Computerized Texture Analysis of Optical Coherence Tomography Angiography of Choriocapillaris in Normal Eyes of Young and Healthy Subjects
Abstract
1. Introduction
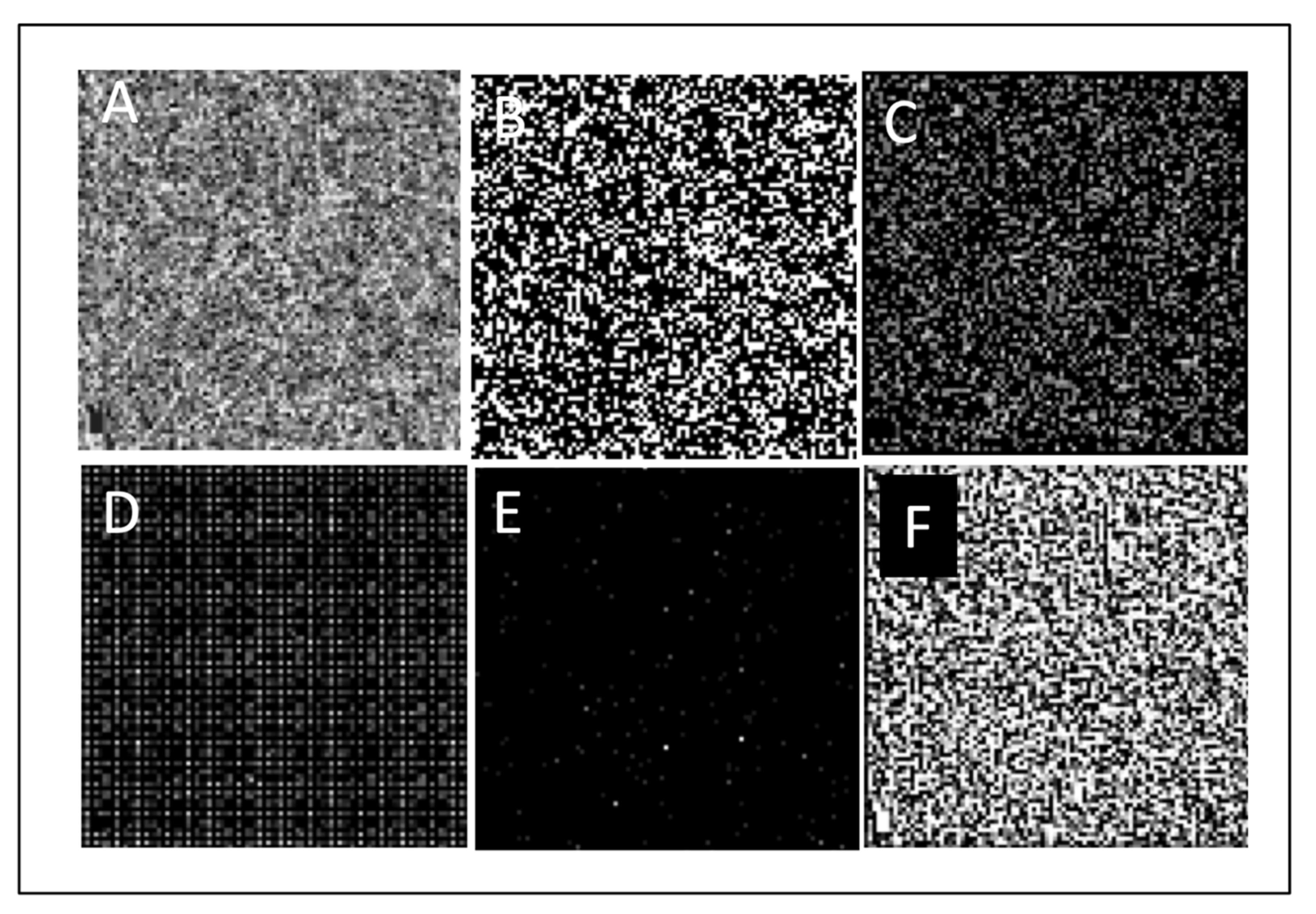
2. Materials and Methods
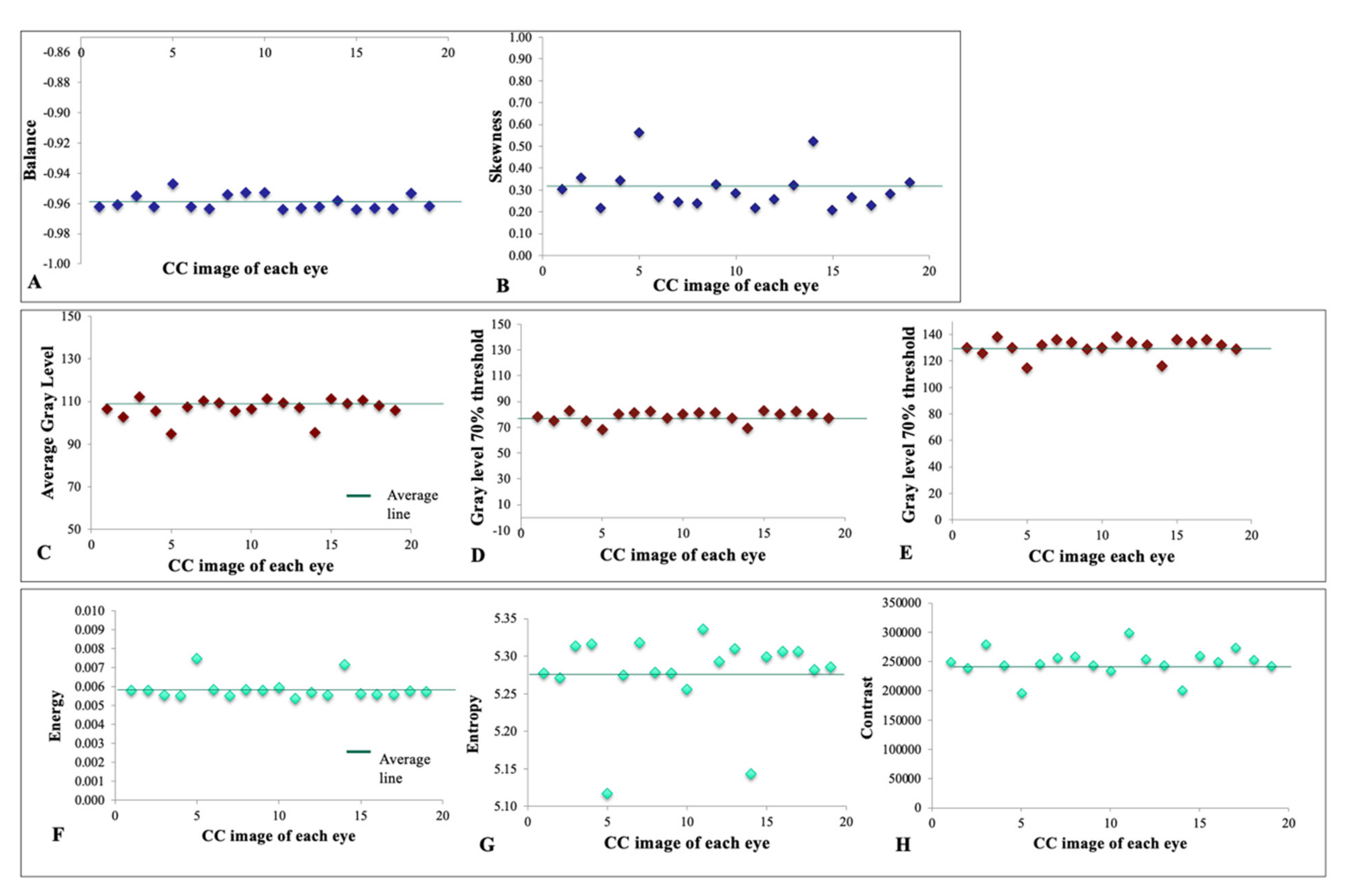
- Absolute value of gray levels: including average gray level, gray level yielding 70% threshold, and gray level yielding 30% threshold of the area under the histogram.
- Gray-level histogram analysis, including balance and skewness parameters.
- Spatial relationship among gray levels: including coarseness, contrast, energy, and entropy parameters.
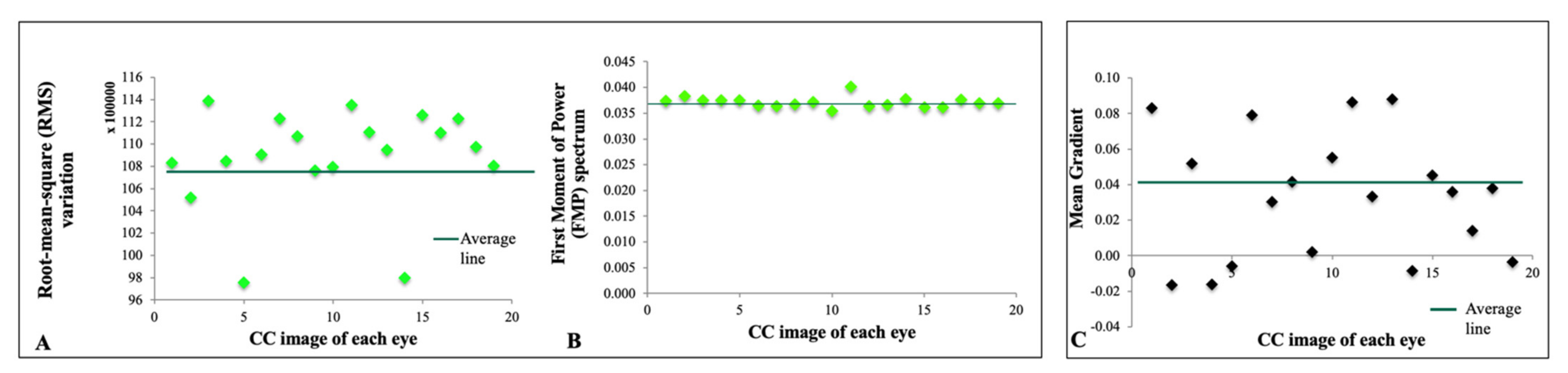
- Edge frequency: defined as mean gradient as a function of distance between pixels.
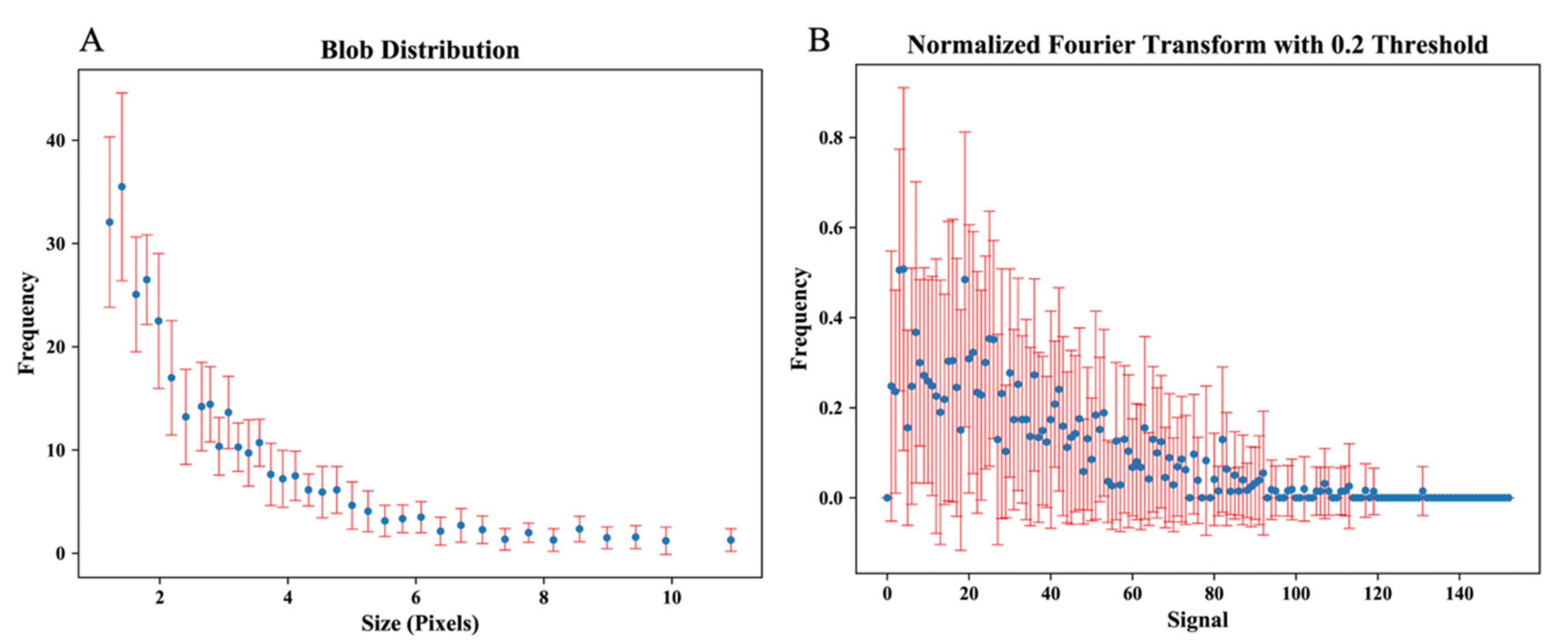
- Fourier transform analysis, including root-mean-square variation (RMS) and first moment of power spectrum (FMP).
3. Results
3.1. Demographics
3.2. Textural Features
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Financial Disclosure
Appendix A

References
- Biesemeier, A.; Taubitz, T.; Julien, S.; Yoeruek, E.; Schraermeyer, U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol. Aging 2014, 35, 2562–2573. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.; Grunwald, J.; Majji, A.B.; Uyama, M.; Yoneya, S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol. Vis. 1999, 5, 35. [Google Scholar] [PubMed]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Taomoto, M.; Otsuji, T.; Green, W.R.; Sunness, J.S.; Lutty, G.A. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1986–1993. [Google Scholar]
- Mullins, R.F.; Johnson, M.N.; Faidley, E.A.; Skeie, J.M.; Huang, J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Ramrattan, R.S.; van der Schaft, T.L.; Mooy, C.M.; De Bruijn, W.C.; Mulder, P.G.; De Jong, P.T. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2857–2864. [Google Scholar]
- Alten, F.; Heiduschka, P.; Clemens, C.R.; Eter, N. Exploring choriocapillaris under reticular pseudodrusen using OCT-Angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 2165–2173. [Google Scholar] [CrossRef]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Adhi, M. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. Am. J. Ophthalmol. 2014, 157, 1272–1281. [Google Scholar] [CrossRef]
- Choi, W. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS ONE 2013, 8, e81499. [Google Scholar] [CrossRef]
- Unterhuber, A. In vivo retinal optical coherence tomography at 1040 nm-enhanced penetration into the choroid. Opt. Express 2005, 13, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chan, S.; Yang, J.Y.; You, B.; Wang, Y.X.; Jonas, J.B.; Wei, W.B. Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am. J. Ophthalmol. 2016, 168, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Montesano, G.; Allegrini, D.; Colombo, L.; Rossetti, L.M.; Pece, A. Features of the normal choriocapillaris with OCT-angiography: Density estimation and textural properties. PLoS ONE 2017, 12, e0185256. [Google Scholar] [CrossRef] [PubMed]
- Ploner, S.B. Toward quantitative optical coherence tomography angiography: Visualizing blood flow speeds in ocular pathology using variable interscan time analysis. Retina 2016, 36, S118–S126. [Google Scholar] [CrossRef]
- Choi, W. Ultrahigh-speed, swept-source optical coherence tomography angiography in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmology 2015, 122, 2532–2544. [Google Scholar] [CrossRef]
- Moult, E. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surgery, Lasers and Imaging. Retina 2014, 45, 496–505. [Google Scholar]
- Miere, A.; Querques, G.; Semoun, O.; Capuano, V.; Souied, E.H. Optical coherence tomography angiography in early type 3 neovascularization. Retina 2015, 35, 2236–2241. [Google Scholar] [CrossRef]
- Chatziralli, I.; Theodossiadis, G.; Panagiotidis, D.; Pousoulidi, P.; Theodossiadis, P. Choriocapillaris’ alterations in the presence of reticular pseudodrusen compared to drusen: Study based on OCTA findings. Int. Ophthalmol. 2018, 38, 1887–1893. [Google Scholar] [CrossRef]
- Dodo, Y. Clinical relevance of reduced decorrelation signals in the diabetic inner choroid on optical coherence tomography angiography. Sci. Rep. 2017, 7, 5227. [Google Scholar] [CrossRef]
- Fujita, K.; Kawamura, A.; Yuzawa, M. Choriocapillaris changes imaged by OCT angiography after half-dose photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmic Surg. Lasers Imaging Retina 2017, 48, 302–310. [Google Scholar] [CrossRef]
- Borrelli, E.; Shi, Y.; Uji, A.; Balasubramanian, S.; Nassisi, M.; Sarraf, D.; Sadda, S.R. Topographic analysis of the choriocapillaris in intermediate age-related macular degeneration. Am. J. Ophthalmol. 2018, 196, 34–43. [Google Scholar] [CrossRef]
- Borrelli, E. Reduced choriocapillaris flow in eyes with type 3 neovascularization and age-related macular degeneration. Retina 2018, 38, 1968–1976. [Google Scholar] [CrossRef]
- Thulliez, M. Correlations between choriocapillaris flow deficits around geographic atrophy and enlargement rates based on swept-source OCT imaging. Ophthalmol. Retina 2019, 3, 478–488. [Google Scholar] [CrossRef]
- Zheng, F. Age-dependent changes in the macular choriocapillaris of normal eyes imaged with swept-source optical coherence tomography angiography. Am. J. Ophthalmol. 2019, 200, 110–122. [Google Scholar] [CrossRef]
- Shi, Y. Correlations between different choriocapillaris flow deficit parameters in normal eyes using swept source OCT angiography. Am. J. Ophthalmol. 2020, 209, 18–26. [Google Scholar] [CrossRef]
- Alagorie, A.R.; Verma, A.; Nassisi, M.; Sadda, S.R. Quantitative assessment of choriocapillaris flow deficits in eyes with advanced age-related macular degeneration versus healthy eyes. Am. J. Ophthalmol. 2019, 205, 132–139. [Google Scholar] [CrossRef]
- Chinander, M.R.; Giger, M.L.; Martell, J.M.; Jiang, C.; Favus, M.J. Computerized radiographic texture measures for characterizing bone strength: A simulated clinical setup using femoral neck specimens. Med. Phys. 1999, 26, 2295–2300. [Google Scholar] [CrossRef]
- Katsuragawa, S. Image feature analysis and computer-aided diagnosis and computer-aided diagnosis in digital radiography: Detection and characterization of interstitial lung disease in digital chest radiographs. Med. Phys. 1988, 15, 311–319. [Google Scholar] [CrossRef]
- Tourassi, G.D.; Frederick, E.D.; Floyd, C.E., Jr.; Coleman, R.E. Multifractal texture analysis of perfusion lung scans as a potential diagnostic tool for acute pulmonary embolism. Comput. Biol. Med. 2001, 31, 15–25. [Google Scholar] [CrossRef]
- Mathias, J.M.; Tofts, P.S.; Losseff, N.A. Texture analysis of spinal cord pathology in multiple sclerosis. Magn. Reson. Med. 1999, 42, 929–935. [Google Scholar] [CrossRef]
- Jirak, D.; Dezortová, M.; Taimr, P.; Hájek, M. Texture analysis of human liver. J. Magn. Reason. Imaging 2002, 15, 68–74. [Google Scholar] [CrossRef]
- Herlidou, S.; Rolland, Y.; Bansard, J.Y.; Le Rumeur, E.; De Certaines, J.D. Comparison of automated and visual texture analysis in MRI: Characterization of normal and diseased skeletal muscle. Magn. Reson. Imaging 1999, 17, 1393–1397. [Google Scholar] [CrossRef]
- Huo, Z.; Giger, M.L.; Wolverton, D.E.; Zhong, W.; Cumming, S.; Olopade, O.I. Computerized analysis of mammographic parenchymal patterns for breast cancer risk assessment: Feature selection. Med. Phys. 2000, 27, 4–12. [Google Scholar] [CrossRef]
- Kakkos, S.K. Computerized texture analysis of carotid plaque ultrasonic images can identify unstable plaques associated with ipsilateral neurological symptoms. Angiology 2011, 62, 317–328. [Google Scholar] [CrossRef]
- Chae, H.D.; Park, C.M.; Park, S.J.; Lee, S.M.; Kim, K.G.; Goo, J.M. Computerized texture analysis of persistent part-solid ground-glass nodules: Differentiation of preinvasive lesions from invasive pulmonary adenocarcinomas. Radiology 2014, 273, 285–293. [Google Scholar] [CrossRef]
- Holli-Helenius, K.; Salminen, A.; Rinta-Kiikka, I.; Koskivuo, I.; Brück, N.; Boström, P.; Parkkola, R. MRI texture analysis in differentiating luminal A and luminal B breast cancer molecular subtypes-a feasibility study. BMC Med. Imaging 2017, 17, 69. [Google Scholar] [CrossRef]
- Niu, X.K.; Chen, Z.F.; Chen, L.; Li, J.; Peng, T.; Li, X. Clinical application of biparametric MRI texture analysis for detection and evaluation of high-grade prostate cancer in zone-specific regions. Am. J. Roentgenol. 2018, 210, 549–556. [Google Scholar] [CrossRef]
- Kontos, D.; Ikejimba, L.C.; Bakic, P.R.; Troxel, A.B.; Conant, E.F.; Maidment, A.D. Analysis of parenchymal texture with digital breast tomosynthesis: Comparison with digital mammography and implications for cancer risk assessment. Radiology 2011, 261, 80–91. [Google Scholar] [CrossRef]
- Yang, X.; Tridandapani, S.; Beitler, J.J.; Yu, D.S.; Yoshida, E.J.; Curran, W.J.; Liu, T. Ultrasound GLCM texture analysis of radiation-induced parotid-gland injury in head-and-neck cancer radiotherapy: An in vivo study of late toxicity. Med. Phys. 2012, 39, 5732–5739. [Google Scholar] [CrossRef]
- Julesz, B.; Gilbert, E.N.; Shepp, L.A.; Frisch, H.L. Inability of humans to discriminate between visual textures that agree in second-order statistics—Revisited. Perception 1973, 2, 391–405. [Google Scholar] [CrossRef]
- Harlow, C.A.; Eisenbeis, S.A. The analysis of radiographic images. IEEE Trans. Comput. 1973, 100, 678–689. [Google Scholar] [CrossRef]
- Wijesinghe, R.E. Biophotonic approach for the characterization of initial bitter-rot progression on apple specimens using optical coherence tomography assessments. Sci. Rep. 2018, 8, 15816. [Google Scholar] [CrossRef]
- Li, Y. 1.7-Micron optical coherence tomography angiography for characterization of skin lesions—A feasibility study. IEEE Trans. Med. Imaging 2021, 40, 2507–2512. [Google Scholar] [CrossRef]
- Tomai, F. Optical coherence tomography for characterization of cardiac allograft vasculopathy in late survivors of pediatric heart transplantation. J. Heart Lung Transplant. 2015, 35, 74–79. [Google Scholar] [CrossRef]
- Grondin, C. Identification and characterization of epivascular glia using en face optical coherence tomography. Am. J. Ophthalmol. 2021, 229, 108–119. [Google Scholar] [CrossRef]
- Liu, Y.Y. Computerized macular pathology diagnosis in spectral domain optical coherence tomography scans based on multiscale texture and shape features. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8316–8322. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, M.; Ishikawa, H.; Wollstein, G.; Schuman, J.S.; Rehg, J.M. Automated macular pathology diagnosis in retinal OCT images using multi-scale spatial pyramid and local binary patterns in texture and shape encoding. Med. Image Anal. 2011, 15, 748–759. [Google Scholar] [CrossRef]
- Sauvola, J.; Pietikäinen, M. Adaptive document image binarization. Pattern Recog. 2000, 33, 225–236. [Google Scholar] [CrossRef]
- Laiginhas, R.; Cabral, D.; Falcão, M. Evaluation of the different thresholding strategies for quantifying choriocapillaris using optical coherence tomography angiography. Quant. Imaging Med. Surg. 2020, 10, 1994. [Google Scholar] [CrossRef]
- Spaide, R.F. Choriocapillaris flow features follow a power law distribution: Implications for characterization and mechanisms of disease progression. Am. J. Ophthalmol. 2016, 170, 58–67. [Google Scholar] [CrossRef]
- Chu, Z.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Quantification of choriocapillaris with optical coherence tomography angiography: A comparison study. Am. J. Ophthalmol. 2019, 208, 111–123. [Google Scholar] [CrossRef]
- Chu, Z. Quantification of choriocapillaris with phansalkar local thresholding: Pitfalls to avoid. Am. J. Ophthalmol. 2020, 213, 161–176. [Google Scholar] [CrossRef]
- Chu, Z. Quantifying choriocapillaris flow deficits using global and localized thresholding methods: A correlation study. Quant. Imaging Med. Surg. 2018, 8, 1102. [Google Scholar] [CrossRef]
- Lei, J. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. 2017, 135, 1092–1098. [Google Scholar] [CrossRef]
- Uji, A.; Balasubramanian, S.; Lei, J.; Baghdasaryan, E.; Al-Sheikh, M.; Sadda, S.R. Choriocapillaris imaging using multiple en face optical coherence tomography angiography image averaging. JAMA Ophthalmol. 2017, 135, 1197–1204. [Google Scholar] [CrossRef]
- Khan, H.M.; Gentle, A.; Armitage, J.A.; To, C.H.; Lam, A.K. Multiple scan averaging to yield accurate quantitative analysis of optical coherence tomography angiograms. Sci. Rep. 2020, 10, 6194. [Google Scholar] [CrossRef]
- Gendelman, I. Topographic analysis of macular choriocapillaris flow deficits in diabetic retinopathy using swept–source optical coherence tomography angiography. Int. J. Retina Vitreous 2020, 6, 6. [Google Scholar] [CrossRef]
- Sacconi, R.; Corbelli, E.; Carnevali, A.; Querques, L.; Bandello, F.; Querques, G. Optical coherence tomography angiography in geographic atrophy. Retina 2018, 38, 2350–2355. [Google Scholar] [CrossRef]
| Texture Feature | Average Gray Level 30% Threshold | Average Gray Level 70% Threshold | Balance | Skewness | Energy | Entropy | Contrast | Mean Gradient | RMS * | FMP ** |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 106.80 ± 4.8 | 130.89 ± 6.3 | −0.96 ± 0.005 | 0.30 ± 0.09 | 0.0058 ± 0.00053 | 5.28 ± 0.055 | 248,156.4 ± 23,327.2 | 0.033 ± 0.035 | 16,086,715.5 ± 447,937 | 0.0371 ± 0.001 |
| Pearson’s correlation with subfoveal choroidal thickness | −0.557 | −0.625 | −0.519 | 0.010 | 0.576 | 0.454 | −0.418 | −0.402 | −0.366 | −0.523 |
| Pearson’s correlation with 3 mm choriocapillaris flow | 0.520 | 0.556 | 0.526 | −0.442 | −0.579 | −0.480 | 0.471 | 0.599 | 0.063 | 0.509 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Movahedan, A.; Vargas, P.; Moir, J.; Kaufmann, G.; Chun, L.; Smith, C.; Massamba, N.; La Riviere, P.; Skondra, D. Computerized Texture Analysis of Optical Coherence Tomography Angiography of Choriocapillaris in Normal Eyes of Young and Healthy Subjects. Cells 2022, 11, 1934. https://doi.org/10.3390/cells11121934
Movahedan A, Vargas P, Moir J, Kaufmann G, Chun L, Smith C, Massamba N, La Riviere P, Skondra D. Computerized Texture Analysis of Optical Coherence Tomography Angiography of Choriocapillaris in Normal Eyes of Young and Healthy Subjects. Cells. 2022; 11(12):1934. https://doi.org/10.3390/cells11121934
Chicago/Turabian StyleMovahedan, Asadolah, Phillip Vargas, John Moir, Gabriel Kaufmann, Lindsay Chun, Claire Smith, Nathalie Massamba, Patrick La Riviere, and Dimitra Skondra. 2022. "Computerized Texture Analysis of Optical Coherence Tomography Angiography of Choriocapillaris in Normal Eyes of Young and Healthy Subjects" Cells 11, no. 12: 1934. https://doi.org/10.3390/cells11121934
APA StyleMovahedan, A., Vargas, P., Moir, J., Kaufmann, G., Chun, L., Smith, C., Massamba, N., La Riviere, P., & Skondra, D. (2022). Computerized Texture Analysis of Optical Coherence Tomography Angiography of Choriocapillaris in Normal Eyes of Young and Healthy Subjects. Cells, 11(12), 1934. https://doi.org/10.3390/cells11121934








