Oral Administration of Clostridium butyricum GKB7 Ameliorates Signs of Osteoarthritis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
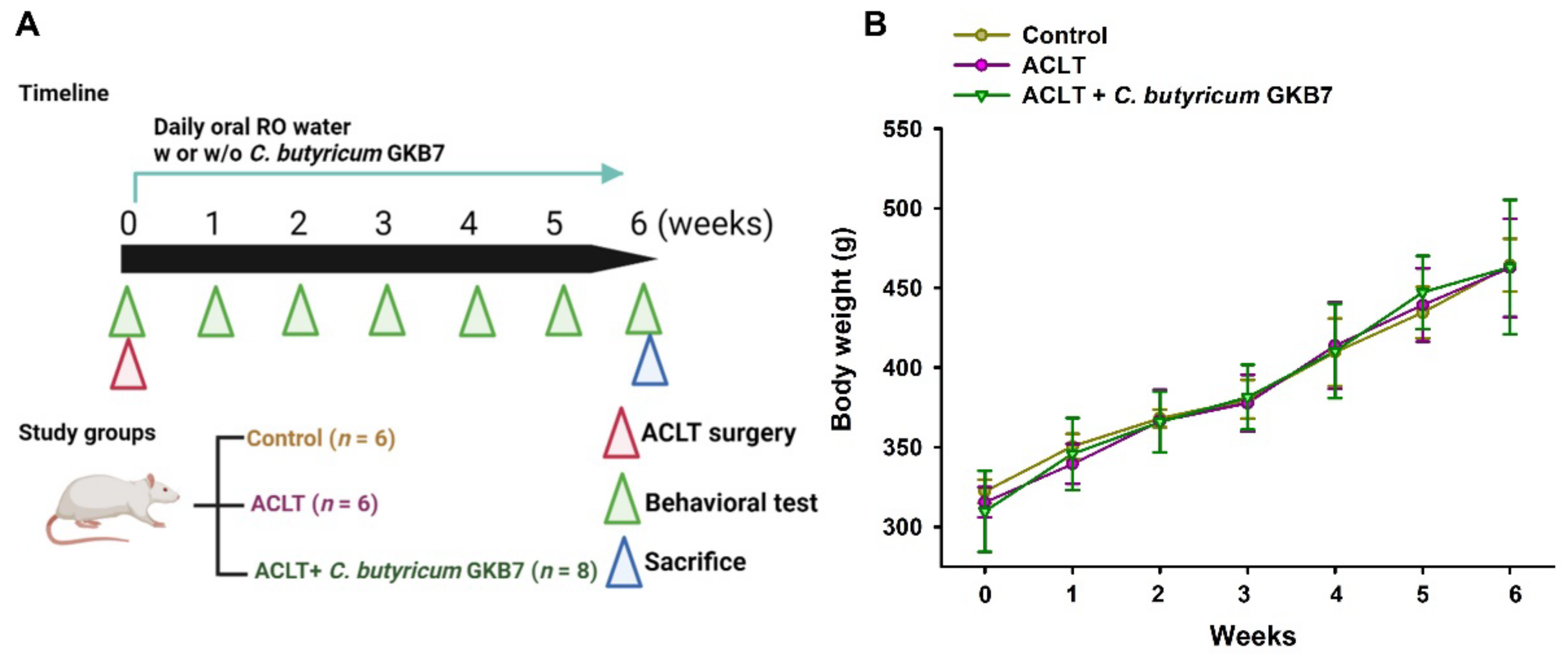
2.2. OA Protocol
2.3. Behavioral Testing
2.4. Micro-CT Analysis
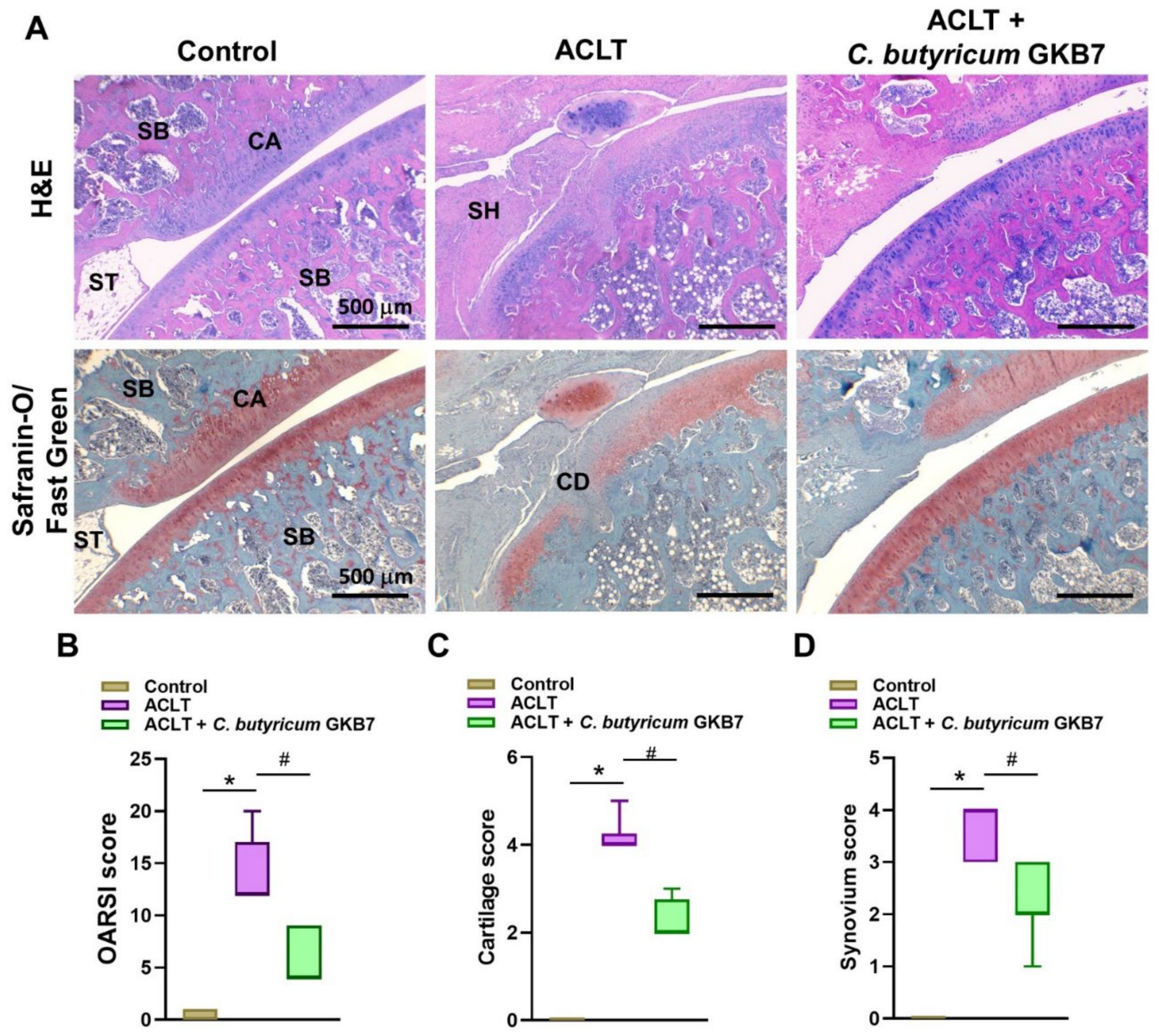
2.5. Histopathological Analysis
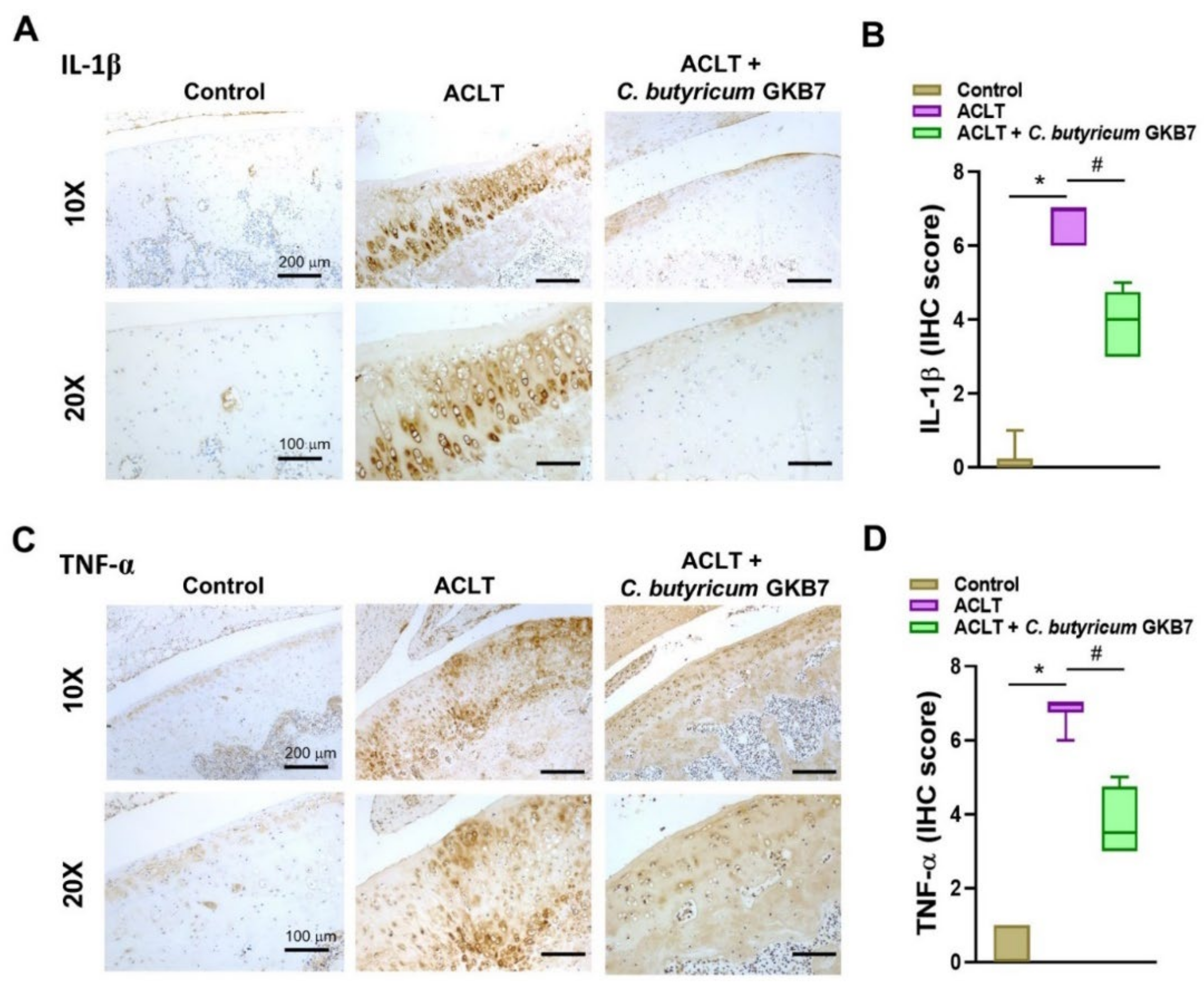
2.6. Immunohistochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Oral C. butyricum GKB7 Does Not Affect Body Weight
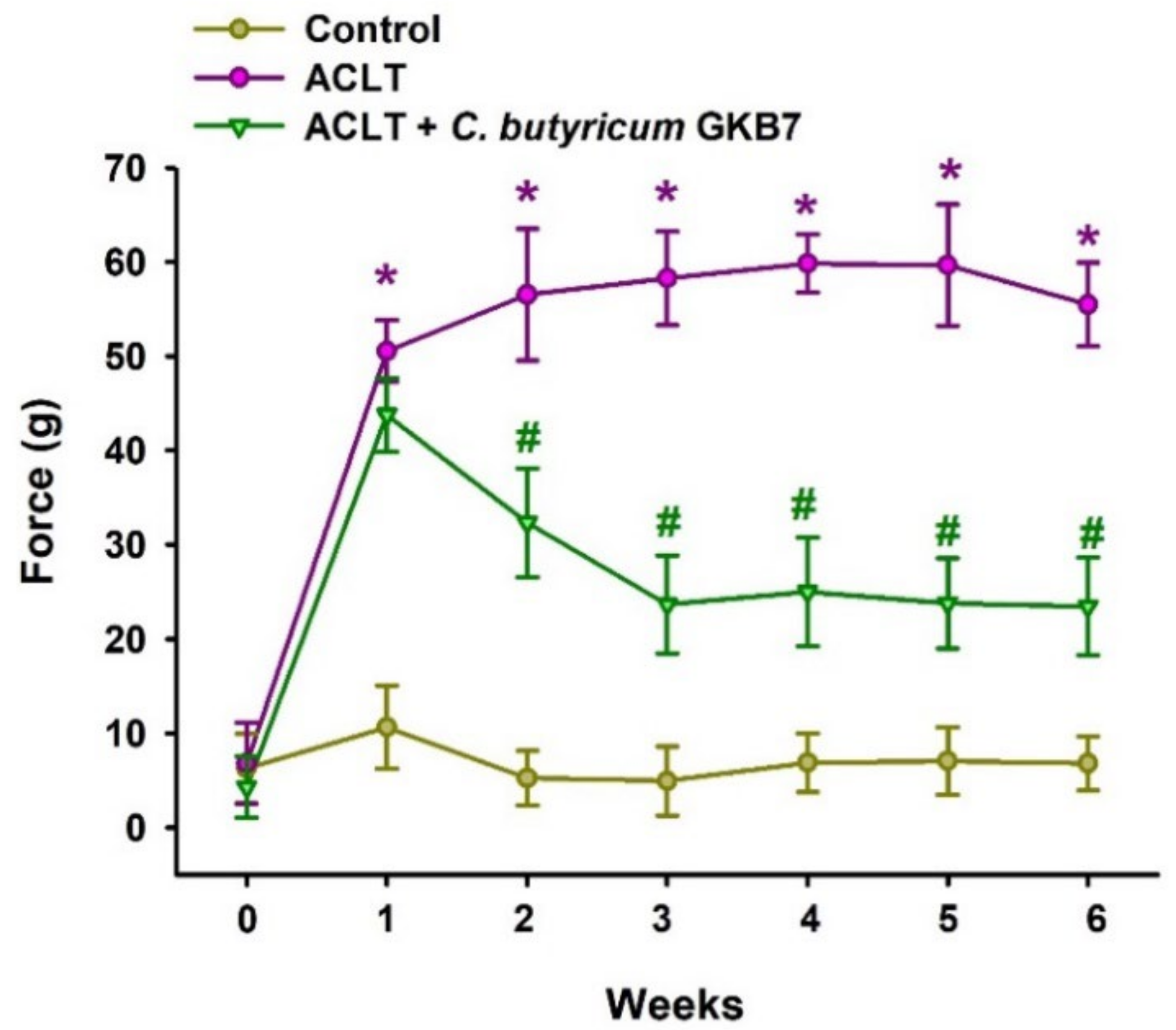
3.2. Oral C. butyricum GKB7 Ameliorates OA Pain
3.3. Oral C. butyricum GKB7 Protects against or Repairs Osseous Damage in OA
3.4. Oral C. butyricum GKB7 Protects against ACLT-Induced Articular Cartilage Damage
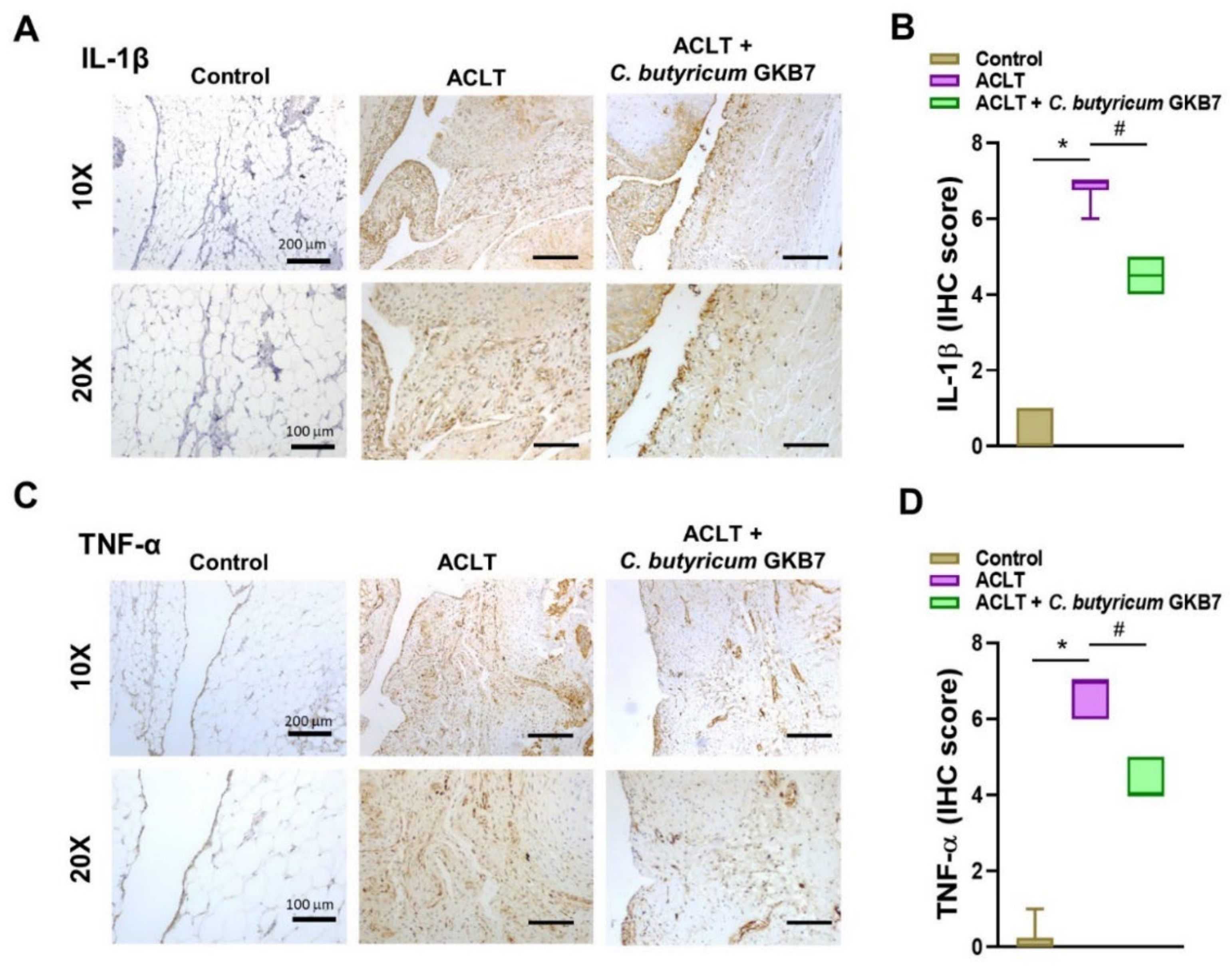
3.5. Oral C. butyricum GKB7 Downregulates TNF-α and IL-1β Expression in OA Cartilage and Synovium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H. Research of Pathogenesis and Novel Therapeutics in Arthritis. Int. J. Mol. Sci. 2019, 20, 1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawker, G.A.; King, L.K. The Burden of Osteoarthritis in Older Adults. Clin. Geriatr. Med. 2022, 38, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Luo, L.; Liu, S.; Guan, Z.; Zhang, Q.; Li, X.; Tao, K. The Role of Depletion of Gut Microbiota in Osteoporosis and Osteoarthritis: A Narrative Review. Front. Endocrinol. 2022, 13, 847401. [Google Scholar] [CrossRef]
- Fang, T.; Zhou, X.; Jin, M.; Nie, J.; Li, X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 2021, 45, 1125–1136. [Google Scholar] [CrossRef]
- Takahata, Y.; Murakami, T.; Hata, K.; Nishimura, R. Molecular Mechanisms Involved in the Progression and Protection of Osteoarthritis. Curr. Mol. Pharmacol. 2021, 14, 165–169. [Google Scholar] [CrossRef]
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthritis Cartilage 2022, 30, 196–206. [Google Scholar] [CrossRef]
- Billesberger, L.M.; Fisher, K.M.; Qadri, Y.J.; Boortz-Marx, R.L. Procedural Treatments for Knee Osteoarthritis: A Review of Current Injectable Therapies. Pain Res. Manag. 2020, 2020, 3873098. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Almeida Paz, F.A.; Braga, S.S. Bisphosphonates, Old Friends of Bones and New Trends in Clinics. J. Med. Chem. 2021, 64, 1260–1282. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Wei, Z.; Li, F.; Pi, G. Association Between Gut Microbiota and Osteoarthritis: A Review of Evidence for Potential Mechanisms and Therapeutics. Front. Cell. Infect. Microbiol. 2022, 12, 812596. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Singh, G.; InSug, O.; Ma, K.; Natarajan Anbazhagan, A.; Votta-Velis, E.G.; Bruce, B.; Richard, R.; van Wijnen, A.J.; Im, H.J. Gut-microbiota modulation: The impact of thegut-microbiotaon osteoarthritis. Gene 2021, 785, 145619. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.Y.; Choi, H.J.; Kim, M.G.; Jeong, D.G.; Lee, D.G.; Yoon, J.M.; Kang, D.J.; Park, S.; Ji, J.G.; Joo, I.H.; et al. Effects of ID-CBT5101 in Preventing and Alleviating Osteoarthritis Symptoms in a Monosodium Iodoacetate-Induced Rat Model. J. Microbiol. Biotechnol. 2018, 28, 1199–1208. [Google Scholar] [CrossRef]
- Tan, T.C.; Chong, T.K.Y.; Low, A.H.L.; Leung, Y.Y. Microbiome and osteoarthritis: New insights from animal and human studies. Int. J. Rheum. Dis. 2021, 24, 984–1003. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [Green Version]
- Ling, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Yuan, L.; Li, L.; Xiang, C. Clostridium butyricum combined with Bifidobacterium infantis probiotic mixture restores fecal microbiota and attenuates systemic inflammation in mice with antibiotic-associated diarrhea. Biomed. Res. Int. 2015, 2015, 582048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borody, T.J.; Paramsothy, S.; Agrawal, G. Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Curr. Gastroenterol. Rep. 2013, 15, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.B.; Kim, H.W.; Lee, H.S.; Jee, S.R.; Lee, J.H.; Kim, T.O. Clinical Efficacy of Probiotic Therapy on Bowel-Related Symptoms in Patients with Ulcerative Colitis during Endoscopic Remission: An Observational Study. Gastroenterol. Res. Pr. 2022, 2022, 9872230. [Google Scholar] [CrossRef]
- Zhou, M.; Yuan, W.; Yang, B.; Pei, W.; Ma, J.; Feng, Q. Clostridium butyricum inhibits the progression of colorectal cancer and alleviates intestinal inflammation via the myeloid differentiation factor 88 (MyD88)-nuclear factor-kappa B (NF-kappaB) signaling pathway. Ann. Transl. Med. 2022, 10, 478. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Liu, Y.; Ma, L.; Chen, Z.; Lin, X.; Si, L.; Ma, X.; Chen, X. Gut Microbiota Interventions With Clostridium butyricum and Norfloxacin Modulate Immune Response in Experimental Autoimmune Encephalomyelitis Mice. Front. Immunol. 2019, 10, 1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Li, H.; Jin, Y.; Yu, J.; Mao, S.; Su, K.P.; Ling, Z.; Liu, J. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav. Immun. 2021, 91, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Jing, J.; Wang, Z.; Wu, D.; Huang, Y. Chondroprotective Effects of Ginsenoside Rg1 in Human Osteoarthritis Chondrocytes and a Rat Model of Anterior Cruciate Ligament Transection. Nutrients 2017, 9, 263. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.C.; Tsai, C.H.; Wang, Y.H.; Su, C.M.; Wu, H.C.; Fong, Y.C.; Yang, S.F.; Tang, C.H. Melatonin abolished proinflammatory factor expression and antagonized osteoarthritis progression in vivo. Cell Death Dis. 2022, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Ko, C.Y.; Liu, S.C.; Wang, Y.H.; Hsu, C.J.; Tsai, C.H.; Wu, T.J.; Tang, C.H. miR-144-3p ameliorates the progression of osteoarthritis by targeting IL-1beta: Potential therapeutic implications. J. Cell Physiol. 2021, 236, 6988–7000. [Google Scholar] [CrossRef]
- Tsai, C.H.; Liu, S.C.; Chung, W.H.; Wang, S.W.; Wu, M.H.; Tang, C.H. Visfatin Increases VEGF-dependent Angiogenesis of Endothelial Progenitor Cells during Osteoarthritis Progression. Cells 2020, 9, 1315. [Google Scholar] [CrossRef]
- Wang, Y.H.; Kuo, S.J.; Liu, S.C.; Wang, S.W.; Tsai, C.H.; Fong, Y.C.; Tang, C.H. Apelin Affects the Progression of Osteoarthritis by Regulating VEGF-Dependent Angiogenesis and miR-150-5p Expression in Human Synovial Fibroblasts. Cells 2020, 9, 594. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhang, D.; Lin, Y.; Yuan, Q.; Zhou, X. Anterior Cruciate Ligament Transection-Induced Cellular and Extracellular Events in Menisci: Implications for Osteoarthritis. Am. J. Sports Med. 2018, 46, 1185–1198. [Google Scholar] [CrossRef]
- Hou, P.W.; Liu, S.C.; Tsay, G.J.; Tang, C.H.; Chang, H.H. The Traditional Chinese Medicine “Hu-Qian-Wan” Attenuates Osteoarthritis-Induced Signs and Symptoms in an Experimental Rat Model of Knee Osteoarthritis. Evid. Based Complement. Alternat. Med. 2022, 2022, 5367494. [Google Scholar] [CrossRef]
- Bethapudi, B.; Murugan, S.; Illuri, R.; Mundkinajeddu, D.; Velusami, C.C. Bioactive Turmerosaccharides from Curcuma longa Extract (NR-INF-02): Potential Ameliorating Effect on Osteoarthritis Pain. Pharmacogn. Mag. 2017, 13, S623–S627. [Google Scholar] [CrossRef]
- Lee, K.T.; Su, C.H.; Liu, S.C.; Chen, B.C.; Chang, J.W.; Tsai, C.H.; Huang, W.C.; Hsu, C.J.; Chen, W.C.; Wu, Y.C.; et al. Cordycerebroside A inhibits ICAM-1-dependent M1 monocyte adhesion to osteoarthritis synovial fibroblasts. J. Food Biochem. 2022, 15, e14108. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.Y.; Tsai, C.H.; Liu, S.C.; Huang, C.C.; Lin, T.H.; Yang, Y.Z.; Tang, C.H. Noggin Inhibits IL-1beta and BMP-2 Expression, and Attenuates Cartilage Degeneration and Subchondral Bone Destruction in Experimental Osteoarthritis. Cells 2020, 9, 927. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Chiu, C.P.; Tsai, C.H.; Hung, C.Y.; Li, T.M.; Wu, Y.C.; Tang, C.H. Soya-cerebroside, an extract of Cordyceps militaris, suppresses monocyte migration and prevents cartilage degradation in inflammatory animal models. Sci. Rep. 2017, 7, 43205. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wang, S.W.; Chen, Y.L.; Chou, W.Y.; Lin, T.Y.; Chen, W.C.; Yang, C.Y.; Liu, S.C.; Hsieh, C.C.; Fong, Y.C.; et al. Brain-derived neurotrophic factor promotes VEGF-C-dependent lymphangiogenesis by suppressing miR-624-3p in human chondrosarcoma cells. Cell Death Dis. 2017, 8, e2964. [Google Scholar] [CrossRef]
- Achudhan, D.; Liu, S.C.; Lin, Y.Y.; Lee, H.P.; Wang, S.W.; Huang, W.C.; Wu, Y.C.; Kuo, Y.H.; Tang, C.H. Antcin K inhibits VEGF-dependent angiogenesis in human rheumatoid arthritis synovial fibroblasts. J. Food Biochem. 2022, 46, e14022. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lin, C.; Tsai, C.; Lee, H.; Lo, L.; Huang, W.; YC, W.; Hsieh, C.; Tang, C. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. J. Funct. Foods 2021, 86, 104729. [Google Scholar] [CrossRef]
- Lee, H.-P.; Liu, S.-C.; Wang, Y.-H.; Chen, B.-C.; Chen, H.-T.; Li, T.-M.; Huang, W.-C.; Hsu, C.-J.; Wu, Y.-C.; Tang, C.-H. Cordycerebroside A suppresses VCAM-dependent monocyte adhesion in osteoarthritis synovial fibroblasts by inhibiting MEK/ERK/AP-1 signaling. J. Funct. Foods 2021, 86, 104712. [Google Scholar] [CrossRef]
- Jhun, J.; Cho, K.H.; Lee, D.H.; Kwon, J.Y.; Woo, J.S.; Kim, J.; Na, H.S.; Park, S.H.; Kim, S.J.; Cho, M.L. Oral Administration of Lactobacillus rhamnosus Ameliorates the Progression of Osteoarthritis by Inhibiting Joint Pain and Inflammation. Cells 2021, 10, 1057. [Google Scholar] [CrossRef]
- So, J.S.; Song, M.K.; Kwon, H.K.; Lee, C.G.; Chae, C.S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011, 88, 358–366. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C. Body mass index and risk of knee osteoarthritis: Systematic review and meta-analysis of prospective studies. BMJ Open 2015, 5, e007568. [Google Scholar] [CrossRef]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci. Rep. 2020, 10, 3601. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Ding, T.T.; Zhao, J.S.; Yang, X.; Zhang, H.X.; Zhang, J.J.; Cui, Y.L. Therapeutic effects of Clostridium butyricum on experimental colitis induced by oxazolone in rats. World J. Gastroenterol. 2009, 15, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhang, X.; Du, C.; Ma, Z.; Jin, S.; Ao, N.; Yang, J.; Du, J. Clostridium butyricum Alleviates Gut Microbiota Alteration-Induced Bone Loss after Bariatric Surgery by Promoting Bone Autophagy. J. Pharmacol. Exp. Ther. 2021, 377, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, N.; Corrado, A.; Cantatore, F.P. Osteoblast role in osteoarthritis pathogenesis. J. Cell Physiol. 2017, 232, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Brion, A.; Zhang, G.; Dossot, M.; Moby, V.; Dumas, D.; Hupont, S.; Piet, M.H.; Bianchi, A.; Mainard, D.; Galois, L.; et al. Nacre extract restores the mineralization capacity of subchondral osteoarthritis osteoblasts. J. Struct. Biol. 2015, 192, 500–509. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.L.-Y.; Lin, Y.-Y.; Liu, S.-C.; Tsai, Y.-S.; Lin, S.-W.; Chen, Y.-L.; Chen, C.-C.; Ko, C.-Y.; Chen, H.-T.; Chen, W.-C.; et al. Oral Administration of Clostridium butyricum GKB7 Ameliorates Signs of Osteoarthritis in Rats. Cells 2022, 11, 2169. https://doi.org/10.3390/cells11142169
Chang SL-Y, Lin Y-Y, Liu S-C, Tsai Y-S, Lin S-W, Chen Y-L, Chen C-C, Ko C-Y, Chen H-T, Chen W-C, et al. Oral Administration of Clostridium butyricum GKB7 Ameliorates Signs of Osteoarthritis in Rats. Cells. 2022; 11(14):2169. https://doi.org/10.3390/cells11142169
Chicago/Turabian StyleChang, Sunny Li-Yun, Yen-You Lin, Shan-Chi Liu, You-Shan Tsai, Shih-Wei Lin, Yen-Lien Chen, Chin-Chu Chen, Chih-Yuan Ko, Hsien-Te Chen, Wei-Cheng Chen, and et al. 2022. "Oral Administration of Clostridium butyricum GKB7 Ameliorates Signs of Osteoarthritis in Rats" Cells 11, no. 14: 2169. https://doi.org/10.3390/cells11142169
APA StyleChang, S. L.-Y., Lin, Y.-Y., Liu, S.-C., Tsai, Y.-S., Lin, S.-W., Chen, Y.-L., Chen, C.-C., Ko, C.-Y., Chen, H.-T., Chen, W.-C., & Tang, C.-H. (2022). Oral Administration of Clostridium butyricum GKB7 Ameliorates Signs of Osteoarthritis in Rats. Cells, 11(14), 2169. https://doi.org/10.3390/cells11142169





