The Time Sequence of Gene Expression Changes after Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedures and Tissue Preparation
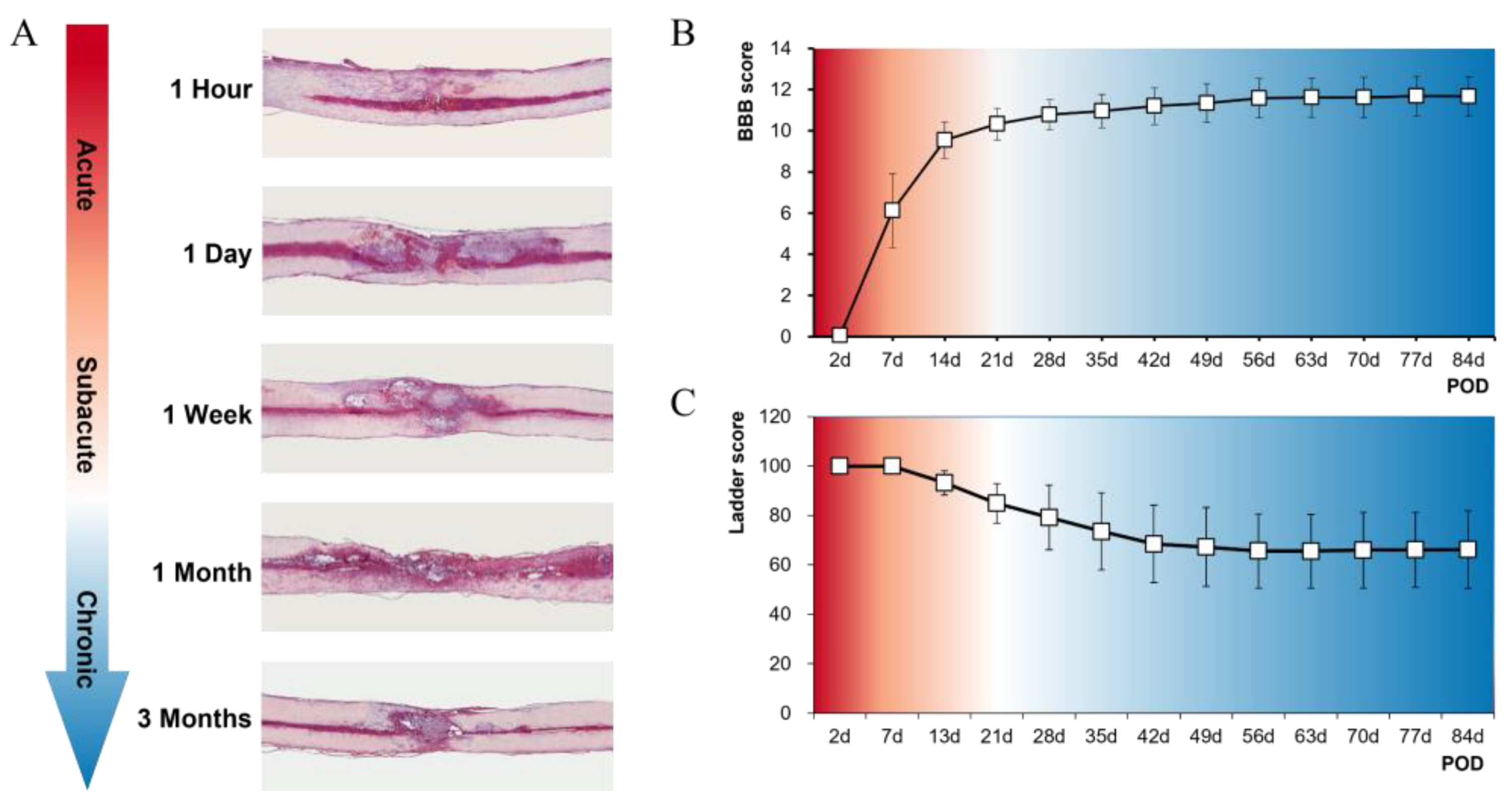
2.3. Histology
2.4. Locomotor Assessment
2.5. RNA Sample Preparation
2.6. RNA Sequencing Library Construction
2.7. Data Analysis
2.7.1. Quality Control
2.7.2. Read Mapping and Differentially Expressed Genes (DEG) Analysis
2.7.3. Statistical Analysis and Visualization of Data
2.7.4. Integrative Function Classification Analysis for DEGs
2.7.5. Time-Series DPGP Clustering Analysis
2.7.6. Gene–Drug Network Analysis
3. Results
3.1. Histological and Functional Results of SCI Models at Five Time Points
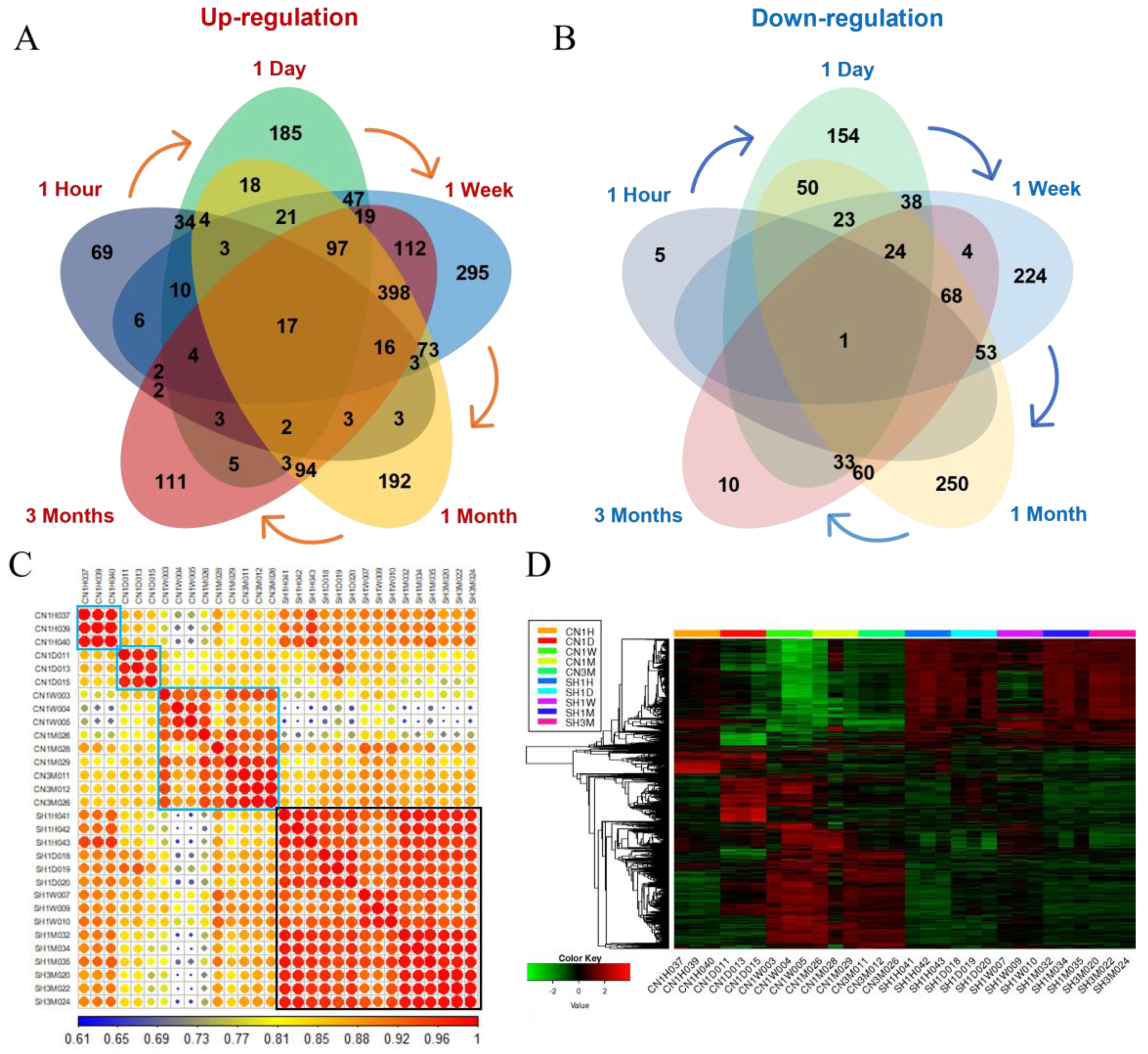
3.2. Transcriptome Sequencing Analysis
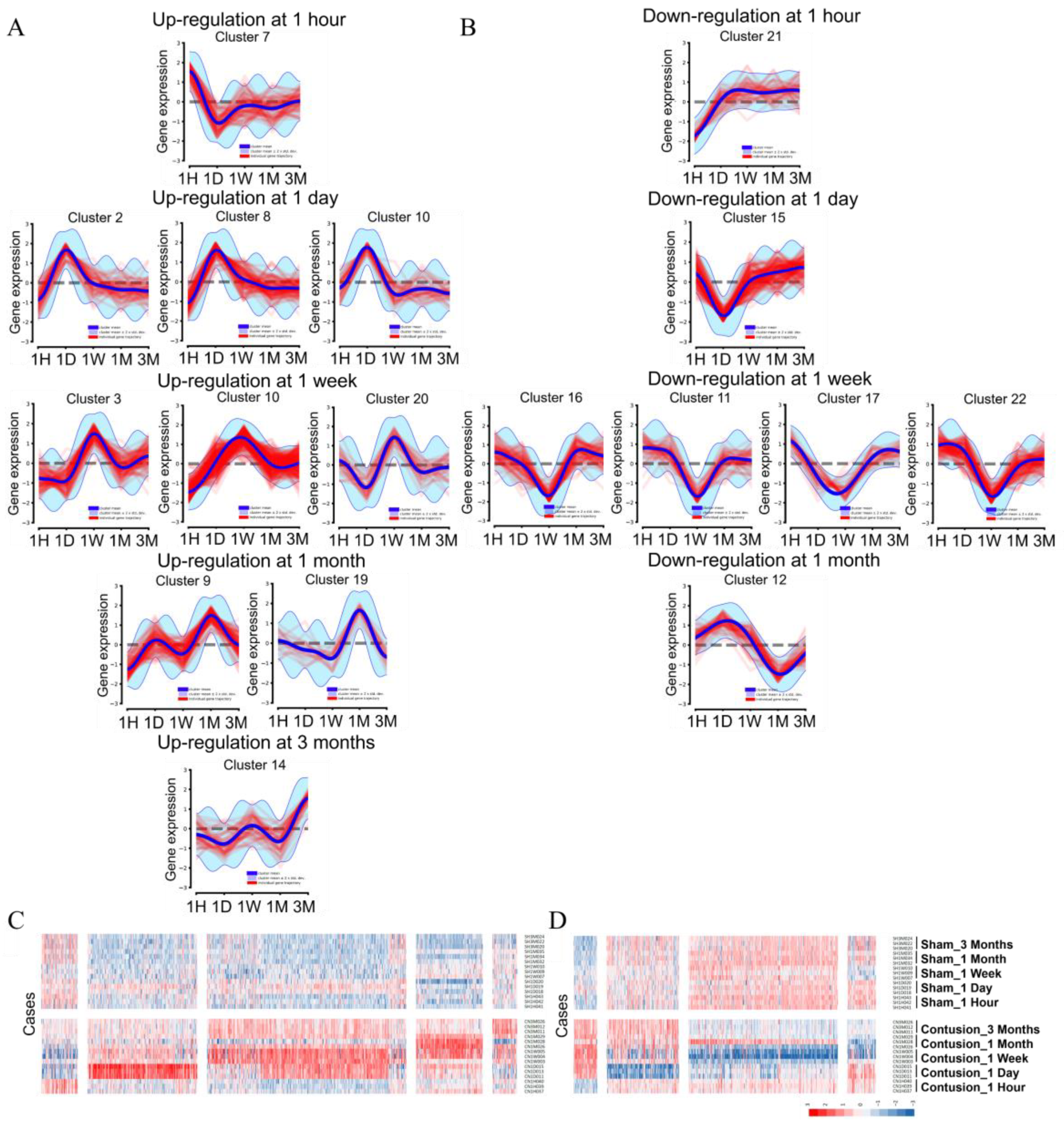
3.3. Transcriptional Waves According to the Time after SCI
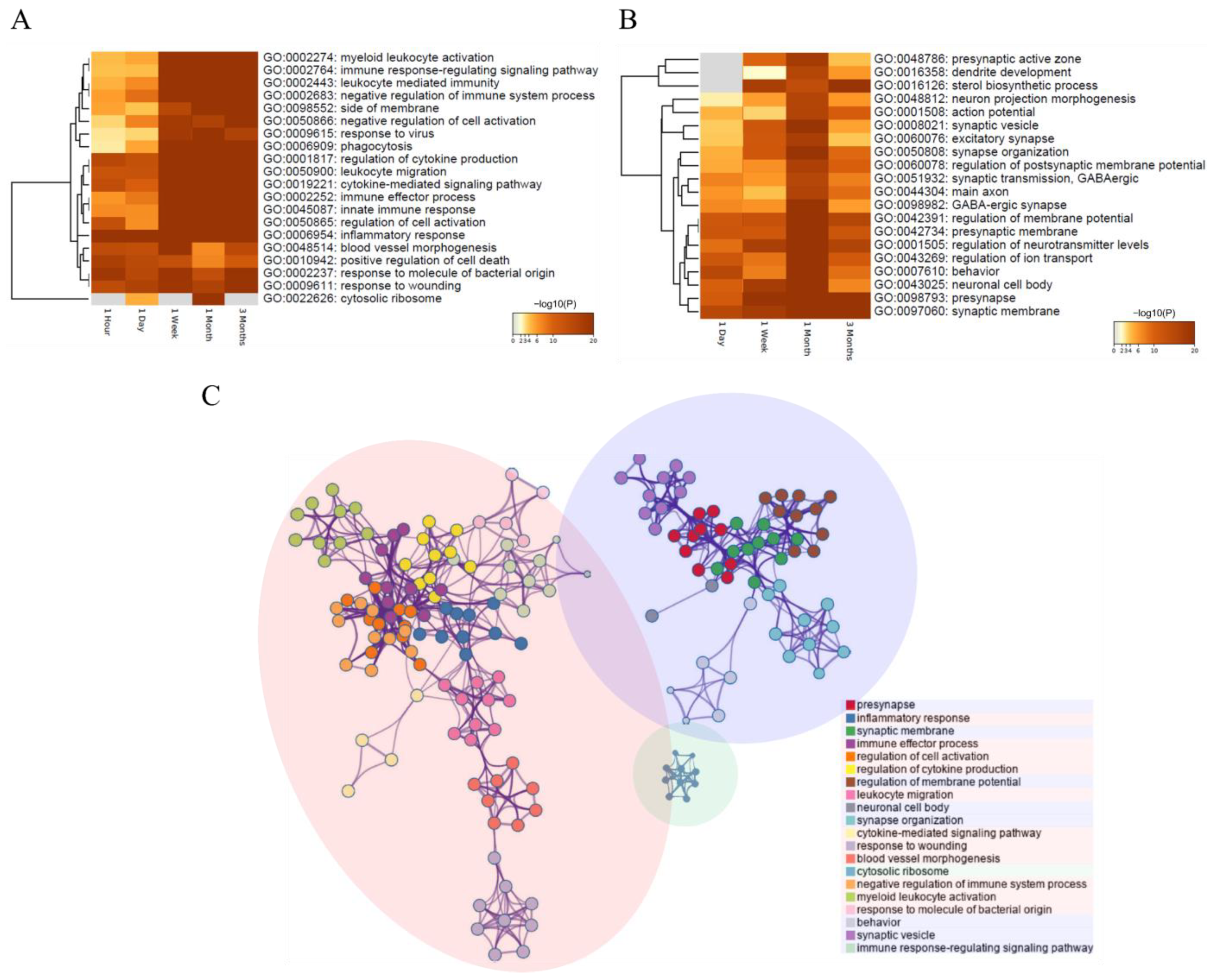
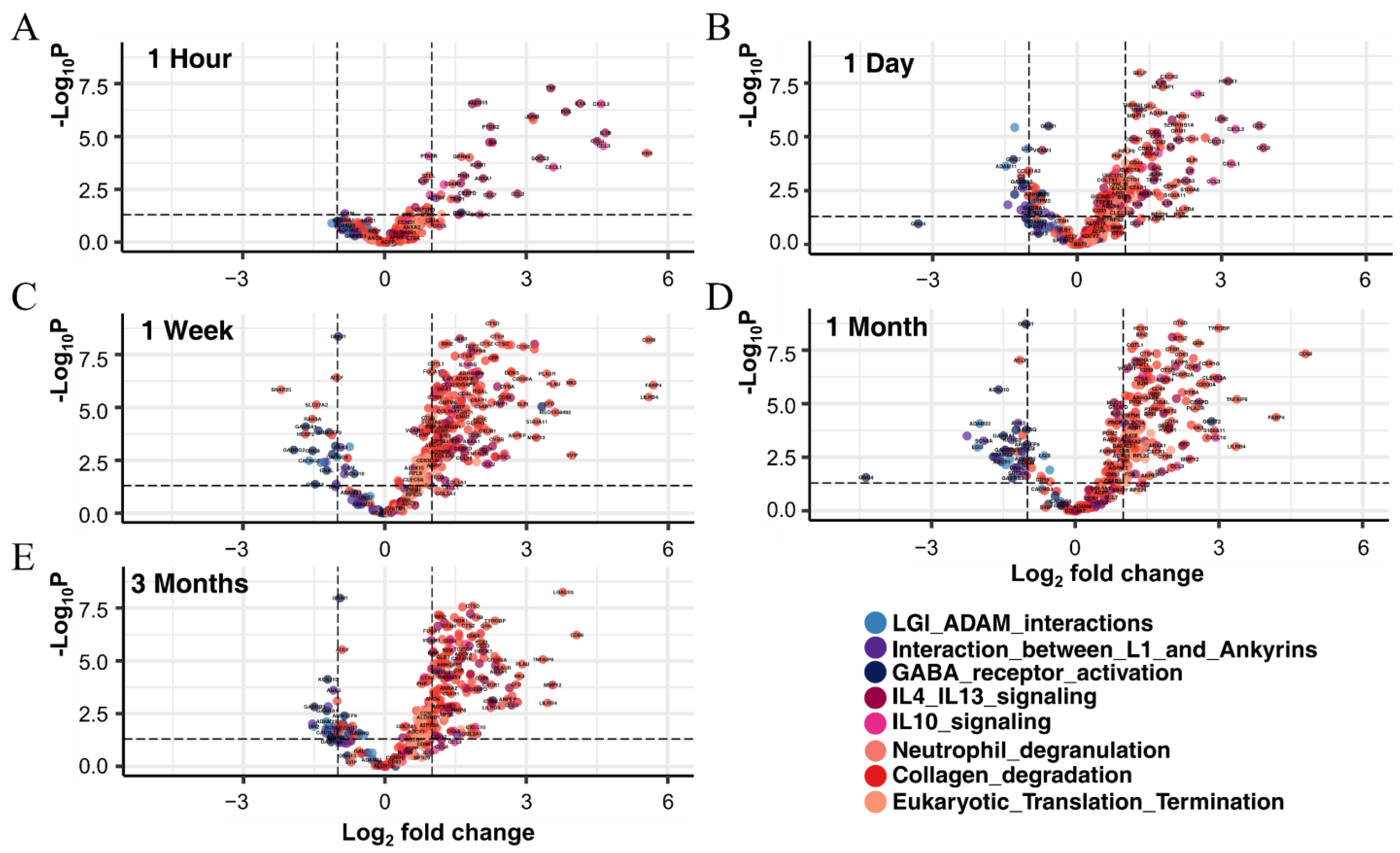
3.3.1. Identification of DEGs in Each Period and Their Functional Prediction
3.3.2. Systematic DEG Classification Using the DPGP Clustering Method for Time Series Analysis
3.4. Molecular Changes Caused by SCI
3.5. Exploration of the Molecular Biological Cascade through Integrated Analysis of the Continuous Period after SCI
3.5.1. Interleukin Signaling
3.5.2. Neutrophil Degranulation, Eukaryotic Translation, and Collagen Degradation
3.5.3. LGI–ADAM Interactions, GABA Receptor, and L1CAM–Ankyrin Interactions
3.6. Gene–Drug Network Analysis Provides Potential Drug or Chemical Candidates for the Treatment of SCI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vismara, I.; Papa, S.; Rossi, F.; Forloni, G.; Veglianese, P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol. Med. 2017, 23, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, A.; Hooshmand, S.; Gokmen, A.; Zarrabi, A.; Mostafavi, E. Spinal Cord Injury Management through the Combination of Stem Cells and Implantable 3D Bioprinted Platforms. Cells 2021, 10, 3189. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Wychowaniec, J.K.; Brougham, D.F.; Dooley, D. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol. Ther. 2022, 234, 108043. [Google Scholar] [CrossRef]
- Chhabra, H.S.; Sarda, K. Clinical translation of stem cell based interventions for spinal cord injury-Are we there yet? Adv. Drug Deliv. Rev. 2017, 120, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Levenberg, S. The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review. Int. J. Mol. Sci. 2022, 23, 1244. [Google Scholar] [CrossRef]
- Sultan, I.; Lamba, N.; Liew, A.; Doung, P.; Tewarie, I.; Amamoo, J.J.; Gannu, L.; Chawla, S.; Doucette, J.; Cerecedo-Lopez, C.D.; et al. The safety and efficacy of steroid treatment for acute spinal cord injury: A Systematic Review and meta-analysis. Heliyon 2020, 6, e03414. [Google Scholar] [CrossRef]
- Fan, B.; Wei, Z.; Feng, S. Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Res. 2022, 10, 35. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Y.; Hertwig, F.; Thierry-Mieg, J.; Zhang, W.; Thierry-Mieg, D.; Wang, J.; Furlanello, C.; Devanarayan, V.; Cheng, J.; et al. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome Biol. 2015, 16, 133. [Google Scholar] [CrossRef]
- Marco-Puche, G.; Lois, S.; Benitez, J.; Trivino, J.C. RNA-Seq Perspectives to Improve Clinical Diagnosis. Front. Genet. 2019, 10, 1152. [Google Scholar] [CrossRef]
- Chen, K.; Deng, S.; Lu, H.; Zheng, Y.; Yang, G.; Kim, D.; Cao, Q.; Wu, J.Q. RNA-seq characterization of spinal cord injury transcriptome in acute/subacute phases: A resource for understanding the pathology at the systems level. PLoS ONE 2013, 8, e72567. [Google Scholar] [CrossRef]
- Wang, X.-D.; Li, Y.; Chen, Y.; Li, X.; Wu, J.; Pan, J.-Y.; Cai, R.-X.; Yang, R.-Y. RNA sequencing screening of differentially expressed genes after spinal cord injury. Neural. Regen. Res. 2019, 14, 1583. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Zhang, N.; Xie, X.M.; Chen, Y.J.; Wang, R.; Shen, L.; Zhou, J.S.; Hu, J.G.; Lu, H.Z. Transcriptome profile of rat genes in injured spinal cord at different stages by RNA-sequencing. BMC Genom. 2017, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Davaa, G.; Yoo, H.; Hong, K.; Hyun, J.K. Ascorbic Acid Promotes Functional Restoration after Spinal Cord Injury Partly by Epigenetic Modulation. Cells 2020, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Mahapatra, C.; Hong, J.Y.; Kim, M.S.; Leong, K.W.; Kim, H.W.; Hyun, J.K. Functional Recovery of Contused Spinal Cord in Rat with the Injection of Optimal-Dosed Cerium Oxide Nanoparticles. Adv. Sci. 2017, 4, 1700034. [Google Scholar] [CrossRef]
- Martin, J.A.; Wang, Z. Next-generation transcriptome assembly. Nat. Rev. Genet. 2011, 12, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Sun, J.; Nishiyama, T.; Shimizu, K.; Kadota, K. TCC: An R package for comparing tag count data with robust normalization strategies. BMC Bioinform. 2013, 14, 219. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudie, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- McDowell, I.C.; Manandhar, D.; Vockley, C.M.; Schmid, A.K.; Reddy, T.E.; Engelhardt, B.E. Clustering gene expression time series data using an infinite Gaussian process mixture model. PLoS Comput. Biol. 2018, 14, e1005896. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Bittker, J.A.; Liu, Z.; Gould, J.; McCarren, P.; Hirschman, J.E.; Johnston, S.E.; Vrcic, A.; Wong, B.; Khan, M.; et al. The Drug Repurposing Hub: A next-generation drug library and information resource. Nat. Med. 2017, 23, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [CrossRef]
- Henriques, A.; Croixmarie, V.; Bouscary, A.; Mosbach, A.; Keime, C.; Boursier-Neyret, C.; Walter, B.; Spedding, M.; Loeffler, J.P. Sphingolipid Metabolism Is Dysregulated at Transcriptomic and Metabolic Levels in the Spinal Cord of an Animal Model of Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2017, 10, 433. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, J.A.; Reinke, A.W.; Bhimsaria, D.; Keating, A.E.; Ansari, A.Z. Combinatorial bZIP dimers display complex DNA-binding specificity landscapes. eLife 2017, 6, e19272. [Google Scholar] [CrossRef]
- Hunt, D.; Raivich, G.; Anderson, P.N. Activating transcription factor 3 and the nervous system. Front. Mol. Neurosci. 2012, 5, 7. [Google Scholar] [CrossRef]
- Schousboe, A.; Scafidi, S.; Bak, L.K.; Waagepetersen, H.S.; McKenna, M.C. Glutamate metabolism in the brain focusing on astrocytes. Adv. Neurobiol. 2014, 11, 13–30. [Google Scholar] [CrossRef]
- Croft, D.; O’Kelly, G.; Wu, G.; Haw, R.; Gillespie, M.; Matthews, L.; Caudy, M.; Garapati, P.; Gopinath, G.; Jassal, B.; et al. Reactome: A database of reactions, pathways and biological processes. Nucleic Acids Res. 2011, 39, D691–D697. [Google Scholar] [CrossRef]
- Su, Y.; Gao, J.; Kaur, P.; Wang, Z. Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases. Pharmaceutics 2020, 12, 1222. [Google Scholar] [CrossRef]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Dixon, B.; Jones, L.; Gorbet, M. The Differential Reactive Oxygen Species Production of Tear Neutrophils in Response to Various Stimuli In Vitro. Int. J. Mol. Sci. 2021, 22, 2899. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.A.; Pagano, P.J.; Colucci, W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 2001, 280, C53–C60. [Google Scholar] [CrossRef]
- Ghosh, A.; Shcherbik, N. Effects of Oxidative Stress on Protein Translation: Implications for Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 2661. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, B.; Li, X.; Barik, S. Translation control: A multifaceted regulator of inflammatory response. J. Immunol. 2010, 184, 3311–3319. [Google Scholar] [CrossRef] [PubMed]
- Fukata, Y.; Yokoi, N.; Miyazaki, Y.; Fukata, M. The LGI1-ADAM22 protein complex in synaptic transmission and synaptic disorders. Neurosci. Res. 2017, 116, 39–45. [Google Scholar] [CrossRef]
- Kegel, L.; Aunin, E.; Meijer, D.; Bermingham, J.R. LGI proteins in the nervous system. ASN Neuro 2013, 5, 167–181. [Google Scholar] [CrossRef]
- Sagane, K.; Ishihama, Y.; Sugimoto, H. LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int. J. Biol. Sci. 2008, 4, 387–396. [Google Scholar] [CrossRef]
- Kegel, L.; Jaegle, M.; Driegen, S.; Aunin, E.; Leslie, K.; Fukata, Y.; Watanabe, M.; Fukata, M.; Meijer, D. Functional phylogenetic analysis of LGI proteins identifies an interaction motif crucial for myelination. Development 2014, 141, 1749–1756. [Google Scholar] [CrossRef]
- Platek, R.; Grycz, K.; Wieckowska, A.; Czarkowska-Bauch, J.; Skup, M. L1 Cell Adhesion Molecule Overexpression Down Regulates Phosphacan and Up Regulates Structural Plasticity-Related Genes Rostral and Caudal to the Complete Spinal Cord Transection. J. Neurotrauma 2020, 37, 534–554. [Google Scholar] [CrossRef]
- Hortsch, M.; Nagaraj, K.; Godenschwege, T.A. The interaction between L1-type proteins and ankyrins--a master switch for L1-type CAM function. Cell. Mol. Biol. Lett. 2009, 14, 57–69. [Google Scholar] [CrossRef]
- Fleming, J.; Hutton, C.F.; Heiser, D.M.; Youngquist, S.; Hutton, K.C.; Barton, E.D. Spinal Cord Injuries and Helicopter Emergency Medical Services, 6,929 Patients: A Multicenter Analysis. Air. Med. J. 2016, 35, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Harrop, J.S.; Ghobrial, G.M.; Chitale, R.; Krespan, K.; Odorizzi, L.; Fried, T.; Maltenfort, M.; Cohen, M.; Vaccaro, A. Evaluating initial spine trauma response: Injury time to trauma center in PA, USA. J. Clin. Neurosci. 2014, 21, 1725–1729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fehlings, M.G.; Perrin, R.G. The timing of surgical intervention in the treatment of spinal cord injury: A systematic review of recent clinical evidence. Spine 2006, 31, S28–S35, discussion S36. [Google Scholar] [CrossRef] [PubMed]
- El Tecle, N.E.; Dahdaleh, N.S.; Hitchon, P.W. Timing of Surgery in Spinal Cord Injury. Spine 2016, 41, E995–E1004. [Google Scholar] [CrossRef]
- Dvorak, M.F.; Noonan, V.K.; Fallah, N.; Fisher, C.G.; Finkelstein, J.; Kwon, B.K.; Rivers, C.S.; Ahn, H.; Paquet, J.; Tsai, E.C.; et al. The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: An observational Canadian cohort study. J. Neurotrauma 2015, 32, 645–654. [Google Scholar] [CrossRef]
- Croce, M.A.; Bee, T.K.; Pritchard, E.; Miller, P.R.; Fabian, T.C. Does optimal timing for spine fracture fixation exist? Ann. Surg. 2001, 233, 851–858. [Google Scholar] [CrossRef]
- Konieczny, M.R.; Struwer, J.; Jettkant, B.; Schinkel, C.; Kalicke, T.; Muhr, G.; Frangen, T.M. Early versus late surgery of thoracic spine fractures in multiple injured patients: Is early stabilization always recommendable? Spine J. 2015, 15, 1713–1718. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Priebe, M.M.; Ho, C.H.; Scelza, W.M.; Chiodo, A.E.; Wuermser, L.A. Spinal cord injury medicine. 3. Rehabilitation phase after acute spinal cord injury. Arch. Phys. Med. Rehabil. 2007, 88, S62–S70. [Google Scholar] [CrossRef]
- Brown, A.K.; Woller, S.A.; Moreno, G.; Grau, J.W.; Hook, M.A. Exercise therapy and recovery after SCI: Evidence that shows early intervention improves recovery of function. Spinal Cord 2011, 49, 623–628. [Google Scholar] [CrossRef]
- Dijkers, M.P.; Zanca, J.M. Factors complicating treatment sessions in spinal cord injury rehabilitation: Nature, frequency, and consequences. Arch. Phys. Med. Rehabil. 2013, 94, S115–S124. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Curt, A.; Steeves, J.D.; Coleman, W.P.; Tuszynski, M.H.; Lammertse, D.; Bartlett, P.F.; Blight, A.R.; Dietz, V.; Ditunno, J.; et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007, 45, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Seo, Y.; Davaa, G.; Kim, H.W.; Kim, S.H.; Hyun, J.K. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 2020, 101, 357–371. [Google Scholar] [CrossRef]
- Zivkovic, S.; Ayazi, M.; Hammel, G.; Ren, Y. For Better or for Worse: A Look Into Neutrophils in Traumatic Spinal Cord Injury. Front. Cell. Neurosci. 2021, 15, 648076. [Google Scholar] [CrossRef]
- Xie, Y.J.; Zhou, L.; Wang, Y.; Jiang, N.W.; Cao, S.; Shao, C.Y.; Wang, X.T.; Li, X.Y.; Shen, Y.; Zhou, L. Leucine-Rich Glioma Inactivated 1 Promotes Oligodendrocyte Differentiation and Myelination via TSC-mTOR Signaling. Front. Mol. Neurosci. 2018, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Romaus-Sanjurjo, D.; Ledo-Garcia, R.; Fernandez-Lopez, B.; Hanslik, K.; Morgan, J.R.; Barreiro-Iglesias, A.; Rodicio, M.C. GABA promotes survival and axonal regeneration in identifiable descending neurons after spinal cord injury in larval lampreys. Cell Death Dis. 2018, 9, 663. [Google Scholar] [CrossRef]
- Bilchak, J.N.; Caron, G.; Cote, M.P. Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 4858. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.P.; Yeh, G.C.; Huang, E.Y.; Law, P.Y.; Loh, H.H.; Tao, P.L. Effects of dextromethorphan and oxycodone on treatment of neuropathic pain in mice. J. Biomed. Sci. 2015, 22, 81. [Google Scholar] [CrossRef]
- Li, D.; Yan, Y.; Yu, L.; Duan, Y. Procaine Attenuates Pain Behaviors of Neuropathic Pain Model Rats Possibly via Inhibiting JAK2/STAT3. Biomol. Ther. 2016, 24, 489–494. [Google Scholar] [CrossRef]
- Imai, T.; Katoh, H.; Suyama, K.; Kuroiwa, M.; Yanagisawa, S.; Watanabe, M. Amiloride Promotes Oligodendrocyte Survival and Remyelination after Spinal Cord Injury in Rats. J. Clin. Med. 2018, 7, 46. [Google Scholar] [CrossRef]
- Paramos-de-Carvalho, D.; Martins, I.; Cristovao, A.M.; Dias, A.F.; Neves-Silva, D.; Pereira, T.; Chapela, D.; Farinho, A.; Jacinto, A.; Saude, L. Targeting senescent cells improves functional recovery after spinal cord injury. Cell Rep. 2021, 36, 109334. [Google Scholar] [CrossRef]
- Arbo, B.D.; Ribeiro, F.S.; Ribeiro, M.F. Astrocyte Neuroprotection and Dehydroepiandrosterone. Vitam. Horm. 2018, 108, 175–203. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; DeFinis, J.H.; Daugherty, S.L.; Tang, C.; Weinberger, J.; de Groat, W.C. Deciphering Spinal Endogenous Dopaminergic Mechanisms That Modulate Micturition Reflexes in Rats with Spinal Cord Injury. eNeuro 2021, 8, ENEURO.0157-21.2021. [Google Scholar] [CrossRef] [PubMed]
- Martineau, M.; Parpura, V.; Mothet, J.P. Cell-type specific mechanisms of D-serine uptake and release in the brain. Front. Synaptic Neurosci. 2014, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ye, J.; Chen, X.; Shi, J.; Wu, W.; Lin, W.; Lin, W.; Li, Y.; Fu, H.; Li, S. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-kappaB pathway dependent of HDAC3. J. Neuroinflamm. 2018, 15, 150. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, S.; Botchway, B.O.A.; Zhang, Y.; Liu, X. Valproic Acid: A Potential Therapeutic for Spinal Cord Injury. Cell. Mol. Neurobiol. 2021, 41, 1441–1452. [Google Scholar] [CrossRef]
- Pu, B.; Xue, Y.; Wang, Q.; Hua, C.; Li, X. Dextromethorphan provides neuroprotection via anti-inflammatory and anti-excitotoxicity effects in the cortex following traumatic brain injury. Mol. Med. Rep. 2015, 12, 3704–3710. [Google Scholar] [CrossRef]
- Chen, C.L.; Cheng, M.H.; Kuo, C.F.; Cheng, Y.L.; Li, M.H.; Chang, C.P.; Wu, J.J.; Anderson, R.; Wang, S.; Tsai, P.J.; et al. Dextromethorphan Attenuates NADPH Oxidase-Regulated Glycogen Synthase Kinase 3beta and NF-kappaB Activation and Reduces Nitric Oxide Production in Group A Streptococcal Infection. Antimicrob. Agents Chemother. 2018, 62, e02045-17. [Google Scholar] [CrossRef]
- O’Neill, S.; Brault, J.; Stasia, M.J.; Knaus, U.G. Genetic disorders coupled to ROS deficiency. Redox Biol. 2015, 6, 135–156. [Google Scholar] [CrossRef]
- Carlsson, K.C.; Hoem, N.O.; Moberg, E.R.; Mathisen, L.C. Analgesic effect of dextromethorphan in neuropathic pain. Acta Anaesthesiol. Scand. 2004, 48, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.N. Clinical Neuropharmacology of Pain in Spinal Cord Injury-Dextromethorphan Dose Response Clinical Trial. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01435798 (accessed on 7 June 2022).
- Premkumar, L.S.; Abooj, M. TRP channels and analgesia. Life Sci. 2013, 92, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Durham-Lee, J.C.; Mokkapati, V.U.; Johnson, K.M.; Nesic, O. Amiloride improves locomotor recovery after spinal cord injury. J. Neurotrauma 2011, 28, 1319–1326. [Google Scholar] [CrossRef]
- Gartside, S.E.; Griffith, N.C.; Kaura, V.; Ingram, C.D. The neurosteroid dehydroepiandrosterone (DHEA) and its metabolites alter 5-HT neuronal activity via modulation of GABAA receptors. J. Psychopharmacol. 2010, 24, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.; Inman, D.M.; Hirose, S.; Noble, L.J.; Igarashi, T.; Compagnone, N.A. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J. Neurosci. Res. 2004, 75, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.; Allard, J.; Rampin, O.; Droupy, S.; Benoit, G.; Alexandre, L.; Bernabe, J. Spinal proerectile effect of apomorphine in the anesthetized rat. Int. J. Impot. Res. 2001, 13, 110–115. [Google Scholar] [CrossRef]
- Ishizuka, O.; Gu, B.J.; Nishizawa, O.; Mizusawa, H.; Andersson, K.E. Effect of apomorphine on intracavernous pressure and blood pressure in conscious, spinalized rats. Int. J. Impot. Res. 2002, 14, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, J.T.; Woods, S.W.; Petkova, E.; Cornblatt, B.; Corcoran, C.M.; Chen, H.; Silipo, G.; Javitt, D.C. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: A pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry 2015, 2, 403–412. [Google Scholar] [CrossRef]
- Valproic Acid. Available online: https://medlineplus.gov/druginfo/meds/a682412.html#how (accessed on 7 June 2022).
- Drewes, A.M.; Andreasen, A.; Poulsen, L.H. Valproate for treatment of chronic central pain after spinal cord injury. A double-blind cross-over study. Paraplegia 1994, 32, 565–569. [Google Scholar] [CrossRef]
- Warner, F.M.; Jutzeler, C.R.; Cragg, J.J.; Tong, B.; Grassner, L.; Bradke, F.; Geisler, F.; Kramer, J.K. The Effect of Non-Gabapentinoid Anticonvulsants on Sensorimotor Recovery After Human Spinal Cord Injury. CNS Drugs 2019, 33, 503–511. [Google Scholar] [CrossRef]
- Barbiellini Amidei, C.; Salmaso, L.; Bellio, S.; Saia, M. Epidemiology of traumatic spinal cord injury: A large population-based study. Spinal Cord 2022, 1–8. [Google Scholar] [CrossRef]
- Mattson, M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasser, M.N.; Mellor, I.R.; Carter, W.G. Is L-Glutamate Toxic to Neurons and Thereby Contributes to Neuronal Loss and Neurodegeneration? A Systematic Review. Brain Sci. 2022, 12, 577. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.N.; MacLean, S.M.; Stromberg, A.J.; Whelan, J.P.; Bailey, W.M.; Gensel, J.C.; Wilson, M.E. Considerations for Studying Sex as a Biological Variable in Spinal Cord Injury. Front. Neurol. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Stephens, K.E.; Zhou, W.; Ji, Z.; Chen, Z.; He, S.; Ji, H.; Guan, Y.; Taverna, S.D. Sex differences in gene regulation in the dorsal root ganglion after nerve injury. BMC Genom. 2019, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Tansley, S.; Uttam, S.; Urena Guzman, A.; Yaqubi, M.; Pacis, A.; Parisien, M.; Deamond, H.; Wong, C.; Rabau, O.; Brown, N.; et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat. Commun. 2022, 13, 843. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, P.; Wang, Q.; Chiang, C.W.; Zhou, Y.; Hou, Y.; Xu, J.; Chen, R.; Zhang, B.; Lewis, S.J.; et al. Artificial intelligence framework identifies candidate targets for drug repurposing in Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 7. [Google Scholar] [CrossRef]
- Paranjpe, M.D.; Taubes, A.; Sirota, M. Insights into Computational Drug Repurposing for Neurodegenerative Disease. Trends Pharmacol. Sci. 2019, 40, 565–576. [Google Scholar] [CrossRef]
- Donner, Y.; Kazmierczak, S.; Fortney, K. Drug Repurposing Using Deep Embeddings of Gene Expression Profiles. Mol. Pharm. 2018, 15, 4314–4325. [Google Scholar] [CrossRef]
- Santos, S.S.; Torres, M.; Galeano, D.; Sanchez, M.D.M.; Cernuzzi, L.; Paccanaro, A. Machine learning and network medicine approaches for drug repositioning for COVID-19. Patterns 2022, 3, 100396. [Google Scholar] [CrossRef]


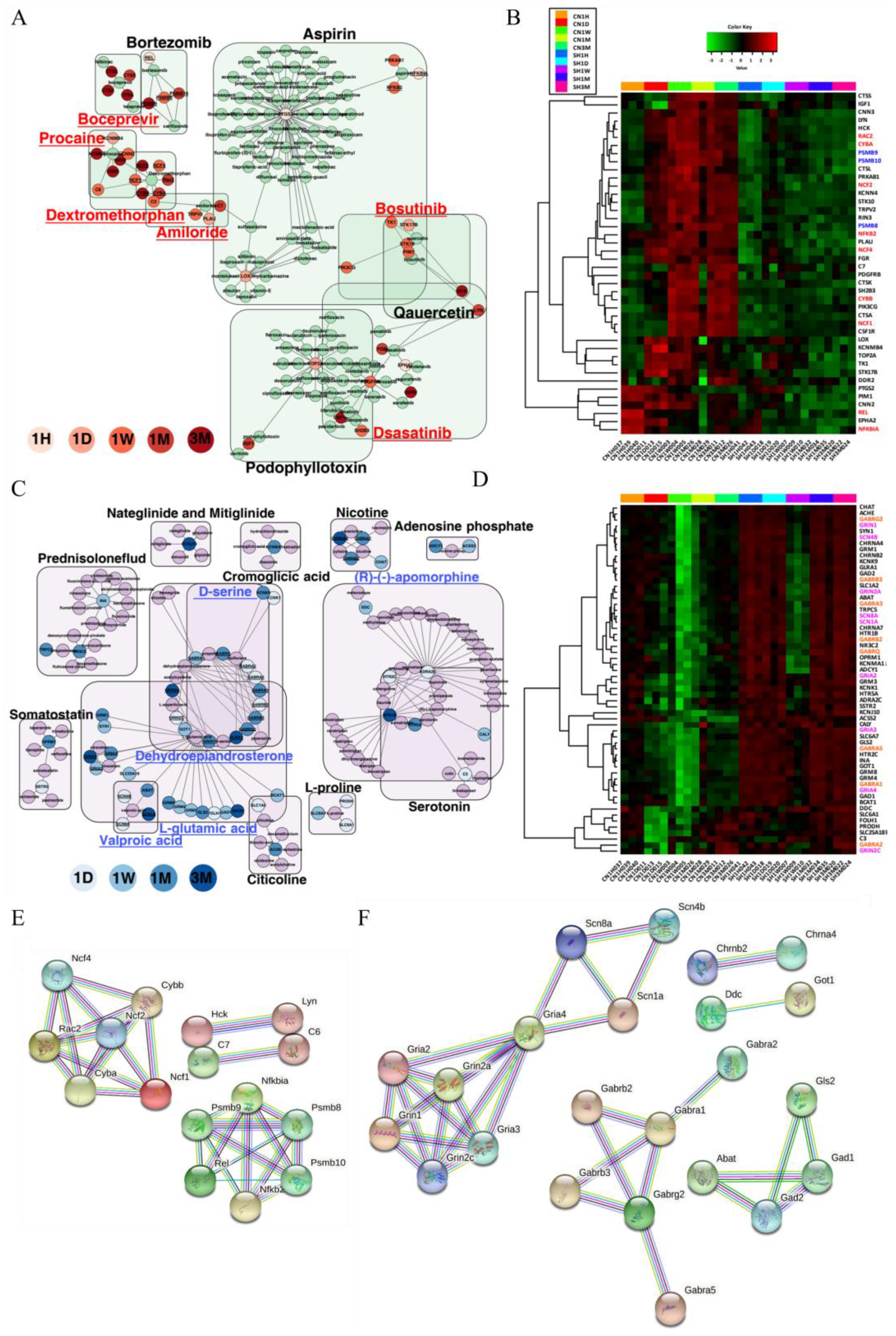
| Mechanism of Action (MOA) | DEGs | Launched Drugs | Description | Genes List | Effective Period |
|---|---|---|---|---|---|
| Antagonist | Upregulated | Dextromethorphan | noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist | C2, C6, CYBA, CYBB, NCF1, NCF2, NCF4, RAC2, RIN3 | 1 d–3 m |
| Procaine | HMGCR inhibitor, sodium channel blocker | CNN2, KCNMB4, C6, KCNN4, RIN3, CNN3 | 1 d–3 m | ||
| Amiloride | sodium channel blocker | PLAU, C2, TRPV2, C7 | 1 d–1 m | ||
| Dasatinib | Bcr-Abl kinase inhibitor, ephrin inhibitor, KIT inhibitor, PDGFR tyrosine kinase receptor inhibitor, SRC inhibitor, tyrosine kinase inhibitor | EPHA2, HCK, LYN, PDGFRB, FGR | 1 h–3 m | ||
| Boceprevir | HCV inhibitor | CTSA, CTSK, CTSL, CTSS | 1 m–3 m | ||
| Bosutinib | Abl kinase inhibitor, Bcr-Abl kinase inhibitor, SRC inhibitor | HCK, LYN, STK10, TK1 | 1 d–1w | ||
| Agonist | Downregulated | L-glutamic acid | glutamate receptor agonist | FOLH1, GLS2, GRIN2C, GRM1, GRM3, GRM4, SLC1A2, SLC25A18, BCAT1, GAD1, GOT1, GRIA2, GRIA4, GRIN1, GRM8, SYN1, ABAT, GAD2, GRIA3, GRIN2A | 1 d–3 m |
| Dehydroepiandrosterone (DHEA) | protein synthesis stimulant | GABRA2, GABRA5, GABRB2, GRIN2C, GABRA1, GABRG2, GRIN1, GABRA3, GABRB3, GABRQ, GRIN2A | 1 d–3 m | ||
| (R)-(-)-apomorphine | dopamine receptor agonist | ADRA2C, HTR2C, CALY, HTR1B, HTR5A | 1 d–3 m | ||
| D-serine | glutamate receptor agonist | GLRA1, GRIN2C, GRIN1, GLRA1, GRIN2A | 1 d–3 m | ||
| Valproic acid | benzodiazepine receptor agonist, HDAC inhibitor | SCN1A, SCN4B, SCN8A, ABAT | 1 d, 1 m–3 m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, S.; Han, K.; Hyun, J.K. The Time Sequence of Gene Expression Changes after Spinal Cord Injury. Cells 2022, 11, 2236. https://doi.org/10.3390/cells11142236
Mun S, Han K, Hyun JK. The Time Sequence of Gene Expression Changes after Spinal Cord Injury. Cells. 2022; 11(14):2236. https://doi.org/10.3390/cells11142236
Chicago/Turabian StyleMun, Seyoung, Kyudong Han, and Jung Keun Hyun. 2022. "The Time Sequence of Gene Expression Changes after Spinal Cord Injury" Cells 11, no. 14: 2236. https://doi.org/10.3390/cells11142236
APA StyleMun, S., Han, K., & Hyun, J. K. (2022). The Time Sequence of Gene Expression Changes after Spinal Cord Injury. Cells, 11(14), 2236. https://doi.org/10.3390/cells11142236






