Regulatory T Cells from Patients with Rheumatoid Arthritis Are Characterized by Reduced Expression of Ikaros Zinc Finger Transcription Factors
Abstract
:1. Background
2. Methods
2.1. Blood Samples
2.2. Isolation of Primary CD4+ T-Cells
2.3. Flow Cytometry
2.4. Statistical Analysis
3. Results
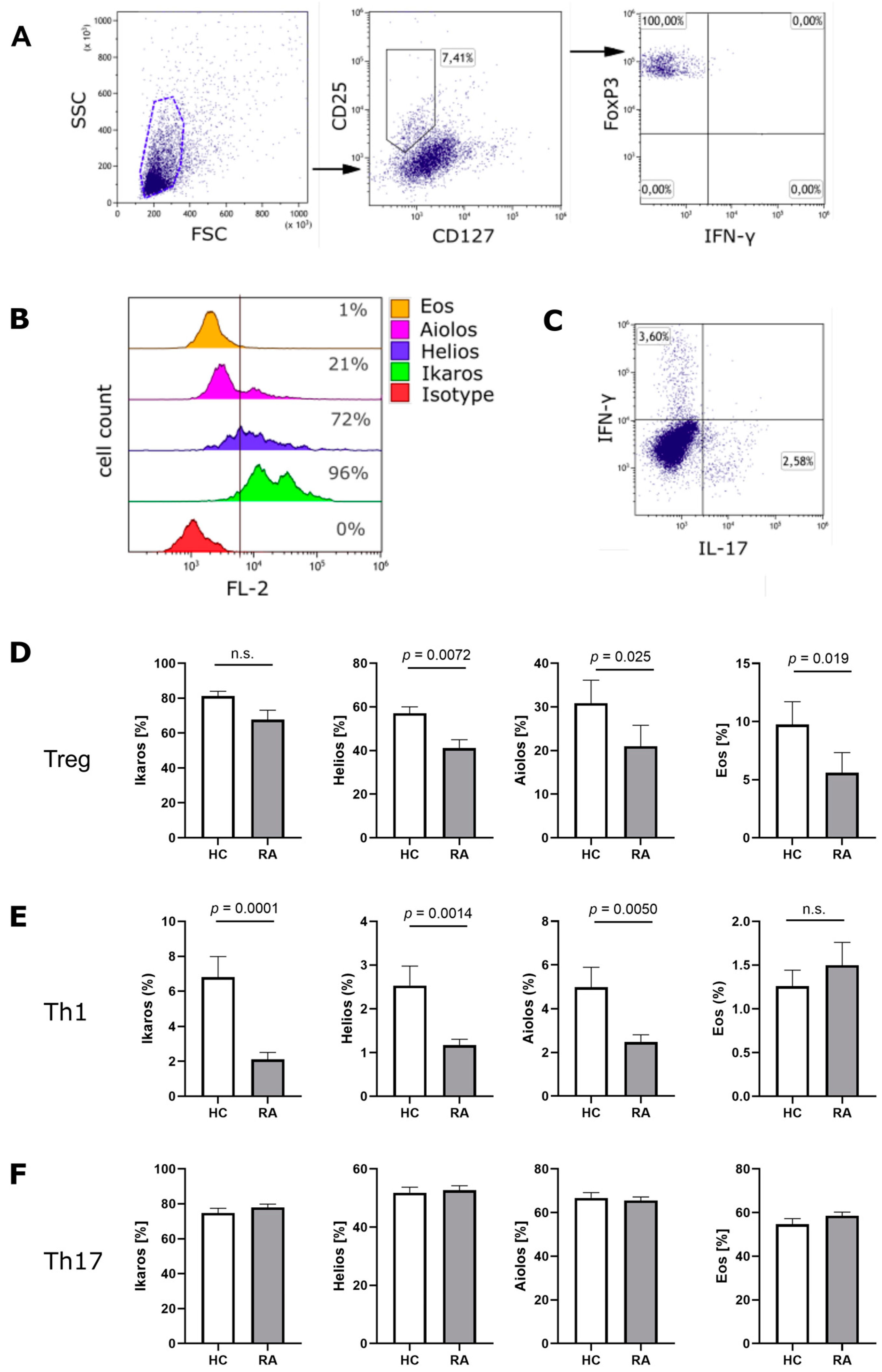
3.1. Ikaros Zinc Finger Transcription Factors in Treg Cells from RA Patients
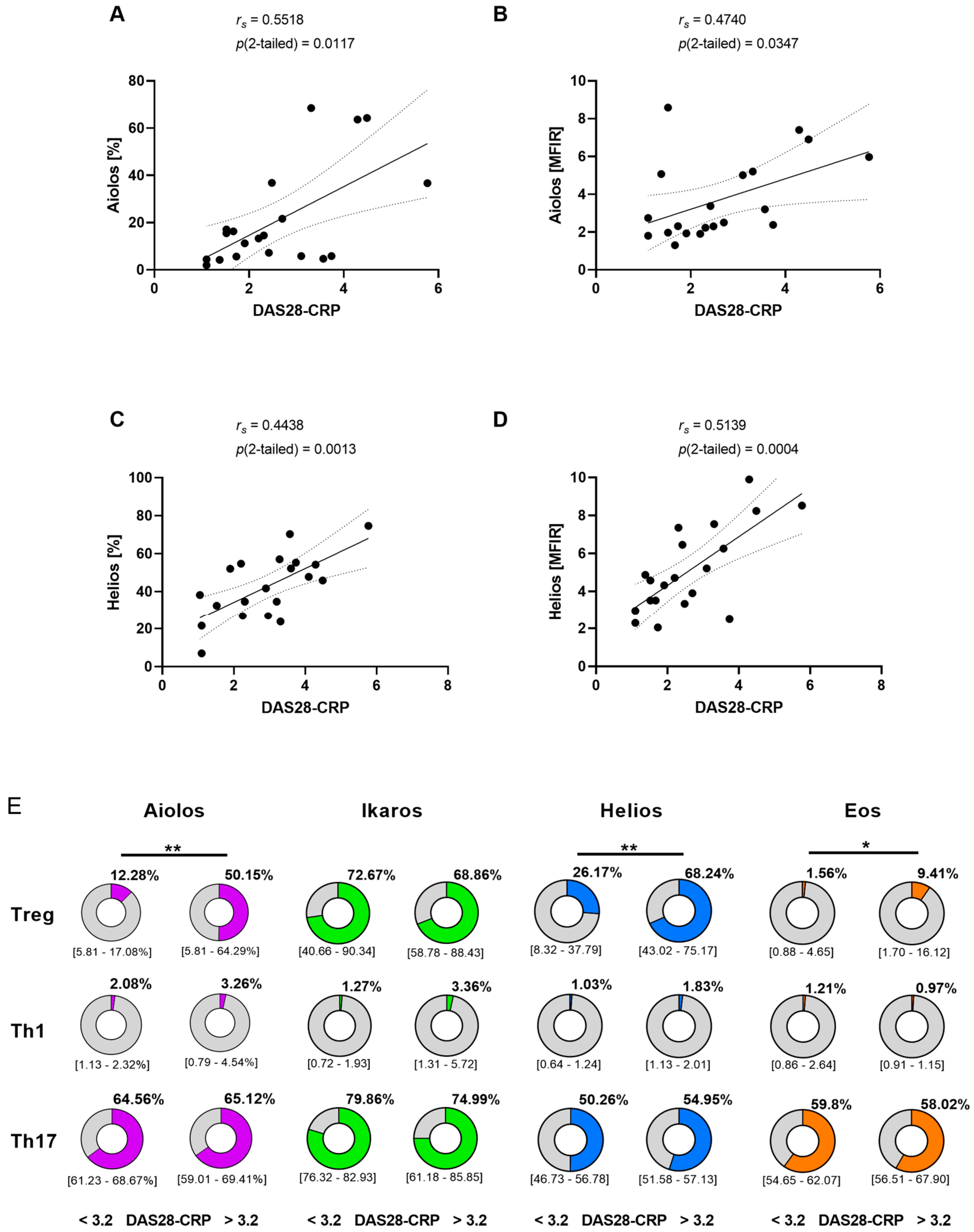
3.2. Correlation between Disease Activity and Aiolos or Helios Expression in Treg Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Powell, M.D.; Read, K.A.; Sreekumar, B.K.; Oestreich, K.J. Ikaros Zinc Finger Transcription Factors: Regulators of Cytokine Signaling Pathways and CD4. Front. Immunol. 2019, 10, 1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, J.L.; Seng, A.; Yankee, T.M. Expression and splicing of Ikaros family members in murine and human thymocytes. Mol. Immunol. 2017, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Read, K.A.; Jones, D.M.; Freud, A.G.; Oestreich, K.J. Established and emergent roles for Ikaros transcription factors in lymphoid cell development and function. Immunol. Rev. 2021, 300, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.L.; Seng, A.; Yankee, T.M. Expression patterns of Ikaros family members during positive selection and lineage commitment of human thymocytes. Immunology 2016, 149, 400–412. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Seng, A.; Yankee, T.M. Ikaros, Helios, and Aiolos protein levels increase in human thymocytes after β selection. Immunol. Res. 2016, 64, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Liu, X.; Du, S.; Xu, X.; Liu, A.; Ge, X.; Qiao, Y.; Jiang, Y. Overexpression of Aiolos in Peripheral Blood Mononuclear Cell Subsets from Patients with Systemic Lupus Erythematosus and Rheumatoid Arthritis. Biochem. Genet. 2016, 54, 73–82. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Chang, J.; Yamashita, M.; Niemela, J.E.; Zou, C.; Okuyama, K.; Harada, J.; Stoddard, J.L.; Nunes-Santos, C.J.; Boast, B.; et al. T and B cell abnormalities, pneumocystis pneumonia, and chronic lymphocytic leukemia associated with an AIOLOS defect in patients. J. Exp. Med. 2021, 218, 2021118. [Google Scholar] [CrossRef]
- Yamashita, M.; Kuehn, H.S.; Okuyama, K.; Okada, S.; Inoue, Y.; Mitsuiki, N.; Imai, K.; Takagi, M.; Kanegane, H.; Takeuchi, M.; et al. A variant in human AIOLOS impairs adaptive immunity by interfering with IKAROS. Nat. Immunol. 2021, 22, 893–903. [Google Scholar] [CrossRef]
- Cortés, M.; Georgopoulos, K. Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity. J. Exp. Med. 2004, 199, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Quintana, F.J.; Jin, H.; Burns, E.J.; Nadeau, M.; Yeste, A.; Kumar, D.; Rangachari, M.; Zhu, C.; Xiao, S.; Seavitt, J.; et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat. Immunol. 2012, 13, 770–777. [Google Scholar] [CrossRef]
- Evans, H.G.; Roostalu, U.; Walter, G.J.; Gullick, N.J.; Frederiksen, K.S.; Roberts, C.A.; Sumner, J.; Baeten, D.L.; Gerwien, J.G.; Cope, A.P.; et al. TNF-α blockade induces IL-10 expression in human CD4+ T cells. Nat. Commun. 2014, 5, 3199. [Google Scholar] [CrossRef] [PubMed]
- Ridley, M.L.; Fleskens, V.; Roberts, C.A.; Lalnunhlimi, S.; Alnesf, A.; O’Byrne, A.M.; Steel, K.J.; Povoleri, G.A.; Sumner, J.; Lavender, P.; et al. IKZF3/Aiolos Is Associated with but Not Sufficient for the Expression of IL-10 by CD4. J. Immunol. 2020, 204, 2940–2948. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, C.M.; Celse, C.; Pignon, P.; Ayyoub, M.; Valmori, D. Differential expression of the immunosuppressive enzyme IL4I1 in human induced Aiolos+, but not natural Helios+, FOXP3+ Treg cells. Eur. J. Immunol. 2015, 45, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Kumar, D.; Burns, E.J.; Nadeau, M.; Dake, B.; Laroni, A.; Kozoriz, D.; Weiner, H.L.; Quintana, F.J. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat. Immunol. 2010, 11, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffin, C.; Pignon, P.; Celse, C.; Debien, E.; Valmori, D.; Ayyoub, M. Human memory Helios- FOXP3+ regulatory T cells (Tregs) encompass induced Tregs that express Aiolos and respond to IL-1β by downregulating their suppressor functions. J. Immunol. 2013, 191, 4619–4627. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.M.; Chen, C.; Chunder, N.; Ma, L.; Taylor, J.; Pearce, E.J.; Wells, A.D. Ikaros silences T-bet expression and interferon-gamma production during T helper 2 differentiation. J. Biol. Chem. 2010, 285, 2545–2553. [Google Scholar] [CrossRef] [Green Version]
- Heller, J.J.; Schjerven, H.; Li, S.; Lee, A.; Qiu, J.; Chen, Z.M.E.; Smale, S.T.; Zhou, L. Restriction of IL-22-producing T cell responses and differential regulation of regulatory T cell compartments by zinc finger transcription factor Ikaros. J. Immunol. 2014, 193, 3934–3946. [Google Scholar] [CrossRef] [Green Version]
- Schafer, P.H.; Ye, Y.; Wu, L.; Kosek, J.; Ringheim, G.; Yang, Z.; Liu, L.; Thomas, M.; Palmisano, M.; Chopra, R. Cereblon modulator iberdomide induces degradation of the transcription factors Ikaros and Aiolos: Immunomodulation in healthy volunteers and relevance to systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Barnitz, R.A.; Kreslavsky, T.; Brown, F.D.; Moffett, H.; Lemieux, M.E.; Kaygusuz, Y.; Meissner, T.; Holderried, T.A.; Chan, S.; et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 2015, 350, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Baine, I.; Basu, S.; Ames, R.; Sellers, R.S.; Macian, F. Helios induces epigenetic silencing of IL2 gene expression in regulatory T cells. J. Immunol. 2013, 190, 1008–1016. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.D.; Huang, L.; Choi, J.H.; Lee, E.J.; Wilson, J.M.; Lemos, H.; Pan, F.; Blazar, B.R.; Pardoll, D.M.; Mellor, A.L.; et al. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity 2013, 38, 998–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, F.; Yu, H.; Dang, E.V.; Barbi, J.; Pan, X.; Grosso, J.F.; Jinasena, D.; Sharma, S.M.; McCadden, E.M.; Getnet, D.; et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science 2009, 325, 1142–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Herrath, J.; Malmström, V. IL-1R1 is expressed on both Helios(+) and Helios(-) FoxP3(+) CD4(+) T cells in the rheumatic joint. Clin. Exp. Immunol. 2015, 182, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokhale, A.S.; Gangaplara, A.; Lopez-Occasio, M.; Thornton, A.M.; Shevach, E.M. Selective deletion of Eos (Ikzf4) in T-regulatory cells leads to loss of suppressive function and development of systemic autoimmunity. J. Autoimmun. 2019, 105, 102300. [Google Scholar] [CrossRef]
- Rieder, S.A.; Metidji, A.; Glass, D.D.; Thornton, A.M.; Ikeda, T.; Morgan, B.A.; Shevach, E.M. Eos Is Redundant for Regulatory T Cell Function but Plays an Important Role in IL-2 and Th17 Production by CD4+ Conventional T Cells. J. Immunol. 2015, 195, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Mola, E.M. IL-6 targeting compared to TNF targeting in rheumatoid arthritis: Studies of olokizumab, sarilumab and sirukumab. Ann. Rheum. Dis. 2014, 73, 1595–1597. [Google Scholar] [CrossRef] [Green Version]
- Gloyer, L.; Golumba-Nagy, V.; Meyer, A.; Yan, S.; Schiller, J.; Breuninger, M.; Jochimsen, D.; Kofler, D.M. Adenosine receptor A2a blockade by caffeine increases IFN-gamma production in Th1 cells from patients with rheumatoid arthritis. Scand. J. Rheumatol. 2022, 51, 279–283. [Google Scholar] [CrossRef]
- Kotschenreuther, K.; Waqué, I.; Yan, S.; Meyer, A.; Haak, T.; von Tresckow, J.; Schiller, J.; Gloyer, L.; Dittrich-Salamon, M.; Kofler, D.M. Cannabinoids drive Th17 cell differentiation in patients with rheumatic autoimmune diseases. Cell. Mol. Immunol. 2020, 18, 764–766. [Google Scholar] [CrossRef]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef]
- Brück, C.; Golumba-Nagy, V.; Yan, S.; Esser, R.L.; Thiele, J.; Stahl, D.; tho Pesch, C.; Steinbach-Knödgen, E.; Kofler, D.M. Th1 and Th17 cells are resistant towards T cell activation-induced downregulation of CD6. Clin. Immunol. 2022, 238, 109025. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Yan, S.; Golumba-Nagy, V.; Esser, R.L.; Barbarino, V.; Blakemore, S.J.; Rusyn, L.; Nikiforov, A.; Seeger-Nukpezah, T.; Gruell, H.; et al. Kinase activity profiling reveals contribution of G-protein signaling modulator 2 deficiency to impaired regulatory T cell migration in rheumatoid arthritis. J. Autoimmun. 2021, 124, 102726. [Google Scholar] [CrossRef] [PubMed]
- Klasen, C.; Meyer, A.; Wittekind, P.S.; Waqué, I.; Nabhani, S.; Kofler, D.M. Prostaglandin receptor EP4 expression by Th17 cells is associated with high disease activity in ankylosing spondylitis. Arthritis Res. Ther. 2019, 21, 159. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Golumba-Nagy, V.; Kotschenreuther, K.; Thiele, J.; Refaian, N.; Shuya, D.; Gloyer, L.; Dittrich-Salamon, M.; Meyer, A.; Heindl, L.M.; et al. Membrane-bound IL-6R is upregulated on Th17 cells and inhibits Treg cell migration by regulating post-translational modification of VASP in autoimmune arthritis. Cell. Mol. Life Sci. 2021, 79, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Huang, J.; Luo, D.; Lv, S.; Lu, X.; Xiao, C. Dysfunctions, Molecular Mechanisms, and Therapeutic Strategies of Regulatory T Cells in Rheumatoid Arthritis. Front. Pharmacol. 2021, 12, 716081. [Google Scholar] [CrossRef]
- Meyer, A.; Wittekind, P.S.; Kotschenreuther, K.; Schiller, J.; von Tresckow, J.; Haak, T.H.; Kofler, D.M. Regulatory T cell frequencies in patients with rheumatoid arthritis are increased by conventional and biological DMARDs but not by JAK inhibitors. Ann. Rheum. Dis. 2021, 80, e196. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Liu, Y.; Mo, B.; Xue, Y.; Ye, C.; Jiang, Y.; Bi, X.; Liu, M.; Wu, Y.; Wang, J.; et al. Helios but not CD226, TIGIT and Foxp3 is a Potential Marker for CD4. Cell Physiol. Biochem. 2019, 52, 1178–1192. [Google Scholar]
- Elkord, E.; al Samid, M.A.; Chaudhary, B. Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget 2015, 6, 20026–20036. [Google Scholar] [CrossRef] [Green Version]
- Takatori, H.; Kawashima, H.; Matsuki, A.; Meguro, K.; Tanaka, S.; Iwamoto, T.; Sanayama, Y.; Nishikawa, N.; Tamachi, T.; Ikeda, K.; et al. Helios Enhances Treg Cell Function in Cooperation With FoxP3. Arthritis. Rheumatol. 2015, 67, 1491–1502. [Google Scholar] [CrossRef]
- Golding, A.; Hasni, S.; Illei, G.; Shevach, E.M. The percentage of FoxP3+Helios+ Treg cells correlates positively with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 2898–2906. [Google Scholar] [CrossRef] [Green Version]
- Alexander, T.; Sattler, A.; Templin, L.; Kohler, S.; Groß, C.; Meisel, A.; Sawitzki, B.; Burmester, G.R.; Arnold, R.; Radbruch, A.; et al. Foxp3+ Helios+ regulatory T cells are expanded in active systemic lupus erythematosus. Ann. Rheum. Dis. 2013, 72, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.; Sun, L.; Avitahl, N.; Andrikopoulos, K.; Ikeda, T.; Gonzales, E.; Wu, P.; Neben, S.; Georgopoulos, K. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997, 16, 2004–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toomer, K.H.; Lui, J.B.; Altman, N.H.; Ban, Y.; Chen, X.; Malek, T.R. Essential and non-overlapping IL-2Rα-dependent processes for thymic development and peripheral homeostasis of regulatory T cells. Nat. Commun. 2019, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Yuliasih, Y.; Rahmawati, L.D.; Putri, R.M. Th17/Treg Ratio and Disease Activity in Systemic Lupus Erythematosus. Casp. J. Intern. Med. 2019, 10, 65–72. [Google Scholar]
- Van der Graaff, W.L.; Prins, A.P.; Dijkmans, B.A.; van Lier, R.A. Prognostic value of Th1/Th2 ratio in rheumatoid arthritis. Lancet 1998, 351, 1931. [Google Scholar] [CrossRef]
- Johnson, T.M.; Register, K.A.; Schmidt, C.M.; O’Dell, J.R.; Mikuls, T.R.; Michaud, K.; England, B.R. Correlation of the Multi-Biomarker Disease Activity Score With Rheumatoid Arthritis Disease Activity Measures: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2019, 71, 1459–1472. [Google Scholar] [CrossRef] [Green Version]
| RA (n = 20) | HC (n = 12) | |
|---|---|---|
| Age | 57.5 ± 6.27 | 52.9 ± 4.14 |
| Sex (%female) | 68.2% | 67.5% |
| Disease duration 1 | 6.3 ± 4.9 | n/a |
| Treatment 2 | ||
| cDMARDs | 6 | n/a |
| bDMARDs | 8 | |
| tsDMARDs | 5 | |
| untreated | 1 | |
| DAS28-CRP | 3.1 ± 2.2 | n/a |
| RF+ | 70% | n/a |
| ACPA+ | 67.5% | n/a |
| CRP | 11.8 ± 4.2 | n/a |
| ESR | 19.8 ± 6.9 | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittrich-Salamon, M.; Meyer, A.; Yan, S.; Steinbach-Knödgen, E.; Kotschenreuther, K.; Stahl, D.; tho Pesch, C.; Schiller, J.; Byrtus, F.; Jochimsen, D.; et al. Regulatory T Cells from Patients with Rheumatoid Arthritis Are Characterized by Reduced Expression of Ikaros Zinc Finger Transcription Factors. Cells 2022, 11, 2171. https://doi.org/10.3390/cells11142171
Dittrich-Salamon M, Meyer A, Yan S, Steinbach-Knödgen E, Kotschenreuther K, Stahl D, tho Pesch C, Schiller J, Byrtus F, Jochimsen D, et al. Regulatory T Cells from Patients with Rheumatoid Arthritis Are Characterized by Reduced Expression of Ikaros Zinc Finger Transcription Factors. Cells. 2022; 11(14):2171. https://doi.org/10.3390/cells11142171
Chicago/Turabian StyleDittrich-Salamon, Mara, Anja Meyer, Shuaifeng Yan, Eva Steinbach-Knödgen, Konstantin Kotschenreuther, David Stahl, Carola tho Pesch, Joanna Schiller, Franziska Byrtus, Dorothee Jochimsen, and et al. 2022. "Regulatory T Cells from Patients with Rheumatoid Arthritis Are Characterized by Reduced Expression of Ikaros Zinc Finger Transcription Factors" Cells 11, no. 14: 2171. https://doi.org/10.3390/cells11142171
APA StyleDittrich-Salamon, M., Meyer, A., Yan, S., Steinbach-Knödgen, E., Kotschenreuther, K., Stahl, D., tho Pesch, C., Schiller, J., Byrtus, F., Jochimsen, D., Golumba-Nagy, V., & Kofler, D. M. (2022). Regulatory T Cells from Patients with Rheumatoid Arthritis Are Characterized by Reduced Expression of Ikaros Zinc Finger Transcription Factors. Cells, 11(14), 2171. https://doi.org/10.3390/cells11142171






