IDO1, FAT10, IFI6, and GILT Are Involved in the Antiretroviral Activity of γ-Interferon and IDO1 Restricts Retrovirus Infection by Autophagy Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Cell
2.3. cDNA Isolation of γ-IFN-Induced Factors
2.4. Construction of Retroviral Vector
2.5. Polymerase Chain Reaction of Unintegrated MLV-Based Vector Genome
2.6. Western Immunoblotting
2.7. Microarray Analysis
2.8. Statistics
3. Results
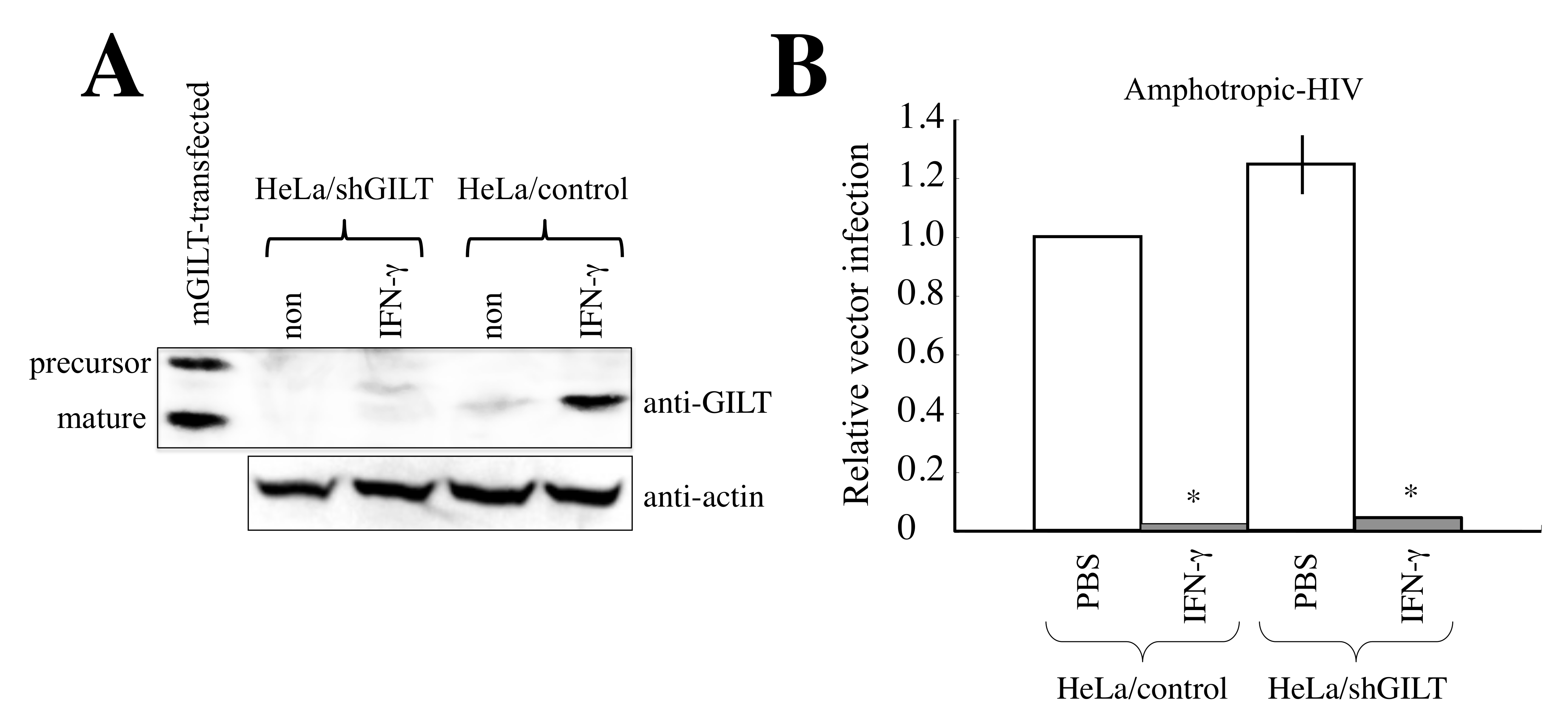
3.1. Unknown Cellular Factors Other than GILT Are Involved in γ-IFN-Mediated Restriction of HIV-1-Based Vector Infection in HeLa Cells
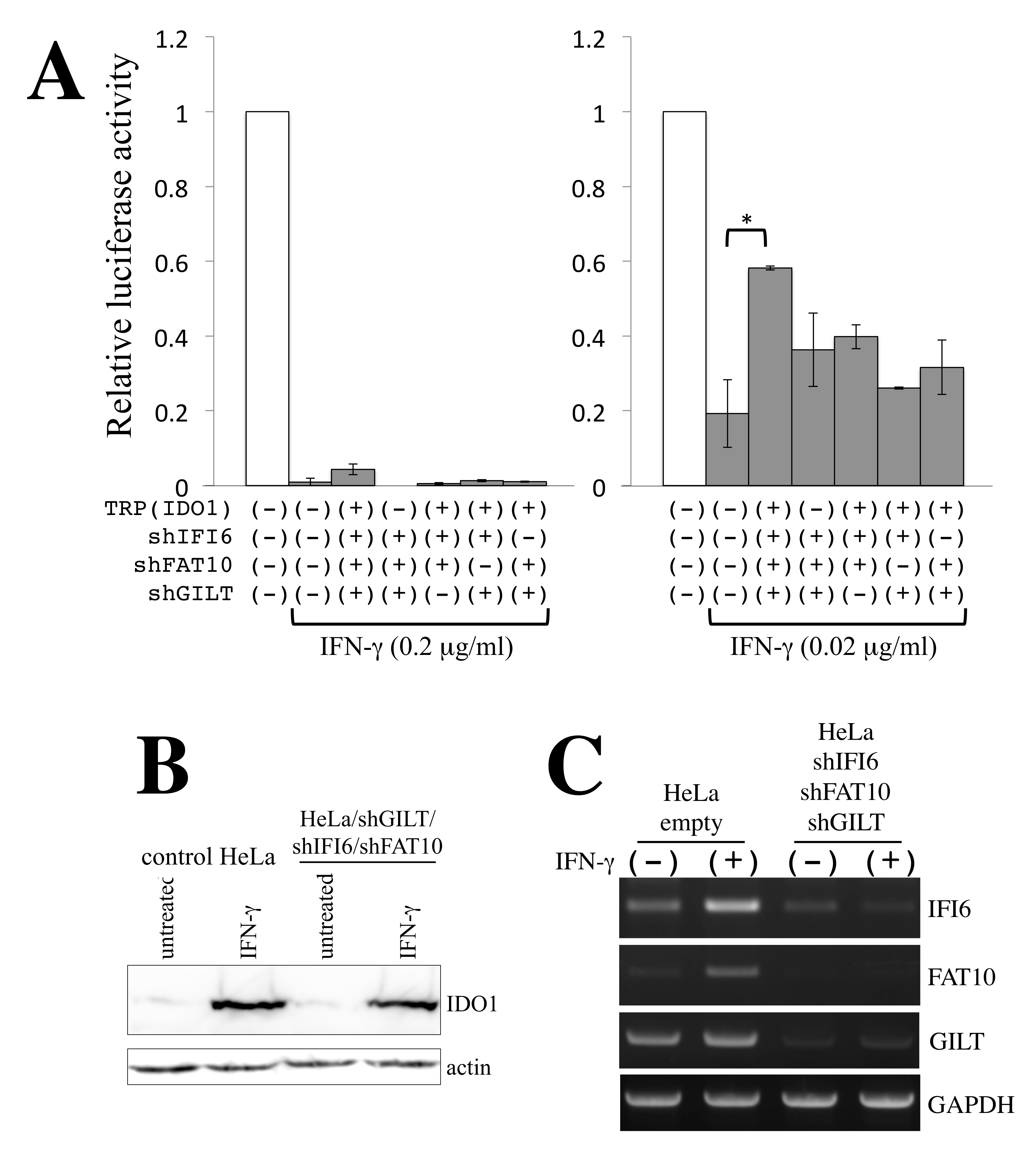
3.2. Identification of Antiretroviral Host Factors
3.3. Restriction Factors Involved in the Antiretroviral Activity of γ-IFN
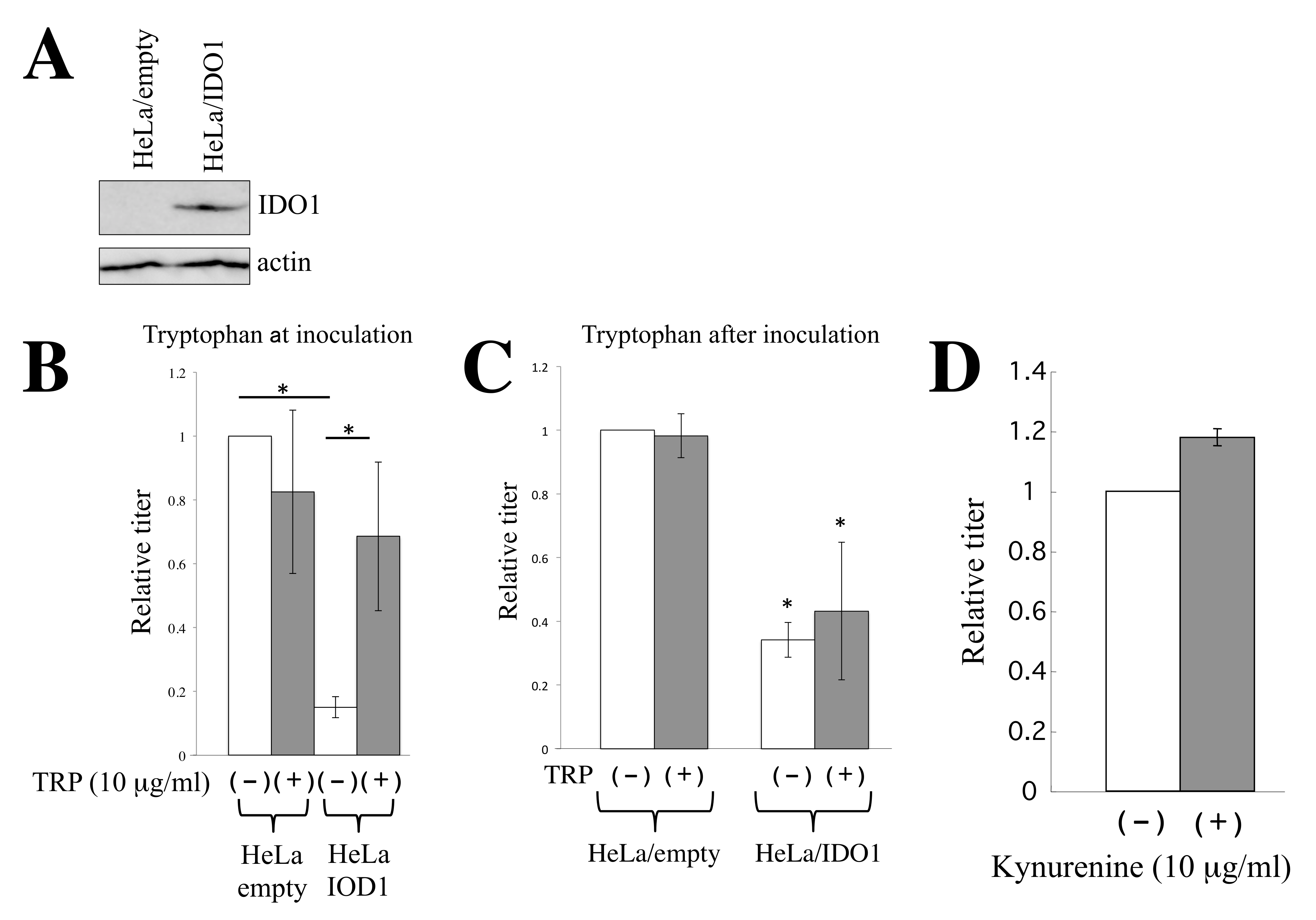
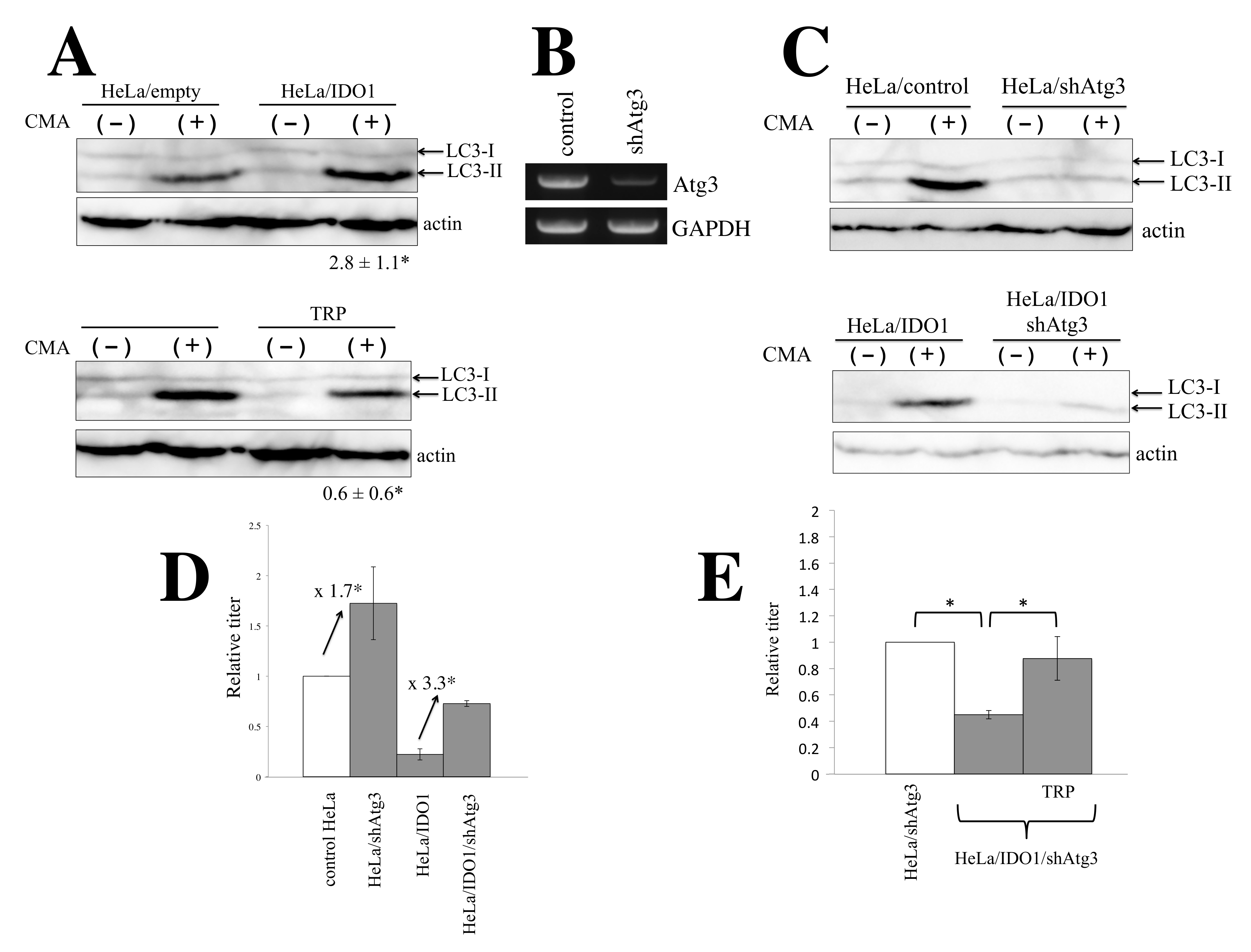
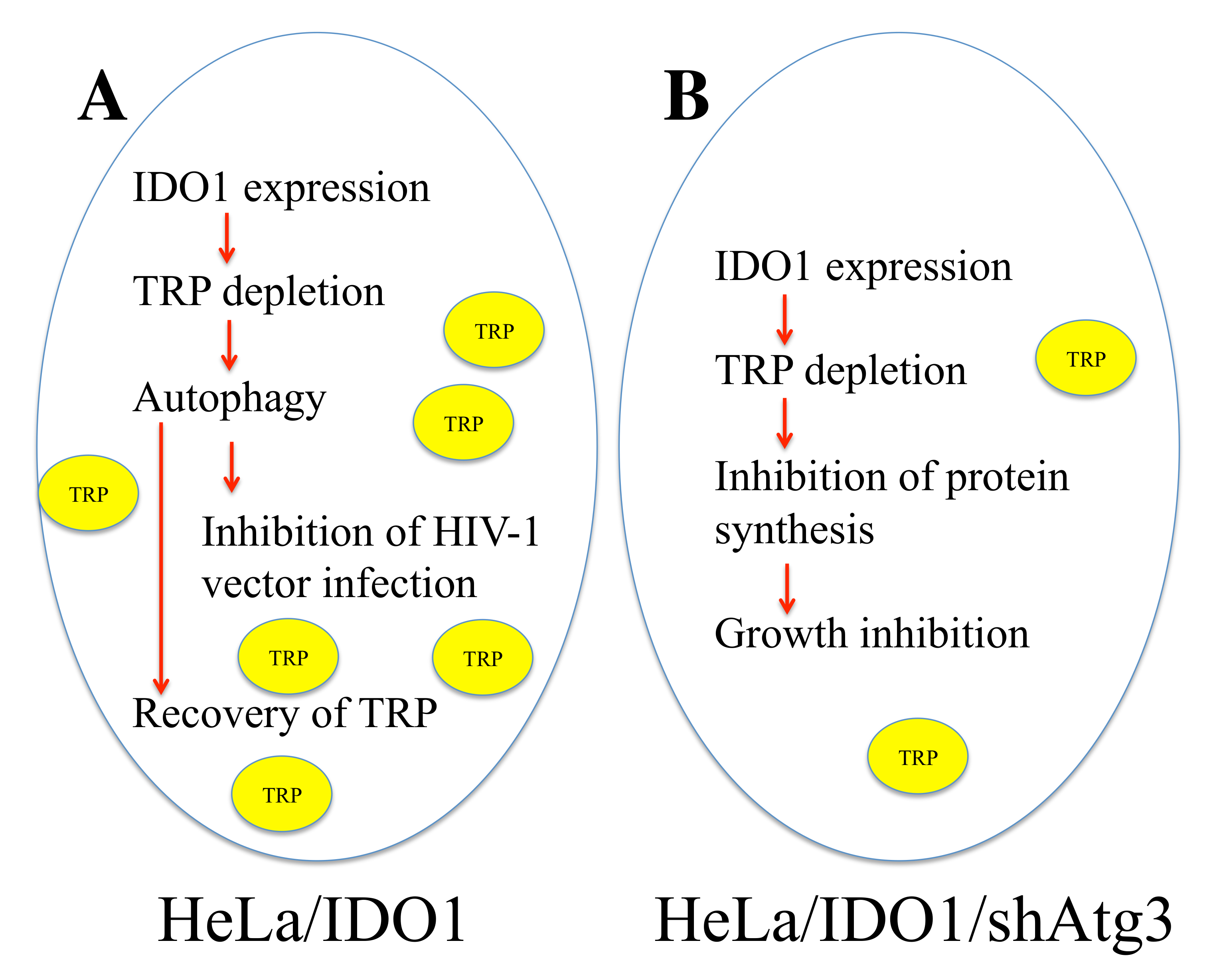
3.4. IDO1 Inhibits HIV-1-Based Vector Infection through Autophagy Enhanced by Tryptophan Depletion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiang, H.S.; Liu, H.M. The molecular basis of viral inhibition of IRF- and STAT-dependent immune responses. Front. Immunol. 2019, 9, 3086. [Google Scholar] [CrossRef] [PubMed]
- Bergantz, L.; Subra, F.; Deprez, E.; Delelis, O.; Richetta, C. Interplay between intrinsic and innate immunity during HIV infection. Cells 2019, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 2002, 418, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Lei, K.J.; Jin, W.; Greenwell-Wild, T.; Wahl, S.M. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J. Exp. Med. 2006, 203, 41–46. [Google Scholar] [CrossRef]
- Argyris, E.G.; Acheampong, E.; Wang, F.; Huang, J.; Chen, K.; Mukhtar, M.; Zhang, H. The interferon-induced expression of APOBEC3G in human blood-brain barrier exerts a potent intrinsic immunity to block HIV-1 entry to central nervous system. Virology 2007, 367, 440–451. [Google Scholar] [CrossRef]
- Goujon, C.; Moncorge, O.; Bauby, H.; Doyle, T.; Ward, C.C.; Schaller, T.; Hue, S.; Barclay, W.S.; Schulz, R.; Malim, M.H. Human MX2 is an interferon-induced post entry inhibitor of HIV-1 infection. Nature 2013, 502, 559–562. [Google Scholar] [CrossRef]
- Kane, M.; Yadav, S.S.; Bitzegeio, J.; Kutluay, S.B.; Zang, T.; Wilson, S.J.; Schoggins, J.W.; Rice, C.M.; Yamashita, M.; Hatziioannou, T.; et al. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 2013, 502, 563–566. [Google Scholar] [CrossRef]
- Lu, J.; Pan, Q.; Rong, L.; He, W.; Liu, S.L.; Liang, C. The IFITM protrins inhibit HIV-1 infection. J. Virol. 2011, 85, 2126–2137. [Google Scholar] [CrossRef]
- Amet, T.; Byrd, D.; Hu, N.; Sun, Q.; Li, F.; Zhao, Y.; Hu, S.; Grantham, A.; Yu, Q. BST-2 expression in human hepatocytes is inducible by all three types of interferons and restricts production of hepatitis C virus. Curr. Mol. Med. 2014, 14, 349–360. [Google Scholar] [CrossRef]
- Kubo, Y.; Izumida, M.; Yashima, Y.; Yoshii-Kamiyama, H.; Tanaka, Y.; Yasui, K.; Hayashi, H.; Matsuyama, T. Gamma-interferon-inducible, lysosome/endosome-localized thiolreductase, GILT, has anti-retroviral activity and its expression is counteracted by HIV-1. Oncotarget 2016, 7, 71255–71273. [Google Scholar] [CrossRef]
- Chen, D.; Hou, Z.; Jiang, D.; Zheng, M.; Li, G.; Zhang, Y.; Li, R.; Lin, H.; Chang, J.; Zeng, H.; et al. GILT restricts the cellular entry mediated by the envelope glycoproteins of SARS-CoV, Ebola virus and Lassa fever virus. Emerg. Microbes Infect. 2019, 8, 1511–1523. [Google Scholar] [CrossRef]
- Thipwong, J.; Saelim, H.; Panrat, T.; Phongdara, A. Penaeus monodon GILT enzyme restricts WSSV infectivity by reducing disulfide bonds in WSSV proteins. Dis. Aquat. Organ. 2019, 135, 59–70. [Google Scholar] [CrossRef]
- Teramoto, T.; Chiang, H.S.; Takhampunya, R.; Manzano, M.; Padmanabhan, R.; Maric, M. Gamma interferon-inducible lysosomal thioreductase (GILT) ablation renders mouse fibroblasts sensitive to dengue virus replication. Virology 2013, 441, 146–151. [Google Scholar] [CrossRef][Green Version]
- Naldini, L.; Blomer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef]
- Chang, L.J.; Urlacher, V.; Iwakuma, T.; Cui, Y.; Zucali, J. Efficacy and safety analysis of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 1999, 6, 715–728. [Google Scholar] [CrossRef]
- Kamiyama, H.; Kakoki, K.; Yoshii, H.; Iwao, M.; Igawa, T.; Sakai, H.; Hayashi, H.; Matsuyama, T.; Yamamoto, N.; Kubo, Y. Infection of XC cells by MLVs and ebola virus is endosome-dependent but acidification-independent. PLoS ONE 2011, 6, e26180. [Google Scholar] [CrossRef]
- Cosset, F.L.; Takeuchi, Y.; Battini, J.L.; Weiss, R.A.; Collins, M.K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 1995, 69, 7430–7436. [Google Scholar] [CrossRef]
- Kubo, Y.; Ishimoto, A.; Amanuma, H. N-linked glycosylation is required for XC-cell specific syncytium formation by the R peptide-containing envelope protein of ecotropic murine leukemia viruses. J. Virol. 2003, 77, 7510–7516. [Google Scholar] [CrossRef][Green Version]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Segeral, E.; Yatim, A.; Emiliani, S.; Schwarts, O.; Benkirane, M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011, 474, 654–657. [Google Scholar] [CrossRef]
- Usami, Y.; Wu, Y.; Gottlinger, H.G. SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef. Nature 2015, 526, 218–223. [Google Scholar] [CrossRef]
- Rosa, A.; Chande, A.; Ziglio, S.; De Sanctis, V.; Bertorelli, R.; Goh, S.L.; McCauley, S.M.; Nowosielska, A.; Antonarakis, S.E.; Luban, J.; et al. HIV-1 Nef promotes infection by excluding SERINC5 from virion incorporation. Nature 2015, 526, 212–217. [Google Scholar] [CrossRef]
- Siddiqui, R.; Suzu, S.; Ueno, M.; Nasser, H.; Koba, R.; Bhuyan, F.; Noyori, O.; Hamidi, S.; Sheng, G.; Yasuda-Inoue, M.; et al. Apolipoprotein E is an HIV-1-inducible inhibitor of viral production and infectivity in macrophages. PLoS Pathog. 2018, 14, e1007372. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Y.; Wang, Q.; Li, M.; Zhou, Z.; Dabbagh, D.; Fu, C.; Zhang, H.; Li, S.; Zhang, T.; et al. Proteomic profiling of HIV-1 infection of human CD4+ T cells identifies PSGL-1 as an HIV restriction factor. Nat. Microbiol. 2019, 4, 813–825. [Google Scholar] [CrossRef]
- Kane, M.; Zang, T.M.; Rihn, S.J.; Zhang, F.; Kueck, T.; Alim, M.; Schoggins, J.; Rice, C.M.; Wilson, S.J.; Bieniasz, P.D. Identification of interferon-stimulated genes with antiretroviral activity. Cell Host Microbe 2016, 20, 392–405. [Google Scholar] [CrossRef]
- Yang, S.L.; Tan, H.X.; Niu, T.T.; Liu, Y.K.; Gu, C.J.; Li, D.J.; Li, M.Q.; Wang, H.Y. The IFN-gamma-IDO1-kynurenine pathway-induced autophagy in cervical cancer cell promotes phagocytosis of macrophage. Int. J. Biol. Sci. 2021, 17, 339–352. [Google Scholar] [CrossRef]
- Chaudhary, K.; Shinde, R.; Liu, H.; Gnana-Prakasam, J.P.; Veeranan-Karmegam, R.; Huang, L.; Ravishankar, B.; Bradley, J.; Kvirkvelia, N.; McMenamin, M.; et al. Amino acid metabolism inhibits antibody-driven kidney injury by inducing autophagy. J. Immunol. 2015, 194, 5713–5724. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kiminami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Dalvi, P.; Sharma, H.; Chinnappan, M.; Sanderson, M.; Allen, J.; Zeng, R.; Choi, A.; O’Brien-Ladner, A.; Dhillon, N.K. Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: Role in HIV-related pulmonary arterial hypertension. Autophagy 2016, 12, 2420–2438. [Google Scholar] [CrossRef]
- Tang, S.W.; Ducroux, A.; Jeang, K.T.; Neuveut, C. Impact of cellular autophagy on viruses: Insights from hepatitis B virus and human retroviruses. J. Biomed. Sci. 2012, 19, 92. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. Inhibition of human immunodeficiency virus type-1 through autophagy. Curr. Opin. Microbiol. 2013, 16, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sagnier, S.; Daussy, C.F.; Borel, S.; Robert-Hebmann, V.; Faure, M.; Blanchet, F.P.; Beaumelle, B.; Biard-Piechaczyk, M.; Espert, L. Autophagy restricts HIV-1 infection by selectively degrading Tat in CD4+ T lymphocytes. J. Virol. 2015, 89, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Murrow, L.; Debnath, J. ATG12-ATG3 connects basal autophagy and late endosome function. Autophagy 2015, 11, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Now, H.; Kim, W.J.; Kim, N.; Yoo, J.Y. Ubiquitin-like modifier FAT10 attenuates RIG-I mediated antiviral signaling by segregating activated RIG-I from its signaling platform. Sci. Rep. 2016, 6, 23377. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Yang, N.; Liu, Q.; Zhang, Q.; Zhang, Y.; Li, N.; Zhao, Y.; Li, S.; Liu, S.; et al. FAT10 is critical in influenza A virus replication by inhibiting type I IFN. J. Immunol. 2016, 197, 824–833. [Google Scholar] [CrossRef]
- Meyer, K.; Kwon, Y.C.; Liu, S.; Hagedorn, C.H.; Ray, R.B.; Ray, R. Interferon-alpha inducible protein 6 impairs EGFR activation by CD81 and inhibits hepatitis C virus infection. Sci. Rep. 2015, 5, 9012. [Google Scholar] [CrossRef]
- Sajid, M.; Ullah, H.; Yan, K.; He, M.; Feng, J.; Shereen, M.A.; Hao, R.; Li, Q.; Guo, D.; Chen, Y.; et al. The functional and antiviral activity of interferon alpha-inducible IFI6 against hepatitis B virus replication and gene expression. Front. Immunol. 2021, 12, 634937. [Google Scholar] [CrossRef]
- Dukhovny, A.; Lamkiewicz, K.; Chen, Q.; Fricke, M.; Jabrane-Ferrat, N.; Marz, M.; Jung, J.U.; Sklan, E.H. A CRISPR activation screen identifies genes that protect against Zika virus infection. J. Virol. 2019, 93, e00211–e00219. [Google Scholar] [CrossRef]
- Richardson, R.B.; Ohlson, M.B.; Eltson, J.L.; Kumar, A.; McDougal, M.B.; Boys, I.N.; Mar, K.B.; De La Cruz-Rivera, P.C.; Douglas, C.; Konopka, G.; et al. A CRISPR screen identifies IFI6 as an ER-resident interferon effector that blocks flavivirus replication. Nat. Microbiol. 2018, 3, 1214–1223. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; Chen, L. Interferon-inducible protein 6-16 (IFI-6-16, ISG16) promotes hepatitis C virus replication in vitro. J. Med. Virol. 2016, 88, 109–114. [Google Scholar] [CrossRef]
- Adams, O.; Besken, K.; Oberdorfer, C.; MacKenzie, C.R.; Takikawa, O.; Daubener, W. Role of indolamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J. Virol. 2004, 78, 2632–2636. [Google Scholar] [CrossRef]
- Obojes, K.; Andres, O.; Kim, K.S.; Daubener, W.; Schneider-Schaulies, J. Indolamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J. Virol. 2005, 79, 7768–7776. [Google Scholar] [CrossRef]
- Terajima, M.; Leporati, A.M. Role of indolamine 2,3-dioxygenase in antiviral activity of interferon-gamma against vaccinia virus. Viral Immunol. 2005, 18, 722–729. [Google Scholar] [CrossRef]
- Esmaeoli, S.A.; Hajavi, J. The role of indoleamine 2,3-dioxygenase in allergic disorders. Mol. Biol. Rep. 2022, 49, 3297–3306. [Google Scholar] [CrossRef]
- Cao, Y.; Li, B.; Ismail, N.; Smith, K.; Li, T.; Dai, R.; Deng, Y. Neurotoxicity and underlying mechanisms of endogenous neurotoxins. Int. J. Mol. Sci. 2021, 22, 12805. [Google Scholar] [CrossRef]
- Ramirez, P.W.; Sharma, S.; Singh, R.; Stoneham, C.A.; Vollbrecht, T.; Guatelli, J. Plasma membrane-associated restriction factors and their counteraction by HIV-1 accessory proteins. Cells 2019, 8, 1020. [Google Scholar] [CrossRef]


| Host Factors | Untreated | γ-IFN | Fold Induction |
|---|---|---|---|
| TRIM5a | 39 | 33 | 0.8 |
| MX2 | 115 | 97 | 0.8 |
| SAMHD1 | 251 | 297 | 1.1 |
| SERINC3 | 4059 | 2691 | 0.7 |
| SERINC5 | 94 | 115 | 1.2 |
| IFITM1 | 3553 | 18,345 | 5.2 |
| IFITM2 | 38,265 | 46,579 | 1.2 |
| IFITM3 | 22,554 | 23,043 | 1 |
| ApoE | 2062 | 3193 | 1.5 |
| PSGL-1 | 104 | 73 | 0.7 |
| GILT | 22147 | 17304 | 0.8 |
| Host Factors | Untreated | γ-IFN | Fold Induction |
|---|---|---|---|
| FAT10 | 9 | 7862 | 898.3 |
| IDO1 | 10 | 4038 | 413.3 |
| GBP1 | 9 | 2481 | 280.8 |
| TRIM22 | 11 | 829 | 76.3 |
| IFI27 | 111 | 6136 | 55.2 |
| SERPING1 | 57 | 2882 | 50.2 |
| GBP2 | 7 | 323 | 44.8 |
| RARRES3 | 761 | 10,985 | 14.3 |
| APOL6 | 1086 | 14,886 | 13.7 |
| IFI6 | 215 | 2860 | 13.3 |
| SECTM | 2645 | 11,344 | 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, Y.; Yasui, K.; Izumida, M.; Hayashi, H.; Matsuyama, T. IDO1, FAT10, IFI6, and GILT Are Involved in the Antiretroviral Activity of γ-Interferon and IDO1 Restricts Retrovirus Infection by Autophagy Enhancement. Cells 2022, 11, 2240. https://doi.org/10.3390/cells11142240
Kubo Y, Yasui K, Izumida M, Hayashi H, Matsuyama T. IDO1, FAT10, IFI6, and GILT Are Involved in the Antiretroviral Activity of γ-Interferon and IDO1 Restricts Retrovirus Infection by Autophagy Enhancement. Cells. 2022; 11(14):2240. https://doi.org/10.3390/cells11142240
Chicago/Turabian StyleKubo, Yoshinao, Kiyoshi Yasui, Mai Izumida, Hideki Hayashi, and Toshifumi Matsuyama. 2022. "IDO1, FAT10, IFI6, and GILT Are Involved in the Antiretroviral Activity of γ-Interferon and IDO1 Restricts Retrovirus Infection by Autophagy Enhancement" Cells 11, no. 14: 2240. https://doi.org/10.3390/cells11142240
APA StyleKubo, Y., Yasui, K., Izumida, M., Hayashi, H., & Matsuyama, T. (2022). IDO1, FAT10, IFI6, and GILT Are Involved in the Antiretroviral Activity of γ-Interferon and IDO1 Restricts Retrovirus Infection by Autophagy Enhancement. Cells, 11(14), 2240. https://doi.org/10.3390/cells11142240







