitative Imaging of Haematopoietic Stem and Progenitor Cell Localization and Hypoxic Status in the Bone Marrow Microenvironment. Nat. Cell Biol. 2013, 15, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Frenette, P.S. Haematopoietic Stem Cell Activity and Interactions with the Niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Luis, T.C.; Naber, B.A.E.; Roozen, P.P.C.; Brugman, M.H.; de Haas, E.F.E.; Ghazvini, M.; Fibbe, W.E.; van Dongen, J.J.M.; Fodde, R.; Staal, F.J.T. Canonical Wnt Signaling Regulates Hematopoiesis in a Dosage-Dependent Fashion. Cell Stem Cell 2011, 9, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Ribatti, D. Bone Niches, Hematopoietic Stem Cells, and Vessel Formation. Int. J. Mol. Sci. 2017, 18, 151. [Google Scholar] [CrossRef]
- Notta, F.; Doulatov, S.; Laurenti, E.; Poeppl, A.; Jurisica, I.; Dick, J.E. Isolation of Single Human Hematopoietic Stem Cells Capable of Long-Term Multilineage Engraftment. Science 2011, 333, 218–221. [Google Scholar] [CrossRef]
- Anjos-Afonso, F.; Currie, E.; Palmer, H.G.; Foster, K.E.; Taussig, D.C.; Bonnet, D. CD34− Cells at the Apex of the Human Hematopoietic Stem Cell Hierarchy Have Distinctive Cellular and Molecular Signatures. Cell Stem Cell 2013, 13, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Anjos-Afonso, F.; Buettner, F.; Mian, S.A.; Rhys, H.; Perez-Lloret, J.; Garcia-Albornoz, M.; Rastogi, N.; Ariza-McNaughton, L.; Bonnet, D. Single Cell Analyses Identify a Highly Regenerative and Homogenous Human CD34+ Hematopoietic Stem Cell Population. Nat. Commun. 2022, 13, 2048. [Google Scholar] [CrossRef]
- Knapp, D.J.H.F.; Hammond, C.A.; Hui, T.; van Loenhout, M.T.J.; Wang, F.; Aghaeepour, N.; Miller, P.H.; Moksa, M.; Rabu, G.M.; Beer, P.A.; et al. Single-Cell Analysis Identifies a CD33+ Subset of Human Cord Blood Cells with High Regenerative Potential. Nat. Cell Biol. 2018, 20, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Tavian, M.; Hallais, M.F.; Peault, B. Emergence of Intraembryonic Hematopoietic Precursors in the Pre-Liver Human Embryo. Development 1999, 126, 793–803. [Google Scholar] [CrossRef]
- Palis, J.; Robertson, S.; Kennedy, M.; Wall, C.; Keller, G. Development of Erythroid and Myeloid Progenitors in the Yolk Sac and Embryo Proper of the Mouse. Development 1999, 126, 5073–5084. [Google Scholar] [CrossRef] [PubMed]
- Vink, C.S.; Mariani, S.A.; Dzierzak, E. Embryonic Origins of the Hematopoietic System: Hierarchies and Heterogeneity. Hemasphere 2022, 6, e737. [Google Scholar] [CrossRef]
- Ajami, B.; Samusik, N.; Wieghofer, P.; Ho, P.P.; Crotti, A.; Bjornson, Z.; Prinz, M.; Fantl, W.J.; Nolan, G.P.; Steinman, L. Single-Cell Mass Cytometry Reveals Distinct Populations of Brain Myeloid Cells in Mouse Neuroinflammation and Neurodegeneration Models. Nat. Neurosci. 2018, 21, 541–551. [Google Scholar] [CrossRef]
- Soares-da-Silva, F.; Freyer, L.; Elsaid, R.; Burlen-Defranoux, O.; Iturri, L.; Sismeiro, O.; Pinto-do-Ó, P.; Gomez-Perdiguero, E.; Cumano, A. Yolk Sac, but Not Hematopoietic Stem Cell–Derived Progenitors, Sustain Erythropoiesis throughout Murine Embryonic Life. J. Exp. Med. 2021, 218, e20201729. [Google Scholar] [CrossRef]
- McGrath, K.E.; Frame, J.M.; Fegan, K.H.; Bowen, J.R.; Conway, S.J.; Catherman, S.C.; Kingsley, P.D.; Koniski, A.D.; Palis, J. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell Rep. 2015, 11, 1892–1904. [Google Scholar] [CrossRef]
- Tavian, M.; Coulombel, L.; Luton, D.; Clemente, H.S.; Dieterlen-Lièvre, F.; Péault, B. Aorta-Associated CD34+ Hematopoietic Cells in the Early Human Embryo. Blood 1996, 87, 67–72. [Google Scholar] [CrossRef]
- Bertrand, J.Y.; Chi, N.C.; Santoso, B.; Teng, S.; Stainier, D.Y.R.; Traver, D. Haematopoietic Stem Cells Derive Directly from Aortic Endothelium during Development. Nature 2010, 464, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Boisset, J.-C.; van Cappellen, W.; Andrieu-Soler, C.; Galjart, N.; Dzierzak, E.; Robin, C. In Vivo Imaging of Haematopoietic Cells Emerging from the Mouse Aortic Endothelium. Nature 2010, 464, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Hemmati, H.D.; Wandycz, A.M.; Weissman, I.L. The Purification and Characterization of Fetal Liver Hematopoietic Stem Cells. Proc. Natl. Acad. Sci. USA 1995, 92, 10302–10306. [Google Scholar] [CrossRef]
- Gao, X.; Xu, C.; Asada, N.; Frenette, P.S. The Hematopoietic Stem Cell Niche: From Embryo to Adult. Development 2018, 145, dev139691. [Google Scholar] [CrossRef] [PubMed]
- Soares-da-Silva, F.; Peixoto, M.; Cumano, A.; Pinto-do-Ó, P. Crosstalk Between the Hepatic and Hematopoietic Systems During Embryonic Development. Front. Cell Dev. Biol. 2020, 8, 612. [Google Scholar] [CrossRef]
- Hirsch, E.; Iglesias, A.; Potocnik, A.J.; Hartmann, U.; Fässler, R. Impaired Migration but Not Differentiation of Haematopoietic Stem Cells in the Absence of Β1 Integrins. Nature 1996, 380, 171–175. [Google Scholar] [CrossRef]
- Roy, V.; Verfaillie, C.M. Expression and Function of Cell Adhesion Molecules on Fetal Liver, Cord Blood and Bone Marrow Hematopoietic Progenitors. Exp. Hematol. 1999, 27, 302–312. [Google Scholar] [CrossRef]
- Chen, J.Y.; Miyanishi, M.; Wang, S.K.; Yamazaki, S.; Sinha, R.; Kao, K.S.; Seita, J.; Sahoo, D.; Nakauchi, H.; Weissman, I.L. Hoxb5 Marks Long-Term Haematopoietic Stem Cells and Reveals a Homogenous Perivascular Niche. Nature 2016, 530, 223–227. [Google Scholar] [CrossRef]
- Ara, T.; Tokoyoda, K.; Sugiyama, T.; Egawa, T.; Kawabata, K.; Nagasawa, T. Long-Term Hematopoietic Stem Cells Require Stromal Cell-Derived Factor-1 for Colonizing Bone Marrow during Ontogeny. Immunity 2003, 19, 257–267. [Google Scholar] [CrossRef]
- Ema, H.; Nakauchi, H. Expansion of Hematopoietic Stem Cells in the Developing Liver of a Mouse Embryo. Blood 2000, 95, 2284–2288. [Google Scholar] [CrossRef]
- Borge, O.; Ramsfjell, V.; Veiby, O.; Murphy, M.J.; Lok, S.; Jacobsen, S. Thrombopoietin, but Not Erythropoietin Promotes Viability and Inhibits Apoptosis of Multipotent Murine Hematopoietic Progenitor Cells in Vitro. Blood 1996, 88, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Petit-Cocault, L.; Volle-Challier, C.; Fleury, M.; Péault, B.; Souyri, M. Dual Role of Mpl Receptor during the Establishment of Definitive Hematopoiesis. Development 2007, 134, 3031–3040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, K.S.; Kulkeaw, K.; Nakanishi, Y.; Sugiyama, D. Expression of Cytokine and Extracellular Matrix MRNAs in Fetal Hepatic Stellate Cells. Genes Cells 2017, 22, 836–844. [Google Scholar] [CrossRef]
- Chou, S.; Flygare, J.; Lodish, H.F. Fetal Hepatic Progenitors Support Long-Term Expansion of Hematopoietic Stem Cells. Exp. Hematol. 2013, 41, 479–490.e4. [Google Scholar] [CrossRef]
- Ciriza, J.; Thompson, H.; Petrosian, R.; Manilay, J.O.; García-Ojeda, M.E. The Migration of Hematopoietic Progenitors from the Fetal Liver to the Fetal Bone Marrow: Lessons Learned and Possible Clinical Applications. Exp. Hematol. 2013, 41, 411–423. [Google Scholar] [CrossRef]
- Wittig, O.; Paez-Cortez, J.; Cardier, J.E. Liver Sinusoidal Endothelial Cells Promote B Lymphopoiesis from Primitive Hematopoietic Cells. Stem Cells Dev. 2010, 19, 341–350. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Cai, X. Liver Regeneration and Cell Transplantation for End-Stage Liver Disease. Biomolecules 2021, 11, 1907. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.A.; Glorioso, J.M.; Nyberg, S.L. Liver Regeneration. Transl. Res. 2014, 163, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Liver Regeneration. J. Cell. Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ma, K.; Zhang, J.; Qatanani, M.; Cuvillier, J.; Liu, J.; Dong, B.; Huang, X.; Moore, D.D. Nuclear Receptor-Dependent Bile Acid Signaling is Required for Normal Liver Regeneration. Science 2006, 312, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.H.; Kalinichenko, V.V.; Holterman, A.-X.L.; Wang, X. Transcription Factors in Liver Development, Differentiation, and Regeneration. Hepatology 2003, 38, ajhep09034. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, Y.V.; Antonyan, S.Z.; Zharikova, T.S.; Tupikin, K.A.; Kalinin, D.V.; Zharikov, Y.O. Molecular Pathways of Liver Regeneration: A Comprehensive Review. World J. Hepatol. 2021, 13, 270–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Cho, G.-S.; Han, C.; Park, D.-H.; Park, H.-K.; Woo, D.-H.; Kim, J.-H. Current Understanding of Stem Cell and Secretome Therapies in Liver Diseases. Tissue Eng. Regen. Med. 2017, 14, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Steiling, H.; Wüstefeld, T.; Bugnon, P.; Brauchle, M.; Fässler, R.; Teupser, D.; Thiery, J.; Gordon, J.I.; Trautwein, C.; Werner, S. Fibroblast Growth Factor Receptor Signalling is Crucial for Liver Homeostasis and Regeneration. Oncogene 2003, 22, 4380–4388. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, E.; Sakisaka, S.; Matsuo, K.; Tanikawa, K.; Sata, M. Expression and Role of Vascular Endothelial Growth Factor in Liver Regeneration after Partial Hepatectomy in Rats. J. Histochem. Cytochem. 2001, 49, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Desbois-Mouthon, C.; Wendum, D.; Cadoret, A.; Rey, C.; Leneuve, P.; Blaise, A.; Housset, C.; Tronche, F.; le Bouc, Y.; Holzenberger, M. Hepatocyte Proliferation during Liver Regeneration is Impaired in Mice with Liver-specific IGF-1R Knockout. FASEB J. 2006, 20, 773–775. [Google Scholar] [CrossRef]
- Nelsen, C.J.; Rickheim, D.G.; Timchenko, N.A.; Stanley, M.W.; Albrecht, J.H. Transient Expression of Cyclin D1 is Sufficient to Promote Hepatocyte Replication and Liver Growth in Vivo. Cancer Res. 2001, 61, 8564–8568. [Google Scholar] [PubMed]
- Tan, X.; Behari, J.; Cieply, B.; Michalopoulos, G.K.; Monga, S.P.S. Conditional Deletion of β-Catenin Reveals Its Role in Liver Growth and Regeneration. Gastroenterology 2006, 131, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.-M.; Hou, L.-H.; Dou, G.-R.; Wang, Y.-C.; Hu, X.-B.; He, F.; Feng, F.; Zhang, H.-W.; Liang, Y.-M.; et al. Disruption of the Transcription Factor Recombination Signal-Binding Protein-Jκ (RBP-J) Leads to Veno-Occlusive Disease and Interfered Liver Regeneration in Mice. Hepatology 2009, 49, 268–277. [Google Scholar] [CrossRef]
- Karkampouna, S.; ten Dijke, P.; Dooley, S.; Kruithof-de Julio, M. TGFβ Signaling in Liver Regeneration. Curr. Pharm. Des. 2012, 18, 4103–4113. [Google Scholar] [CrossRef]
- Huck, I.; Gunewardena, S.; Espanol-Suner, R.; Willenbring, H.; Apte, U. Hepatocyte Nuclear Factor 4 Alpha Activation is Essential for Termination of Liver Regeneration in Mice. Hepatology 2019, 70, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-P.; Jiang, Y.-Z.; Sun, L.-Y.; Zhu, Z.-J. Therapeutic Effect and Safety of Stem Cell Therapy for Chronic Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Stem Cell Res. Ther. 2020, 11, 419. [Google Scholar] [CrossRef]
- Moya, I.M.; Halder, G. Hippo–YAP/TAZ Signalling in Organ Regeneration and Regenerative Medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef] [PubMed]
- De Silvestro, G.; Vicarioto, M.; Donadel, C.; Menegazzo, M.; Marson, P.; Corsini, A. Mobilization of Peripheral Blood Hematopoietic Stem Cells Following Liver Resection Surgery. Hepatogastroenterology 2004, 51, 805–810. [Google Scholar] [PubMed]
- Fujii, H.; Hirose, T.; Oe, S.; Yasuchika, K.; Azuma, H.; Fujikawa, T.; Nagao, M.; Yamaoka, Y. Contribution of Bone Marrow Cells to Liver Regeneration after Partial Hepatectomy in Mice. J. Hepatol. 2002, 36, 653–659. [Google Scholar] [CrossRef]
- Kollet, O.; Shivtiel, S.; Chen, Y.-Q.; Suriawinata, J.; Thung, S.N.; Dabeva, M.D.; Kahn, J.; Spiegel, A.; Dar, A.; Samira, S.; et al. HGF, SDF-1, and MMP-9 are Involved in Stress-Induced Human CD34+ Stem Cell Recruitment to the Liver. J. Clin. Investig. 2003, 112, 160–169. [Google Scholar] [CrossRef]
- Crosby, H.A.; Lalor, P.F.; Ross, E.; Newsome, P.N.; Adams, D.H. Adhesion of Human Haematopoietic (CD34+) Stem Cells to Human Liver Compartments is Integrin and CD44 Dependent and Modulated by CXCR3 and CXCR4. J. Hepatol. 2009, 51, 734–749. [Google Scholar] [CrossRef]
- King, A.; Houlihan, D.D.; Kavanagh, D.; Haldar, D.; Luu, N.; Owen, A.; Suresh, S.; Than, N.N.; Reynolds, G.; Penny, J.; et al. Sphingosine-1-Phosphate Prevents Egress of Hematopoietic Stem Cells from Liver to Reduce Fibrosis. Gastroenterology 2017, 153, 233–248.e16. [Google Scholar] [CrossRef]
- Forbes, S.J.; Newsome, P.N. Liver Regeneration—Mechanisms and Models to Clinical Application. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 473–485. [Google Scholar] [CrossRef]
- Petersen, B.E.; Bowen, W.C.; Patrene, K.D.; Mars, W.M.; Sullivan, A.K.; Murase, N.; Boggs, S.S.; Greenberger, J.S.; Goff, J.P. Bone Marrow as a Potential Source of Hepatic Oval Cells. Science 1999, 284, 1168–1170. [Google Scholar] [CrossRef]
- Lagasse, E.; Connors, H.; Al-Dhalimy, M.; Reitsma, M.; Dohse, M.; Osborne, L.; Wang, X.; Finegold, M.; Weissman, I.L.; Grompe, M. Purified Hematopoietic Stem Cells Can Differentiate into Hepatocytes In Vivo. Nat. Med. 2000, 6, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.R.; Poulsom, R.; Jeffery, R.; Dhillon, A.P.; Quaglia, A.; Jacob, J.; Novelli, M.; Prentice, G.; Williamson, J.; Wright, N.A. Hepatocytes from Non-Hepatic Adult Stem Cells. Nature 2000, 406, 257. [Google Scholar] [CrossRef] [PubMed]
- Theise, N.D.; Nimmakayalu, M.; Gardner, R.; Illei, P.B.; Morgan, G.; Teperman, L.; Henegariu, O.; Krause, D.S. Liver from Bone Marrow in Humans. Hepatology 2000, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wagers, A.J.; Sherwood, R.I.; Christensen, J.L.; Weissman, I.L. Little Evidence for Developmental Plasticity of Adult Hematopoietic Stem Cells. Science 2002, 297, 2256–2259. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulos, G.; Wang, P.-R.; Russell, D.W. Transplanted Bone Marrow Regenerates Liver by Cell Fusion. Nature 2003, 422, 901–904. [Google Scholar] [CrossRef]
- Wang, X.; Willenbring, H.; Akkari, Y.; Torimaru, Y.; Foster, M.; Al-Dhalimy, M.; Lagasse, E.; Finegold, M.; Olson, S.; Grompe, M. Cell Fusion is the Principal Source of Bone-Marrow-Derived Hepatocytes. Nature 2003, 422, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Kashofer, K.; Siapati, E.K.; Bonnet, D. In Vivo Formation of Unstable Heterokaryons after Liver Damage and Hematopoietic Stem Cell/Progenitor Transplantation. Stem Cells 2006, 24, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Jaiswal, A.K.; Mukhopadhyay, A. Hepatocyte Nuclear Factor-4α Induces Transdifferentiation of Hematopoietic Cells into Hepatocytes. J. Biol. Chem. 2010, 285, 4725–4731. [Google Scholar] [CrossRef]
- Jang, Y.-Y.; Collector, M.I.; Baylin, S.B.; Diehl, A.M.; Sharkis, S.J. Hematopoietic Stem Cells Convert into Liver Cells within Days without Fusion. Nat. Cell Biol. 2004, 6, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Muraca, M.; Ferraresso, C.; Vilei, M.T.; Granato, A.; Quarta, M.; Cozzi, E.; Rugge, M.; Pauwelyn, K.A.; Caruso, M.; Avital, I.; et al. Liver Repopulation with Bone Marrow Derived Cells Improves the Metabolic Disorder in the Gunn Rat. Gut 2007, 56, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Johannessen, I.; Boyle, S.; Dalakas, E.; Mcaulay, K.A.; Samuel, K.; Rae, F.; Forrester, L.; Turner, M.L.; Hayes, P.C.; et al. Human Cord Blood-Derived Cells Can Differentiate into Hepatocytes in the Mouse Liver with No Evidence of Cellular Fusion. Gastroenterology 2003, 124, 1891–1900. [Google Scholar] [CrossRef]
- Tang, X.-P. Differentiation of Human Umbilical Cord Blood Stem Cells into Hepatocytes in Vivo and in Vitro. World J. Gastroenterol. 2006, 12, 4014. [Google Scholar] [CrossRef]
- Pedone, E.; Olteanu, V.-A.; Marucci, L.; Muñoz-Martin, M.I.; Youssef, S.A.; de Bruin, A.; Cosma, M.P. Modeling Dynamics and Function of Bone Marrow Cells in Mouse Liver Regeneration. Cell Rep. 2017, 18, 107–121. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Wang, D.; Estrov, Z.; Raj, S.; Willerson, J.T.; Yeh, E.T.H. Both Cell Fusion and Transdifferentiation Account for the Transformation of Human Peripheral Blood CD34-Positive Cells into Cardiomyocytes In Vivo. Circulation 2004, 110, 3803–3807. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Porada, G.; Porada, C.D.; Chamberlain, J.; Torabi, A.; Zanjani, E.D. Formation of Human Hepatocytes by Human Hematopoietic Stem Cells in Sheep. Blood 2004, 104, 2582–2590. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, Y.N.; Kim, J.S.; Park, M.-S.; Paik, Y.H.; Seok, J.-Y.; Chung, Y.E.; Kim, H.O.; Kim, K.S.; Ahn, S.H.; et al. Autologous Bone Marrow Infusion Activates the Progenitor Cell Compartment in Patients with Advanced Liver Cirrhosis. Cell Transplant. 2010, 19, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B. Mouse A6–Positive Hepatic Oval Cells Also Express Several Hematopoietic Stem Cell Markers. Hepatology 2003, 37, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Weimar, I.S.; Miranda, N.; Muller, E.J.; Hekman, A.; Kerst, J.M.; de Gast, G.C.; Gerritsen, W.R. Hepatocyte Growth Factor/Scatter Factor (HGF/SF) is Produced by Human Bone Marrow Stromal Cells and Promotes Proliferation, Adhesion and Survival of Human Hematopoietic Progenitor Cells (CD34+). Exp. Hematol. 1998, 26, 885–894. [Google Scholar]
- Zachman, D.K.; Goldman, D.C.; Hamlin, K.L.; Guha, C.; Fleming, W.H. Role of Hepatocyte Growth Factor in Endothelial-Dependent Hematopoietic Stem Cell Regeneration. Blood 2014, 124, 4369. [Google Scholar] [CrossRef]
- Goff, J.P.; Shields, D.S.; Petersen, B.E.; Zajac, V.F.; Michalopoulos, G.K.; Greenberger, J.S. Synergistic Effects of Hepatocyte Growth Factor on Human Cord Blood CD34 + Progenitor Cells are the Result of C-met Receptor Expression. Stem Cells 1996, 14, 592–602. [Google Scholar] [CrossRef]
- Baccin, C.; Al-Sabah, J.; Velten, L.; Helbling, P.M.; Grünschläger, F.; Hernández-Malmierca, P.; Nombela-Arrieta, C.; Steinmetz, L.M.; Trumpp, A.; Haas, S. Combined Single-Cell and Spatial Transcriptomics Reveal the Molecular, Cellular and Spatial Bone Marrow Niche Organization. Nat. Cell Biol. 2020, 22, 38–48. [Google Scholar] [CrossRef]
- Fujii, K.; Ishimaru, F.; Kozuka, T.; Matsuo, K.; Nakase, K.; Kataoka, I.; Tabayashi, T.; Shinagawa, K.; Ikeda, K.; Harada, M.; et al. Elevation of Serum Hepatocyte Growth Factor during Granulocyte Colony-Stimulating Factor-Induced Peripheral Blood Stem Cell Mobilization. Br. J. Haematol. 2004, 124, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Carstanjen, D. Interleukin-6 is a Major Effector Molecule of Short-Term G-CSF Treatment Inducing Bone Metabolism and an Acute-Phase Response. Exp. Hematol. 2001, 29, 812–821. [Google Scholar] [CrossRef]
- Garg, V.; Garg, H.; Khan, A.; Trehanpati, N.; Kumar, A.; Sharma, B.C.; Sakhuja, P.; Sarin, S.K. Granulocyte Colony–Stimulating Factor Mobilizes CD34+ Cells and Improves Survival of Patients with Acute-on-Chronic Liver Failure. Gastroenterology 2012, 142, 505–512.e1. [Google Scholar] [CrossRef] [PubMed]
- Ieda, Y.; Fujita, J.; Ieda, M.; Yagi, T.; Kawada, H.; Ando, K.; Fukuda, K. G-CSF and HGF: Combination of Vasculogenesis and Angiogenesis Synergistically Improves Recovery in Murine Hind Limb Ischemia. J. Mol. Cell. Cardiol. 2007, 42, 540–548. [Google Scholar] [CrossRef]
- Piscaglia, A.C.; Shupe, T.D.; Oh, S.; Gasbarrini, A.; Petersen, B.E. Granulocyte–Colony Stimulating Factor Promotes Liver Repair and Induces Oval Cell Migration and Proliferation in Rats. Gastroenterology 2007, 133, 619–631. [Google Scholar] [CrossRef]
- Tsolaki, E.; Athanasiou, E.; Gounari, E.; Zogas, N.; Siotou, E.; Yiangou, M.; Anagnostopoulos, A.; Yannaki, E. Hematopoietic Stem Cells and Liver Regeneration: Differentially Acting Hematopoietic Stem Cell Mobilization Agents Reverse Induced Chronic Liver Injury. Blood Cells Mol. Dis. 2014, 53, 124–132. [Google Scholar] [CrossRef]
- Zekri, A.-R.N.; Salama, H.; Medhat, E.; Musa, S.; Abdel-Haleem, H.; Ahmed, O.S.; Khedr, H.A.H.; Lotfy, M.M.; Zachariah, K.S.; Bahnassy, A.A. The Impact of Repeated Autologous Infusion of Haematopoietic Stem Cells in Patients with Liver Insufficiency. Stem Cell Res. Ther. 2015, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Terai, S.; Ishikawa, T.; Omori, K.; Aoyama, K.; Marumoto, Y.; Urata, Y.; Yokoyama, Y.; Uchida, K.; Yamasaki, T.; Fujii, Y.; et al. Improved Liver Function in Patients with Liver Cirrhosis after Autologous Bone Marrow Cell Infusion Therapy. Stem Cells 2006, 24, 2292–2298. [Google Scholar] [CrossRef]
- Yannaki, E.; Anagnostopoulos, A.; Kapetanos, D.; Xagorari, A.; Iordanidis, F.; Batsis, I.; Kaloyannidis, P.; Athanasiou, E.; Dourvas, G.; Kitis, G.; et al. Lasting Amelioration in the Clinical Course of Decompensated Alcoholic Cirrhosis with Boost Infusions of Mobilized Peripheral Blood Stem Cells. Exp. Hematol. 2006, 34, 1583–1587. [Google Scholar] [CrossRef]
- Gaia, S.; Smedile, A.; Omedè, P.; Olivero, A.; Sanavio, F.; Balzola, F.; Ottobrelli, A.; Abate, M.L.; Marzano, A.; Rizzetto, M.; et al. Feasibility and Safety of G-CSF Administration to Induce Bone Marrow-Derived Cells Mobilization in Patients with End Stage Liver Disease. J. Hepatol. 2006, 45, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.Y.; Levičar, N.; Pai, M.; Bachellier, P.; Dimarakis, I.; Al-Allaf, F.; M’Hamdi, H.; Thalji, T.; Welsh, J.P.; Marley, S.B.; et al. Characterization and Clinical Application of Human CD34+ Stem/Progenitor Cell Populations Mobilized into the Blood by Granulocyte Colony-Stimulating Factor. Stem Cells 2006, 24, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M. Phase 1 Human Trial of Autologous Bone Marrow-Hematopoietic Stem Cell Transplantation in Patients with Decompensated Cirrhosis. World J. Gastroenterol. 2007, 13, 3359. [Google Scholar] [CrossRef] [PubMed]
- Lyra, A.C. Feasibility and Safety of Autologous Bone Marrow Mononuclear Cell Transplantation in Patients with Advanced Chronic Liver Disease. World J. Gastroenterol. 2007, 13, 1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, L.; Han, Y.; Wang, J.; Liu, J.; Hong, L.; Fan, D. Peripheral Blood Monocytes from Patients with HBV Related Decompensated Liver Cirrhosis Can Differentiate into Functional Hepatocytes. Am. J. Hematol. 2007, 82, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Levičar, N.; Pai, M.; Habib, N.A.; Tait, P.; Jiao, L.R.; Marley, S.B.; Davis, J.; Dazzi, F.; Smadja, C.; Jensen, S.L.; et al. Long-Term Clinical Results of Autologous Infusion of Mobilized Adult Bone Marrow Derived CD34+ Cells in Patients with Chronic Liver Disease. Cell Prolif. 2007, 41, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Parveen, N.; Mahaboob, V.S.; Rajendraprasad, A.; Ravindraprakash, H.R.; Venkateswarlu, J.; Rao, S.G.A.; Narusu, M.L.; Khaja, M.N.; Pramila, R.; et al. Safety and Efficacy of Autologous Bone Marrow Stem Cell Transplantation Through Hepatic Artery for the Treatment of Chronic Liver Failure: A Preliminary Study. Transplant. Proc. 2008, 40, 1140–1144. [Google Scholar] [CrossRef]
- Han, Y.; Yan, L.; Han, G.; Zhou, X.; Hong, L.; Yin, Z.; Zhang, X.; Wang, S.; Wang, J.; Sun, A.; et al. Controlled Trials in Hepatitis B Virus-Related Decompensate Liver Cirrhosis: Peripheral Blood Monocyte Transplant versus Granulocyte–Colony-Stimulating Factor Mobilization Therapy. Cytotherapy 2008, 10, 390–396. [Google Scholar] [CrossRef]
- Pai, M.; Zacharoulis, D.; Milicevic, M.N.; Helmy, S.; Jiao, L.R.; Levičar, N.; Tait, P.; Scott, M.; Marley, S.B.; Jestice, K.; et al. Autologous Infusion of Expanded Mobilized Adult Bone Marrow-Derived CD34+ Cells into Patients with Alcoholic Liver Cirrhosis. Am. J. Gastroenterol. 2008, 103, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Salama, H. Autologous CD34 + and CD133 + Stem Cells Transplantation in Patients with End Stage Liver Disease. World J. Gastroenterol. 2010, 16, 5297. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.; Zekri, A.-R.; Zern, M.; Bahnassy, A.; Loutfy, S.; Shalaby, S.; Vigen, C.; Burke, W.; Mostafa, M.; Medhat, E.; et al. Autologous Hematopoietic Stem Cell Transplantation in 48 Patients with End-Stage Chronic Liver Diseases. Cell Transplant. 2010, 19, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Lyra, A.C.; Soares, M.B.P.; da Silva, L.F.M.; Braga, E.L.; Oliveira, S.A.; Fortes, M.F.; Silva, A.G.P.; Brustolim, D.; Genser, B.; dos Santos, R.R.; et al. Infusion of Autologous Bone Marrow Mononuclear Cells through Hepatic Artery Results in a Short-Term Improvement of Liver Function in Patients with Chronic Liver Disease: A Pilot Randomized Controlled Study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Okumoto, K.; Haga, H.; Nishise, Y.; Ishii, R.; Sato, C.; Watanabe, H.; Okada, A.; Ikeda, M.; Togashi, H.; et al. Potential Therapeutic Application of Intravenous Autologous Bone Marrow Infusion in Patients with Alcoholic Liver Cirrhosis. Stem Cells Dev. 2011, 20, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Spahr, L.; Chalandon, Y.; Terraz, S.; Kindler, V.; Rubbia-Brandt, L.; Frossard, J.-L.; Breguet, R.; Lanthier, N.; Farina, A.; Passweg, J.; et al. Autologous Bone Marrow Mononuclear Cell Transplantation in Patients with Decompensated Alcoholic Liver Disease: A Randomized Controlled Trial. PLoS ONE 2013, 8, e53719. [Google Scholar] [CrossRef]
- Bai, Y.-Q. Outcomes of Autologous Bone Marrow Mononuclear Cell Transplantation in Decompensated Liver Cirrhosis. World J. Gastroenterol. 2014, 20, 8660. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.; Zhou, J.; Huang, L.W.; He, C.P.; Ling, K.; Zhou, H.C.; Wen, Q.M.; Wang, X.M. Curative Effect of Combined Lamivudine, Adefovir Dipivoxil, and Stem Cell Transplantation on Decompensated Hepatitis B Cirrhosis. Genet. Mol. Res. 2014, 13, 9336–9342. [Google Scholar] [CrossRef]
- Andreone, P.; Catani, L.; Margini, C.; Brodosi, L.; Lorenzini, S.; Sollazzo, D.; Nicolini, B.; Giordano, R.; Montemurro, T.; Rizzi, S.; et al. Reinfusion of Highly Purified CD133+ Bone Marrow-Derived Stem/Progenitor Cells in Patients with End-Stage Liver Disease: A Phase I Clinical Trial. Dig. Liver Dis. 2015, 47, 1059–1066. [Google Scholar] [CrossRef]
- Sharma, M.; Rao, P.N.; Sasikala, M.; Kuncharam, M.R.; Reddy, C.; Gokak, V.; Raju, B.; Singh, J.R.; Nag, P.; Reddy, D.N. Autologous Mobilized Peripheral Blood CD34 + Cell Infusion in Non-Viral Decompensated Liver Cirrhosis. World J. Gastroenterol. 2015, 21, 7264–7271. [Google Scholar] [CrossRef]
- Deng, Q.; Cai, T.; Zhang, S.; Hu, A.; Zhang, X.; Wang, Y.; Huang, J. Autologous Peripheral Blood Stem Cell Transplantation Improves Portal Hemodynamics in Patients with Hepatitis B Virus-Related Decompensated Cirrhosis. Hepat. Mon. 2015, 15, e32498. [Google Scholar] [CrossRef]
- Al Tayeb, H.; El Dorry, A.; Amer, N.; Mowafy, N.; Zimaity, M.; Bayoumy, E.; Saleh, A.S.A. Autologous Stem Cells Transplantation in Egyptian Patients with Liver Cirrhosis on Top of Hepatitis C Virus. Int. J. Stem Cells 2015, 8, 209–218. [Google Scholar] [CrossRef][Green Version]
- Mohamadnejad, M.; Vosough, M.; Moossavi, S.; Nikfam, S.; Mardpour, S.; Akhlaghpoor, S.; Ashrafi, M.; Azimian, V.; Jarughi, N.; Hosseini, S.-E.; et al. Intraportal Infusion of Bone Marrow Mononuclear or CD133+ Cells in Patients with Decompensated Cirrhosis: A Double-Blind Randomized Controlled Trial. Stem Cells Transl. Med. 2016, 5, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Yoon, J.-H.; Kim, W.; Lee, J.M.; Bin Lee, Y.; Cho, Y.; Lee, D.H.; Lee, M.; Yoo, J.-J.; Cho, E.J.; et al. Ultrasound-Guided Percutaneous Portal Transplantation of Peripheral Blood Monocytes in Patients with Liver Cirrhosis. Korean J. Intern. Med. 2017, 32, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Fox, R.; King, A.L.; Barton, D.; Than, N.-N.; Moore, J.; Corbett, C.; Townsend, S.; Thomas, J.; Guo, K.; et al. Granulocyte Colony-Stimulating Factor and Autologous CD133-Positive Stem-Cell Therapy in Liver Cirrhosis (REALISTIC): An Open-Label, Randomised, Controlled Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2018, 3, 25–36. [Google Scholar] [CrossRef]
- Esmaeilzadeh, A.; Ommati, H.; Kooshyar, M.M.; Jarahi, L.; Akhavan Rezayat, K.; Saberi, S.; Vosough, M.; Ghassemi, A. Autologous Bone Marrow Stem Cell Transplantation in Liver Cirrhosis after Correcting Nutritional Anomalies, A Controlled Clinical Study. Cell J. 2019, 21, 268–273. [Google Scholar] [CrossRef]
- Cui, L.N.; Wang, X.F.; Sun, R.Q.; Deng, J.; Gao, Z.J.; Zhou, X.M.; Guo, C.C.; Jia, G.; Shang, Y.L.; Yang, C.M.; et al. Study of the Effects of Long-Term Outcomes of Autologous Peripheral Blood Stem Cell Reinfusion in Patients with Decompensated Cirrhosis. Zhonghua Gan Zang Bing Za Zhi 2022, 30, 279–284. [Google Scholar] [CrossRef]
- Chavez-Tapia, N.C.; Mendiola-Pastrana, I.; Ornelas-Arroyo, V.J.; Noreña-Herrera, C.; Vidaña-Perez, D.; Delgado-Sanchez, G.; Uribe, M.; Barrientos-Gutierrez, T. Granulocyte-Colony Stimulating Factor for Acute-on-Chronic Liver Failure: Systematic Review and Meta-Analysis. Ann. Hepatol. 2015, 14, 631–641. [Google Scholar] [CrossRef]
- Zhu, C.-H.; Zhang, D.-H.; Zhu, C.-W.; Xu, J.; Guo, C.-L.; Wu, X.-G.; Cao, Q.-L.; Di, G.-H. Adult Stem Cell Transplantation Combined with Conventional Therapy for the Treatment of End-Stage Liver Disease: A Systematic Review and Meta-Analysis. Stem Cell Res. Ther. 2021, 12, 558. [Google Scholar] [CrossRef]
- Wu, C.-X.; Wang, D.; Cai, Y.; Luo, A.-R.; Sun, H. Effect of Autologous Bone Marrow Stem Cell Therapy in Patients with Liver Cirrhosis: A Meta-Analysis. J. Clin. Transl. Hepatol. 2019, 7, 238–248. [Google Scholar] [CrossRef]
- Yannaki, E.; Athanasiou, E.; Xagorari, A.; Constantinou, V.; Batsis, I.; Kaloyannidis, P.; Proya, E.; Anagnostopoulos, A.; Fassas, A. G-CSF–Primed Hematopoietic Stem Cells or G-CSF per Se Accelerate Recovery and Improve Survival after Liver Injury, Predominantly by Promoting Endogenous Repair Programs. Exp. Hematol. 2005, 33, 108–119. [Google Scholar] [CrossRef]
- Hu, X.-M.; Zhang, Q.; Zhou, R.-X.; Wu, Y.-L.; Li, Z.-X.; Zhang, D.-Y.; Yang, Y.-C.; Yang, R.-H.; Hu, Y.-J.; Xiong, K. Programmed Cell Death in Stem Cell-Based Therapy: Mechanisms and Clinical Applications. World J. Stem Cells 2021, 13, 386–415. [Google Scholar] [CrossRef]
- García Martínez, J.J.; Bendjelid, K. Artificial Liver Support Systems: What is New over the Last Decade? Ann. Intensive Care 2018, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Azparren-Angulo, M.; Royo, F.; Gonzalez, E.; Liebana, M.; Brotons, B.; Berganza, J.; Goñi-de-Cerio, F.; Manicardi, N.; Abad-Jordà, L.; Gracia-Sancho, J.; et al. Extracellular Vesicles in Hepatology: Physiological Role, Involvement in Pathogenesis, and Therapeutic Opportunities. Pharmacol. Ther. 2021, 218, 107683. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tan, W.-F.; Yang, M.-Q.; Li, J.-Y.; Geller, D.A. The Therapeutic Potential of Exosomes Derived from Different Cell Sources in Liver Diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G397–G404. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Country | Condition | Design | Patients (Treated/Control) | Cell Type | Cells Injection | Follow-Up Period | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Terai S. [129] | 2006 | Japan | Liver cirrhosis | Case-control | 9/0 | BM-MNCs (94% CD45+) | 5.2 × 109 | 24 weeks | Improved liver function and ALB levels. A trend towards ascites improvement |
| Yannaki E. [130] | 2006 | Greece | Alcohol-induced liver cirrhosis | Case-control | 2/0 | Autologous BM-HSCs (mobilised CD34+) | 2 × 106/kg | 120 weeks | Improvement of baseline CTP and MELD scores |
| Gaia S. [131] | 2006 | Italy | Severe liver cirrhosis | Case-control | 8/0 | GCSF mobilisation | N/A | 32 weeks | Improvement of baseline CTP and MELD scores in 50% of patients |
| Gordon M. [132] | 2006 | UK | Chronic liver failure | Case-control | 5/0 | Autologous BM-HSCs (mobilised CD34+) | 106–2 × 108 | 60 days | Ν/A |
| Mohamadnejad M. [133] | 2007 | Iran | Decompensated cirrhosis | Case-control | 4/0 | BM-HSCs | 2.5–8 × 106 | 24 weeks | Improvement in ALB levels in 2 patients and MELD scores in 1 patient. |
| Lyra A.C. [134] | 2007 | Brazil | Chronic liver disease | Case-control | 10/0 | BM-MNCs | 108 | 16 weeks | Overall improvement in ALB, TBIL and INR |
| Yan L. [135] | 2007 | China | HBV-related decompensated liver cirrhosis | Case-control | 2/0 | Autologous BM-HSCs (GCSF-mobilised) | 107–108/kg | 18 months | Improvement of baseline CTP score |
| Levicar N. [136] LCER No. 2004/6746 | 2008 | UK | Chronic liver disease | Case-control | 5/0 | Autologous BM-HSCs (GCSF-mobilised CD34+) | 106–2 × 108 | 18 months | Improvement in ALB and AFP levels |
| Khan A.A. [137] | 2008 | India | Liver cirrhosis | Case-control | 4/0 | Autologous BM-HSCs (GCSF-mobilised CD34+) | 0.1 × 108 | 26 weeks | Improvement of baseline CTP and MELD scores |
| Han Y. [138] | 2008 | China | HBV-related decompensated liver cirrhosis | RCT | 20/20 | Autologous BM-MNCs (GCSF-mobilised) | 107–108/kg | 6 months | Improved ALB and CTP score in patients receiving cell transplant |
| Pai M. [139] | 2008 | UK | Severe alcoholic liver cirrhosis | Case-control | 9/0 | Autologous BM-HSCs (GCSF-mobilised CD34+) | 2.3 × 108 | 3 months | Improved TBIL, ALT, AST and CTP score. Some improvement in ascites formation |
| Salama H. [140] | 2010 | Egypt | End-stage liver disease | RCT | 90/50 | Autologous BM-HSCs (mobilised CD34+ and CD133+) | 0.5 × 108 | 24 weeks | Improved liver function and ALB levels |
| Salama H. [141] | 2010 | Egypt | End-stage liver disease | Case-control | 48 | Autologous BM-HSCs (GCSF-mobilised CD34+) | 1 × 109 | 48 weeks | Decrease in ascites; Improvement in ALB, TBIL, INR, ALT |
| Kim J.K. [116] | 2010 | China | Advanced liver cirrhosis | Case-control | 10/0 | Autologous BM-MNCs (80% CD45+) | 0.5–1.5 × 108 | 6 months | Improvement in CPT score and ascites formation |
| Lyra A.C. [142] | 2010 | Brazil | Chronic liver disease | RCT | 15/15 | Autologous BM cells | 3.8 × 108 | 12 months | The MELD score remained stable in treated patients while it increased in the control group. Improvement in ALB and TBIL in the treated group |
| Saito S. [143] | 2011 | Japan | Alcoholic liver cirrhosis | Case-control | 5/5 | Autologous BM-MNCs | 8.0–7.3 × 109 | 24 weeks | Higher ALB and PTA; improved CTP score |
| Garg V. [124] NCT01036932 | 2012 | India | Acute-on-chronic liver failure | RCT | 23/24 | GCSF mobilisation | N/A | 2 months | Improvement in survival, CTP and MELD scores |
| Spahr L. [144] ISRCTN83972743 | 2013 | Switzerland | Decompensated alcoholic liver disease | RCT | 28/30 | Autologous BM-MNCs (GCSF-mobilised) | 0.47 ± 0.15 × 108/kg | 3 months | No improvement in liver function |
| Bai Y.Q. [145] | 2014 | China | HBV-related liver cirrhosis | Case-control | 32/15 | Autologous BM-MNCs | Not reported | 24 months | Improvement in ALB, PTA, fibrinogen, PLT, TBIL and reduction of adverse effects |
| Liu L. [146] | 2014 | China | Hepatitis B and decompensated liver cirrhosis | RCT | 40/37 | Autologous BM-MNCs (GCSF-mobilised) | 3.2 +/−1.6 × 1011 | 4 weeks | Improvement in serum AST, ALT, ALB, and TBIL levels |
| Andreone P. [147] NCT01025622 | 2015 | Italy | End-stage liver disease | Case-control | 12/0 | Autologous BM-HSCs (GCSF-mobilised CD133+) | 5 × 104/kg up to 1 × 106/kg | 12 months | Temporary improvement in MELD score; worsening of CTP score |
| Zekri A.R. [128] NCT01729221 | 2015 | Egypt | HCV-associated liver cirrhosis | RCT | 60/30 | Autologous BM-HSCs (mobilised CD34+) followed by MSC infusion | 0.5 × 108 | 52 weeks | Improvement in baseline CTP in 40% patients. Improvement in ALB, TBIL and INR |
| Sharma M. [148] | 2015 | India | Non-viral decompensated cirrhosis | RCT | 22/23 | Autologous BM-HSCs (GCSF-mobilised CD34+) | N/A | 3 months | Improvement in serum creatinin and MELD scores |
| Deng Q. [149] | 2015 | China | HBV-related decompensated cirrhosis | RCT | 33/35 | Autologous BM-HSCs (GCSF-mobilised CD34+) | 2–4 × 107 | 48 weeks | improvements in liver function (ALB, PTA) and portal vein hemodynamics |
| TayebH. [150] | 2015 | Egypt | HCV-associated liver cirrhosis | RCT | 10/10 | Autologous BM-MNCs (GCSF-mobilised) | 25 × 106–191 × 106 | 3 months | Improvement in ALB levels 1 month post BMT; γ-GT improvement at 3 months; Improved CTP score at 3 months; No statistical improvement in any other liver parameter at 3 months |
| Mohamadnejad M. [151] | 2016 | Iran | Decompensated cirrhosis | RCT | 8 (CD133) 10 (MNC) 9 (CONTROL) | Autologous BM-CD133+ versus BM-MNCs | 2–13 × 108 MNC/2–7 106 CD133+ | 12 months | Improved MELD score in the CD133+ group at 3 mo |
| Yu S.J. [152] NCT01503749 | 2016 | Korea | Decompensated cirrhosis | RCT | 3 (GSCF + CELLS) 3 (GCSF) 3 (CONTROL) | Autologous BM-MNCs (GCSF-mobilised) | 1.67 × 109–2 × 1010 | 6 months | Small improvement in CTP scores at 24 weeks |
| Newsome [153] ISRCTN 91288089 | 2018 | UK | Compensated liver cirrhosis | RCT | 28/26 GCSF/27 control | Autologous BM-HSCs (GCSF-mobilised CD133+) | 0.2 × 10⁶ | 90 days | No improvement in liver dysfunction or fibrosis while adverse events may occur compared with standard care |
| Esmaeilzadeh A. [154] IRCT2014091919217N1 | 2019 | Iran | Decompensated liver cirrhosis | RCT | 10/10 | Autologous BM-MNCs | 8.06 ± 2.5×106 cells/kg | 6 months | Improvement in MELD socre, INR, TBIL, ALB levels after cell transplantation (6 months) |
| Cui L. [155] | 2022 | China | Decompensated liver cirrhosis | 10 yr follow up study | 287/151 | PBSC | Survival was higher in treated group alongside ALB levels, CTP and MELD scores |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siapati, E.K.; Roubelakis, M.G.; Vassilopoulos, G. Liver Regeneration by Hematopoietic Stem Cells: Have We Reached the End of the Road? Cells 2022, 11, 2312. https://doi.org/10.3390/cells11152312
Siapati EK, Roubelakis MG, Vassilopoulos G. Liver Regeneration by Hematopoietic Stem Cells: Have We Reached the End of the Road? Cells. 2022; 11(15):2312. https://doi.org/10.3390/cells11152312
Chicago/Turabian StyleSiapati, Elena Konstantina, Maria G. Roubelakis, and George Vassilopoulos. 2022. "Liver Regeneration by Hematopoietic Stem Cells: Have We Reached the End of the Road?" Cells 11, no. 15: 2312. https://doi.org/10.3390/cells11152312
APA StyleSiapati, E. K., Roubelakis, M. G., & Vassilopoulos, G. (2022). Liver Regeneration by Hematopoietic Stem Cells: Have We Reached the End of the Road? Cells, 11(15), 2312. https://doi.org/10.3390/cells11152312







