Neutrophils Promote Glioblastoma Tumor Cell Migration after Biopsy
Abstract
:1. Introduction
2. Materials & Methods
2.1. Tumor Cell Lines
2.2. Mice
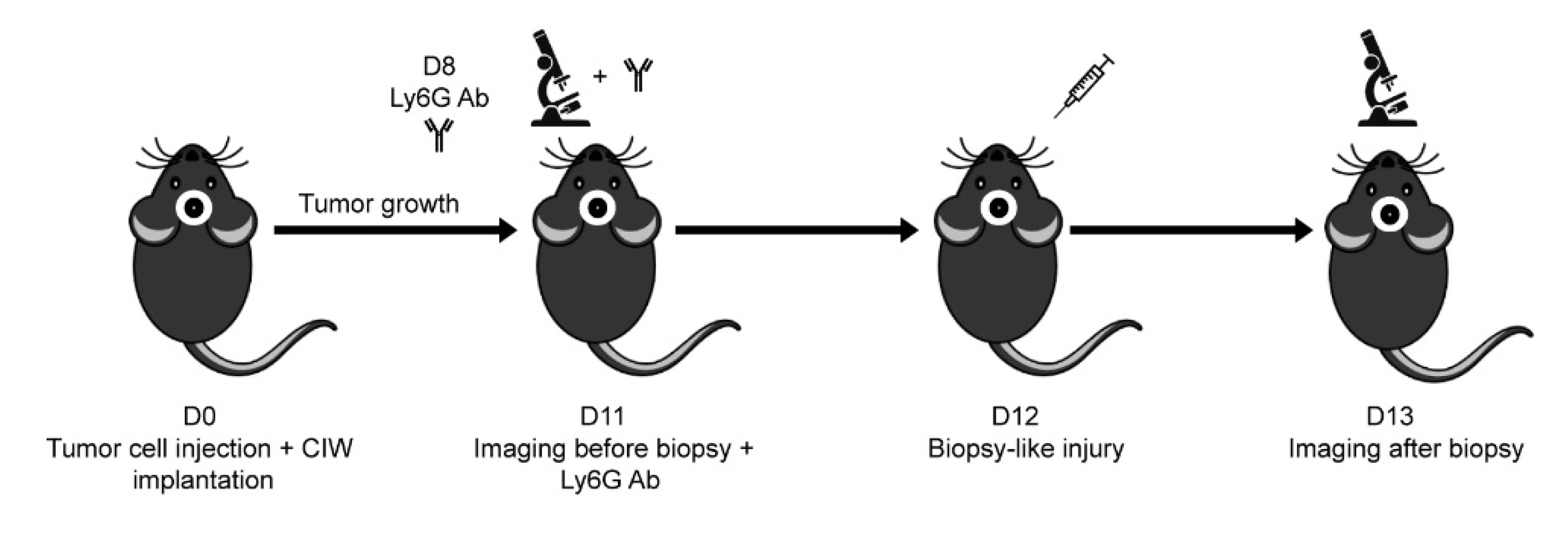
2.3. Mouse Intravital Imaging & In Vivo Cell Tracking
2.4. Immunostaining of Mouse Brain Slices
2.5. Flow Cytometry of Mouse Blood Cells
2.6. Human Blood and Neutrophil Isolation
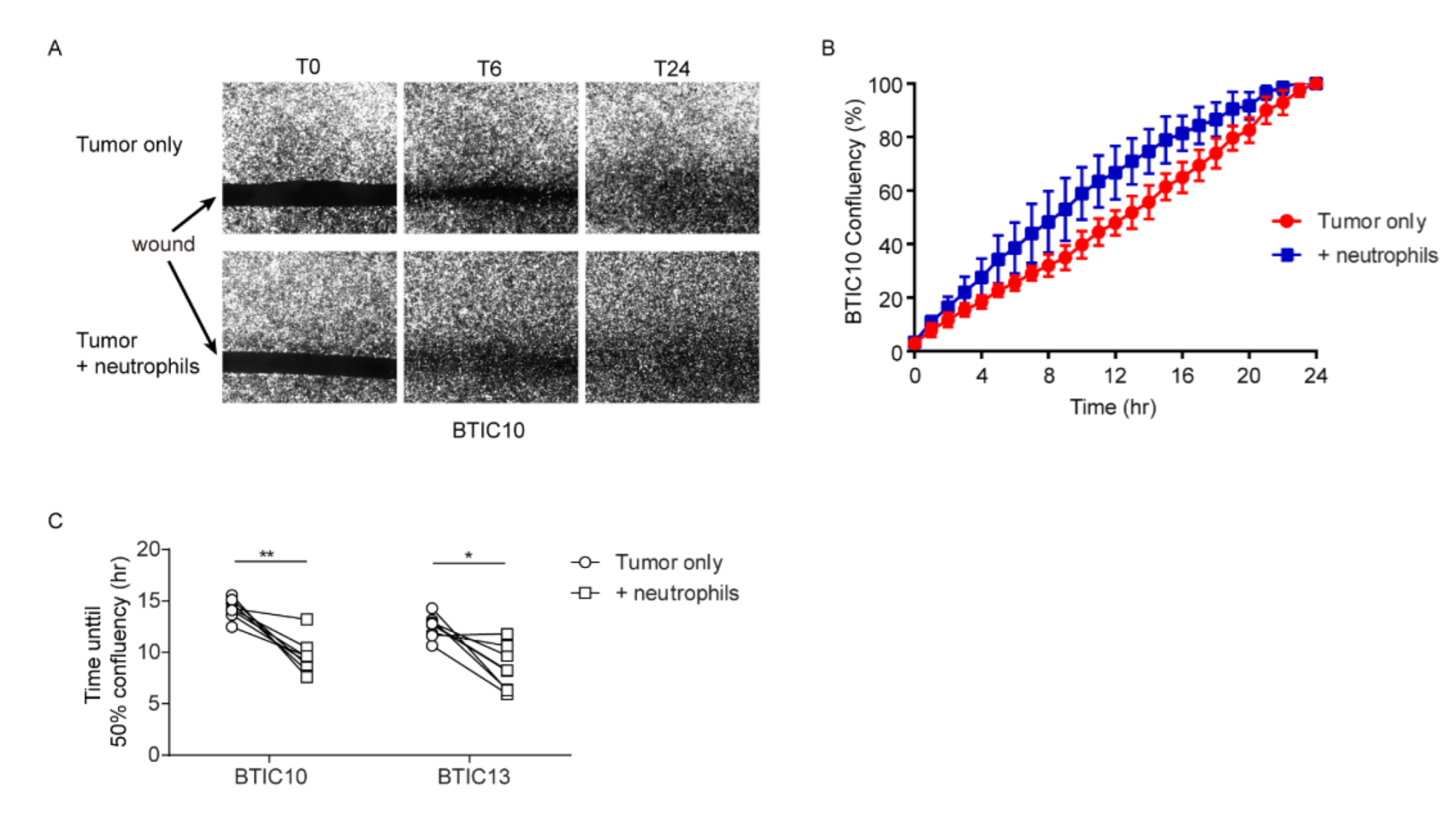
2.7. Wound-Healing Assay
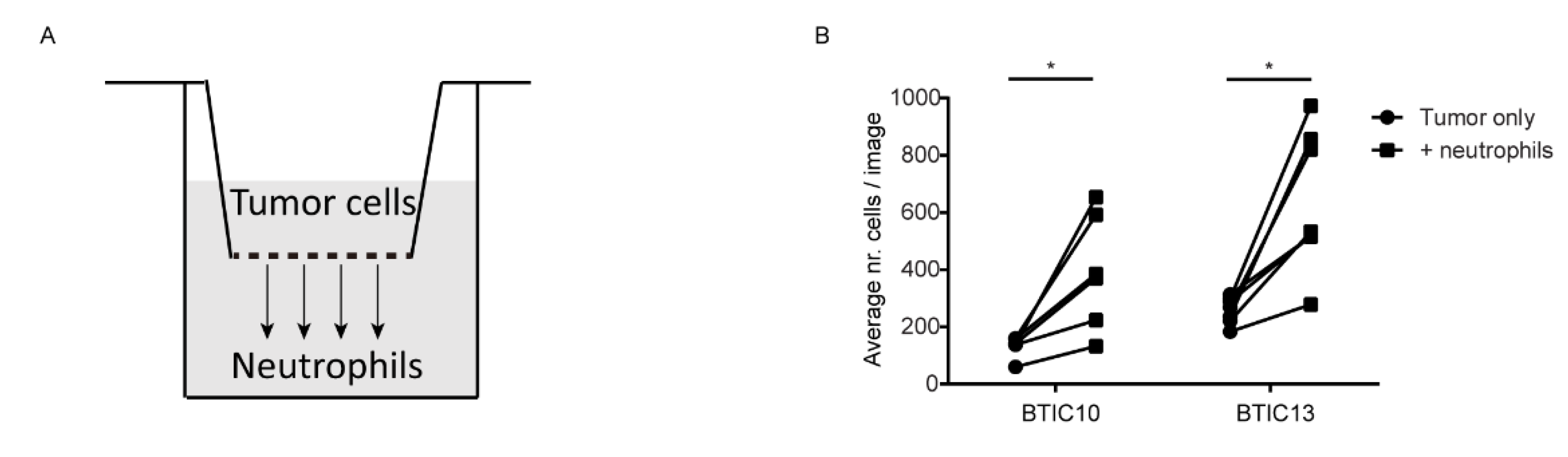
2.8. Transwell Migration Assay
2.9. Statistics
3. Results
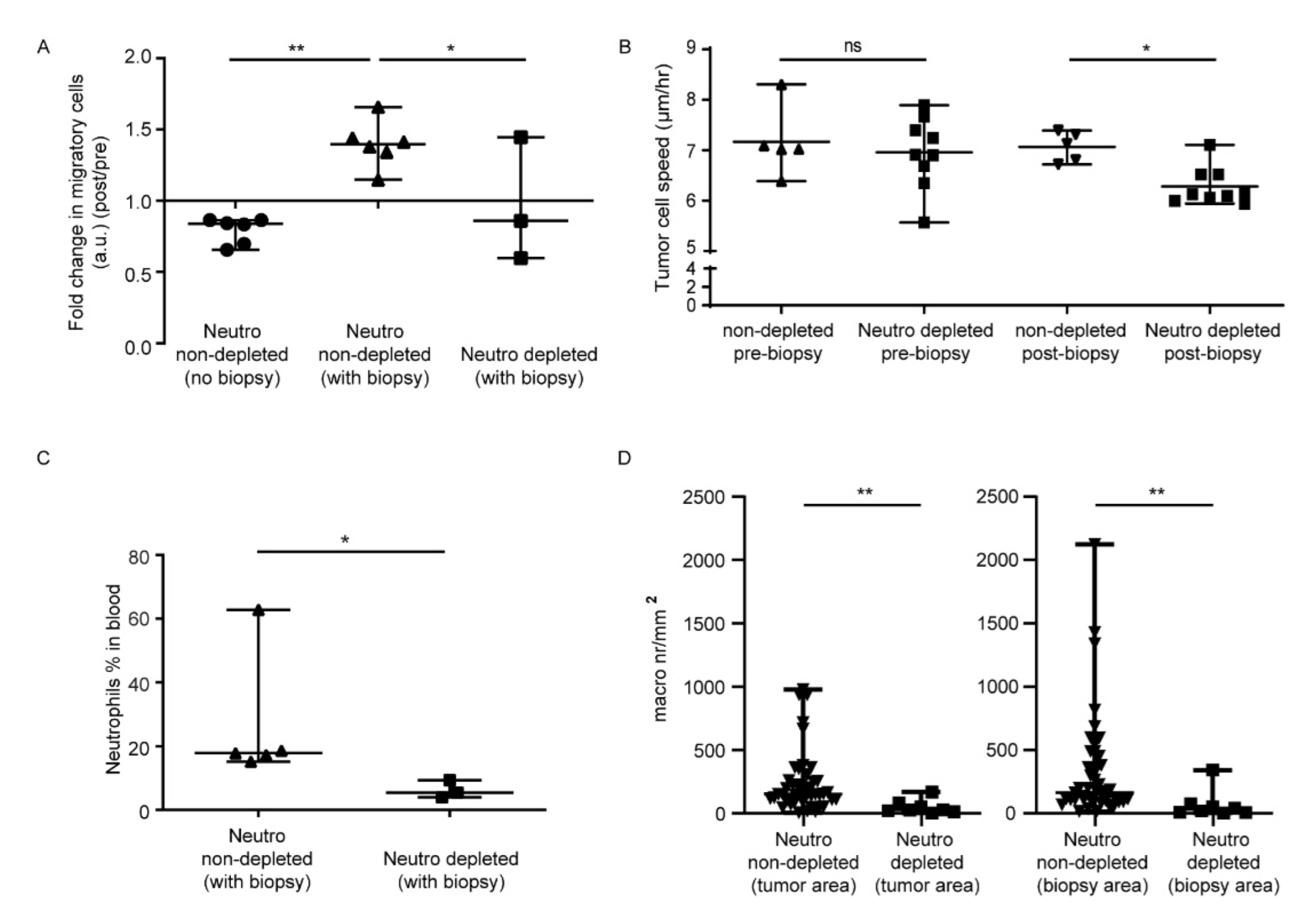
3.1. In Vivo Biopsy Induces a Neutrophil-Dependent Increase in Motility of Mouse Glioma Tumor Cells
3.2. Neutrophils Promote In Vitro Wound-Closure of Human Glioblastoma Tumor Cells
3.3. Soluble Factor(s) from Neutrophils Increase Transmigration of Human Glioblastoma Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Adamson, C.; Kanu, O.O.; Mehta, A.I.; Di, C.; Lin, N.; Mattox, A.K.; Bigner, D.D. Glioblastoma multiforme: A review of where we have been and where we are going. Expert Opin. Investig. Drugs 2009, 18, 1061–1083. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours: WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Massara, M.; Persico, P.; Bonavita, O.; Poeta, V.M.; Locati, M.; Simonelli, M.; Bonecchi, R. Neutrophils in gliomas. Front. Immunol. 2017, 8, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsa, A.T.; Wachhorst, S.; Lamborn, K.R.; Prados, M.D.; McDermott, M.W.; Berger, M.S.; Chang, S.M. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J. Neurosurg. 2005, 102, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Al-Sahaf, O.; Wang, J.H.; Browne, T.J.; Cotter, T.G.; Redmond, H.P. Surgical injury enhances the expression of genes that mediate breast cancer metastasis to the lung. Ann. Surg. 2010, 252, 1037–1043. [Google Scholar] [CrossRef]
- Mathenge, E.G.; Dean, C.A.; Clements, D.; Vaghar-Kashani, A.; Photopoulos, S.; Coyle, K.M.; Giacomantonio, M.; Malueth, B.; Nunokawa, A.; Jordan, J.; et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia 2014, 16, 950–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alieva, M.; Margarido, A.S.; Wieles, T.; Abels, E.R.; Colak, B.; Boquetale, C.; Noordmans, H.J.; Snijders, T.J.; Broekman, M.L.; Van Rheenen, J. Preventing inflammation inhibits biopsy-mediated changes in tumor cell behavior. Sci. Rep. 2017, 7, 7529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Predina, J.; Eruslanov, E.; Judy, B.; Kapoor, V.; Cheng, G.; Wang, L.-C.; Sun, J.; Moon, E.K.; Fridlender, Z.G.; Albelda, S.; et al. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proc. Natl. Acad. Sci. USA 2013, 110, E415–E424. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.; Moebius, P.; Büttner-Herold, M.; Amann, K.; Preidl, R.; Neukam, F.W.; Wehrhan, F. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas—An immunohistochemical study. Br. J. Cancer 2015, 113, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Szalayova, G.; Ogrodnik, A.; Spencer, B.; Wade, J.; Bunn, J.; Ambaye, A.; James, T.; Rincon, M. Human breast cancer biopsies induce eosinophil recruitment and enhance adjacent cancer cell proliferation. Breast Cancer Res. Treat. 2016, 157, 461–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.M.; Recht, L.; Strober, S. The promise of targeting macrophages in cancer therapy. Clin. Cancer Res. 2017, 23, 3241–3250. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Colotta, F.; Re, F.; Polentarutti, N.; Sozzani, S.; Mantovani, A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 1992, 80, 2012–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trellakis, S.; Bruderek, K.; Dumitru, C.A.; Gholaman, H.; Gu, X.; Bankfalvi, A.; Scherag, A.; Hütte, J.; Dominas, N.; Lehnerdt, G.F.; et al. Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int. J. Cancer 2011, 129, 2183–2193. [Google Scholar] [CrossRef]
- Jensen, H.K.; Donskov, F.; Marcussen, N.; Nordsmark, M.; Lundbeck, F.; von der Maase, H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J. Clin. Oncol. 2009, 27, 4709–4717. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Q.; Peng, C.; Sun, L.; Li, X.-F.; Kuang, D.-M. Neutrophils promote motility of cancer cells via a hyaluronan-mediated tlr4/pi3k activation loop. J. Pathol. 2011, 225, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.-M.; Zhao, Q.; Wu, Y.; Peng, C.; Wang, J.; Xu, Z.; Yin, X.-Y.; Zheng, L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 2011, 54, 948–955. [Google Scholar] [CrossRef]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-Associated Neutrophils as a New Prognostic Factor in Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e98259. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Liu, Y.; Li, Q.; Li, Z.; Hou, H.; Wu, A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and t-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer 2015, 15, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by tgf-beta: “N1” versus “n2” tan. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Pillay, J.; Tak, T.; Kamp, V.M.; Koenderman, L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: Similarities and differences. Cell. Mol. Life Sci. 2013, 70, 3813–3827. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Talbot, J.; Bianchini, F.J.; Nascimento, D.C.; Oliveira, R.D.R.; Souto, F.O.; Pinto, L.G.; Peres, R.S.; Silva, J.R.; Almeida, S.C.L.; Louzada-Junior, P.; et al. CCR2 Expression in Neutrophils Plays a Critical Role in Their Migration into the Joints in Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 1751–1759. [Google Scholar] [CrossRef] [Green Version]
- Onken, J.; Moeckel, S.; Leukel, P.; Leidgens, V.; Baumann, F.; Bogdahn, U.; Vollmann-Zwerenz, A.; Hau, P. Versican isoform V1 regulates proliferation and migration in high-grade gliomas. J. Neuro-Oncol. 2014, 120, 73–83. [Google Scholar] [CrossRef]
- Seliger, C.; Meyer, A.L.; Renner, K.; Leidgens, V.; Moeckel, S.; Jachnik, B.; Dettmer, K.; Tischler, U.; Gerthofer, V.; Rauer, L.; et al. Metformin inhibits proliferation and migration of glioblastoma cells independently of tgf-beta. Cell Cycle 2016, 15, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Leidgens, V.; Proske, J.; Rauer, L.; Moeckel, S.; Renner, K.; Bogdahn, U.; Riemenschneider, M.J.; Proescholdt, M.; Vollmann-Zwerenz, A.; Hau, P.; et al. Stattic and metformin inhibit brain tumor initiating cells by reducing STAT3-phosphorylation. Oncotarget 2016, 8, 8250–8263. [Google Scholar] [CrossRef] [Green Version]
- Moses, K.; Klein, J.C.; Mann, L.; Klingberg, A.; Gunzer, M.; Brandau, S. Survival of residual neutrophils and accelerated myelopoiesis limit the efficacy of anti-body-mediated depletion of ly-6g+ cells in tumor-bearing mice. J. Leukoc. Biol. 2016, 99, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Ribechini, E.; Leenen, P.J.M.; Lutz, M.B. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur. J. Immunol. 2009, 39, 3538–3551. [Google Scholar] [CrossRef]
- Ho, I.A.W.; Shim, W.S.N. Contribution of the microenvironmental niche to glioblastoma heterogeneity. BioMed Res. Int. 2017, 2017, 9634172. [Google Scholar] [CrossRef]
- Ma, C.; Kapanadze, T.; Gamrekelashvili, J.; Manns, M.P.; Korangy, F.; Greten, T.F. Anti-gr-1 antibody depletion fails to eliminate hepatic myeloid-derived sup-pressor cells in tumor-bearing mice. J. Leukoc. Biol. 2012, 92, 1199–1206. [Google Scholar] [CrossRef] [Green Version]
- Daley, J.M.; Thomay, A.A.; Connolly, M.D.; Reichner, J.S.; Albina, J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2007, 83, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soehnlein, O.; Lindbom, L.; Weber, C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood 2009, 114, 4613–4623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-L.; Zhou, Z.-J.; Hu, Z.-Q.; Huang, X.-W.; Wang, Z.; Chen, E.-B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016, 150, 1646–1658.e17. [Google Scholar] [CrossRef] [Green Version]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Piao, Y.; Holmes, L.; Fuller, G.N.; Henry, V.; Tiao, N.; de Groot, J.F. Neutrophils promote the malignant glioma phenotype through S100a. Clin. Cancer Res. 2014, 20, 187–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertaut, A.; Truntzer, C.; Madkouri, R.; Kaderbhai, C.G.; Derangère, V.; Vincent, J.; Chauffert, B.; Aubriot-Lorton, M.H.; Farah, W.; Mourier, K.L.; et al. Blood baseline neutrophil count predicts bevacizumab efficacy in glioblastoma. Oncotarget 2016, 7, 70948–70958. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Alieva, M.; van der Most, T.; Klazen, J.A.Z.; Vollmann-Zwerenz, A.; Hau, P.; Vrisekoop, N. Neutrophils Promote Glioblastoma Tumor Cell Migration after Biopsy. Cells 2022, 11, 2196. https://doi.org/10.3390/cells11142196
Chen N, Alieva M, van der Most T, Klazen JAZ, Vollmann-Zwerenz A, Hau P, Vrisekoop N. Neutrophils Promote Glioblastoma Tumor Cell Migration after Biopsy. Cells. 2022; 11(14):2196. https://doi.org/10.3390/cells11142196
Chicago/Turabian StyleChen, Na, Maria Alieva, Tom van der Most, Joelle A. Z. Klazen, Arabel Vollmann-Zwerenz, Peter Hau, and Nienke Vrisekoop. 2022. "Neutrophils Promote Glioblastoma Tumor Cell Migration after Biopsy" Cells 11, no. 14: 2196. https://doi.org/10.3390/cells11142196
APA StyleChen, N., Alieva, M., van der Most, T., Klazen, J. A. Z., Vollmann-Zwerenz, A., Hau, P., & Vrisekoop, N. (2022). Neutrophils Promote Glioblastoma Tumor Cell Migration after Biopsy. Cells, 11(14), 2196. https://doi.org/10.3390/cells11142196








