The Role of the Fibronectin Synergy Site for Skin Wound Healing
Abstract
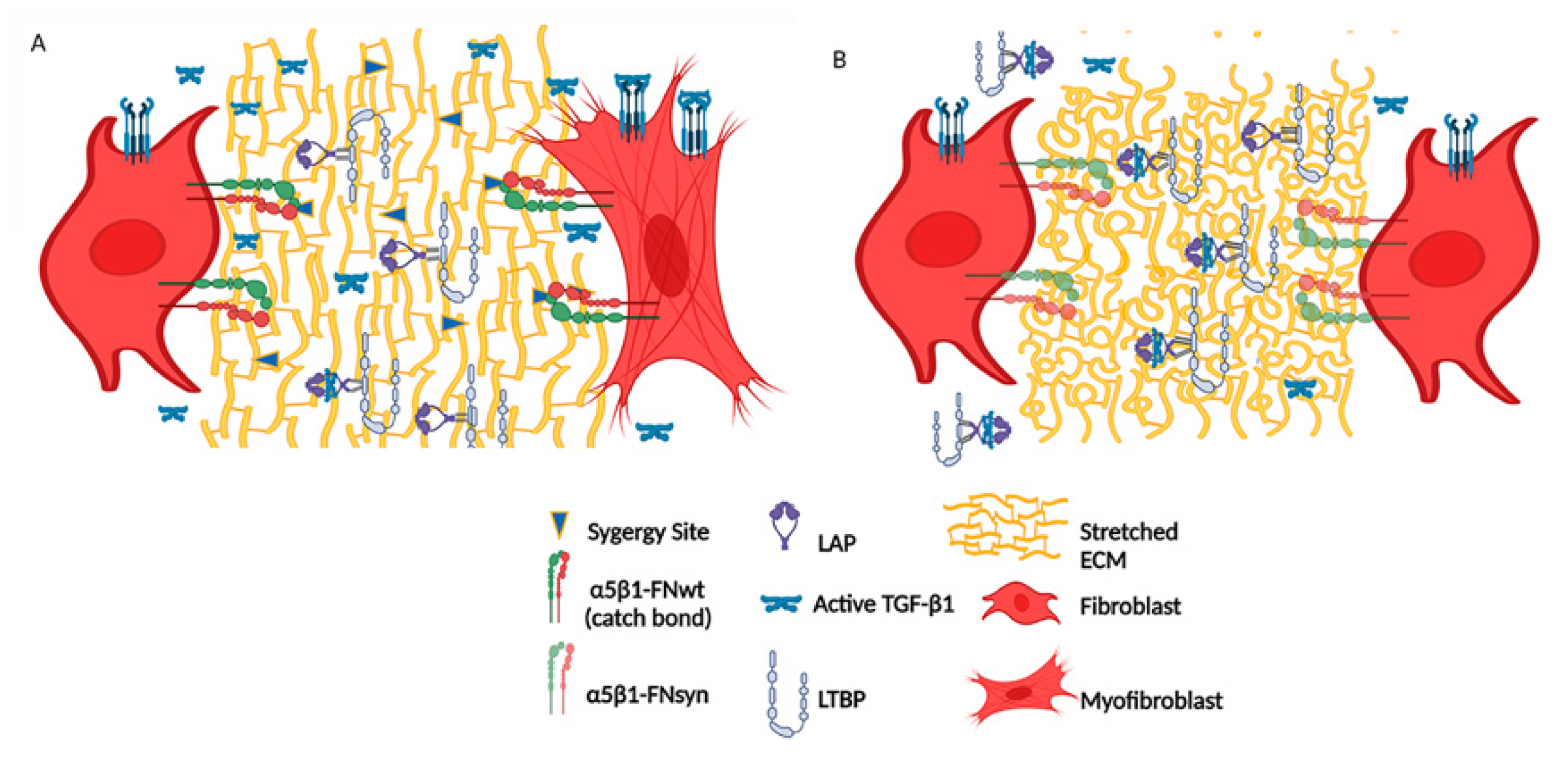
:1. Introduction
2. Materials and Methods
2.1. Mouse Strain
2.2. Cell Lines
2.3. Antibodies
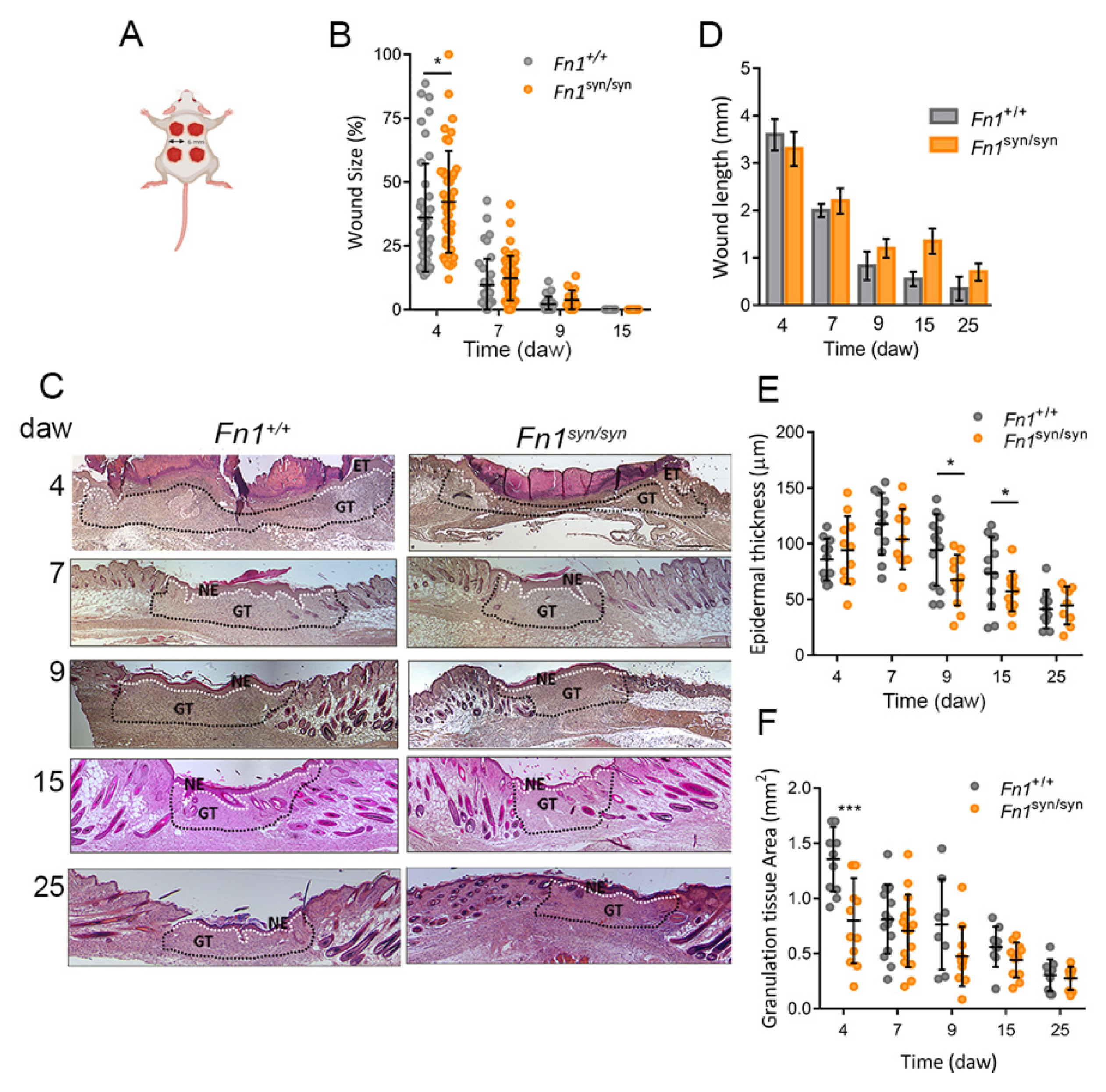
2.4. Wound Healing Assay
2.5. Tissue Processing and Histological Analysis
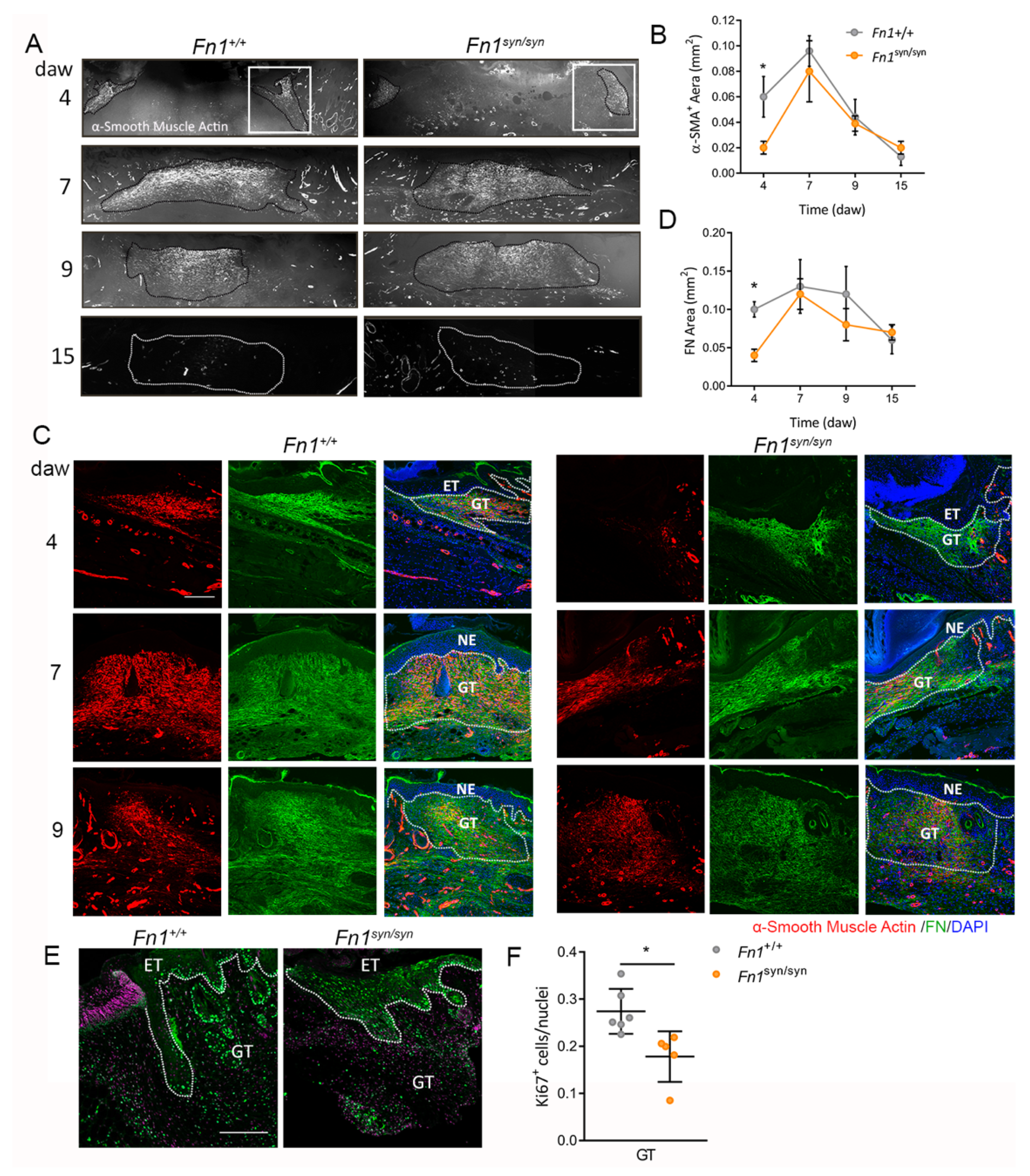
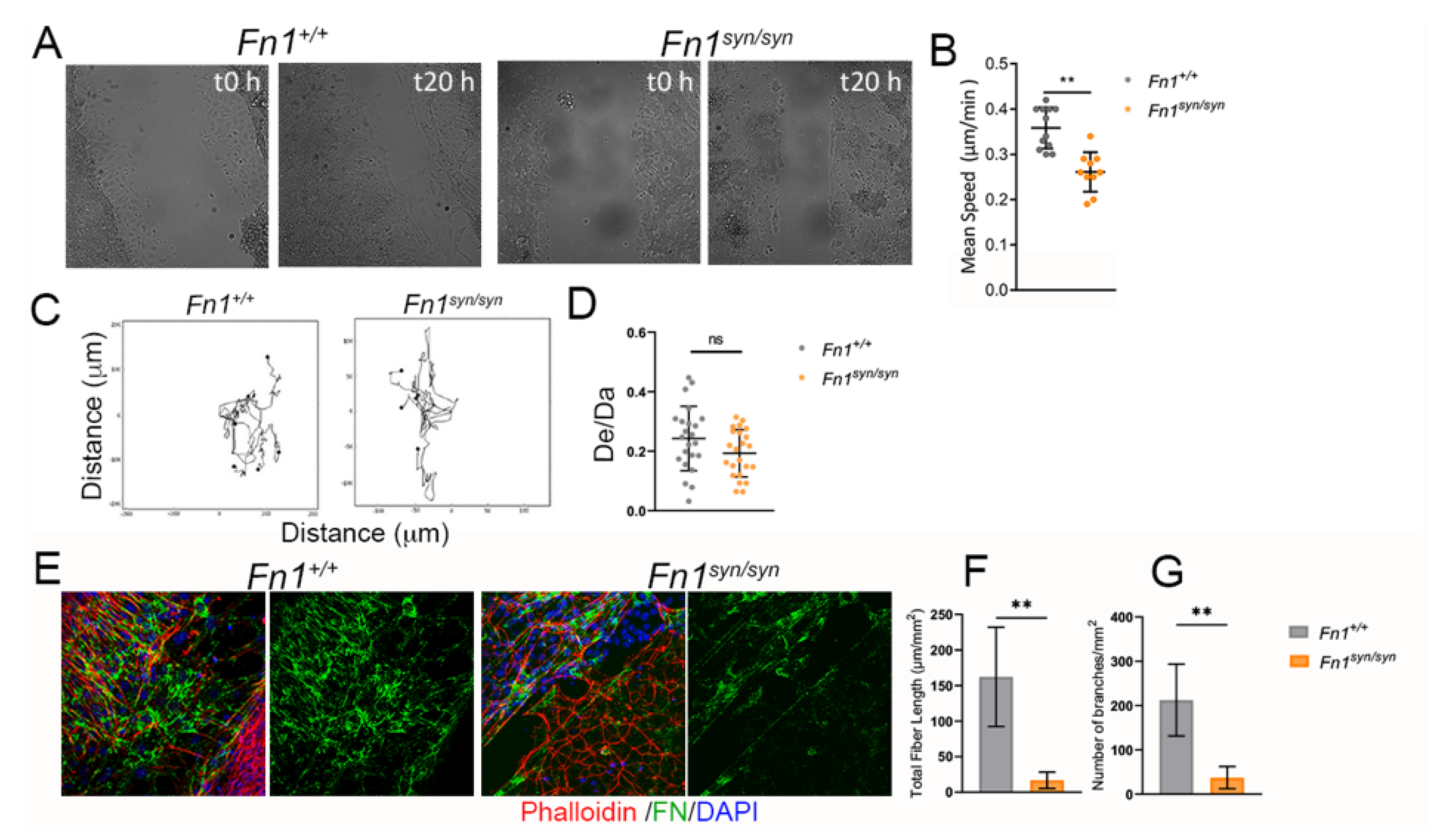
2.6. Cell Culture and Immunostaining
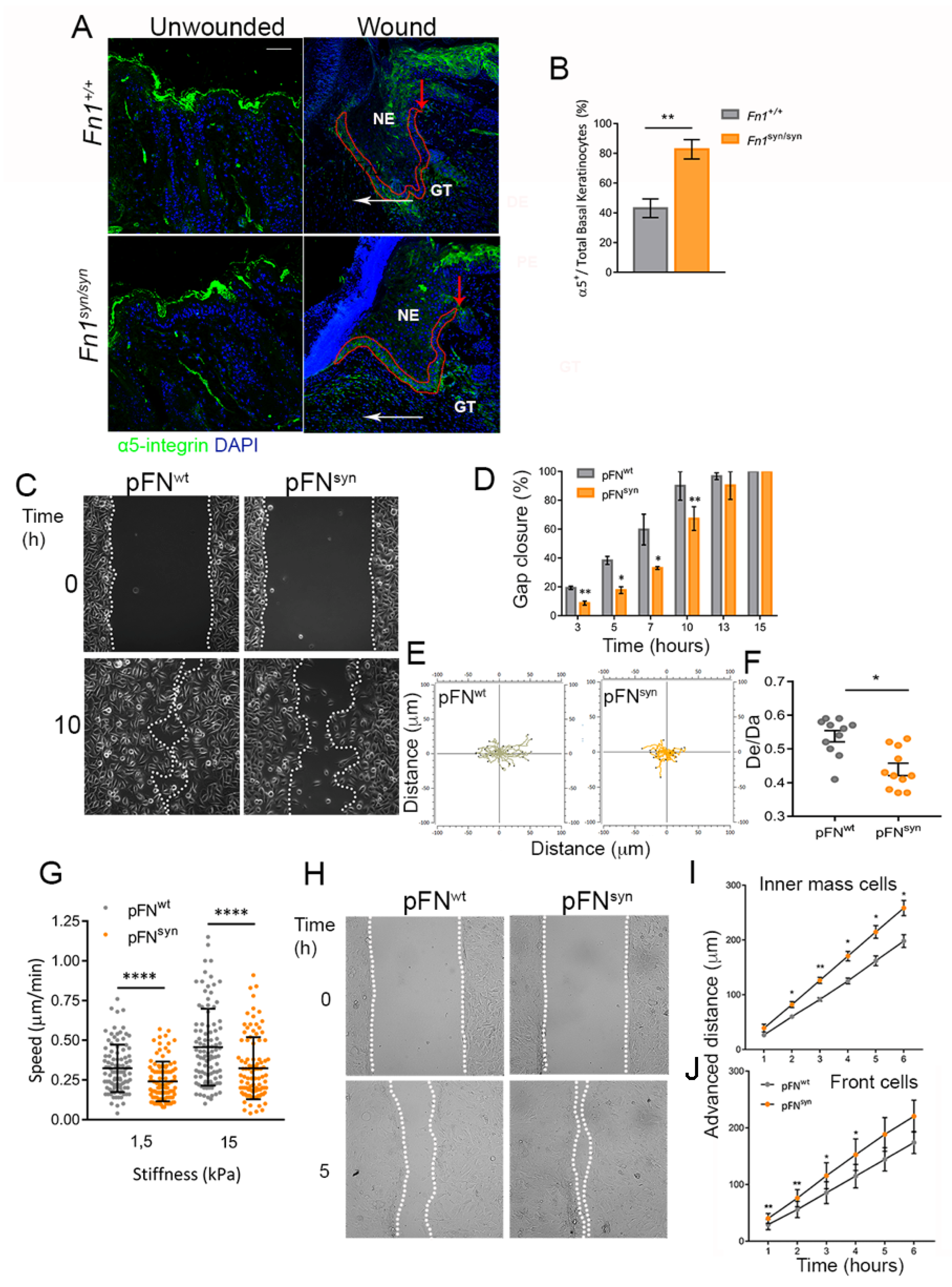
2.7. In-Vitro Wound Healing Assays
2.8. Keratinocyte Migration on PDMS Gels of Different Stiffness
2.9. Scratch Assays
2.10. Cell Migration Analysis
2.11. Dermal Fibroblasts Conversion into Myofibroblasts on Compliant Substrates
2.12. TGF-β Bioassay
2.13. Flow Cytometry
2.14. Statistical Analysis
3. Results
3.1. Skin Wound Closure in Fn1syn/syn Mice
3.2. Characterization of The Re-Epithelialization Process on FNsyn
3.3. Formation of the Granulation Tissue in Fn1syn/syn Mice
3.4. Myofibroblast Conversion Is Reduced in the Absence of the FN Synergy Site
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B. Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; Echeverri, K. Learning from Regeneration Research Organisms: The Circuitous Road to Scar Free Wound Healing. Dev. Biol. 2018, 433, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.L.; Marshall, C.D.; Barnes, L.A.; Murphy, M.P.; Ransom, R.C.; Longaker, M.T. Scarless wound healing: Transitioning from fetal to regenerative healing. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, 10. [Google Scholar] [CrossRef]
- Mascharak, S.; Desjardins-Park, H.E.; Davitt, M.F.; Guardino, N.J.; Gurtner, G.C.; Wan, D.C.; Longaker, M.T. Modulating Cellular Responses to Mechanical Forces to Promote Wound Regeneration. Adv. Wound Care 2022, 11, 479–495. [Google Scholar] [CrossRef]
- Jiang, D.; Correa-Gallegos, D.; Christ, S.; Stefanska, A.; Liu, J.; Ramesh, P.; Rajendran, V.; de Santis, M.M.; Wagner, D.E.; Rinkevich, Y. Two Succeeding Fibroblastic Lineages Drive Dermal Development and the Transition from Regeneration to Scarring. Nat. Cell Biol. 2018, 20, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Campbell, I.D.; Humphries, M.J. Integrin Structure, Activation, and Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sottile, J.; Shi, F.; Rublyevska, I.; Chiang, H.-Y.; Lust, J.; Chandler, J. Fibronectin-Dependent Collagen I Deposition Modulates the Cell Response to Fibronectin. Am. J. Physiol. Cell Physiol. 2007, 293, C1934–C1946. [Google Scholar] [CrossRef] [Green Version]
- Sabatier, L.; Chen, D.; Fagotto-Kaufmann, C.; Hubmacher, D.; McKee, M.D.; Annis, D.S.; Mosher, D.F.; Reinhardt, D.P. Fibrillin Assembly Requires Fibronectin. Mol. Biol. Cell 2009, 20, 846–858. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Rinkevich, Y. Distinct Fibroblasts in Scars and Regeneration. Curr. Opin. Genet. Dev. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Correa-Gallegos, D.; Jiang, D.; Christ, S.; Ramesh, P.; Ye, H.; Wannemacher, J.; Gopal, S.K.; Yu, Q.; Aichler, M.; Walch, A.; et al. Patch Repair of Deep Wounds by Mobilized Fascia. Nature 2019, 576, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Engler, A.J. The Provisional Matrix: Setting the Stage for Tissue Repair Outcomes. Matrix Biol. 2017, 60–61, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiss, M.; Beckmann, K.; Girós, A.; Costell, M.; Fässler, R. The Role of Integrin Binding Sites in Fibronectin Matrix Assembly In Vivo. Curr. Opin. Cell Biol. 2008, 20, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Danen, E.H.; Aota, S.I.; van Kraats, A.A.; Yamada, K.M.; Ruiter, D.J.; van Muijen, G.N. Requirement for the Synergy Site for Cell Adhesion to Fibronectin Depends on the Activation State of Integrin Alpha 5 Beta 1. J. Biol. Chem. 1995, 270, 21612–21618. [Google Scholar] [CrossRef] [Green Version]
- Bowditch, R.D.; Hariharan, M.; Tominna, E.F.; Smith, J.W.; Yamada, K.M.; Getzoff, E.D.; Ginsberg, M.H. Identification of a Novel Integrin Binding Site in Fibronectin. Differential Utilization by Beta 3 Integrins. J. Biol. Chem. 1994, 269, 10856–10863. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical Regulation of a Molecular Clutch Defines Force Transmission and Transduction in Response to Matrix Rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Benito-Jardón, M.; Klapproth, S.; Gimeno-LLuch, I.; Petzold, T.; Bharadwaj, M.; Müller, D.J.; Zuchtriegel, G.; Reichel, C.A.; Costell, M. The Fibronectin Synergy Site Re-Enforces Cell Adhesion and Mediates a Crosstalk between Integrin Classes. eLife 2017, 6, e22264. [Google Scholar] [CrossRef]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascré, G.; Simons, B.D.; Blanpain, C. Defining Stem Cell Dynamics and Migration during Wound Healing in Mouse Skin Epidermis. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [Green Version]
- Haydont, V.; Neiveyans, V.; Perez, P.; Busson, É.; Lataillade, J.J.; Asselineau, D.; Fortunel, N.O. Fibroblasts from the Human Skin Dermo-Hypodermal Junction Are Distinct from Dermal Papillary and Reticular Fibroblasts and from Mesenchymal Stem Cells and Exhibit a Specific Molecular Profile Related to Extracellular Matrix Organization and Modeling. Cells 2020, 9, 368. [Google Scholar] [CrossRef] [Green Version]
- Walraven, M.; Hinz, B. Therapeutic Approaches to Control Tissue Repair and Fibrosis: Extracellular Matrix as a Game Changer. Matrix Biol. 2018, 71–72, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, F.; Chow, M.L.; Koehler, A.; Boo, S.; Buscemi, L.; Quinn, T.M.; Costell, M.; Alman, B.A.; Genot, E.; Hinz, B. Prestress in the Extracellular Matrix Sensitizes Latent TGF-Β1 for Activation. J. Cell Biol. 2014, 207, 283. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, E.; Widmaier, M.; Jakobson, M.; Ruppert, R.; Ussar, S.; Katsougkri, D.; Böttcher, R.T.; Lai-Cheong, J.E.; Rifkin, D.B.; McGrath, J.A.; et al. Kindlin-1 controls Wnt and TGF-β availability to regulate cutaneous epithelial stem cell proliferation. Nat. Med. 2014, 20, 350–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.M.; Kumra, H.; Reinhardt, D.P. Quantification of Extracellular Matrix Fiber Systems Related to ADAMTS Proteins. Methods Mol. Biol. 2020, 2043, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Benito-Jardón, M.; Strohmeyer, N.; Ortega-Sanchís, S.; Bharadwaj, M.; Moser, M.; Müller, D.J.; Fässler, R.; Costell, M. αv-Class integrin binding to fibronectin is solely mediated by RGD and unaffected by an RGE mutation. J. Cell Biol. 2020, 219, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Harpel, J.G.; Metz, C.N.; Nunes, I.; Loskutoff, D.J.; Rifkin, D.B. An Assay for Transforming Growth Factor-Beta Using Cells Transfected with a Plasminogen Activator Inhibitor-1 Promoter-Luciferase Construct. Anal. Biochem. 1994, 216, 276–284. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Mizutani, T.; Kawabata, K.; Haga, H. Leader cells regulate collective cell migration via Rac activation in the downstream signaling of integrin β1 and PI3K. Sci. Rep. 2015, 5, 7656. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B. The Role of Myofibroblasts in Wound Healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Bielefeld, K.A.; Amini-Nik, S.; Whetstone, H.; Poon, R.; Youn, A.; Wang, J.; Alman, B.A. Fibronectin and β-Catenin Act in a Regulatory Loop in Dermal Fibroblasts to Modulate Cutaneous Healing. J. Biol. Chem. 2011, 286, 27687–27697. [Google Scholar] [CrossRef] [Green Version]
- To, W.S.; Midwood, K.S. Plasma and Cellular Fibronectin: Distinct and Independent Functions during Tissue Repair. Fibrogenesis Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognoni, E.; Pisco, A.O.; Hiratsuka, T.; Sipilä, K.H.; Belmonte, J.M.; Mobasseri, S.A.; Philippeos, C.; Dilão, R.; Watt, F.M. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 2018, 14, e8174. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, V.F.; Buscemi, L.; Diekmann, H.; Smith-Clerc, J.; Schwengler, H.; Meister, J.-J.; Wenck, H.; Gallinat, S.; Hinz, B. The Nano-Scale Mechanical Properties of the Extracellular Matrix Regulate Dermal Fibroblast Function. J. Investig. Dermatol. 2014, 134, 1862–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unsöld, C.; Hyytiäinen, M.; Bruckner-Tuderman, L.; Keski-Oja, J. Latent TGF-Beta Binding Protein LTBP-1 Contains Three Potential Extracellular Matrix Interacting Domains. J. Cell Sci. 2001, 114, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Annes, J.P.; Munger, J.S.; Rifkin, D.B. Making Sense of Latent TGFbeta Activation. J. Cell Sci. 2003, 116, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Hyytiäinen, M.; Penttinen, C.; Keski-Oja, J. Latent TGF-Beta Binding Proteins: Extracellular Matrix Association and Roles in TGF-Beta Activation. Crit. Rev. Clin. Lab. Sci. 2004, 41, 233–264. [Google Scholar] [CrossRef]
- Koli, K.; Wempe, F.; Sterner-Kock, A.; Kantola, A.; Komor, M.; Hofmann, W.K.; von Melchner, H.; Keski-Oja, J. Disruption of LTBP-4 Function Reduces TGF-Beta Activation and Enhances BMP-4 Signaling in the Lung. J. Cell Biol. 2004, 167, 123–133. [Google Scholar] [CrossRef]
- Samarakoon, R.; Higgins, P.J. Integration of non-SMAD and SMAD signaling in TGF-β1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb. Haemost. 2008, 100, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A. Wound Repair. Curr. Opin. Cell. Biol. 1989, 1, 1000–1008. [Google Scholar] [CrossRef]
- Sakai, T.; Johnson, K.J.; Murozono, M.; Sakai, K.; Magnuson, M.A.; Wieloch, T.; Cronberg, T.; Isshiki, A.; Erickson, H.P.; Fässler, R. Plasma Fibronectin Supports Neuronal Survival and Reduces Brain Injury Following Transient Focal Cerebral Ischemia but Is Not Essential for Skin-Wound Healing and Hemostasis. Nat. Med. 2001, 7, 324–330. [Google Scholar] [CrossRef]
- Shiu, J.-Y.; Aires, L.; Lin, Z.; Vogel, V. Nanopillar Force Measurements Reveal Actin-Cap-Mediated YAP Mechanotransduction. Nat. Cell Biol. 2018, 20, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Schiller, H.B.; Hermann, M.-R.; Polleux, J.; Vignaud, T.; Zanivan, S.; Friedel, C.C.; Sun, Z.; Raducanu, A.; Gottschalk, K.-E.; Théry, M.; et al. Β1- and Av-Class Integrins Cooperate to Regulate Myosin II during Rigidity Sensing of Fibronectin-Based Microenvironments. Nat. Cell Biol. 2013, 15, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Ladoux, B.; Mège, R.M. Mechanobiology of Collective Cell Behaviours. Nat. Rev. Mol. Cell Biol. 2017, 18, 743–757. [Google Scholar] [CrossRef]
- Vedula, S.R.K.; Leong, M.C.; Lai, T.L.; Hersen, P.; Kabla, A.J.; Lim, C.T.; Ladoux, B. Emerging Modes of Collective Cell Migration Induced by Geometrical Constraints. Proc. Natl. Acad. Sci. USA. 2012, 109, 12974–12979. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.W.; Rustad, K.C.; Akaishi, S.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Nelson, E.R.; Levi, K.; Paterno, J.; Vial, I.N.; et al. Focal Adhesion Kinase Links Mechanical Force to Skin Fibrosis via Inflammatory Signaling. Nat. Med. 2012, 18, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pensalfini, M.; Ehret, A.E.; Stüdeli, S.; Marino, D.; Kaech, A.; Reichmann, E.; Mazza, E. Factors affecting the mechanical behavior of collagen hydrogels for skin tissue engineering. J. Mech. Behav. Biomed. Mater. 2017, 69, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and Mechano-Regulation of Connective Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Wipff, P.-J.; Rifkin, D.B.; Meister, J.-J.; Hinz, B. Myofibroblast Contraction Activates Latent TGF-Β1 from the Extracellular Matrix. J. Cell Biol. 2007, 179, 1311–1323. [Google Scholar] [CrossRef] [Green Version]
- Miyazono, K.; Olofsson, A.; Colosetti, P.; Heldin, C.H. A Role of the Latent TGF-Beta 1-Binding Protein in the Assembly and Secretion of TGF-Beta 1. EMBO J. 1991, 10, 1091–1101. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimeno-LLuch, I.; Benito-Jardón, M.; Guerrero-Barberà, G.; Burday, N.; Costell, M. The Role of the Fibronectin Synergy Site for Skin Wound Healing. Cells 2022, 11, 2100. https://doi.org/10.3390/cells11132100
Gimeno-LLuch I, Benito-Jardón M, Guerrero-Barberà G, Burday N, Costell M. The Role of the Fibronectin Synergy Site for Skin Wound Healing. Cells. 2022; 11(13):2100. https://doi.org/10.3390/cells11132100
Chicago/Turabian StyleGimeno-LLuch, Irene, María Benito-Jardón, Gemma Guerrero-Barberà, Natalia Burday, and Mercedes Costell. 2022. "The Role of the Fibronectin Synergy Site for Skin Wound Healing" Cells 11, no. 13: 2100. https://doi.org/10.3390/cells11132100
APA StyleGimeno-LLuch, I., Benito-Jardón, M., Guerrero-Barberà, G., Burday, N., & Costell, M. (2022). The Role of the Fibronectin Synergy Site for Skin Wound Healing. Cells, 11(13), 2100. https://doi.org/10.3390/cells11132100





