Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing
Abstract
:1. Introduction
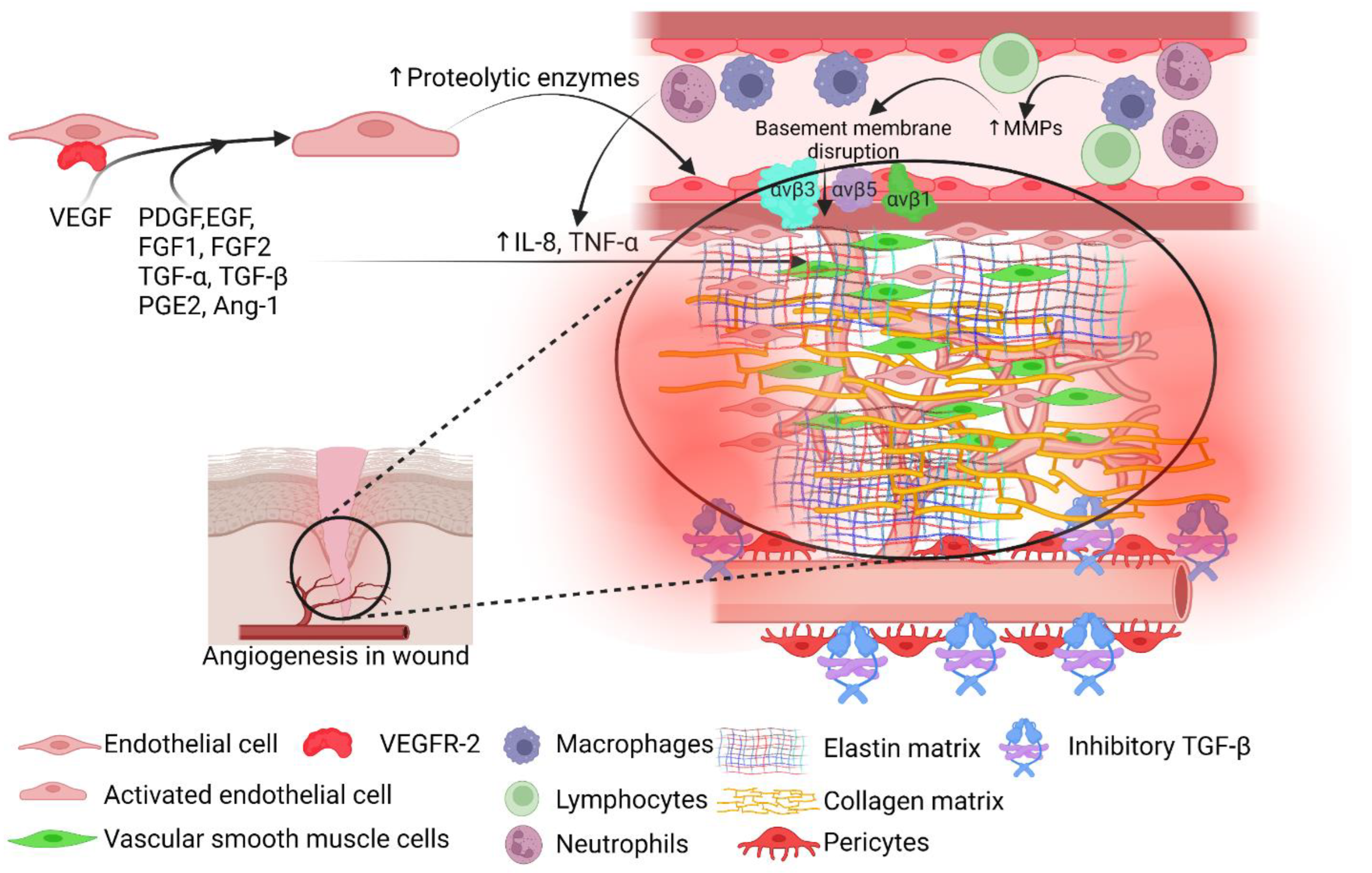
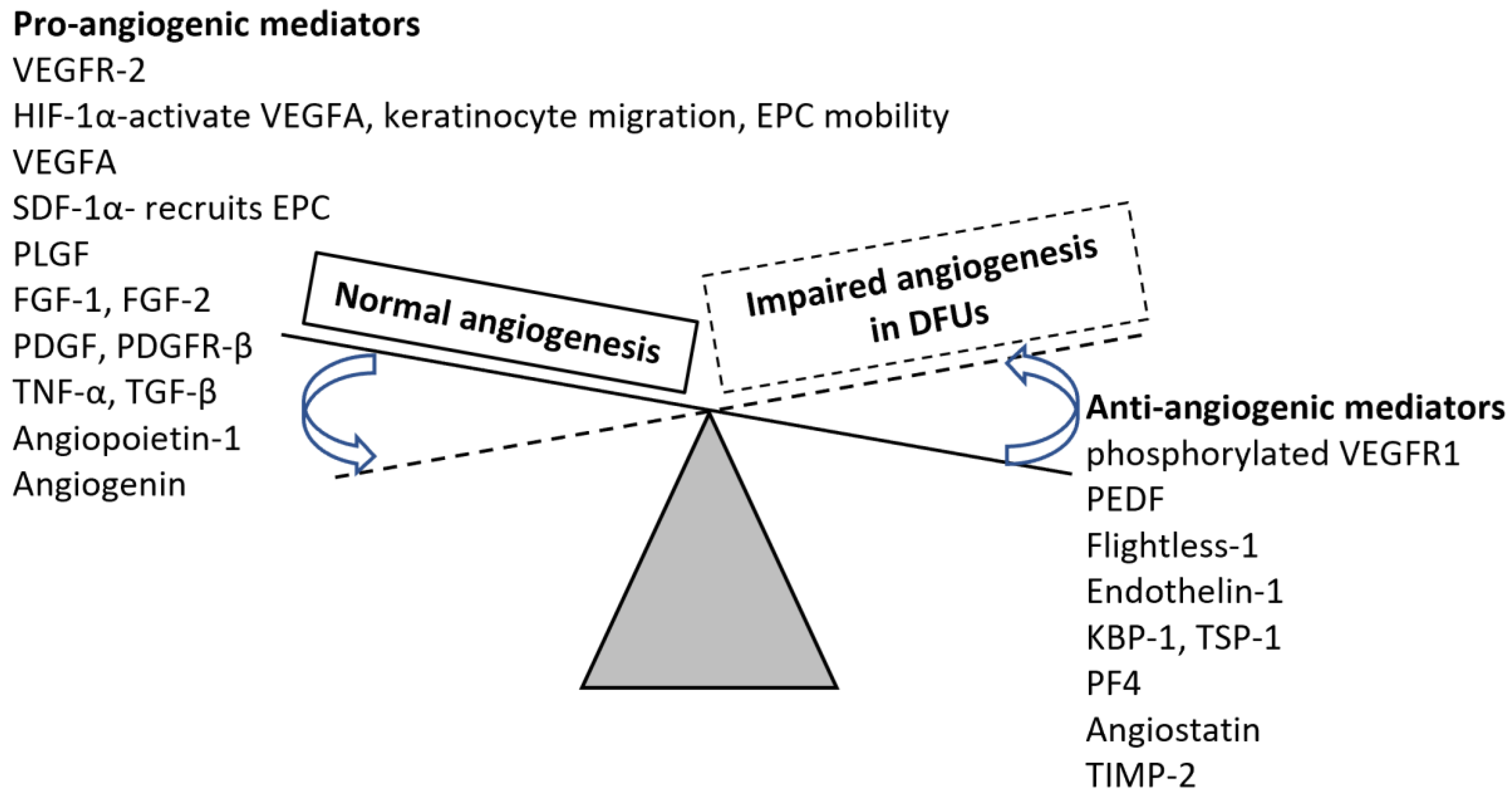
2. Angiogenesis and Wound Healing
3. Stem Cells and Angiogenesis in DFUs
4. Stem-Cell Derived Exosomes and Combinational Strategies to Enhance Diabetic Wound Healing
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J. Orthop. 2015, 6, 62–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, T.I.; Mutluoglu, M. Diabetic Foot Ulcer. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537328/ (accessed on 1 July 2022).
- Uccioli, L.; Izzo, V.; Meloni, M.; Vainieri, E.; Ruotolo, V.; Giurato, L. Non-healing foot ulcers in diabetic patients: General and local interfering conditions and management options with advanced wound dressings. J. Wound Care 2015, 24, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Pei, Q.; Yu, T.; Chang, Q.; Wang, D.; Gao, M.; Zhang, X.; Liu, Y. Compromised Wound Healing in Ischemic Type 2 Diabetic Rats. PLoS ONE 2016, 11, e0152068. [Google Scholar] [CrossRef] [Green Version]
- Shofler, D.; Rai, V.; Mansager, S.; Cramer, K.; Agrawal, D.K. Impact of resolvin mediators in the immunopathology of diabetes and wound healing. Expert Rev. Clin. Immunol. 2021, 17, 681–690. [Google Scholar] [CrossRef]
- Theoret, C.L. The pathophysiology of wound repair. Vet. Clin. N. Am. Equine Pract. 2005, 21, 1–13. [Google Scholar] [CrossRef]
- Honnegowda, T.M.; Kumar, P.; Udupa, E.G.P.; Kumar, S.; Kumar, U.; Rao, P. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast. Aesthet. Res. 2015, 2, 243–249. [Google Scholar]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; Chen, L.; Ma, D.; Haywood, V.A.; Barakat, M.; Urao, N.; DiPietro, L.A. Compromised angiogenesis and vascular Integrity in impaired diabetic wound healing. PLoS ONE 2020, 15, e0231962. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [Green Version]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Aramideh Khouy, R.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, C.I. Becaplermin (Regranex) for diabetic foot ulcers. Am. Fam. Physician 2008, 78, 255. [Google Scholar]
- Verdi, J.; Shirian, S.; Saleh, M.; Khadem Haghighian, H.; Kavianpour, M. Mesenchymal Stem Cells Regenerate Diabetic Foot Ulcers: A Review Article. World J. Plast. Surg 2022, 11, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gang, X.; Sun, C.; Wang, G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J. Diabetes Res. 2017, 2017, 9328347. [Google Scholar] [CrossRef]
- Yu, Q.; Qiao, G.H.; Wang, M.; Yu, L.; Sun, Y.; Shi, H.; Ma, T.L. Stem Cell-Based Therapy for Diabetic Foot Ulcers. Front. Cell Dev. Biol. 2022, 10, 812262. [Google Scholar] [CrossRef]
- Lopes, L.; Setia, O.; Aurshina, A.; Liu, S.; Hu, H.; Isaji, T.; Liu, H.; Wang, T.; Ono, S.; Guo, X.; et al. Stem cell therapy for diabetic foot ulcers: A review of preclinical and clinical research. Stem Cell Res. Ther. 2018, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Krasilnikova, O.A.; Baranovskii, D.S.; Lyundup, A.V.; Shegay, P.V.; Kaprin, A.D.; Klabukov, I.D. Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action. Stem Cell Rev. Rep. 2022. [Google Scholar] [CrossRef]
- Zarei, F.; Negahdari, B.; Eatemadi, A. Diabetic ulcer regeneration: Stem cells, biomaterials, growth factors. Artif. Cells Nanomed. Biotechnol. 2018, 46, 26–32. [Google Scholar] [CrossRef]
- Nolan, G.S.; Smith, O.J.; Jell, G.; Mosahebi, A. Fat grafting and platelet-rich plasma in wound healing: A review of histology from animal studies. Adipocyte 2021, 10, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, S.; Heidarpour, M.; Sabbagh, S.; Amini, E.; Saffari, H.; Dolati, S.; Meamar, R. Stem cell transplantation therapy for diabetic foot ulcer: A narrative review. Asian Biomed. 2021, 15, 3–18. [Google Scholar] [CrossRef]
- Nour, S.; Imani, R.; Chaudhry, G.R.; Sharifi, A.M. Skin wound healing assisted by angiogenic targeted tissue engineering: A comprehensive review of bioengineered approaches. J. Biomed. Mater. Res. A 2021, 109, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Schonborn, M.; Laczak, P.; Pasieka, P.; Borys, S.; Plotek, A.; Maga, P. Pro- and Anti-Angiogenic Factors: Their Relevance in Diabetic Foot Syndrome-A Review. Angiology 2022, 73, 299–311. [Google Scholar] [CrossRef]
- O’Loughlin, A.; O’Brien, T. Topical stem and progenitor cell therapy for diabetic foot ulcers. In Stem Cells in Clinic and Research; IntechOpen: London, UK, 2011. [Google Scholar]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Riedl, J.; Popp, C.; Eide, C.; Ebens, C.; Tolar, J. Mesenchymal stromal cells in wound healing applications: Role of the secretome, targeted delivery and impact on recessive dystrophic epidermolysis bullosa treatment. Cytotherapy 2021, 23, 961–973. [Google Scholar] [CrossRef]
- Yamada, H.; Naito, R.; Nishimura, M.; Kawakami, R.; Morinaga, E.; Morita, Y.; Shimizu, M.; Yoshimatsu, G.; Sawamoto, O.; Matsumoto, S.; et al. Xenotransplantation of neonatal porcine bone marrow-derived mesenchymal stem cells improves diabetic wound healing by promoting angiogenesis and lymphangiogenesis. Xenotransplantation 2022, 29, e12739. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, T.; He, Z. ADSCs enhance VEGFR3-mediated lymphangiogenesis via METTL3-mediated VEGF-C m(6)A modification to improve wound healing of diabetic foot ulcers. Mol. Med. 2021, 27, 146. [Google Scholar] [CrossRef]
- Gorecka, J.; Gao, X.; Fereydooni, A.; Dash, B.C.; Luo, J.; Lee, S.R.; Taniguchi, R.; Hsia, H.C.; Qyang, Y.; Dardik, A. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen. Med. 2020, 15, 1277–1293. [Google Scholar] [CrossRef] [Green Version]
- Irons, R.F.; Cahill, K.W.; Rattigan, D.A.; Marcotte, J.H.; Fromer, M.W.; Chang, S.; Zhang, P.; Behling, E.M.; Behling, K.C.; Caputo, F.J. Acceleration of diabetic wound healing with adipose-derived stem cells, endothelial-differentiated stem cells, and topical conditioned medium therapy in a swine model. J. Vasc. Surg 2018, 68, 115S–125S. [Google Scholar] [CrossRef]
- Elsharawy, M.A.; Naim, M.; Greish, S. Human CD34+ stem cells promote healing of diabetic foot ulcers in rats. Interact. Cardiovasc Thorac. Surg 2012, 14, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, L.S.; Duplaa, C.; Krankel, N.; Graiani, G.; Invernici, G.; Katare, R.; Siragusa, M.; Meloni, M.; Campesi, I.; Monica, M.; et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ. Res. 2009, 104, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluth, M.A.; Kerstan, A.; Dieter, K.; Niebergall-Roth, E.; Klingele, S.; Jünger, M.; Hasslacher, C.; Daeschlein, G.; Stemler, L.; Meyer-Pannwitt, U. Translational Development of ABCB5+ Dermal Mesenchymal Stem Cells for Therapeutic Induction of Angiogenesis in Non-Healing Diabetic Foot Ulcers. 2022. Available online: https://assets.researchsquare.com/files/rs-1508134/v1/012d9e57-5a5b-41c7-bdd5-621c7c557cc5.pdf?c=1649188762 (accessed on 1 July 2022).
- Liu, Y.; Chen, J.; Liang, H.; Cai, Y.; Li, X.; Yan, L.; Zhou, L.; Shan, L.; Wang, H. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Res. Ther. 2022, 13, 258. [Google Scholar] [CrossRef]
- Yan, J.; Liang, J.; Cao, Y.; El Akkawi, M.M.; Liao, X.; Chen, X.; Li, C.; Li, K.; Xie, G.; Liu, H. Efficacy of topical and systemic transplantation of mesenchymal stem cells in a rat model of diabetic ischemic wounds. Stem Cell Res. Ther. 2021, 12, 220. [Google Scholar] [CrossRef]
- Shi, M.; Gao, Y.; Lee, L.; Song, T.; Zhou, J.; Yan, L.; Li, Y. Adaptive Gelatin Microspheres Enhanced Stem Cell Delivery and Integration With Diabetic Wounds to Activate Skin Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 813805. [Google Scholar] [CrossRef]
- Takahashi, H.; Ohnishi, S.; Yamamoto, Y.; Hayashi, T.; Murao, N.; Osawa, M.; Maeda, T.; Ishikawa, K.; Sakamoto, N.; Funayama, E. Topical Application of Conditioned Medium from Hypoxically Cultured Amnion-Derived Mesenchymal Stem Cells Promotes Wound Healing in Diabetic Mice. Plast. Reconstr. Surg 2021, 147, 1342–1352. [Google Scholar] [CrossRef]
- Wang, P.; Theocharidis, G.; Vlachos, I.S.; Kounas, K.; Lobao, A.; Shu, B.; Wu, B.; Xie, J.; Hu, Z.; Qi, S.; et al. Exosomes Derived from Epidermal Stem Cells Improve Diabetic Wound Healing. J. Investig. Dermatol. 2022. [Google Scholar] [CrossRef]
- Las Heras, K.; Royo, F.; Garcia-Vallicrosa, C.; Igartua, M.; Santos-Vizcaino, E.; Falcon-Perez, J.M.; Hernandez, R.M. Extracellular vesicles from hair follicle-derived mesenchymal stromal cells: Isolation, characterization and therapeutic potential for chronic wound healing. Stem Cell Res. Ther. 2022, 13, 147. [Google Scholar] [CrossRef]
- Gondaliya, P.; Sayyed, A.A.; Bhat, P.; Mali, M.; Arya, N.; Khairnar, A.; Kalia, K. Mesenchymal Stem Cell-Derived Exosomes Loaded with miR-155 Inhibitor Ameliorate Diabetic Wound Healing. Mol. Pharm. 2022, 19, 1294–1308. [Google Scholar] [CrossRef]
- Yan, C.; Xv, Y.; Lin, Z.; Endo, Y.; Xue, H.; Hu, Y.; Hu, L.; Chen, L.; Cao, F.; Zhou, W.; et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Accelerate Diabetic Wound Healing via Ameliorating Oxidative Stress and Promoting Angiogenesis. Front. Bioeng. Biotechnol. 2022, 10, 829868. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal. Transduct. Target. Ther. 2021, 6, 354. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, Y.; Liu, D. Extracellular Vesicles from Adipose-Derived Stem Cells Promote Diabetic Wound Healing via the PI3K-AKT-mTOR-HIF-1alpha Signaling Pathway. Tissue Eng. Regen. Med. 2021, 18, 1035–1044. [Google Scholar] [CrossRef]
- Qiu, J.; Shu, C.; Li, X.; Ye, C.; Zhang, W.C. Exosomes from linc00511-overexpressing ADSCs accelerates angiogenesis in diabetic foot ulcers healing by suppressing PAQR3-induced Twist1 degradation. Diabetes Res. Clin. Pract. 2021, 180, 109032. [Google Scholar] [CrossRef]
- Han, Z.F.; Cao, J.H.; Liu, Z.Y.; Yang, Z.; Qi, R.X.; Xu, H.L. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res. Clin. Pract. 2022, 183, 109126. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 150. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Ma, K.; Li, Q.; Li, B.; Hu, W.; Fu, X.; Zhang, C. Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Facilitate Diabetic Wound Healing Through MiR-17-5p-mediated Enhancement of Angiogenesis. Stem Cell Rev. Rep. 2022, 18, 1025–1040. [Google Scholar] [CrossRef]
- Born, L.J.; Chang, K.H.; Shoureshi, P.; Lay, F.; Bengali, S.; Hsu, A.T.W.; Abadchi, S.N.; Harmon, J.W.; Jay, S.M. HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Adv. Healthc. Mater. 2022, 11, e2002070. [Google Scholar] [CrossRef]
- Seo, E.; Lim, J.S.; Jun, J.B.; Choi, W.; Hong, I.S.; Jun, H.S. Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing. J. Transl. Med. 2017, 15, 35. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Sun, N.; Liu, Y. Self-Assembled Nano-Peptide Hydrogels with Human Umbilical Cord Mesenchymal Stem Cell Spheroids Accelerate Diabetic Skin Wound Healing by Inhibiting Inflammation and Promoting Angiogenesis. Int. J. Nanomed. 2022, 17, 2459–2474. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, X.; Shi, X.; Chen, C.; Cao, Y.; Liu, J. Constructing Tissue-Engineered Dressing Membranes with Adipose-Derived Stem Cells and Acellular Dermal Matrix for Diabetic Wound Healing: A Comparative Study of Hypoxia- or Normoxia-Culture Modes. Stem Cells Int. 2022, 2022, 2976185. [Google Scholar] [CrossRef]
- Yang, H.Y.; Fierro, F.; Yoon, D.J.; Gallegos, A.; Osborn, S.L.; Nguyen, A.V.; Peavy, T.R.; Ferrier, W.; Talken, L.; Ma, B.W.; et al. Combination product of dermal matrix, preconditioned human mesenchymal stem cells and timolol promotes wound healing in the porcine wound model. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1615–1623. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, X.; Niu, Y.; Huang, S.; Wang, J.; Luo, M.; Shi, G.; Huang, J. Wharton’s jelly mesenchymal stem cells embedded in PF-127 hydrogel plus sodium ascorbyl phosphate combination promote diabetic wound healing in type 2 diabetic rat. Stem Cell Res. Ther. 2021, 12, 559. [Google Scholar] [CrossRef]
- Ebrahim, N.; Dessouky, A.A.; Mostafa, O.; Hassouna, A.; Yousef, M.M.; Seleem, Y.; El Gebaly, E.; Allam, M.M.; Farid, A.S.; Saffaf, B.A.; et al. Adipose mesenchymal stem cells combined with platelet-rich plasma accelerate diabetic wound healing by modulating the Notch pathway. Stem Cell Res. Ther. 2021, 12, 392. [Google Scholar] [CrossRef]
- Ni, X.; Shan, X.; Xu, L.; Yu, W.; Zhang, M.; Lei, C.; Xu, N.; Lin, J.; Wang, B. Adipose-derived stem cells combined with platelet-rich plasma enhance wound healing in a rat model of full-thickness skin defects. Stem Cell Res. Ther. 2021, 12, 226. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Wu, M.; Zeng, Q.; Wang, X.; Wang, H. Efficacy of the therapy of 5-aminolevulinic acid photodynamic therapy combined with human umbilical cord mesenchymal stem cells on methicillin-resistant Staphylococcus aureus-infected wound in a diabetic mouse model. Photodiagnosis Photodyn. Ther. 2021, 36, 102480. [Google Scholar] [CrossRef]
- Pak, C.S.; Heo, C.Y.; Shin, J.; Moon, S.Y.; Cho, S.W.; Kang, H.J. Effects of a Catechol-Functionalized Hyaluronic Acid Patch Combined with Human Adipose-Derived Stem Cells in Diabetic Wound Healing. Int. J. Mol. Sci. 2021, 22, 2632. [Google Scholar] [CrossRef]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1alpha Gene-Activated Collagen Scaffold Restores Pro-Angiogenic Wound Healing Features in Human Diabetic Adipose-Derived Stem Cells. Biomedicines 2021, 9, 160. [Google Scholar] [CrossRef]
- Chen, J.; Liu, R.; Huang, T.; Sun, H.; Jiang, H. Adipose stem cells-released extracellular vesicles as a next-generation cargo delivery vehicles: A survey of minimal information implementation, mass production and functional modification. Stem Cell Res. Ther. 2022, 13, 182. [Google Scholar] [CrossRef]
- Liu, W.J.; Liu, D.W. Research advances on mesenchymal stem cell-derived extracellular vesicles in promoting angiogenesis of diabetic ulcers. Zhonghua Shao Shang Za Zhi 2022, 38, 393–399. [Google Scholar] [CrossRef]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Zhang, D.; Liu, X.; Zhao, F.; Pang, X.; Wang, Q. Effect of advanced glycosylation end products on apoptosis in human adipose tissue-derived stem cells in vitro. Cell Biosci. 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Assis, A.; Gellman, Y.N.; Cahn, A.; Haze, A.; Camargo, S.; Mitrani, E. Angiogenic potential of mesenchymal stem cells derived from patients with diabetes seeded on decellularized micro fragments. J. Diabetes Complicat. 2021, 35, 108001. [Google Scholar] [CrossRef]
- Mathew, E.; Brannon, A.L.; Del Vecchio, A.; Garcia, P.E.; Penny, M.K.; Kane, K.T.; Vinta, A.; Buckanovich, R.J.; di Magliano, M.P. Mesenchymal Stem Cells Promote Pancreatic Tumor Growth by Inducing Alternative Polarization of Macrophages. Neoplasia 2016, 18, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, H.; Amini, A.; Fadaei Fathabady, F.; Mostafavinia, A.; Zare, F.; Ebrahimpour-Malekshah, R.; Ghalibaf, M.N.; Abrisham, M.; Rezaei, F.; Albright, R.; et al. Transplantation of photobiomodulation-preconditioned diabetic stem cells accelerates ischemic wound healing in diabetic rats. Stem Cell Res. Ther. 2020, 11, 494. [Google Scholar] [CrossRef]
- Moradi, A.; Zare, F.; Mostafavinia, A.; Safaju, S.; Shahbazi, A.; Habibi, M.; Abdollahifar, M.A.; Hashemi, S.M.; Amini, A.; Ghoreishi, S.K.; et al. Photobiomodulation plus Adipose-derived Stem Cells Improve Healing of Ischemic Infected Wounds in Type 2 Diabetic Rats. Sci. Rep. 2020, 10, 1206. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Jeon, H.R.; Kim, S.W.; Kim, Y.H.; Im, G.B.; Im, J.; Um, S.H.; Cho, S.M.; Lee, J.R.; Kim, H.Y.; et al. Lightwave-reinforced stem cells with enhanced wound healing efficacy. J. Tissue Eng. 2021, 12, 20417314211067004. [Google Scholar] [CrossRef]
- Palma, M.B.; Luzzani, C.; Andrini, L.B.; Riccillo, F.; Buero, G.; Pelinski, P.; Inda, A.M.; Errecalde, A.L.; Miriuka, S.; Carosella, E.D.; et al. Wound Healing by Allogeneic Transplantation of Specific Subpopulation From Human Umbilical Cord Mesenchymal Stem Cells. Cell Transplant. 2021, 30, 963689721993774. [Google Scholar] [CrossRef]
- Carstens, M.H.; Quintana, F.J.; Calderwood, S.T.; Sevilla, J.P.; Rios, A.B.; Rivera, C.M.; Calero, D.W.; Zelaya, M.L.; Garcia, N.; Bertram, K.A.; et al. Treatment of chronic diabetic foot ulcers with adipose-derived stromal vascular fraction cell injections: Safety and evidence of efficacy at 1 year. Stem Cells Transl. Med. 2021, 10, 1138–1147. [Google Scholar] [CrossRef]
| Source of Stem Cells | Advantages |
|---|---|
| BM-MSCs | No immunologic restriction, does not stimulate alloreactivity, escape lysis by T-cells and NK cells Reduced formation of cytotoxic lymphocytes suppresses T-cell-derived IFN-γ, intramuscular transplantation |
| UCB-MSCs | Similar morphology and cell surface antigens, the potential of differentiation into BM-MSCs Short doubling time, long viable time, anti-inflammatory activity, intramuscular transplantation |
| AMSCs | Characteristics like BM-MSCs, subcutaneous injection |
| UC-MSCs | Rich, safe, of short doubling time, and easy to collect Fibroblastic morphology, typical immunophenotypic markers, and multiple differentiation potential to BM-MSCs, lower immunogenicity |
| PMSCs | A large number of cells can be isolated, better proliferation capacity, intraperitoneal administration The morphology, size, surface phenotype, and immunosuppressive characteristics are like BM-MSCs |
| AF-MSCs | A large number of cells can be isolated from the small volume, remain stable, have a high proliferative capacity, multilineage differentiation potential, immunomodulatory activity Lack of significant immunogenicity |
| GMSCs | Homogenous, not tumorigenic, easy to isolate, stable phenotype, can be isolated without ethical problems, greater capacity of proliferation and migration than AMSCs and BM-MSCs without growth factors |
| Stem Cell | Strategy | Parameters Checked | Outcome |
|---|---|---|---|
| npBM-MSC [29] | Xenotransplantation in mice model of diabetic wound. | Rate of wound closure and the promotion of neovascularization | The wound closure rate was significantly improved on postoperative days 4 and 7 Promoted angiogenesis and lymphangiogenesis |
| ADSCs [30] | Autologous transplantation in mice | Lymphangiogenesis Wound healing | ADSCs accelerate lymphatic endothelial cells proliferation, migration, and lymphangiogenesis ADSCs enhance VEGFR3-mediated lymphangiogenesis via METTL3-mediated VEGF-C m6A modification to improve wound healing in DFUs |
| hiPSCSMC [31] | Xenotransplantation hiPSC-SMC embedded in 3D collagen scaffolds were applied to diabetic, nude mice with splinted back wounds | To compare angiogenic factor secretion from ADMCs and hiPSCSMC | hiPSC-SMC secretes increased concentration of pro-angiogenic cytokines compared with murine ADMCs. hiPSC-SMC-containing collagen scaffolds accelerate diabetic wound healing hiPSC-SMC increases the number of total and M2 macrophages hiPSC-SMC increases angiogenesis via VEGF-A and TGF-β1 |
| ADSCs [32] | Autologous transplantation in swine | Percentage of wound healing | Increased percentage of wound closure rates with ADSCs and EC-ADSCs, and conditioned media Increased angiogenesis with stem cell therapy Significant decrease in inflammation with stem cells |
| hUCB-CD34+SC [33] | Xenotransplantation in a rat model of diabetic wound locally. | To evaluate the efficacy of stem cells in the healing of wounds | A significant decrease in mean wound surface area, increase in mean epidermal thickness, blood vessel proliferation, and collagen deposition |
| EPCs-BMMNCs | Autologous stem cells transplantation in mice topically | Wound healing and angiogenesis | Accelerated wound healing and induced expression of VEGF and bFGF promoting angiogenesis |
| CD133+ CCM [34] | Xenotransplantation in mice model of diabetic wound | Wound healing and angiogenesis | Stimulation of migration, angiogenesis-like network formation and induction of Wnt expression Stimulate wound healing by paracrine mechanisms |
| ABCB5+MSCs [35] | Human dermal ABCB5+ MSCs were transplanted via intramuscular injection in mice ischemic limb and topically in human DFUs | To evaluate the angiogenic potential of ABCB5+ MSCs | In mice Accelerated perfusion recovery of ischemia Increased angiogenesis Clinical trial in human Reduction in wound surface area in therapy refractory DFUs with topical application |
| hucMSCs [36] | hucMSCs were infused in diabetic rat | Repair of diabetic vascular endothelial cell damage | hucMSCs ameliorated blood glucose and protected vascular endothelium from diabetic damage through paracrine effect involving MAPK/ERK signaling |
| Treatment | Combination/Strategy | Assessing Parameters | Study Outcome |
|---|---|---|---|
| Exosomes from linc00511-overexpressing ADSCs [46] | hADSCs-derived exosomes were injected into Sprague–Dawley (SD) rats along with human blood-derived EPC | Angiogenesis and wound healing Underlying molecular mechanism | Accelerate angiogenesis and wound healing by suppressing PAQR3-induced Twist1degradation |
| BMSC-derived exosomal lncRNA KLF3-AS1 [47] | Exosomes were delivered via tail vein injection in diabetic BALB/C mice | Wound healing Angiogenesis | Induction of angiogenesis to promote diabetic cutaneous wound healing. |
| Exosomes from pioglitazone pretreated MSCs [48] | Exosomes isolated from supernatants of pioglitazone-treated BMSCs (PGZ-Ex) were injected around the wounds by multisite subcutaneous injection | Wound healing Angiogenesis | PGZ-EX accelerates diabetic wound healing via enhanced angiogenesis, increased collagen deposition, ECM remodeling, and increased VEGF and CD31 expression |
| hucMSC-EVs [49] | hucMSC-EVs applied locally to diabetic mice | Angiogenesis Wound healing | hucMSC-EVs have regenerative and protective effects on high glucose-induced endothelial cells involving the transfer of miR-17-5p to target PTEN/AKT/HIF-1α/VEGF pathway hucMSC-EVs promote angiogenesis and accelerate wound healing |
| HOTAIR-MSC EVs [50] | HOTAIR-MSC EVs were injected around the wound in Sprague–Dawley rats | Wound healing Angiogenesis | HOTAIR-MSC EVs promote angiogenesis and wound healing in diabetic (db/db) mice. |
| Exendin-4 with ADSCs [51] | hADSCs were injected intradermally around the wound in db/db mice and Ex-4 was applied topically | Wound size Wound histology Angiogenesis | The combination of topical treatment of Ex-4 and injection of ADSCs has a better effect therapeutically than Ex-4 alone |
| hUC-MSCsp [52] | hUC-MSCsp transplanted into wounded skin of mice model of diabetes | Wound healing Angiogenesis Inflammation Comparison between stem cells alone and in combination | Accelerated wound healing Inhibited inflammation Promotes angiogenesis |
| ADSCs [53] | ADSCs in the acellular dermal matrix under hypoxic and normoxic conditions applied over DFU in a diabetic rat | Stem cell viability under hypoxic and normoxic conditions | The transplanted cells in the hypoxic-ADSCs/ADM membrane can survive longer at the chronic ulcer site and enhance angiogenesis, inhibits inflammation, and increase ECM formation |
| hBM-MSCs [54] | hBM-MSCs/T/H/S administered to porcine wound model | Wound healing Angiogenesis | MSC/T/H/S promoted wound re-epithelialization and angiogenesis and improved wound healing |
| WJMSC [55] | WJMSC with PF-127 hydrogel and SAP were transplanted onto excisional cutaneous wound bed in type II diabetic Sprague–Dawley rats | Wound healing Mitochondrial damage and oxidative stress | Promoted diabetic wound healing Decreased M1 and increased M2 macrophages Increased angiogenesis |
| ADSCs [56] | ADSCs (isolated from rats) alone and ADSCs with PRP was injected at the wound base and edges of diabetic Albino rats | To compare the efficacy of ADSC alone vs. ADSC+ PRP in wound healing and angiogenesis | PRP+ADSCs compared to their individual use are better for re-epithelialization, granulation tissue formation, collagen deposition, epidermal thickness, and angiogenesis by modulating the Notch pathway |
| ADSCs [57] | ADSCs (isolated from rats) combined with PRP were injected to wound in Sprague–Dawley rats | Angiogenesis Wound healing | ADSCs-PRP induced a higher wound closure rate Increases the expression of VEGF, p-STAT3, and SDF-1 Promote ECs proliferation thereby neovascularization |
| hUC-MSCs [58] | hUC-MSCs combined with ALA-PDT- hUC-MSCs were injected intradermally to diabetic C57BL/6J mice after exposing the mice to ALA-PDT with 10% ALA gel and 25 J/cm2 of PDT. | To investigate the efficacy of the combinational approach on wound closure, angiogenesis, and inflammation | Combining ALA-PDT with hUC-MSCs possesses a significantly enhanced therapeutic efficacy in enhancing wound healing, promoting angiogenesis, and attenuating inflammation and bacterial load suggesting its efficacy in healing refractory wounds. |
| ADSCs [59] | ADSCs combined with HA-CA ADSCs were injected around the wound in diabetic C57BL/6 mice and a patch was deposited on the wound | Angiogenesis Wound healing | HA-CA + ADSCs enhanced wound healing and angiogenesis synergistically involving PI3K/AKT pathway. |
| ADSCs [60] | Human ADSCs with SDF-1α gene-activated scaffold were tested in vitro using HUVEC | Pro-angiogenic properties | SDF-1α gene-activated scaffold overcomes the deficiencies associated with diabetic ADSCs and restores pro-angiogenic features ln ADSCs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, V.; Moellmer, R.; Agrawal, D.K. Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells 2022, 11, 2287. https://doi.org/10.3390/cells11152287
Rai V, Moellmer R, Agrawal DK. Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells. 2022; 11(15):2287. https://doi.org/10.3390/cells11152287
Chicago/Turabian StyleRai, Vikrant, Rebecca Moellmer, and Devendra K. Agrawal. 2022. "Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing" Cells 11, no. 15: 2287. https://doi.org/10.3390/cells11152287
APA StyleRai, V., Moellmer, R., & Agrawal, D. K. (2022). Stem Cells and Angiogenesis: Implications and Limitations in Enhancing Chronic Diabetic Foot Ulcer Healing. Cells, 11(15), 2287. https://doi.org/10.3390/cells11152287







