Activation of the Ventrolateral Preoptic Neurons Projecting to the Perifornical-Hypothalamic Area Promotes Sleep: DREADD Activation in Wild-Type Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Subjects
2.2. Surgical Procedures
2.3. Experimental Groups and Viral Vectors
2.4. Recovery and Adaptation
2.5. Data Acquisition
2.6. Histology
2.7. Data Analyses
3. Results
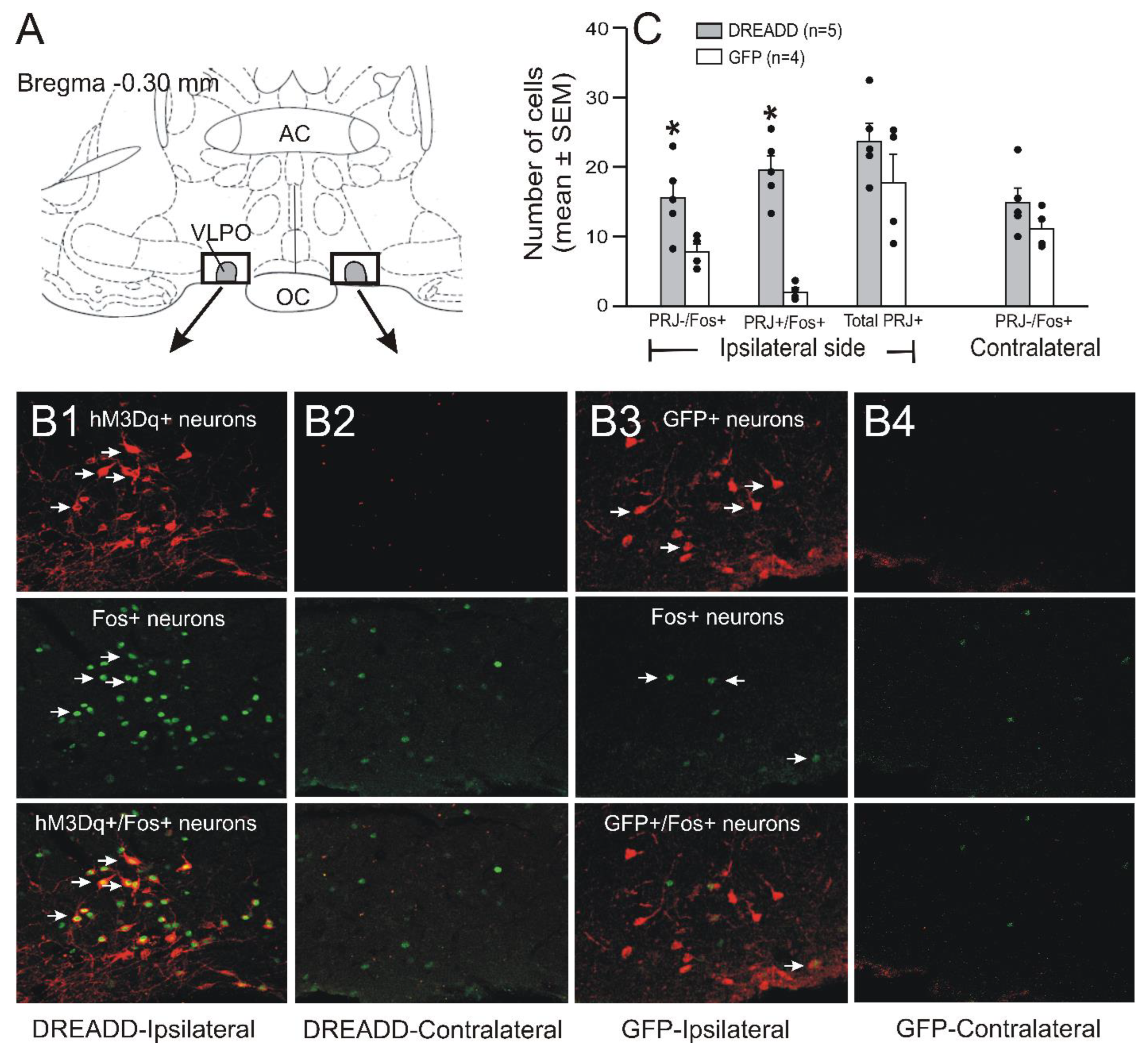
3.1. Labeling of VLPO > PF-HAPRJ Neurons and hM3Dq Receptor Expression
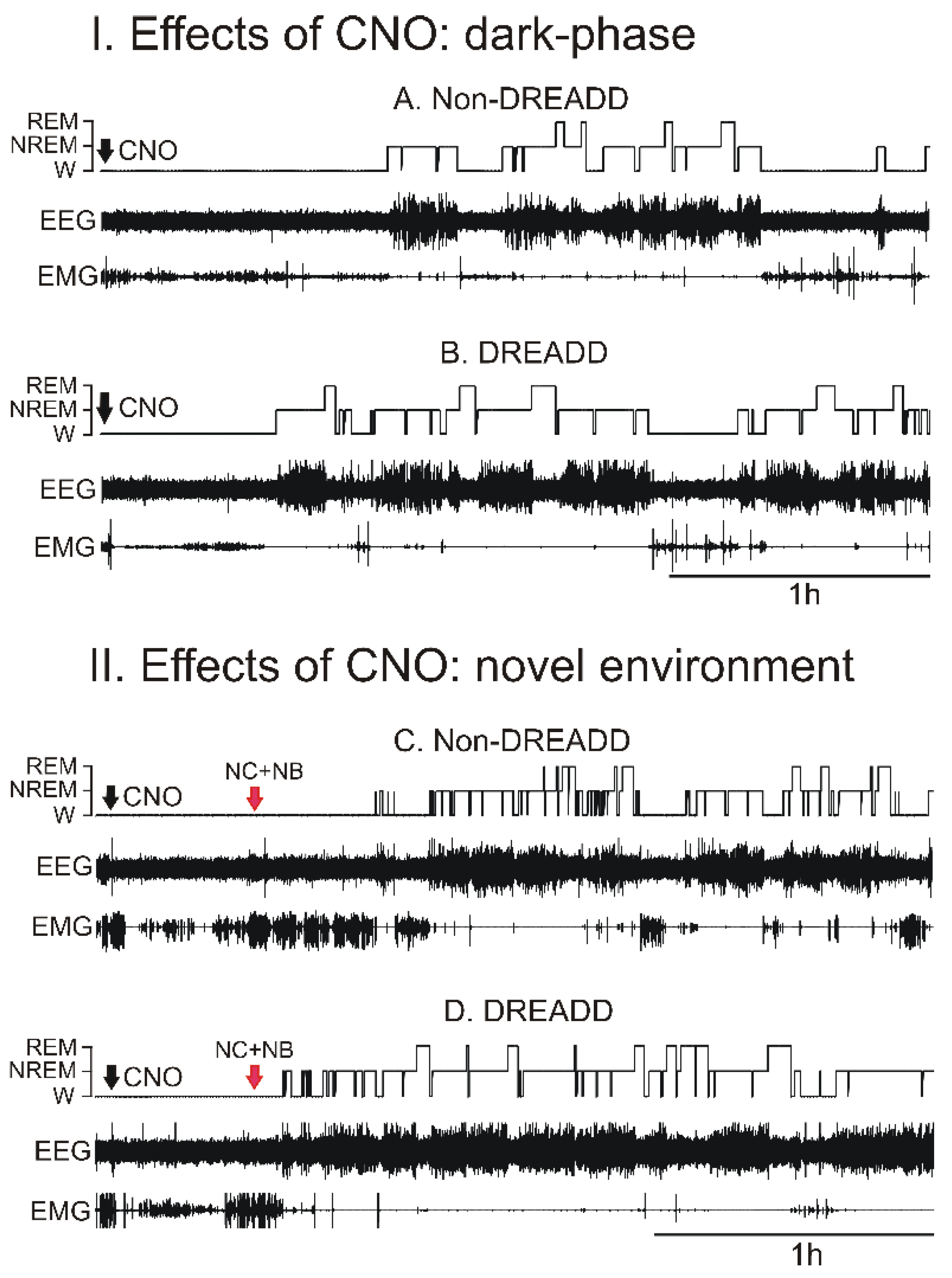
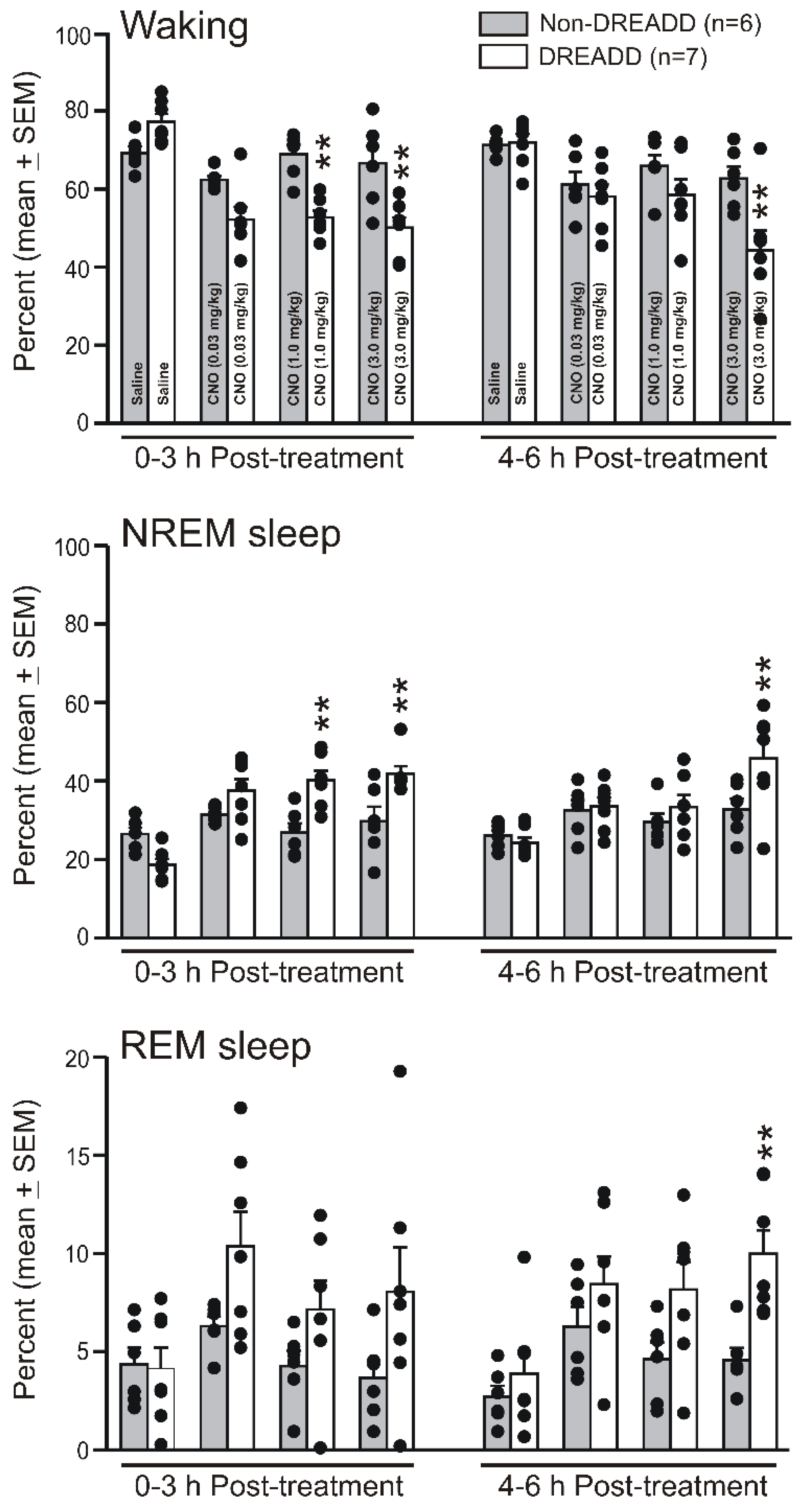
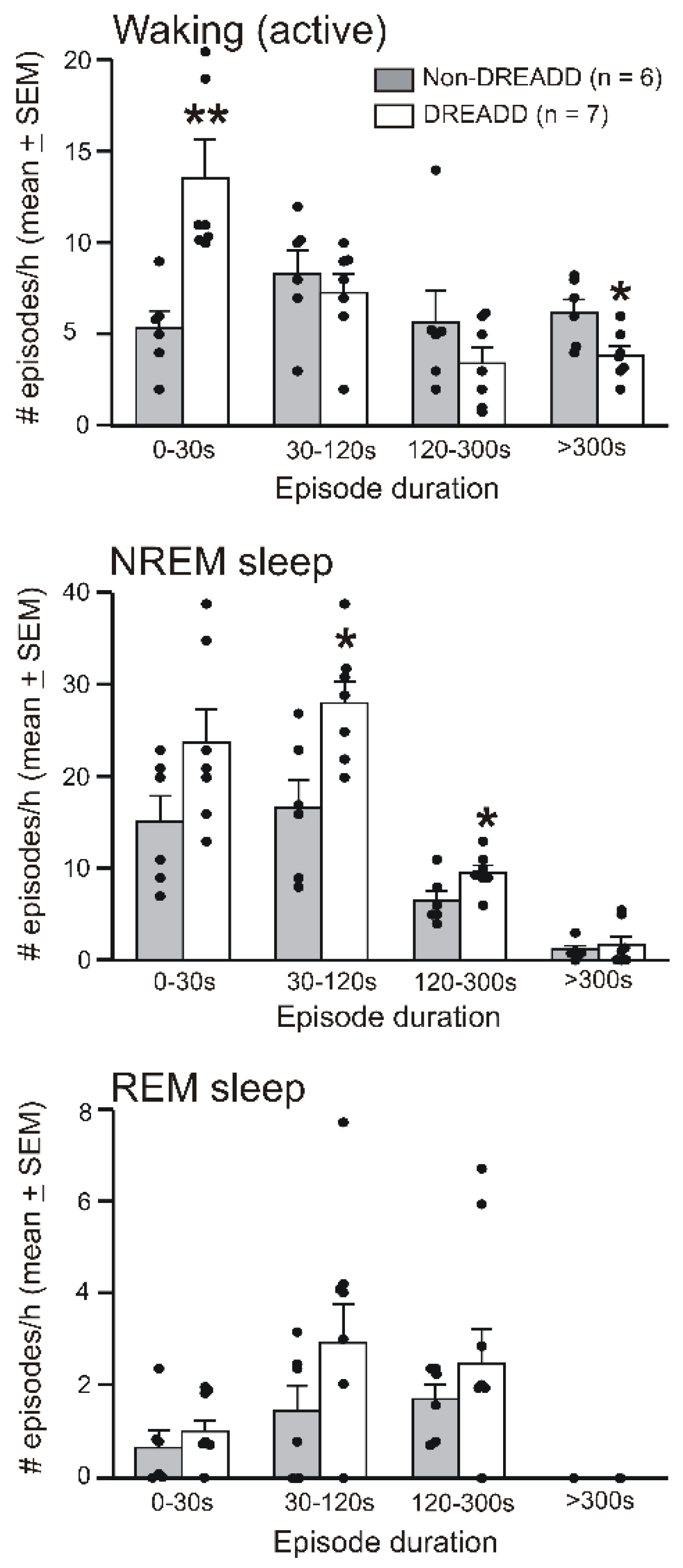
3.2. Chemoactivation of VLPO > PF-HAPRJ Neurons Causes Sleep during the Dark Phase
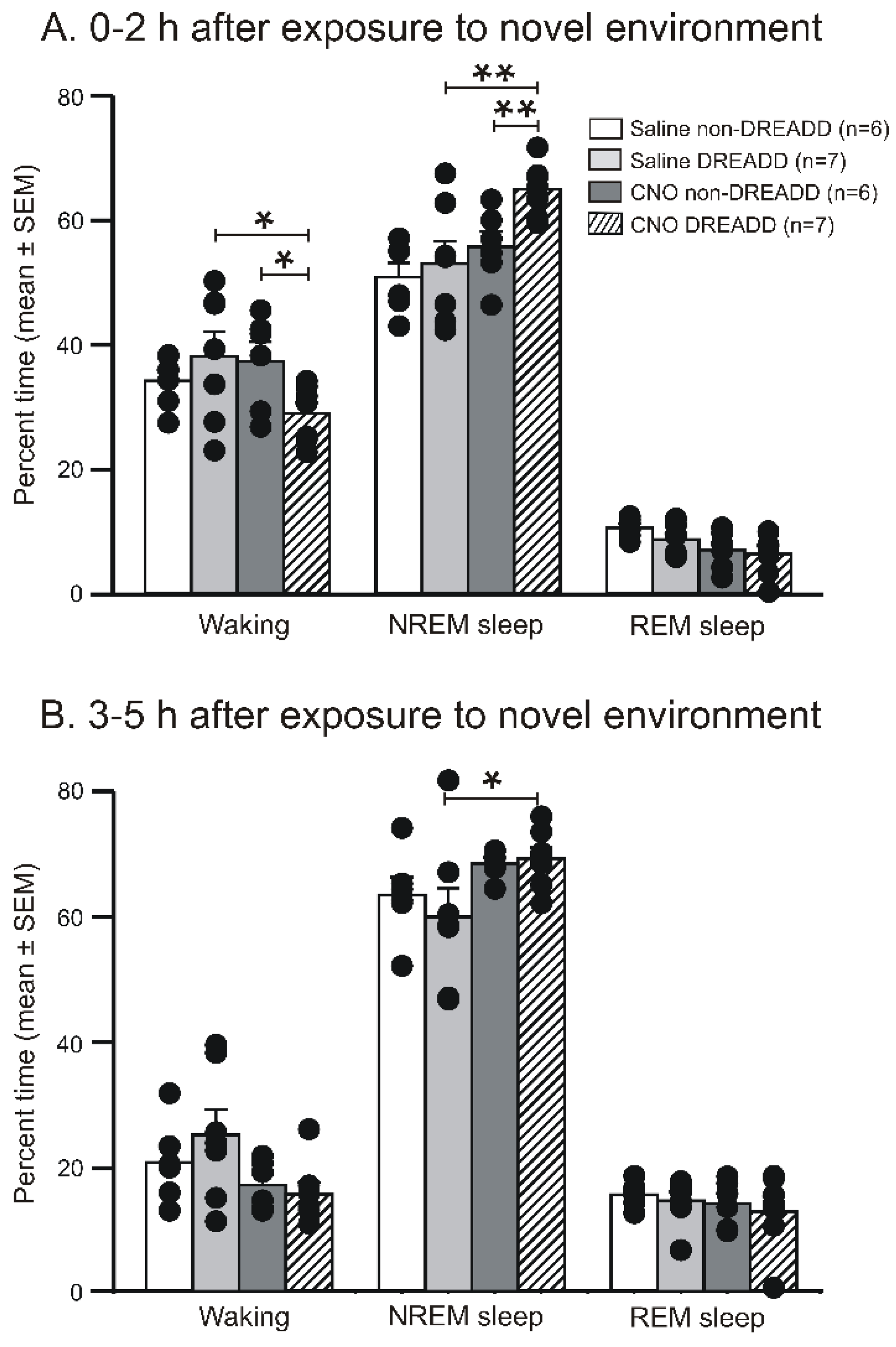
3.3. Chemoactivation of VLPO > PF-HAPRJ Neurons Increases Sleep in an Animal Model of Insomnia
3.4. Chemoactivation Increases Fos-IR in VLPO > PF-HAPRJ Neurons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, M.N. NREM sleep: Anatomy and physiology. In Encyclopaedia of Sleep; Elsevier: Amsterdam, The Netherlands, 2013; pp. 453–459. [Google Scholar]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep state switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef] [PubMed]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Greco, M.A.; Shiromani, P.; Saper, C.B. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 2000, 20, 3830–3842. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, R.; Alam, N.; Steininger, T.L.; McGinty, D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998, 803, 178–188. [Google Scholar] [CrossRef]
- Alam, M.A.; Kumar, S.; McGinty, D.; Alam, M.N.; Szymusiak, R. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J. Neurophysiol. 2014, 111, 287–299. [Google Scholar] [CrossRef]
- Gvilia, I.; Xu, F.; McGinty, D.; Szymusiak, R. Homeostatic regulation of sleep: A role for preoptic area neurons. J. Neurosci. 2006, 26, 9426–9433. [Google Scholar] [CrossRef]
- Sherin, J.E.; Shiromani, P.J.; McCarley, R.W.; Saper, C.B. Activation of ventrolateral preoptic neurons during sleep. Science 1996, 271, 216–219. [Google Scholar] [CrossRef]
- Gong, H.; Szymusiak, R.; King, J.; Steininger, T.; McGinty, D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: Effects of ambient warming. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R2079–R2088. [Google Scholar] [CrossRef]
- Suntsova, N.; Szymusiak, R.; Alam, M.N.; Guzman-Marin, R.; McGinty, D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J. Physiol. 2002, 543 Pt 2, 665–677. [Google Scholar] [CrossRef]
- Gong, H.; McGinty, D.; Guzman-Marin, R.; Chew, K.T.; Stewart, D.; Szymusiak, R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J. Physiol. 2004, 556 Pt 3, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Sherin, J.E.; Elmquist, J.K.; Torrealba, F.; Saper, C.B. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 1998, 18, 4705–4721. [Google Scholar] [CrossRef] [PubMed]
- Gaus, S.E.; Strecker, R.E.; Tate, B.A.; Parker, R.A.; Saper, C.B. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience 2002, 115, 285–294. [Google Scholar] [CrossRef]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.T.; Hörmann, N.; Chang, W.-C.; Zhang, Z.; Do, J.P.; et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Gong, H.; Alam, T.; Jaganath, R.; McGinty, D.; Szymusiak, R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J. Physiol. 2002, 538 Pt 2, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Mileykovskiy, B.Y.; Kiyashchenko, L.I.; Siegel, J.M. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 2005, 46, 787–798. [Google Scholar] [CrossRef]
- Takahashi, K.; Lin, J.S.; Sakai, K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience 2008, 153, 860–870. [Google Scholar] [CrossRef]
- Saito, Y.C.; Maejima, T.; Nishitani, M.; Hasegawa, E.; Yanagawa, Y.; Mieda, M.; Sakurai, T. Monoamines inhibit GABAergic neurons in ventrolateral preoptic area that make direct synaptic connections to hypothalamic arousal neurons. J. Neurosci. 2018, 38, 6366–6378. [Google Scholar] [CrossRef]
- Kroeger, D.; Absi, G.; Gagliardi, C.; Bandaru, S.S.; Madara, J.C.; Ferrari, L.L.; Arrigoni, E.; Münzberg, H.; Scammell, T.E.; Saper, C.B.; et al. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat. Commun. 2018, 9, 4129. [Google Scholar] [CrossRef]
- Lu, J.; Bjorkum, A.A.; Xu, M.; Gaus, S.E.; Shiromani, P.J.; Saper, C.B. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J. Neurosci. 2002, 22, 4568–4576. [Google Scholar] [CrossRef]
- Uschakov, A.; Gong, H.; McGinty, D.; Szymusiak, R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur. J. Neurosci. 2006, 23, 3284–3296. [Google Scholar] [CrossRef]
- Abrahamson, E.E.; Moore, R.Y. The posterior hypothalamic area: Chemoarchitecture and afferent connections. Brain Res. 2001, 889, 1–22. [Google Scholar] [CrossRef]
- McGinty, D.; Szymusiak, R. Hypothalamic regulation of sleep and arousal. Front. Biosci. 2003, 8, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.C.; Tsujino, N.; Hasegawa, E.; Akashi, K.; Abe, M.; Mieda, M.; Sakimura, K.; Sakurai, T. GABAergic neurons in the preoptic area send direct inhibitory projections to orexin neurons. Front. Neural Circuits 2013, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Vanini, G.; Bassana, M.; Mast, M.; Mondino, A.; Cerda, I.; Phyle, M.; Chen, V.; Colmenero, A.V.; Hambrecht-Wiedbusch, V.S.; Mashour, G.A. Activation of Preoptic GABAergic or Glutamatergic Neurons Modulates Sleep-Wake Architecture, but Not Anesthetic State Transitions. Curr. Biol. 2020, 30, 779–787.e4. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.C.; Gvilia, I.; Kumar, S.; Uschakov, A.; McGinty, D.; Alam, M.N.; Szymusiak, R. c-Fos expression in neurons projecting from the preoptic and lateral hypothalamic areas to the ventrolateral periaqueductal gray in relation to sleep states. Neuroscience 2011, 188, 55–67. [Google Scholar] [CrossRef][Green Version]
- Methippara, M.M.; Alam, M.N.; Szymusiak, R.; McGinty, D. Preoptic area warming inhibits wake-active neurons in the perifornical lateral hypothalamus. Brain Res. 2003, 960, 165–173. [Google Scholar] [CrossRef]
- Suntsova, N.; Guzman-Marin, R.; Kumar, S.; Alam, M.N.; Szymusiak, R.; McGinty, D. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. J. Neurosci. 2007, 27, 1616–1630. [Google Scholar] [CrossRef]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Model Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Kostin, A.; Rai, S.; Kumar, S.; Szymusiak, R.; McGinty, D.; Alam, M.N. Nitric oxide production in the perifornical-lateral hypothalamic area and its influences on the modulation of perifornical-lateral hypothalamic area neurons. Neuroscience 2011, 179, 159–169. [Google Scholar] [CrossRef][Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Fenno, L.E.; Mattis, J.; Ramakrishnan, C.; Hyun, M.; Lee, S.Y.; He, M.; Tucciarone, J.; Selimbeyoglu, A.; Berndt, A.; Grosenick, L.; et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 2014, 11, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.; Sabatini, B.L. Cre Activated and Inactivated Recombinant Adeno-Associated Viral Vectors for Neuronal Anatomical Tracing or Activity Manipulation. Curr. Protoc. Neurosci. 2015, 72, 1.24.1–1.24.15. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Ohkura, M.; Imoto, K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001, 19, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Cano, G.; Mochizuki, T.; Saper, C.B. Neural circuitry of stress-induced insomnia in rats. J. Neurosci. 2008, 28, 10167–10184. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.H.; Qu, W.M.; Lazarus, M.; Urade, Y.; Huang, Z.L. A mouse model mimicking human first night effect for the evaluation of hypnotics. Pharmacol. Biochem. Behav. 2014, 116, 129–136. [Google Scholar] [CrossRef]
- Kostin, A.; Alam, M.A.; Siegel, J.M.; McGinty, D.; Alam, M.N. Sex- and Age-dependent Differences in Sleep-wake Characteristics of Fisher-344 Rats. Neuroscience 2020, 427, 29–42. [Google Scholar] [CrossRef]
- Tervo, D.G.; Hwang, B.Y.; Viswanathan, S.; Gaj, T.; Lavzin, M.; Ritola, K.D.; Lindo, S.; Michael, S.; Kuleshova, E.; Ojala, D.; et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92, 372–382. [Google Scholar] [CrossRef]
- Castle, M.J.; Gershenson, Z.T.; Giles, A.R.; Holzbaur, E.L.; Wolfe, J.H. Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Hum. Gene Ther. 2014, 25, 705–720. [Google Scholar] [CrossRef]
- Taymans, J.M.; Vandenberghe, L.H.; Haute, C.V.; Thiry, I.; Deroose, C.M.; Mortelmans, L.; Wilson, J.; Debyser, Z.; Baekelandt, V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum. Gene Ther. 2007, 18, 195–206. [Google Scholar] [CrossRef]
- Kostin, A.; Alam, M.A.; McGinty, D.; Szymusiak, R.; Alam, M.N. Chronic Suppression of Hypothalamic Cell Proliferation and Neurogenesis Induces Aging-Like Changes in Sleep-Wake Organization in Young Mice. Neuroscience 2019, 404, 541–556. [Google Scholar] [CrossRef]
- Saunders, A.; Johnson, C.A.; Sabatini, B.L. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front. Neural Circuits 2012, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Centurion, C.; Luo, S.; Vidal-Ortiz, A.; Swank, C.; Shiromani, P.J. Activity of a subset of vesicular GABA-transporter neurons in the ventral zona incerta anticipate sleep onset. Sleep 2020, 44, zsaa268. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Centurion, C.; Luo, S.; Spergel, D.J.; Vidal-Ortiz, A.; Oprisan, S.A.; Van den Pol, A.N.; Liu, M.; Shiromani, P.J. Dynamic Network Activation of Hypothalamic MCH Neurons in REM Sleep and Exploratory Behavior. J. Neurosci. 2019, 39, 4986–4998. [Google Scholar] [CrossRef] [PubMed]
- Hassani, O.K.; Henny, P.; Lee, M.G.; Jones, B.E. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur. J. Neurosci. 2010, 32, 448–457. [Google Scholar] [CrossRef]
- Sharma, R.; Sahota, P.; Thakkar, M.M. Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. Sleep 2014, 37, 525–533. [Google Scholar] [CrossRef]
- Alam, M.A.; Mallick, B.N. Glutamic acid stimulation of the perifornical-lateral hypothalamic area promotes arousal and inhibits non-REM/REM sleep. Neurosci. Lett. 2008, 439, 281–286. [Google Scholar] [CrossRef]
- Kostin, A.; Siegel, J.M.; Alam, M.N. Lack of hypocretin attenuates behavioral changes produced by glutamatergic activation of the perifornical-lateral hypothalamic area. Sleep 2013, 37, 1011–1020. [Google Scholar] [CrossRef][Green Version]
- Alam, M.N.; McGinty, D.; Szymusiak, R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: Relation to NREM sleep. Am. J. Physiol. 1995, 269 Pt 2, R1240–R1249. [Google Scholar] [CrossRef]
- Guzman-Marin, R.; Alam, M.N.; Szymusiak, R.; Drucker-Colin, R.; Gong, H.; McGinty, D. Discharge modulation of rat dorsal raphe neurons during sleep and waking: Effects of preoptic/basal forebrain warming. Brain Res. 2000, 875, 23–34. [Google Scholar] [CrossRef]
- Hadjimarkou, M.M.; Benham, R.; Schwarz, J.M.; Holder, M.K.; Mong, J.A. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur. J. Neurosci. 2008, 27, 1780–1792. [Google Scholar] [CrossRef] [PubMed]
- Peterfi, Z.; Churchill, L.; Hajdu, I.; Obal, F., Jr.; Krueger, J.M.; Parducz, A. Fos-immunoreactivity in the hypothalamus: Dependency on the diurnal rhythm, sleep, gender, and estrogen. Neuroscience 2004, 124, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Mong, J.A.; Cusmano, D.M. Sex differences in sleep: Impact of biological sex and sex steroids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150110. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostin, A.; Alam, M.A.; Saevskiy, A.; McGinty, D.; Alam, M.N. Activation of the Ventrolateral Preoptic Neurons Projecting to the Perifornical-Hypothalamic Area Promotes Sleep: DREADD Activation in Wild-Type Rats. Cells 2022, 11, 2140. https://doi.org/10.3390/cells11142140
Kostin A, Alam MA, Saevskiy A, McGinty D, Alam MN. Activation of the Ventrolateral Preoptic Neurons Projecting to the Perifornical-Hypothalamic Area Promotes Sleep: DREADD Activation in Wild-Type Rats. Cells. 2022; 11(14):2140. https://doi.org/10.3390/cells11142140
Chicago/Turabian StyleKostin, Andrey, Md. Aftab Alam, Anton Saevskiy, Dennis McGinty, and Md. Noor Alam. 2022. "Activation of the Ventrolateral Preoptic Neurons Projecting to the Perifornical-Hypothalamic Area Promotes Sleep: DREADD Activation in Wild-Type Rats" Cells 11, no. 14: 2140. https://doi.org/10.3390/cells11142140
APA StyleKostin, A., Alam, M. A., Saevskiy, A., McGinty, D., & Alam, M. N. (2022). Activation of the Ventrolateral Preoptic Neurons Projecting to the Perifornical-Hypothalamic Area Promotes Sleep: DREADD Activation in Wild-Type Rats. Cells, 11(14), 2140. https://doi.org/10.3390/cells11142140





