The Roles of TNFR2 Signaling in Cancer Cells and the Tumor Microenvironment and the Potency of TNFR2 Targeted Therapy
Abstract
1. Introduction
2. The Structure and Function of TNF/TNFR
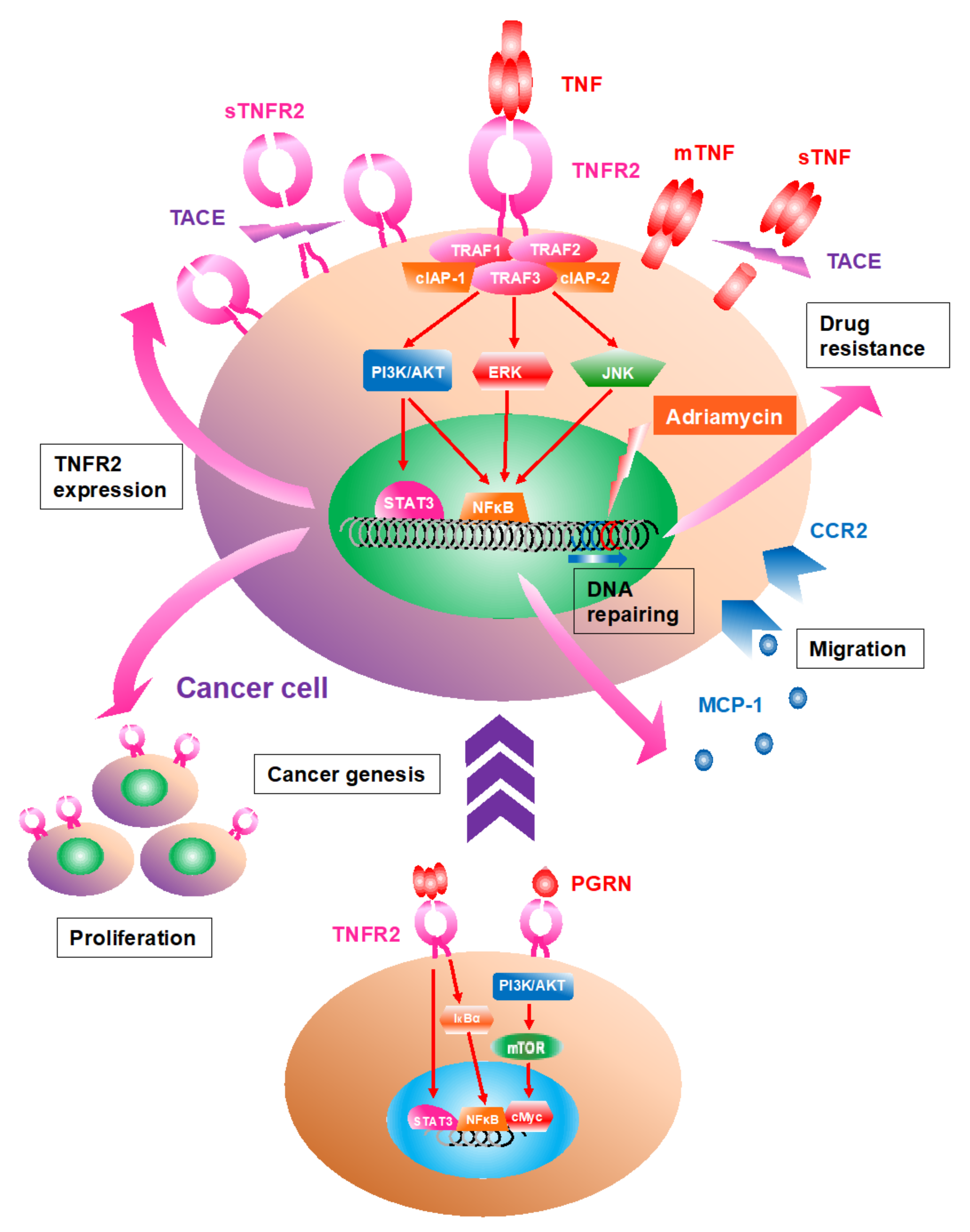
3. TNFR2 Expression Is Upregulated in Cancer Cells; TNFR2 Signaling Accelerates Proliferation
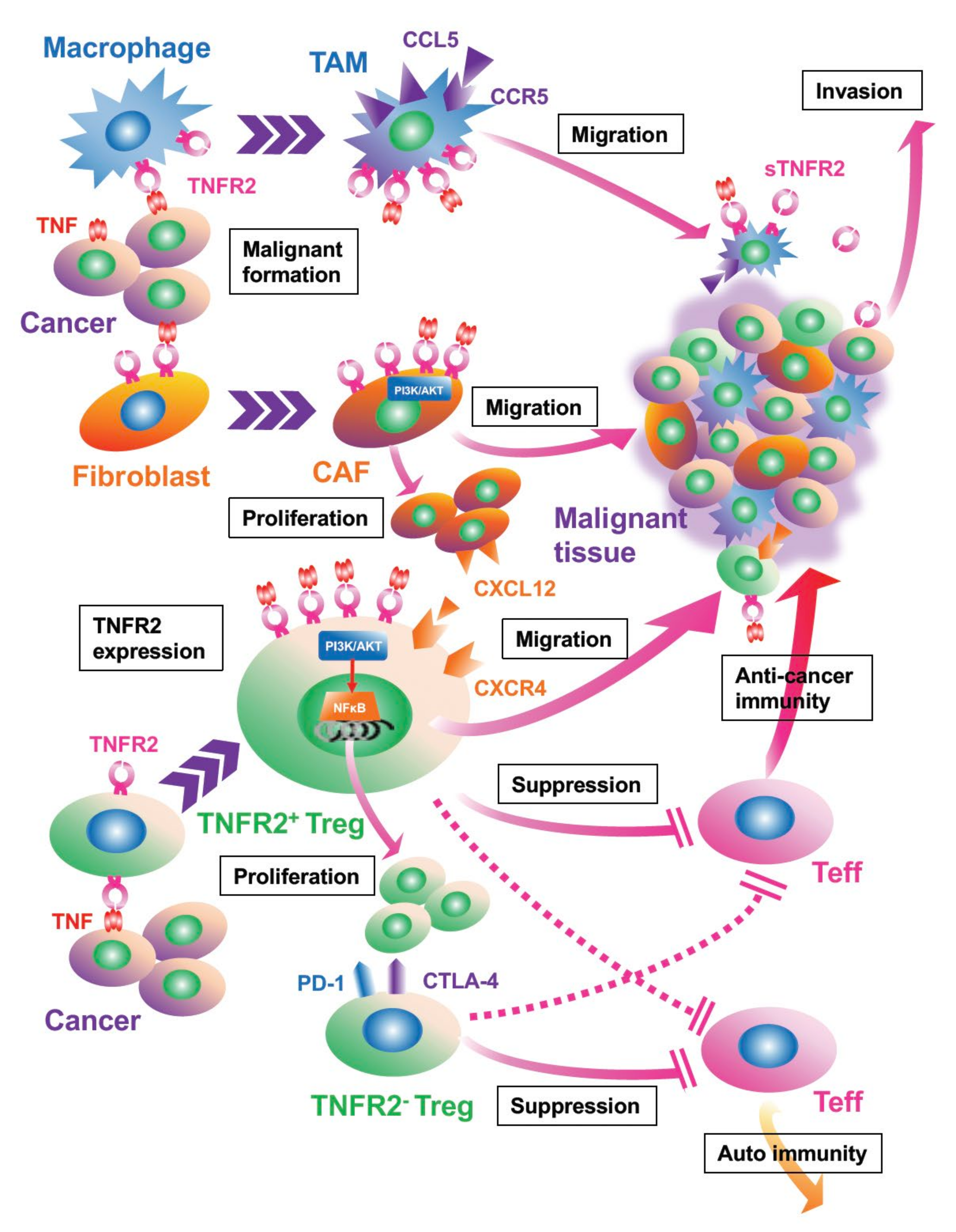
4. Activated TNF/TNFR2 Signaling Is Associated with Proliferation and Recruitment of Stromal Cells in the TME
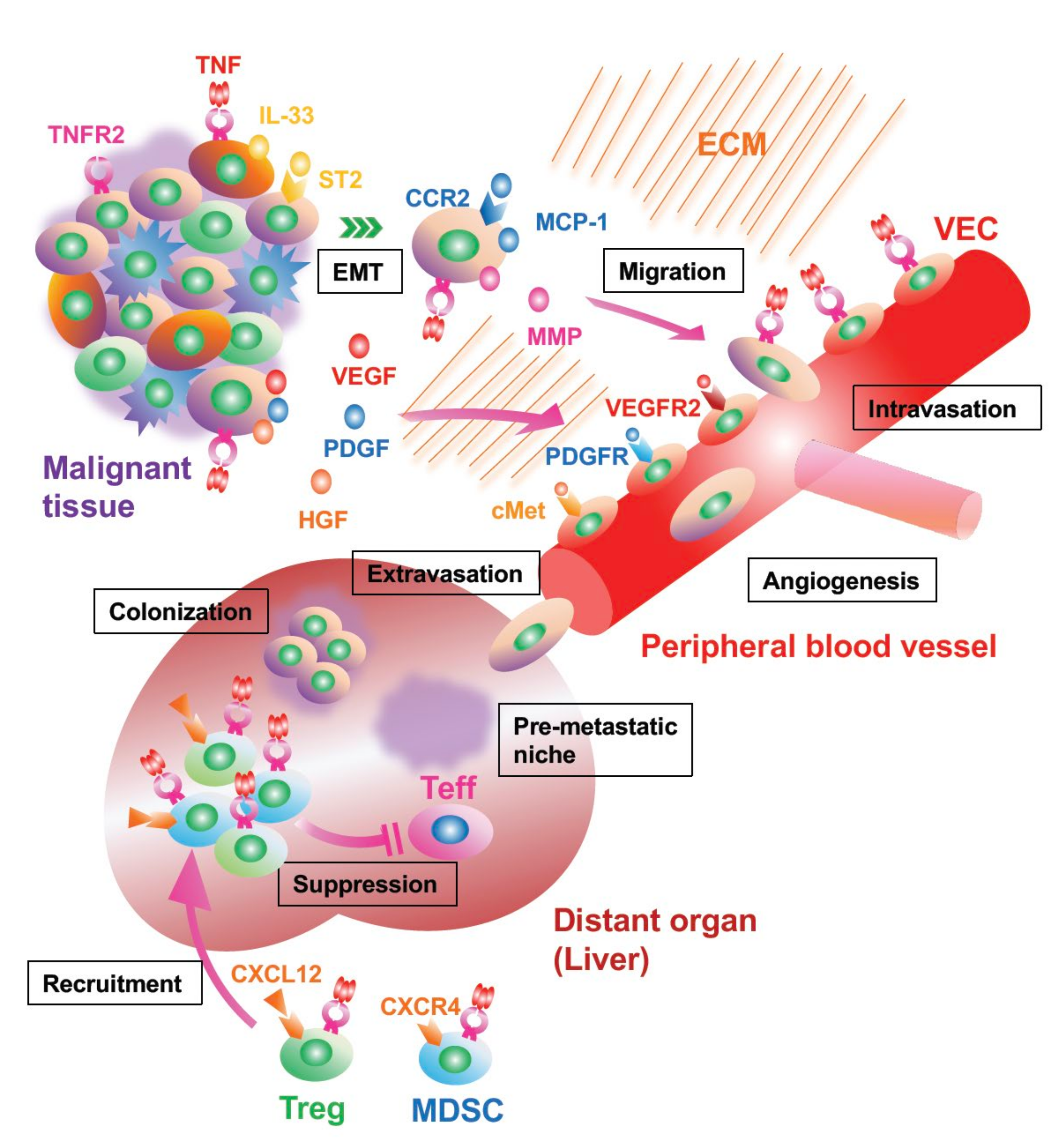
5. Cancer Invasion and Metastasis Are Facilitated by TNFR2 Signaling
6. TNFR Signaling Controls Immunomodulating Cells, Tregs, and MDSCs
7. Oncogenesis Can Be Driven by TNFR2 Signaling
8. TNFR2 Signaling in Cancer Stem Cells: Still Controversial
9. TNFR2 Signaling Can Contribute to Chemotherapy Resistance
10. Anti-Cancer Therapy Targeting TNFR2; TNFR2+ Treg Depletion Leads to Beneficial Outcomes
11. Clinical Evaluation of TNFR2: Higher Levels of TNFR2 Expression Reflect Poor Prognosis and Risk of Cancer Development
12. Discussion
- (1)
- (2)
- (3)
- The dominant antibody inhibits the cleavage of the receptor from the cellular surface [73].
- (4)
- We also expect that this dominant anti-TNFR2 antibody may overcome the drug resistance of CSCs with the expectation of achieving a complete cure in some malignant diseases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anslow, W.P., Jr.; Karnovsky, D.A.; Jager, B.V.; Smith, H.W. The toxicity and pharmacological action of the nitrogen mustards and certain related compounds. J. Pharmacol. Exp. Ther. 1947, 91, 224–235. [Google Scholar] [PubMed]
- Gobbini, E.; Charles, J.; Toffart, A.C.; Leccia, M.T.; Moro-Sibilot, D.; Giaj Levra, M. Current opinions in immune checkpoint inhibitors rechallenge in solid cancers. Crit. Rev. Oncol. Hematol. 2019, 144, 102816. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Mercurio, V.; Bonaduce, D.; Marone, G.; Tocchetti, C.G. Pharmacovigilating cardiotoxicity of immune checkpoint inhibitors. Lancet Oncol. 2018, 19, 1545–1546. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- Tang, J.; Yu, J.X.; Hubbard-Lucey, V.M.; Neftelinov, S.T.; Hodge, J.P.; Lin, Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat. Rev. Drug Discov. 2018, 17, 854–855. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, F.; Qin, Z. TNF Receptor 2 makes tumor necrosis factor a friend of tumors. Front. Immunol. 2018, 9, 1170. [Google Scholar] [CrossRef]
- Naude, P.J.; den Boer, J.A.; Luiten, P.G.; Eisel, U.L. Tumor necrosis factor receptor cross-talk. FEBS J. 2011, 278, 888–898. [Google Scholar] [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Faustman, D.L.; Davis, M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front. Immunol. 2013, 4, 478. [Google Scholar] [CrossRef]
- Hasegawa, S.; Nishikawa, S.; Miura, T.; Saito, Y.; Madarame, H.; Sekikawa, K.; Tagawa, Y.; Iwakura, Y.; Nakane, A. Tumor necrosis factor-alpha is required for gastritis induced by Helicobacter felis infection in mice. Microb. Pathog. 2004, 37, 119–124. [Google Scholar] [CrossRef]
- Vanamee, E.S.; Faustman, D.L. Structural principles of tumor necrosis factor superfamily signaling. Sci. Signal. 2018, 11, eaao4910. [Google Scholar] [CrossRef]
- Wajant, H. Principles of antibody-mediated TNF receptor activation. Cell Death Differ. 2015, 22, 1727–1741. [Google Scholar] [CrossRef]
- Grell, M.; Douni, E.; Wajant, H.; Lohden, M.; Clauss, M.; Maxeiner, B.; Georgopoulos, S.; Lesslauer, W.; Kollias, G.; Pfizenmaier, K.; et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 1995, 83, 793–802. [Google Scholar] [CrossRef]
- De Silva, D.G.; Mendis, L.N.; Sheron, N.; Alexander, G.J.; Candy, D.C.; Chart, H.; Rowe, B. TNF alpha in stool as marker of intestinal inflammation. Lancet 1992, 340, 372. [Google Scholar] [CrossRef]
- Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Lauenborg, B.; Christensen, L.; Ralfkiaer, U.; Kopp, K.L.; Jonson, L.; Dabelsteen, S.; Bonefeld, C.M.; Geisler, C.; Gjerdrum, L.M.; Zhang, Q.; et al. Malignant T cells express lymphotoxin alpha and drive endothelial activation in cutaneous T cell lymphoma. Oncotarget 2015, 6, 15235–15249. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, L.L.; Dong, T.T.; Shen, Y.H.; Guo, X.S.; Liu, C.Y.; Liu, J.; Zhang, P.; Li, J.; Sun, Y.P. Progranulin promotes colorectal cancer proliferation and angiogenesis through TNFR2/Akt and ERK signaling pathways. Am. J. Cancer Res. 2015, 5, 3085–3097. [Google Scholar] [PubMed]
- Collette, Y.; Gilles, A.; Pontarotti, P.; Olive, D. A co-evolution perspective of the TNFSF and TNFRSF families in the immune system. Trends Immunol. 2003, 24, 387–394. [Google Scholar] [CrossRef]
- Vanamee, E.S.; Faustman, D.L. TNFR2: A novel target for cancer immunotherapy. Trends Mol. Med. 2017, 23, 1037–1046. [Google Scholar] [CrossRef]
- Huang, J.; Yu, S.; Ji, C.; Li, J. Structural basis of cell apoptosis and necrosis in TNFR signaling. Apoptosis 2015, 20, 210–215. [Google Scholar] [CrossRef]
- Wajant, H.; Beilhack, A. Targeting regulatory T cells by addressing tumor necrosis factor and its receptors in allogeneic hematopoietic cell transplantation and cancer. Front. Immunol. 2019, 10, 2040. [Google Scholar] [CrossRef]
- Rothe, M.; Sarma, V.; Dixit, V.M.; Goeddel, D.V. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science 1995, 269, 1424–1427. [Google Scholar] [CrossRef]
- Cabal-Hierro, L.; Rodriguez, M.; Artime, N.; Iglesias, J.; Ugarte, L.; Prado, M.A.; Lazo, P.S. TRAF-mediated modulation of NF-kB AND JNK activation by TNFR2. Cell Signal. 2014, 26, 2658–2666. [Google Scholar] [CrossRef]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef]
- Aderka, D.; Engelmann, H.; Shemer-Avni, Y.; Hornik, V.; Galil, A.; Sarov, B.; Wallach, D. Variation in serum levels of the soluble TNF receptors among healthy individuals. Lymphokine Cytokine Res. 1992, 11, 157–159. [Google Scholar]
- Faustman, D.; Davis, M. TNF receptor 2 pathway: Drug target for autoimmune diseases. Nat. Rev. Drug Discov. 2010, 9, 482–493. [Google Scholar] [CrossRef]
- Hamilton, K.E.; Simmons, J.G.; Ding, S.; Van Landeghem, L.; Lund, P.K. Cytokine induction of tumor necrosis factor receptor 2 is mediated by STAT3 in colon cancer cells. Mol. Cancer Res. 2011, 9, 1718–1731. [Google Scholar] [CrossRef]
- Rivas, M.A.; Carnevale, R.P.; Proietti, C.J.; Rosemblit, C.; Beguelin, W.; Salatino, M.; Charreau, E.H.; Frahm, I.; Sapia, S.; Brouckaert, P.; et al. TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp. Cell Res. 2008, 314, 509–529. [Google Scholar] [CrossRef]
- Zhao, T.; Li, H.; Liu, Z. Tumor necrosis factor receptor 2 promotes growth of colorectal cancer via the PI3K/AKT signaling pathway. Oncol. Lett. 2017, 1, 342–346. [Google Scholar] [CrossRef]
- Sasi, S.P.; Yan, X.; Enderling, H.; Park, D.; Gilbert, H.Y.; Curry, C.; Coleman, C.; Hlatky, L.; Qin, G.; Kishore, R.; et al. Breaking the ‘harmony’ of TNF-alpha signaling for cancer treatment. Oncogene 2012, 31, 4117–4127. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Sadler, T.J.; Wang, J.; Reid, M.J.; Warren, A.Y.; Movassagh, M.; Lu, W.; Mills, I.G.; Neal, D.E.; Burge, J.; et al. Tumor necrosis factor receptor expression and signaling in renal cell carcinoma. Am. J. Pathol. 2010, 177, 943–954. [Google Scholar] [CrossRef]
- Feng, T.; Zheng, L.; Liu, F.; Xu, X.; Mao, S.; Wang, X.; Liu, J.; Lu, Y.; Zhao, W.; Yu, X.; et al. Growth factor progranulin promotes tumorigenesis of cervical cancer via PI3K/Akt/mTOR signaling pathway. Oncotarget 2016, 7, 58381–58395. [Google Scholar] [CrossRef]
- Arnott, C.H.; Scott, K.A.; Moore, R.J.; Robinson, S.C.; Thompson, R.G.; Balkwill, F.R. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene 2004, 23, 1902–1910. [Google Scholar] [CrossRef]
- Sasi, S.P.; Bae, S.; Song, J.; Perepletchikov, A.; Schneider, D.; Carrozza, J.; Yan, X.; Kishore, R.; Enderling, H.; Goukassian, D.A. Therapeutic non-toxic doses of TNF induce significant regression in TNFR2-p75 knockdown Lewis lung carcinoma tumor implants. PLoS ONE 2014, 9, e92373. [Google Scholar] [CrossRef]
- Lan, X.; Sun, W.; Dong, W.; Wang, Z.; Zhang, T.; He, L.; Zhang, H. Downregulation of long noncoding RNA H19 contributes to the proliferation and migration of papillary thyroid carcinoma. Gene 2018, 646, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, C.; Rabe, D.; Bainer, R.; Sankarasharma, D.; Chada, K.; Krausz, T.; Gilad, Y.; Becker, L.; Rosner, M.R. Metastasis Suppressors regulate the tumor microenvironment by blocking recruitment of prometastatic tumor-associated macrophages. Cancer Res. 2015, 75, 4063–4073. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, N.; Wu, N. TNFR2 promotes Adriamycin resistance in breast cancer cells by repairing DNA damage. Mol. Med. Rep. 2017, 16, 2962–2968. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, X.; Hamano, R.; Subleski, J.J.; Hurwitz, A.A.; Howard, O.M.; Oppenheim, J.J. Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3- conventional T cells to suppression by CD4+FoxP3+ regulatory T cells. J. Immunol. 2010, 185, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.; Wang, N.; D’Costa, Z.; Fernandez, M.C.; Bourdeau, F.; Auguste, P.; Illemann, M.; Eefsen, R.L.; Hoyer-Hansen, G.; Vainer, B.; et al. TNF Receptor-2 Facilitates an Immunosuppressive Microenvironment in the Liver to Promote the Colonization and Growth of Hepatic Metastases. Cancer Res. 2015, 75, 5235–5247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wu, X.; Wang, X.; Yu, Z.; Pan, T.; Li, Z.; Chang, X.; Jin, Z.; Li, J.; Zhu, Z.; et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-alpha/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene 2019, 39, 1414–1428. [Google Scholar] [CrossRef]
- Jing, Y.; Sun, K.; Liu, W.; Sheng, D.; Zhao, S.; Gao, L.; Wei, L. Tumor necrosis factor-alpha promotes hepatocellular carcinogenesis through the activation of hepatic progenitor cells. Cancer Lett. 2018, 434, 22–32. [Google Scholar] [CrossRef]
- Tanimura, Y.; Kokuryo, T.; Tsunoda, N.; Yamazaki, Y.; Oda, K.; Nimura, Y.; Naing Mon, N.; Huang, P.; Nakanuma, Y.; Chen, M.F.; et al. Tumor necrosis factor alpha promotes invasiveness of cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett. 2005, 219, 205–213. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Tian, J.; Gao, A.; Shen, Y.; Ren, X.; Li, X.; Jiang, G.; Dong, T. Tumor necrosis factor receptor 2/AKT and ERK signaling pathways contribute to the switch from fibroblasts to CAFs by progranulin in microenvironment of colorectal cancer. Oncotarget 2017, 8, 26323–26333. [Google Scholar] [CrossRef]
- Chang, L.Y.; Lin, Y.C.; Chiang, J.M.; Mahalingam, J.; Su, S.H.; Huang, C.T.; Chen, W.T.; Huang, C.H.; Jeng, W.J.; Chen, Y.C.; et al. Blockade of TNF-alpha signaling benefits cancer therapy by suppressing effector regulatory T cell expansion. Oncoimmunology 2015, 4, e1040215. [Google Scholar] [CrossRef]
- Al-Lamki, R.S.; Wang, J.; Yang, J.; Burrows, N.; Maxwell, P.H.; Eisen, T.; Warren, A.Y.; Vanharanta, S.; Pacey, S.; Vandenabeele, P.; et al. Tumor necrosis factor receptor 2-signaling in CD133-expressing cells in renal clear cell carcinoma. Oncotarget 2016, 7, 24111–24124. [Google Scholar] [CrossRef]
- Govindaraj, C.; Scalzo-Inguanti, K.; Madondo, M.; Hallo, J.; Flanagan, K.; Quinn, M.; Plebanski, M. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin. Immunol. 2013, 149, 97–110. [Google Scholar] [CrossRef]
- Chopra, M.; Riedel, S.S.; Biehl, M.; Krieger, S.; von Krosigk, V.; Bauerlein, C.A.; Brede, C.; Jordan Garrote, A.L.; Kraus, S.; Schafer, V.; et al. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis 2013, 34, 1296–1303. [Google Scholar] [CrossRef]
- Johrer, K.; Janke, K.; Krugmann, J.; Fiegl, M.; Greil, R. Transendothelial migration of myeloma cells is increased by tumor necrosis factor (TNF)-alpha via TNF receptor 2 and autocrine up-regulation of MCP-1. Clin. Cancer Res. 2004, 10, 1901–1910. [Google Scholar] [CrossRef]
- Govindaraj, C.; Tan, P.; Walker, P.; Wei, A.; Spencer, A.; Plebanski, M. Reducing TNF receptor 2+ regulatory T cells via the combined action of azacitidine and the HDAC inhibitor, panobinostat for clinical benefit in acute myeloid leukemia patients. Clin. Cancer Res. 2014, 20, 724–735. [Google Scholar] [CrossRef]
- Deb, S.; Amin, S.; Imir, A.G.; Yilmaz, M.B.; Suzuki, T.; Sasano, H.; Bulun, S.E. Estrogen regulates expression of tumor necrosis factor receptors in breast adipose fibroblasts. J. Clin. Endocrinol. Metab. 2004, 89, 4018–4024. [Google Scholar] [CrossRef]
- Kowal, J.; Kornete, M.; Joyce, J.A. Re-education of macrophages as a therapeutic strategy in cancer. Immunotherapy 2019, 11, 677–689. [Google Scholar] [CrossRef]
- Komohara, Y.; Takeya, M. CAFs and TAMs: Maestros of the tumour microenvironment. J. Pathol. 2017, 241, 313–315. [Google Scholar] [CrossRef]
- Dollt, C.; Becker, K.; Michel, J.; Melchers, S.; Weis, C.A.; Schledzewski, K.; Krewer, A.; Kloss, L.; Gebhardt, C.; Utikal, J.; et al. The shedded ectodomain of Lyve-1 expressed on M2-like tumor-associated macrophages inhibits melanoma cell proliferation. Oncotarget 2017, 8, 103682–103692. [Google Scholar] [CrossRef]
- Tai, S.K.; Chang, H.C.; Lan, K.L.; Lee, C.T.; Yang, C.Y.; Chen, N.J.; Chou, T.Y.; Tarng, D.C.; Hsieh, S.L. Decoy receptor 3 enhances tumor progression via induction of tumor-associated macrophages. J. Immunol. 2012, 188, 2464–2471. [Google Scholar] [CrossRef]
- Liu, J.; Kang, S.G.; Wang, P.; Wang, Y.; Lv, X.; Liu, Y.; Wang, F.; Gu, Z.; Yang, Z.; Weber, J.K.; et al. Molecular mechanism of Gd@C82(OH)22 increasing collagen expression: Implication for encaging tumor. Biomaterials 2018, 152, 24–36. [Google Scholar] [CrossRef]
- McAllister, S.S.; Weinberg, R.A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014, 16, 717–727. [Google Scholar] [CrossRef]
- Bowers, E.; Slaughter, A.; Frenette, P.S.; Kuick, R.; Pello, O.M.; Lucas, D. Granulocyte-derived TNFalpha promotes vascular and hematopoietic regeneration in the bone marrow. Nat. Med. 2018, 24, 95–102. [Google Scholar] [CrossRef]
- Baluk, P.; Yao, L.C.; Feng, J.; Romano, T.; Jung, S.S.; Schreiter, J.L.; Yan, L.; Shealy, D.J.; McDonald, D.M. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J. Clin. Investig. 2009, 119, 2954–2964. [Google Scholar] [CrossRef] [PubMed]
- Ieda, T.; Tazawa, H.; Okabayashi, H.; Yano, S.; Shigeyasu, K.; Kuroda, S.; Ohara, T.; Noma, K.; Kishimoto, H.; Nishizaki, M.; et al. Visualization of epithelial-mesenchymal transition in an inflammatory microenvironment-colorectal cancer network. Sci. Rep. 2019, 9, 16378. [Google Scholar] [CrossRef]
- Takahashi, E.; Nagano, O.; Ishimoto, T.; Yae, T.; Suzuki, Y.; Shinoda, T.; Nakamura, S.; Niwa, S.; Ikeda, S.; Koga, H.; et al. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J. Biol. Chem. 2010, 285, 4060–4073. [Google Scholar] [CrossRef]
- Hagemann, T.; Wilson, J.; Kulbe, H.; Li, N.F.; Leinster, D.A.; Charles, K.; Klemm, F.; Pukrop, T.; Binder, C.; Balkwill, F.R. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J. Immunol. 2005, 175, 1197–1205. [Google Scholar] [CrossRef]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef]
- Mahmud, S.A.; Manlove, L.S.; Schmitz, H.M.; Xing, Y.; Wang, Y.; Owen, D.L.; Schenkel, J.M.; Boomer, J.S.; Green, J.M.; Yagita, H.; et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 2014, 15, 473–481. [Google Scholar] [CrossRef]
- Okubo, Y.; Mera, T.; Wang, L.; Faustman, D.L. Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci. Rep. 2013, 3, 3153. [Google Scholar] [CrossRef] [PubMed]
- Urbano, P.C.M.; Koenen, H.; Joosten, I.; He, X. An Autocrine TNFalpha-tumor necrosis factor receptor 2 loop promotes epigenetic effects inducing human treg stability in vitro. Front. Immunol. 2018, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; He, J.; Shirota, H.; Trivett, A.L.; Yang Klinman, D.M.; Oppenheim, J.J.; Chen, X. Blockade of TNFR2 signaling enhances the immunotherapeutic effect of CpG ODN in a mouse model of colon cancer. Sci. Signal. 2018, 11, eaan0790. [Google Scholar] [CrossRef] [PubMed]
- Torrey, H.; Butterworth, J.; Mera, T.; Okubo, Y.; Wang, L.; Baum, D.; Defusco, A.; Plager, S.; Warden, S.; Huang, D.; et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci. Signal. 2017, 10, eaaf8608. [Google Scholar] [CrossRef] [PubMed]
- Torrey, H.; Khodadoust, M.; Tran, L.; Baum, D.; Defusco, A.; Kim, Y.H.; Faustman, D.L. Targeted killing of TNFR2-expressing tumor cells and Tregs by TNFR2 antagonistic antibodies in advanced Sezary syndrome. Leukemia 2019, 33, 1206–1218. [Google Scholar] [CrossRef]
- Yan, F.; Du, R.; Wei, F.; Zhao, H.; Yu, J.; Wang, C.; Zhan, Z.; Ding, T.; Ren, X.; Chen, X.; et al. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol. Immunother. 2015, 64, 1475–1485. [Google Scholar] [CrossRef]
- Chen, X.; Subleski, J.J.; Kopf, H.; Howard, O.M.; Mannel, D.N.; Oppenheim, J.J. Cutting edge: Expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: Applicability to tumor-infiltrating T regulatory cells. J. Immunol. 2008, 180, 6467–6471. [Google Scholar] [CrossRef]
- Kim, E.Y.; Teh, S.J.; Yang, J.; Chow, M.T.; Teh, H.S. TNFR2-deficient memory CD8 T cells provide superior protection against tumor cell growth. J. Immunol. 2009, 183, 6051–6057. [Google Scholar] [CrossRef]
- Nagaraj, S.; Gabrilovich, D.I. Regulation of suppressive function of myeloid-derived suppressor cells by CD4+ T cells. Semin Cancer Biol. 2012, 22, 282–288. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Li, B.; Li, X.; Zhao, X.; Wan, L.; Lin, G.; Yu, M.; Wang, J.; Jiang, X.; Feng, W.; et al. Transmembrane TNF-alpha promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J. Immunol. 2014, 192, 1320–1331. [Google Scholar] [CrossRef]
- Zhao, X.; Rong, L.; Zhao, X.; Li, X.; Liu, X.; Deng, J.; Wu, H.; Xu, X.; Erben, U.; Wu, P.; et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J. Clin. Investig. 2012, 122, 4094–4104. [Google Scholar] [CrossRef]
- Ba, H.; Li, B.; Li, X.; Li, C.; Feng, A.; Zhu, Y.; Wang, J.; Li, Z.; Yin, B. Transmembrane tumor necrosis factor-alpha promotes the recruitment of MDSCs to tumor tissue by upregulating CXCR4 expression via TNFR2. Int. Immunopharmacol. 2017, 44, 143–152. [Google Scholar] [CrossRef]
- Xu, J.; Chakrabarti, A.K.; Tan, J.L.; Ge, L.; Gambotto, A.; Vujanovic, N.L. Essential role of the TNF-TNFR2 cognate interaction in mouse dendritic cell-natural killer cell crosstalk. Blood 2007, 109, 3333–3341. [Google Scholar] [CrossRef]
- Onizawa, M.; Nagaishi, T.; Kanai, T.; Nagano, K.; Oshima, S.; Nemoto, Y.; Yoshioka, A.; Totsuka, T.; Okamoto, R.; Nakamura, T.; et al. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G850–G859. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagaishi, T.; Yamazaki, M.; Onizawa, M.; Watabe, T.; Sakamaki, Y.; Ichinose, S.; Totsuka, M.; Oshima, S.; Okamoto, R.; et al. Myosin light chain kinase expression induced via tumor necrosis factor receptor 2 signaling in the epithelial cells regulates the development of colitis-associated carcinogenesis. PLoS ONE 2014, 9, e88369. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, Q.; An, L.; Jiao, S.; Li, R.; Sang, Y.; Liao, J.; Nie, P.; Wen, F.; Ju, J.; et al. A TNFR2-hnRNPK axis promotes primary liver cancer development via activation of YAP Signaling in hepatic progenitor cells. Cancer Res. 2021, 81, 3036–3050. [Google Scholar] [CrossRef]
- He, L.; Bhat, K.; Duhacheck-Muggy, S.; Ioannidis, A.; Zhang, L.; Nguyen, N.T.; Moatamed, N.A.; Pajonk, F. Tumor necrosis factor receptor signaling modulates carcinogenesis in a mouse model of breast cancer. Neoplasia 2021, 23, 197–209. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bayik, D.; Lathia, J.D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 2021, 21, 526–536. [Google Scholar] [CrossRef]
- Xiang, Q.; Yang, B.; Li, L.; Qiu, B.; Qiu, C.; Gao, X.B.; Zhou, H.J.; Min, W. Critical role of Lin28-TNFR2 signalling in cardiac stem cell activation and differentiation. J. Cell Mol. Med. 2019, 23, 2943–2953. [Google Scholar] [CrossRef]
- Madsen, P.M.; Motti, D.; Karmally, S.; Szymkowski, D.E.; Lambertsen, K.L.; Bethea, J.R.; Brambilla, R. Oligodendroglial TNFR2 Mediates Membrane TNF-Dependent Repair in Experimental Autoimmune Encephalomyelitis by Promoting Oligodendrocyte Differentiation and Remyelination. J. Neurosci. 2016, 36, 5128–5143. [Google Scholar] [CrossRef]
- Paris, J.; Morgan, M.; Campos, J.; Spencer, G.J.; Shmakova, A.; Ivanova, I.; Mapperley, C.; Lawson, H.; Wotherspoon, D.A.; Sepulveda, C.; et al. Targeting the RNA m(6)A Reader YTHDF2 Selectively Compromises Cancer Stem Cells in Acute Myeloid Leukemia. Cell Stem Cell 2019, 25, 137–148.e6. [Google Scholar] [CrossRef]
- Bradley, J.R.; Wang, J.; Pacey, S.; Warren, A.Y.; Pober, J.S.; Al-Lamki, R.S. Tumor necrosis factor receptor-2 signaling pathways promote survival of cancer stem-like CD133(+) cells in clear cell renal carcinoma. FASEB Bioadv. 2020, 2, 126–144. [Google Scholar] [CrossRef]
- Zhang, W.; Ramdas, L.; Shen, W.; Song, S.W.; Hu, L.; Hamilton, S.R. Apoptotic response to 5-fluorouracil treatment is mediated by reduced polyamines, non-autocrine Fas ligand and induced tumor necrosis factor receptor 2. Cancer Biol. Ther. 2003, 2, 572–578. [Google Scholar] [CrossRef]
- Sprowl, J.A.; Reed, K.; Armstrong, S.R.; Lanner, C.; Guo, B.; Kalatskaya, I.; Stein, L.; Hembruff, S.L.; Tam, A.; Parissenti, A.M. Alterations in tumor necrosis factor signaling pathways are associated with cytotoxicity and resistance to taxanes: A study in isogenic resistant tumor cells. Breast Cancer Res. 2012, 14, R2. [Google Scholar] [CrossRef]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H., 2nd; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196. [Google Scholar] [CrossRef]
- DeRogatis, J.M.; Viramontes, K.M.; Neubert, E.N.; Henriquez, M.L.; Guerrero-Juarez, C.F.; Tinoco, R. Targeting the PSGL-1 Immune Checkpoint Promotes Immunity to PD-1-Resistant Melanoma. Cancer Immunol. Res. 2022, 10, 612–625. [Google Scholar] [CrossRef]
- Mayer, L.S.; Orlowski, R.J.; Giles, J.; Benci, J.L.; Ellis, G.; Deng, G.; Attanasio, J.; Chen, Z.; Bengsch, B.; Kahn, O.; et al. Targeting TNFR2 to overcome acquired adaptive resistance to immune checkpoint blockade. J. Immunol. 2020, 204, 165.42. [Google Scholar]
- Case, K.; Tran, L.; Yang, M.; Zheng, H.; Kuhtreiber, W.M.; Faustman, D.L. TNFR2 blockade alone or in combination with PD-1 blockade shows therapeutic efficacy in murine cancer models. J. Leukoc. Biol. 2020, 107, 981–991. [Google Scholar] [CrossRef]
- Chen, X.; Oppenheim, J.J. Targeting TNFR2, an immune checkpoint stimulator and oncoprotein, is a promising treatment for cancer. Sci. Signal. 2017, 10, eaal2328. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, C.; Madondo, M.; Kong, Y.Y.; Tan, P.; Wei, A.; Plebanski, M. Lenalidomide-based maintenance therapy reduces TNF receptor 2 on CD4 T cells and enhances immune effector function in acute myeloid leukemia patients. Am. J. Hematol. 2014, 89, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Goo, K.; Uy, R.; Roswarski, J. Azacitidine-associated pleuropericardial effusion in myelodysplastic syndrome: A case report. J. Oncol. Pharm. Pract. 2019, 25, 1248–1252. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Zhou, Q.; Howard, O.M.; Netea, M.G.; Oppenheim, J.J. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J. Immunol. 2013, 190, 1076–1084. [Google Scholar] [CrossRef]
- Da Silva Almeida, A.C.; Abate, F.; Khiabanian, H.; Martinez-Escala, E.; Guitart, J.; Tensen, C.P.; Vermeer, M.H.; Rabadan, R.; Ferrando, A.; Palomero, T. The mutational landscape of cutaneous T cell lymphoma and Sezary syndrome. Nat. Genet. 2015, 47, 1465–1470. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; Bou-Anak, S.; Burgett, M.E.; Sarmey, N.; Khosla, D.; Dahiya, S.; Weil, R.J.; Bae, E.; Huang, P.; McGraw, M.; et al. Correlation of higher levels of soluble TNF-R1 with a shorter survival, independent of age, in recurrent glioblastoma. J. Neurooncol. 2017, 131, 449–458. [Google Scholar] [CrossRef]
- Xiao, C.; Beitler, J.J.; Higgins, K.A.; Glazer, T.; Huynh, L.K.; Paul, S.; Felger, J.C.; Wommack, E.C.; Saba, N.F.; Shin, D.M.; et al. Associations among human papillomavirus, inflammation, and fatigue in patients with head and neck cancer. Cancer 2018, 124, 3163–3170. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Z.; Zhao, N. Clinical implications of tumor necrosis factor receptor 2 in breast cancer. Oncol. Lett. 2017, 14, 2393–2398. [Google Scholar] [CrossRef]
- Cui, L.F.; Guo, X.J.; Wei, J.; Liu, F.F.; Fan, Y.; Lang, R.G.; Gu, F.; Zhang, X.M.; Fu, L. Overexpression of TNF-alpha and TNFRII in invasive micropapillary carcinoma of the breast: Clinicopathological correlations. Histopathology 2008, 53, 381–388. [Google Scholar] [CrossRef]
- Ghods, A.; Ghaderi, A.; Shariat, M.; Talei, A.R.; Mehdipour, F. TNFR2 but not TNFR1 is the main TNFR expressed by B and T lymphocytes in breast cancer draining lymph nodes. Immunol. Lett. 2019, 209, 36–44. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, C.; Ma, G.; Sun, X.; Liu, J.; Li, S.; Liu, K.; Wang, J.; Yang, D. High expression of tumor necrosis factor receptor 2 in tissue is associated with progression and prognosis of esophageal squamous cell carcinoma. Hum. Pathol. 2018, 80, 179–185. [Google Scholar] [CrossRef]
- Grote, V.A.; Kaaks, R.; Nieters, A.; Tjonneland, A.; Halkjaer, J.; Overvad, K.; Skjelbo Nielsen, M.R.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Racine, A.; et al. Inflammation marker and risk of pancreatic cancer: A nested case-control study within the EPIC cohort. Br. J. Cancer 2012, 106, 1866–1874. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Denda, T.; Kudo, T.; Sugimoto, N.; Ura, T.; Yamazaki, K.; Fujii, H.; Kajiwara, T.; Nakajima, T.E.; Takahashi, S.; et al. Exploration of potential prognostic biomarkers in aflibercept plus FOLFIRI in Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019, 110, 3565–3572. [Google Scholar] [CrossRef]
- Babic, A.; Shah, S.M.; Song, M.; Wu, K.; Meyerhardt, J.A.; Ogino, S.; Yuan, C.; Giovannucci, E.L.; Chan, A.T.; Stampfer, M.J.; et al. Soluble tumour necrosis factor receptor type II and survival in colorectal cancer. Br. J. Cancer 2016, 114, 995–1002. [Google Scholar] [CrossRef]
- Dobrzycka, B.; Terlikowski, S.J.; Garbowicz, M.; Niklinska, W.; Bernaczyk, P.S.; Niklinski, J.; Kinalski, M.; Chyczewski, L. Tumor necrosis factor-alpha and its receptors in epithelial ovarian cancer. Folia Histochem. Cytobiol. 2009, 47, 609–613. [Google Scholar] [CrossRef]
- Zhang, T.; Jiao, J.; Jiao, X.; Zhao, L.; Tian, X.; Zhang, Q.; Ma, D.; Cui, B. Aberrant frequency of TNFR2(+) Treg and related cytokines in patients with CIN and cervical cancer. Oncotarget 2018, 9, 5073–5083. [Google Scholar] [CrossRef]
- Gadducci, A.; Ferdeghini, M.; Fanucchi, A.; Annicchiarico, C.; Prato, B.; Prontera, C.; Facchini, V.; Genazzani, A.R. Serum levels of soluble receptors for tumor necrosis factor (p55 and p75 sTNFr) in endometrial cancer. Anticancer Res. 1996, 16, 3125–3128. [Google Scholar]
- Dossus, L.; Becker, S.; Rinaldi, S.; Lukanova, A.; Tjonneland, A.; Olsen, A.; Overvad, K.; Chabbert-Buffet, N.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; et al. Tumor necrosis factor (TNF)-alpha, soluble TNF receptors and endometrial cancer risk: The EPIC study. Int. J. Cancer 2011, 129, 2032–2037. [Google Scholar] [CrossRef]
- Gu, Y.; Shore, R.E.; Arslan, A.A.; Koenig, K.L.; Liu, M.; Ibrahim, S.; Lokshin, A.E.; Zeleniuch-Jacquotte, A. Circulating cytokines and risk of B-cell non-Hodgkin lymphoma: A prospective study. Cancer Causes Control 2010, 21, 1323–1333. [Google Scholar] [CrossRef]
- Purdue, M.P.; Hofmann, J.N.; Kemp, T.J.; Chaturvedi, A.K.; Lan, Q.; Park, J.H.; Pfeiffer, R.M.; Hildesheim, A.; Pinto, L.A.; Rothman, N. A prospective study of 67 serum immune and inflammation markers and risk of non-Hodgkin lymphoma. Blood 2013, 122, 951–957. [Google Scholar] [CrossRef]
- Yang, M.; Tran, L.; Torrey, H.; Song, Y.; Perkins, H.; Case, K.; Zheng, H.; Takahashi, H.; Kuhtreiber, W.M.; Faustman, D.L. Optimizing TNFR2 antagonism for immunotherapy with tumor microenvironment specificity. J. Leukoc. Biol. 2020, 107, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Tam, E.M.; Fulton, R.B.; Sampson, J.F.; Muda, M.; Camblin, A.; Richards, J.; Koshkaryev, A.; Tang, J.; Kurella, V.; Jiao, Y.; et al. Antibody-mediated targeting of TNFR2 activates CD8(+) T cells in mice and promotes antitumor immunity. Sci. Transl. Med. 2019, 11, eaax0720. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.I.; EARE; Boer, J.C.; Ferji, K.; Six, J.L.; Chen, X.; Elkord, E.; Plebanski, M.; Mohamud, R. Synergistic effects of nanomedicine targeting TNFR2 and DNA demethylation inhibitor-an opportunity for cancer treatment. Cells 2019, 9, 33. [Google Scholar] [CrossRef]
- Ungewickell, A.; Bhaduri, A.; Rios, E.; Reuter, J.; Lee, C.S.; Mah, A.; Zehnder, A.; Ohgami, R.; Kulkarni, S.; Armstrong, R.; et al. Genomic analysis of mycosis fungoides and Sezary syndrome identifies recurrent alterations in TNFR2. Nat. Genet. 2015, 47, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
| Malignant Disease | Human (C, Cancer Cell Line; P, Primary Cancer Cell) | Animal (M, Mouse; R, Rat) |
|---|---|---|
| Thyroid | (P) Non-coding RNA H19 inhibits lymphatic metastasis via TNFR2 downregulation [41]. | |
| Breast | (P) TNFR2 is more highly expressed on TAMs [42]. (C) TNFR2 signaling contributes to Adriamycin resistance by repairing DNA damage via AKT signaling [43]. | (M) TNFR2 signaling promotes cancer cell proliferation via activated p42/p44 MAPK [34]. (M) TNFR2+ Tregs have greater suppressive function over TNFR2+ Teffs [44]. |
| Lung | (M) Tumor growth and angiogenesis are driven via TNFR2 signaling [36]. (M) TNFR2 signaling facilitates MDSCs-mediated immunosuppression and metastasis [45]. (M) TNFR2+ Tregs have greater suppressive function, which overcomes TNFR2+ Teffs [44]. | |
| Stomach | (P) CAFs-derived IL-33 released via TNF/TNFR2 signaling promotes gastric cancer metastasis [46]. | |
| Liver | (R) TNFR2 signaling promotes recurrence of HCC via STAT3 activation [47]. | |
| Gallbladder | (C) MMP secretion and increased invasiveness are driven via TNF/TNFR2 signaling [48]. | |
| Colorectal | (C) IL-6 and TNF induce TNFR2 expression via STAT3 activation [33]. (C) TNFR2 signaling promotes tumor growth via PI3K/AKT pathway [35]. (C) PGRN promotes cancer cell proliferation and angiogenesis via TNFR2/AKT pathway [23]. (C) PGRN/TNFR2 drives proliferation and migration of CAFs via AKT or ERK pathway [49]. | (M) TNFR2 signaling facilitates MDSC-mediated immunosuppression and liver metastasis [45]. (M) TNFR2 signaling enhances Treg’s suppressive function [50]. |
| Kidney | (P) and (C) TNFR2 signaling increases tumor progression via Etk-VEGFR2 cross talk [37]. (P) TNFR2 signaling drives CSC’s proliferation and increases their sensitivity to cytotoxicity [51]. | |
| Ovary | (P) TNFR2+ Tregs in malignant ascites are more suppressive than those in blood [52]. | |
| Uterus | (C) PGRN/TNFR2 signaling is needed for malignant transformation via mTOR signaling [38]. | |
| Skin/Lymphoma | (C) LTα/TNFR2 signaling promotes tumor growth and angiogenesis in cutaneous lymphoma [22]. | (M) Both TNFR1 and TNFR2 are necessary for optimal TNF signaling during skin cancer development [39]. |
| Melanoma | (M) Tumor growth and angiogenesis are driven via TNFR2 signaling [36]. (M) TNFR2 signaling expands Tregs, driving lung metastasis [53]. | |
| Leukemia/Myeloma | (C) Transendothelial migration is driven via TNFR2 signaling and upregulated MCP-1 secretion [54]. (P) Compared to TNFR- Tregs, TNFR2+ Tregs express higher CXCL4, which facilitates migration in AML [55]. |
| Malignant Disease | Peripheral Blood | Primary Region | Metastatic Region |
|---|---|---|---|
| Brain | Plasma sTNFR2 levels are significantly higher in patients with recurrent GBM than healthy donors [104]. | ||
| Head and Neck | Decreased plasma sTNFR2 levels reflect treatment efficacy at 3 months post-IMRT [105]. | ||
| Breast | TNFR2 expression is positively correlated with increased tumor size, advanced clinical stage, poor differentiation, shorter OS, DFS [106], and tumor growth and angiogenesis [107]. | Increased TNFR2+ B cells in metastatic LN are related to good prognosis [108]. | |
| TNFR2 expression on TAMs is upregulated in TNBC compared to non-TNBC [42]. | |||
| Lung | Tregs express higher levels of TNFR2 than Teffs, and higher TNFR2+ Tregs are related to poor prognosis [75]. | ||
| Esophagus | TNFR2 expression is positively related to an advanced clinical stage, poor differentiation, and poor OS [109]. | ||
| Pancreas | Higher plasma sTNFR2 levels are marginally associated with a higher risk of pancreatic cancer [110]. | ||
| Colorectal | A higher plasma TNFR2 level is related to shorter OS in patients with metastasis after second-line chemotherapy [111]. Pre-diagnosis higher plasma sTNFR2 levels are associated with shorter OS [112]. | ||
| Ovary | TNFR2 expression is higher in cancer cells than in benign ovarian cells, and the elevated expression reflects cancer progression [113]. | TNFR2+ Tregs are abundantly present in malignant ascites and show more suppressive characteristics than those in the peripheral blood [52]. | |
| Uterus | Peripheral TNFR2+ Tregs and circulating sTNFR2 are increased, and the percentage of TNFR2+ Tregs is inversely correlated with clinical stage [114]. Higher plasma sTNFR2 levels are associated with advanced clinical stage [115] and cancer risk [116]. | Tumor-infiltrating TNFR2+ Tregs are significantly increased [114]. | |
| Leukemia/Melanoma | Both total and TNFR2+ Treg populations are significantly higher in AML patients as compared with healthy donors [55]. | ||
| TNFR2+ Tregs have higher levels of CTLA-4, Ki67 and CXCR4 as compared with TNFR2- Tregs in AML patients [55]. | |||
| Higher TNFR2+ Tregs and lower TNFR2+ Teffs are observed in AML patients as compared with healthy donors, and increased TNFR2+ Tregs are related to cancer relapse [100]. | |||
| Lymphoma | Higher plasma sTNFR2 levels are related to higher NHL risk [117,118]. |
| Author (Year) [Ref.] | Drug | Specimen/Tissue | Mechanism/Outcomes |
|---|---|---|---|
| Govindaraj, C. et al. (2014) [55] | Combination of azacytidine and panobinostat | NFR2+ Tregs in AML patients |
|
| Govindaraj, C. et al. (2014) [100] | Combination of azacytidine and lenalidomide | NFR2+ Tregs in clinically remitted AML patients |
|
| Torrey, H. et al. (2017) [73] | Anti-TNFR2 antibody | Human Tregs in ovarian cancer ascites Human ovarian cancer cell line |
|
| Nie, Y. et al. (2018) [72] | Combination of anti-TNFR2 antibody and anti-CD25 antibody | TNFR2+ Tregs in murine colorectal and breast cancer model |
|
| Torrey, H. et al. (2019) [74] | Anti-TNFR2 antibody | TNFR2+ cancer cells and Tregs from patients with stage IV SS |
|
| Tam, E. M. et al. (2019) [120] | Anti-TNFR2 antibody | Murine colorectal, breast, fibroblast fibrosarcoma, and B cell lymphoma cell lines |
|
| Murine TNFR2+ Tregs | |||
| Yang, M. et al. (2020) [119] | Anti-TNFR2 antibody | Human colorectal cancer, lymphoma and leukemia cell line |
|
| Human Tregs and Teffs in the blood | |||
| Case, K. et al. (2020) [98] | Combination of anti-TNFR2 antibody and anti-PD-1 antibody | Murine colorectal cancer |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, H.; Yoshimatsu, G.; Faustman, D.L. The Roles of TNFR2 Signaling in Cancer Cells and the Tumor Microenvironment and the Potency of TNFR2 Targeted Therapy. Cells 2022, 11, 1952. https://doi.org/10.3390/cells11121952
Takahashi H, Yoshimatsu G, Faustman DL. The Roles of TNFR2 Signaling in Cancer Cells and the Tumor Microenvironment and the Potency of TNFR2 Targeted Therapy. Cells. 2022; 11(12):1952. https://doi.org/10.3390/cells11121952
Chicago/Turabian StyleTakahashi, Hiroyuki, Gumpei Yoshimatsu, and Denise Louise Faustman. 2022. "The Roles of TNFR2 Signaling in Cancer Cells and the Tumor Microenvironment and the Potency of TNFR2 Targeted Therapy" Cells 11, no. 12: 1952. https://doi.org/10.3390/cells11121952
APA StyleTakahashi, H., Yoshimatsu, G., & Faustman, D. L. (2022). The Roles of TNFR2 Signaling in Cancer Cells and the Tumor Microenvironment and the Potency of TNFR2 Targeted Therapy. Cells, 11(12), 1952. https://doi.org/10.3390/cells11121952





