Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis
Abstract
:1. Introduction
2. Extracellular Vesicles: Current State and Emerging Concepts
3. EVs as Pathophysiological Drivers of Atherothrombosis
3.1. Atherogenesis
3.2. Atheroinflammation
3.3. Lesion Progression
3.3.1. Atherogenic Foam Cell Formation
3.3.2. Vascular Smooth Muscle Cell Proliferation, Migration, and Phenotype Switching
3.3.3. Intravascular Calcification
3.3.4. Necrotic Core
3.4. Advanced Lesion and Plaque Rupture
3.4.1. Arterial Neoangiogenesis and Intraplaque Hemorrhage
3.4.2. Thrombus Formation after Rupture
4. Extracellular Vesicles as Diagnostic and Prognostic Biomarkers
| Clinical Condition/Context | EV Subtype | Cargo | Reference |
|---|---|---|---|
| Cardiovascular risk factors | ↑ eEV (CD144+, CD105+) (Hypertension) | [267] | |
| ↑ lEV (CD3+) (Hypertension) | [235] | ||
| ↑ eEV (CD31+/42−; CD31+/AnnV+), pEV (CD31+/42+) (Diabetes) | miR-126 | [236] | |
| ↑ lEV (CD45+), nEV (CD15+), pEV (CD62P+) (Familial hypercholesterolemia [FH]) | [242] | ||
| ↑ pEV (CD42a+, CD62P+) (Obesity) | [268] | ||
| ↑ pEV (CD41+), eEV (CD62E+), lEV (CD45+) (Smoking) | CD40L | [269] | |
| Pulmonary hypertension | ↑ eEV (CD31+, CD144+, CD62E+), lEV (CD45+) | [260] | |
| Endothelial dysfunction | eEV (CD31+/CD42b−) | [255] | |
| eEV (CD31+/AnnV+) | [256] | ||
| FH and subclinical atherosclerosis | ↑ lEV (CD3+/CD45+) | [50] | |
| ↑ pEV (TSP1+/CD142+) | Tissue factor | [266] | |
| Coronary artery disease (CAD) | ↑ CD31+/Annexin V+ EV | [258] | |
| ↑ eEV (CD31+), pEV (CD42b+) | miR-126, miR-199a | [241] | |
| ↑ eEV (CD31+/AnnV+) | [256] | ||
| ↑ eEV (CD31+ and CD51+) | [238] | ||
| ↑ eEV (CD31+) | [270] | ||
| Arterial hypertension and coronary artery disease | ↑ eEV (CD31+/41−, CD62E+, CD144+) | [271] | |
| Diabetes and CAD | ↑ eEV (CD62E+, CD31+) | ↓LAP(TGF-β1), PD-ECGF, PF4, TSP1 | [233] |
| Coronary heart disease | ↑ eEV (CD31+/CD42−, CD144+) | [272] | |
| ↑ pEV (CD41+), eEV (CD144+) | [273] | ||
| ↑ eEV (CD31+ and CD146+) | [248] | ||
| Stable angina | ↑ eEV (CD31+), pEV (CD41+) | [274] | |
| Acute coronary syndrome | ↑ pEV (CD31+, CD41a+) | [275] | |
| ↑ eEV (CD146+) | [276] | ||
| ↑ eEV (CD144+), ErEV (CD235a+), pEV (CD41a+) | [261] | ||
| Myocardial infarction (MI) | ↑ CM-EV and eEV (CD31+/CD41−) | Caveolin-3, TnT | [185] |
| ↑ ErEV (CD235a+) | [207,252] | ||
| ↑ eEV (CD144+) | [277] | ||
| ↑ l/mEV, eEV, ErEV, activated vascular cell-EV, (CD66b+/62E+/142+ for coronary thrombosis) | [245] | ||
| ↑ eEV (CD154+, CD62E+), pEV (CD62P+) | [278] | ||
| ↑ eEV (CD31+), pEV (GPIbα) | [247] | ||
| ↑ eEV (CD31+, CD146+), pEV (CD42b+) | [254] | ||
| ↑ pEV (CD61+), mEV (CD14+) | Tissue factor | [244] | |
| ↑ lEV(CD45+), pEV (CD61/62P+), ErEV (CD235a+) | Lactadherin, Fbn | [279] | |
| ↑ eEV (CD144+), pEV (CD62P+/41), mEV (CD14+) | Tissue factor | [280] | |
| MI and stable angina | ↑ pEV (CD51+/61+), eEV (CD42−/31+), mEV (CD14+) | Tissue factor | [250] |
| MI in chronic kidney disease | ↑ eEV (CD154+, CD62E+), pEV (CD62P+) | [278] | |
| Ischemic cerebrovascular disease | ↑ eEV (CD144+, CD31+, CD62E+/41a−, AnnV+) | [281] | |
| ↑ eEV (CD62E+, CD31+/, CD42b− and Annexin V+) | [237] | ||
| Stroke | ↑ NPC-EV (CD34+, CD56+) | [282] | |
| ↑ pEV (CD41+) | [283] | ||
| ↑ eEV (CD62E+) | [259] | ||
| ↑ eEV (CD31+, CD144+, CD146+), lEV (CD45+) | [243] | ||
| Peripheral arterial disease | ↑ pEV (CD41+/61+) | Calprotectin | [284] |
| ↑ cEV | Sonic hedgehog | [285] | |
| ↑ eEV (CD144+) | [286] | ||
| Venous thromboembolism | ↑ eEV (CD62E+), mEV (CD14+) | PSGL-1+ | [287] |
| Atrial fibrillation and stroke | ↑ eEV (CD41−/31+), pEV (CD61+) | [288] | |
| Autoimmune chronic inflammatory diseases | ↑ eEV and pEV | [289] |
Pharmacological Modulation of Extracellular Vesicles
5. Extracellular Vesicles as Therapeutic Tools
6. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Buzas, E.I.; Vizio, D.D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lotvall, J.; Raposo, G.; Stahl, P.D.; Thery, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Suades, R.; Arderiu, G.; Pena, E.; Chiva-Blanch, G.; Padro, T. Microvesicles in Atherosclerosis and Angiogenesis: From Bench to Bedside and Reverse. Front. Cardiovasc. Med. 2017, 4, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Freitas, R.C.C.; Hirata, R.D.C.; Hirata, M.H.; Aikawa, E. Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases. Biomolecules 2021, 11, 388. [Google Scholar] [CrossRef]
- Yates, A.G.; Pink, R.C.; Erdbrugger, U.; Siljander, P.R.; Dellar, E.R.; Pantazi, P.; Akbar, N.; Cooke, W.R.; Vatish, M.; Dias-Neto, E.; et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part II: Pathology. J. Extracell Vesicles 2022, 11, e12190. [Google Scholar] [CrossRef]
- Yates, A.G.; Pink, R.C.; Erdbrugger, U.; Siljander, P.R.; Dellar, E.R.; Pantazi, P.; Akbar, N.; Cooke, W.R.; Vatish, M.; Dias-Neto, E.; et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology. J. Extracell Vesicles 2022, 11, e12151. [Google Scholar] [CrossRef]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Hunt, S.; Lambert, D.W. Extracellular vesicles and the extracellular matrix: A new paradigm or old news? Biochem. Soc. Trans. 2020, 48, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sanfrutos, J.; Munoz, J. Unraveling the complexity of the extracellular vesicle landscape with advanced proteomics. Expert Rev. Proteom. 2022, 1–13. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell Biol. 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.; Kang, H. Exosome-Based Treatment for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1002. [Google Scholar] [CrossRef]
- Paone, S.; Baxter, A.A.; Hulett, M.D.; Poon, I.K.H. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell Mol. Life Sci. 2019, 76, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [Green Version]
- Dickhout, A.; Koenen, R.R. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Palviainen, M.; Saraswat, M.; Varga, Z.; Kitka, D.; Neuvonen, M.; Puhka, M.; Joenvaara, S.; Renkonen, R.; Nieuwland, R.; Takatalo, M.; et al. Extracellular vesicles from human plasma and serum are carriers of extravesicular cargo-Implications for biomarker discovery. PLoS ONE 2020, 15, e0236439. [Google Scholar] [CrossRef] [PubMed]
- Toth, E.A.; Turiak, L.; Visnovitz, T.; Cserep, C.; Mazlo, A.; Sodar, B.W.; Forsonits, A.I.; Petovari, G.; Sebestyen, A.; Komlosi, Z.; et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J. Extracell Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Shurtleff, M.J.; Temoche-Diaz, M.M.; Karfilis, K.V.; Ri, S.; Schekman, R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife 2016, 5, e19276. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez Garcia, G.; Galicia Garcia, G.; Zalapa Soto, J.; Izquierdo Medina, A.; Rotzinger-Rodriguez, M.; Casas Aguilar, G.A.; Lopez Pacheco, C.P.; Aguayo, A.; Aguilar-Hernandez, M.M. Analysis of RNA yield in extracellular vesicles isolated by membrane affinity column and differential ultracentrifugation. PLoS ONE 2020, 15, e0238545. [Google Scholar] [CrossRef]
- Nieuwland, R.; Siljander, P.R.; Falcon-Perez, J.M.; Witwer, K.W. Reproducibility of extracellular vesicle research. Eur. J. Cell Biol. 2022, 101, 151226. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F.; et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies—From exosomes to microvesicles. Cardiovasc. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Coly, P.M.; Boulanger, C.M. Role of extracellular vesicles in atherosclerosis: An update. J. Leukoc. Biol. 2022, 111, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Killingsworth, M.C.; Orekhov, A.N. Increased shedding of microvesicles from intimal smooth muscle cells in athero-prone areas of the human aorta: Implications for understanding of the predisease stage. Pathobiology 2013, 80, 24–31. [Google Scholar] [CrossRef]
- Leroyer, A.S.; Isobe, H.; Leseche, G.; Castier, Y.; Wassef, M.; Mallat, Z.; Binder, B.R.; Tedgui, A.; Boulanger, C.M. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J. Am. Coll Cardiol. 2007, 49, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Mayr, M.; Grainger, D.; Mayr, U.; Leroyer, A.S.; Leseche, G.; Sidibe, A.; Herbin, O.; Yin, X.; Gomes, A.; Madhu, B.; et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ. Cardiovasc Genet. 2009, 2, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Canault, M.; Leroyer, A.S.; Peiretti, F.; Leseche, G.; Tedgui, A.; Bonardo, B.; Alessi, M.C.; Boulanger, C.M.; Nalbone, G. Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-alpha converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am. J. Pathol. 2007, 171, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Alexander, Y.; Osto, E.; Schmidt-Trucksass, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Back, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Amabile, N.; Cheng, S.; Renard, J.M.; Larson, M.G.; Ghorbani, A.; McCabe, E.; Griffin, G.; Guerin, C.; Ho, J.E.; Shaw, S.Y.; et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 2014, 35, 2972–2979. [Google Scholar] [CrossRef]
- Diamant, M.; Nieuwland, R.; Pablo, R.F.; Sturk, A.; Smit, J.W.; Radder, J.K. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 2002, 106, 2442–2447. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.C.; Peter, A.A.; Mendez, A.J.; Jimenez, J.J.; Mauro, L.M.; Chirinos, J.A.; Ghany, R.; Virani, S.; Garcia, S.; Horstman, L.L.; et al. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation 2004, 110, 3599–3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, C.; Amabile, N.; Lee, A.C.; Real, W.M.; Schick, S.F.; Lao, D.; Wong, M.L.; Jahn, S.; Angeli, F.S.; Minasi, P.; et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: Sustained vascular injury and blunted nitric oxide production. J. Am. Coll Cardiol. 2008, 51, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Sugiyama, S.; Kugiyama, K.; Watanabe, K.; Fukushima, H.; Tanaka, T.; Sakamoto, T.; Yoshimura, M.; Jinnouchi, H.; Ogawa, H. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Coll Cardiol. 2005, 45, 1622–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugesan, S.; Hussey, H.; Saravanakumar, L.; Sinkey, R.G.; Sturdivant, A.B.; Powell, M.F.; Berkowitz, D.E. Extracellular Vesicles From Women With Severe Preeclampsia Impair Vascular Endothelial Function. Anesth. Analg. 2022, 134, 713–723. [Google Scholar] [CrossRef]
- Nomura, S.; Shouzu, A.; Omoto, S.; Nishikawa, M.; Fukuhara, S.; Iwasaka, T. Losartan and simvastatin inhibit platelet activation in hypertensive patients. J. Thromb. Thrombolysis 2004, 18, 177–185. [Google Scholar] [CrossRef]
- Ousmaal, M.E.F.; Gaceb, A.; Khene, M.A.; Ainouz, L.; Giaimis, J.; Andriantsitohaina, R.; Martinez, M.C.; Baz, A. Circulating microparticles released during dyslipidemia may exert deleterious effects on blood vessels and endothelial function. J. Diabetes Complicat. 2020, 34, 107683. [Google Scholar] [CrossRef]
- Preston, R.A.; Jy, W.; Jimenez, J.J.; Mauro, L.M.; Horstman, L.L.; Valle, M.; Aime, G.; Ahn, Y.S. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003, 41, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Sabatier, F.; Darmon, P.; Hugel, B.; Combes, V.; Sanmarco, M.; Velut, J.G.; Arnoux, D.; Charpiot, P.; Freyssinet, J.M.; Oliver, C.; et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes 2002, 51, 2840–2845. [Google Scholar] [CrossRef] [Green Version]
- Stockelman, K.A.; Hijmans, J.G.; Bammert, T.D.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Circulating endothelial cell derived microvesicles are elevated with hypertension and associated with endothelial dysfunction. Can. J. Physiol. Pharmacol. 2020, 98, 557–561. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Alonso, R.; Lopez-Miranda, J.; Mata, P.; Badimon, L. Circulating CD45+/CD3+ lymphocyte-derived microparticles map lipid-rich atherosclerotic plaques in familial hypercholesterolaemia patients. Thromb. Haemost. 2014, 111, 111–121. [Google Scholar] [CrossRef]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; de Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014, 348, f7412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zhang, Q.; Sun, J.; Liang, Y.; Zhang, M.; Zhao, M.; Zhang, K.; Dong, C.; Ma, Q.; Liu, W.; et al. Extracellular vesicles derived from PM2.5-exposed alveolar epithelial cells mediate endothelial adhesion and atherosclerosis in ApoE(-/-) mice. FASEB J. 2022, 36, e22161. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.G.; Lee, S.H.; Abdelazim, A.M.; Saadeldin, I.M.; Abomughaid, M.M. Role of Extracellular Vesicles in Compromising Cellular Resilience to Environmental Stressors. Biomed. Res. Int 2021, 2021, 9912281. [Google Scholar] [CrossRef]
- Rota, F.; Ferrari, L.; Hoxha, M.; Favero, C.; Antonioli, R.; Pergoli, L.; Greco, M.F.; Mariani, J.; Lazzari, L.; Bollati, V. Blood-derived extracellular vesicles isolated from healthy donors exposed to air pollution modulate in vitro endothelial cells behavior. Sci. Rep. 2020, 10, 20138. [Google Scholar] [CrossRef] [PubMed]
- Agouni, A.; Lagrue-Lak-Hal, A.H.; Ducluzeau, P.H.; Mostefai, H.A.; Draunet-Busson, C.; Leftheriotis, G.; Heymes, C.; Martinez, M.C.; Andriantsitohaina, R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am. J. Pathol. 2008, 173, 1210–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulanger, C.M.; Scoazec, A.; Ebrahimian, T.; Henry, P.; Mathieu, E.; Tedgui, A.; Mallat, Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 2001, 104, 2649–2652. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Zhang, F.; Nasjletti, A.; Goligorsky, M.S. Endothelium-derived microparticles impair endothelial function in vitro. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1910–H1915. [Google Scholar] [CrossRef] [Green Version]
- Jansen, F.; Yang, X.; Franklin, B.S.; Hoelscher, M.; Schmitz, T.; Bedorf, J.; Nickenig, G.; Werner, N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc. Res. 2013, 98, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Tesse, A.; Hugel, B.; Martinez, M.C.; Morel, O.; Freyssinet, J.M.; Andriantsitohaina, R. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation 2004, 109, 1653–1659. [Google Scholar] [CrossRef] [Green Version]
- Amabile, N.; Guerin, A.P.; Leroyer, A.; Mallat, Z.; Nguyen, C.; Boddaert, J.; London, G.M.; Tedgui, A.; Boulanger, C.M. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 2005, 16, 3381–3388. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; El-Gamal, H.; Pasha, M.; Zeidan, A.; Korashy, H.M.; Abdelsalam, S.S.; Hasan, M.; Benameur, T.; Agouni, A. Endoplasmic Reticulum (ER) Stress-Generated Extracellular Vesicles (Microparticles) Self-Perpetuate ER Stress and Mediate Endothelial Cell Dysfunction Independently of Cell Survival. Front. Cardiovasc. Med. 2020, 7, 584791. [Google Scholar] [CrossRef] [PubMed]
- El Habhab, A.; Altamimy, R.; Abbas, M.; Kassem, M.; Amoura, L.; Qureshi, A.W.; El Itawi, H.; Kreutter, G.; Khemais-Benkhiat, S.; Zobairi, F.; et al. Significance of neutrophil microparticles in ischaemia-reperfusion: Pro-inflammatory effectors of endothelial senescence and vascular dysfunction. J. Cell Mol. Med. 2020, 24, 7266–7281. [Google Scholar] [CrossRef] [PubMed]
- Donadee, C.; Raat, N.J.; Kanias, T.; Tejero, J.; Lee, J.S.; Kelley, E.E.; Zhao, X.; Liu, C.; Reynolds, H.; Azarov, I.; et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 2011, 124, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Y.; Li, X.; Du, Y.; Li, L.; Hu, C.; Zhang, J.; Qin, Y.; Wei, Y.; Zhang, H. Extracellular Vesicles Derived from Intermittent Hypoxia-Treated Red Blood Cells Impair Endothelial Function Through Regulating eNOS Phosphorylation and ET-1 Expression. Cardiovasc. Drugs Ther. 2021, 35, 901–913. [Google Scholar] [CrossRef]
- Brewster, L.M.; Bain, A.R.; Garcia, V.P.; Fandl, H.K.; Stone, R.; DeSouza, N.M.; Greiner, J.J.; Tymko, M.M.; Vizcardo-Galindo, G.A.; Figueroa-Mujica, R.J.; et al. Global REACH 2018: Dysfunctional extracellular microvesicles in Andean highlander males with excessive erythrocytosis. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1851–H1861. [Google Scholar] [CrossRef]
- Taguchi, K.; Kaneko, N.; Okudaira, K.; Matsumoto, T.; Kobayashi, T. Endothelial dysfunction caused by circulating microparticles from diabetic mice is reduced by PD98059 through ERK and ICAM-1. Eur. J. Pharmacol. 2021, 913, 174630. [Google Scholar] [CrossRef]
- Abbas, M.; Jesel, L.; Auger, C.; Amoura, L.; Messas, N.; Manin, G.; Rumig, C.; Leon-Gonzalez, A.J.; Ribeiro, T.P.; Silva, G.C.; et al. Endothelial Microparticles From Acute Coronary Syndrome Patients Induce Premature Coronary Artery Endothelial Cell Aging and Thrombogenicity: Role of the Ang II/AT1 Receptor/NADPH Oxidase-Mediated Activation of MAPKs and PI3-Kinase Pathways. Circulation 2017, 135, 280–296. [Google Scholar] [CrossRef]
- Chatterjee, V.; Yang, X.; Ma, Y.; Cha, B.; Meegan, J.E.; Wu, M.; Yuan, S.Y. Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis. Cardiovasc. Res. 2020, 116, 1525–1538. [Google Scholar] [CrossRef] [Green Version]
- Densmore, J.C.; Signorino, P.R.; Ou, J.; Hatoum, O.A.; Rowe, J.J.; Shi, Y.; Kaul, S.; Jones, D.W.; Sabina, R.E.; Pritchard, K.A., Jr.; et al. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock 2006, 26, 464–471. [Google Scholar] [CrossRef]
- Huber, L.C.; Jungel, A.; Distler, J.H.; Moritz, F.; Gay, R.E.; Michel, B.A.; Pisetsky, D.S.; Gay, S.; Distler, O. The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis 2007, 12, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Abid Hussein, M.N.; Boing, A.N.; Sturk, A.; Hau, C.M.; Nieuwland, R. Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thromb. Haemost. 2007, 98, 1096–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boing, A.N.; Hau, C.M.; Sturk, A.; Nieuwland, R. Platelet microparticles contain active caspase 3. Platelets 2008, 19, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Mitra, S.; Mehta, S.; Raices, R.; Wewers, M.D. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS ONE 2009, 4, e7140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid Hussein, M.N.; Nieuwland, R.; Hau, C.M.; Evers, L.M.; Meesters, E.W.; Sturk, A. Cell-derived microparticles contain caspase 3 in vitro and in vivo. J. Thromb. Haemost. 2005, 3, 888–896. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Hoyer, F.F.; Paul, K.; Heiermann, N.; Becher, M.U.; Abu Hussein, N.; Kebschull, M.; Bedorf, J.; Franklin, B.S.; et al. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1925–1935. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, H.; Zhang, J.; Chen, X.; Zhang, Z.; Li, Q. Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov. 2021, 7, 235. [Google Scholar] [CrossRef]
- Bao, H.; Chen, Y.X.; Huang, K.; Zhuang, F.; Bao, M.; Han, Y.; Chen, X.H.; Shi, Q.; Yao, Q.P.; Qi, Y.X. Platelet-derived microparticles promote endothelial cell proliferation in hypertension via miR-142-3p. FASEB J. 2018, 32, 3912–3923. [Google Scholar] [CrossRef] [Green Version]
- Zu, L.; Ren, C.; Pan, B.; Zhou, B.; Zhou, E.; Niu, C.; Wang, X.; Zhao, M.; Gao, W.; Guo, L.; et al. Endothelial microparticles after antihypertensive and lipid-lowering therapy inhibit the adhesion of monocytes to endothelial cells. Int. J. Cardiol. 2016, 202, 756–759. [Google Scholar] [CrossRef]
- Guerrero, F.; Carmona, A.; Obrero, T.; Jimenez, M.J.; Soriano, S.; Moreno, J.A.; Martin-Malo, A.; Aljama, P. Role of endothelial microvesicles released by p-cresol on endothelial dysfunction. Sci. Rep. 2020, 10, 10657. [Google Scholar] [CrossRef]
- Sanz-Rubio, D.; Khalyfa, A.; Qiao, Z.; Ullate, J.; Marin, J.M.; Kheirandish-Gozal, L.; Gozal, D. Cell-Selective Altered Cargo Properties of Extracellular Vesicles Following In Vitro Exposures to Intermittent Hypoxia. Int. J. Mol. Sci. 2021, 22, 5604. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Du, Y.; Peng, L.; Qin, Y.; Liu, H.; Ma, X.; Wei, Y. Extracellular vesicle microRNA cargoes from intermittent hypoxia-exposed cardiomyocytes and their effect on endothelium. Biochem. Biophys. Res. Commun. 2021, 548, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, X.; Wang, Y.; Jian, D.; Li, M. Monocyte-derived extracellular vesicles upon treated by palmitate promote endothelial migration and monocytes attachment to endothelial cells. Biochem. Biophys. Res. Commun. 2020, 523, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, A.; Long, M.B.; Heath, P.R.; Wharton, S.B.; Ince, P.G.; Ridger, V.C.; Simpson, J.E. Neutrophil-Derived Microvesicle Induced Dysfunction of Brain Microvascular Endothelial Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 5227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Mesri, M.; Altieri, D.C. Endothelial cell activation by leukocyte microparticles. J. Immunol. 1998, 161, 4382–4387. [Google Scholar] [PubMed]
- Mesri, M.; Altieri, D.C. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J. Biol. Chem. 1999, 274, 23111–23118. [Google Scholar] [CrossRef] [Green Version]
- Robbins, P.D.; Dorronsoro, A.; Booker, C.N. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Investig. 2016, 126, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Burger, D.; Turner, M.; Xiao, F.; Munkonda, M.N.; Akbari, S.; Burns, K.D. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia 2017, 60, 1791–1800. [Google Scholar] [CrossRef] [Green Version]
- Barry, O.P.; Pratico, D.; Savani, R.C.; FitzGerald, G.A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Investig. 1998, 102, 136–144. [Google Scholar] [CrossRef]
- Nomura, S.; Tandon, N.N.; Nakamura, T.; Cone, J.; Fukuhara, S.; Kambayashi, J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis 2001, 158, 277–287. [Google Scholar] [CrossRef]
- Forlow, S.B.; McEver, R.P.; Nollert, M.U. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood 2000, 95, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Mause, S.F.; von Hundelshausen, P.; Zernecke, A.; Koenen, R.R.; Weber, C. Platelet microparticles: A transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1512–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasina, E.M.; Cauwenberghs, S.; Feijge, M.A.; Heemskerk, J.W.; Weber, C.; Koenen, R.R. Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis. 2011, 2, e211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimen, M.; Evryviadou, A.; Box, C.L.; Harrison, M.J.; Hazeldine, J.; Dib, L.H.; Kuravi, S.J.; Payne, H.; Price, J.M.J.; Kavanagh, D.; et al. Appropriation of GPIbalpha from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica 2020, 105, 1248–1261. [Google Scholar] [CrossRef]
- Mause, S.F.; Ritzel, E.; Liehn, E.A.; Hristov, M.; Bidzhekov, K.; Muller-Newen, G.; Soehnlein, O.; Weber, C. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation 2010, 122, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Wadey, R.M.; Connolly, K.D.; Mathew, D.; Walters, G.; Rees, D.A.; James, P.E. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis 2019, 283, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Pitanga, T.N.; de Aragao Franca, L.; Rocha, V.C.; Meirelles, T.; Borges, V.M.; Goncalves, M.S.; Pontes-de-Carvalho, L.C.; Noronha-Dutra, A.A.; dos-Santos, W.L. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol. 2014, 15, 21. [Google Scholar] [CrossRef] [Green Version]
- Rossaint, J.; Kuhne, K.; Skupski, J.; Van Aken, H.; Looney, M.R.; Hidalgo, A.; Zarbock, A. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat. Commun. 2016, 7, 13464. [Google Scholar] [CrossRef]
- Gomez, I.; Ward, B.; Souilhol, C.; Recarti, C.; Ariaans, M.; Johnston, J.; Burnett, A.; Mahmoud, M.; Luong, L.A.; West, L.; et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat. Commun. 2020, 11, 214. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Sun, B.; Gupta, A.; Rempel, H.; Pulliam, L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-kappaB in endothelial cells. FASEB J. 2016, 30, 3097–3106. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.G.; Williams, J.C.; Davis, B.K.; Jacobson, K.; Doerschuk, C.M.; Ting, J.P.; Mackman, N. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood 2011, 118, 2366–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470 e413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017, 17, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J. Clin. Investig. 2016, 126, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Soubannier, V.; Rippstein, P.; Kaufman, B.A.; Shoubridge, E.A.; McBride, H.M. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS ONE 2012, 7, e52830. [Google Scholar] [CrossRef] [Green Version]
- Puhm, F.; Afonyushkin, T.; Resch, U.; Obermayer, G.; Rohde, M.; Penz, T.; Schuster, M.; Wagner, G.; Rendeiro, A.F.; Melki, I.; et al. Mitochondria Are a Subset of Extracellular Vesicles Released by Activated Monocytes and Induce Type I IFN and TNF Responses in Endothelial Cells. Circ. Res. 2019, 125, 43–52. [Google Scholar] [CrossRef]
- Holvoet, P.; Vanhaverbeke, M.; Bloch, K.; Baatsen, P.; Sinnaeve, P.; Janssens, S. Low MT-CO1 in Monocytes and Microvesicles Is Associated With Outcome in Patients With Coronary Artery Disease. J. Am. Heart Assoc. 2016, 5, e004207. [Google Scholar] [CrossRef]
- Boudreau, L.H.; Duchez, A.C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Pare, A.; Rousseau, M.; Naika, G.S.; Levesque, T.; et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014, 124, 2173–2183. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Wu, C.; Xiao, J.; Li, D.; Sun, Z.; Li, M. Endothelial extracellular vesicles modulate the macrophage phenotype: Potential implications in atherosclerosis. Scand. J. Immunol. 2018, 87, e12648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, Q.; Hosen, M.R.; Zietzer, A.; Flender, A.; Levermann, P.; Schmitz, T.; Fruhwald, D.; Goody, P.; Nickenig, G.; et al. Atherosclerotic Conditions Promote the Packaging of Functional MicroRNA-92a-3p Into Endothelial Microvesicles. Circ. Res. 2019, 124, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Walker, S.A.; Aguilar Diaz de Leon, J.S.; Davidovich, I.; Broad, K.; Talmon, Y.; Borges, C.R.; Wolfram, J. Extracellular vesicle glucose transporter-1 and glycan features in monocyte-endothelial inflammatory interactions. Nanomedicine 2022, 42, 102515. [Google Scholar] [CrossRef]
- Perez-Casal, M.; Downey, C.; Cutillas-Moreno, B.; Zuzel, M.; Fukudome, K.; Toh, C.H. Microparticle-associated endothelial protein C receptor and the induction of cytoprotective and anti-inflammatory effects. Haematologica 2009, 94, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Luquero, A.; Vilahur, G.; Crespo, J.; Badimon, L.; Borrell-Pages, M. Microvesicles carrying LRP5 induce macrophage polarization to an anti-inflammatory phenotype. J. Cell Mol. Med. 2021, 25, 7935–7947. [Google Scholar] [CrossRef] [PubMed]
- Hergenreider, E.; Heydt, S.; Treguer, K.; Boettger, T.; Horrevoets, A.J.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Gasser, O.; Schifferli, J.A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004, 104, 2543–2548. [Google Scholar] [CrossRef]
- Dalli, J.; Norling, L.V.; Renshaw, D.; Cooper, D.; Leung, K.Y.; Perretti, M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 2008, 112, 2512–2519. [Google Scholar] [CrossRef] [Green Version]
- Jansen, F.; Yang, X.; Baumann, K.; Przybilla, D.; Schmitz, T.; Flender, A.; Paul, K.; Alhusseiny, A.; Nickenig, G.; Werner, N. Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism. J. Cell Mol. Med. 2015, 19, 2202–2214. [Google Scholar] [CrossRef]
- Njock, M.S.; Cheng, H.S.; Dang, L.T.; Nazari-Jahantigh, M.; Lau, A.C.; Boudreau, E.; Roufaiel, M.; Cybulsky, M.I.; Schober, A.; Fish, J.E. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood 2015, 125, 3202–3212. [Google Scholar] [CrossRef] [Green Version]
- Koppler, B.; Cohen, C.; Schlondorff, D.; Mack, M. Differential mechanisms of microparticle transfer toB cells and monocytes: Anti-inflammatory propertiesof microparticles. Eur. J. Immunol. 2006, 36, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, B.; Kuypers, S.; van den Akker, N.M.S.; Molin, D.G.M.; Michiels, L. Extracellular Vesicles Work as a Functional Inflammatory Mediator Between Vascular Endothelial Cells and Immune Cells. Front. Immunol. 2018, 9, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseinkhani, B.; van den Akker, N.M.S.; Molin, D.G.M.; Michiels, L. (Sub)populations of extracellular vesicles released by TNF-alpha -triggered human endothelial cells promote vascular inflammation and monocyte migration. J. Extracell Vesicles 2020, 9, 1801153. [Google Scholar] [CrossRef] [PubMed]
- Edrissi, H.; Schock, S.C.; Hakim, A.M.; Thompson, C.S. Microparticles generated during chronic cerebral ischemia increase the permeability of microvascular endothelial barriers in vitro. Brain Res. 2016, 1634, 83–93. [Google Scholar] [CrossRef]
- Marcos-Ramiro, B.; Oliva Nacarino, P.; Serrano-Pertierra, E.; Blanco-Gelaz, M.A.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Tunon, A.; Lopez-Larrea, C.; Millan, J.; et al. Microparticles in multiple sclerosis and clinically isolated syndrome: Effect on endothelial barrier function. BMC Neurosci. 2014, 15, 110. [Google Scholar] [CrossRef] [Green Version]
- Lapping-Carr, G.; Gemel, J.; Mao, Y.; Sparks, G.; Harrington, M.; Peddinti, R.; Beyer, E.C. Insights image for “Circulating extracellular vesicles from patients with acute chest syndrome disrupt adherens junctions between endothelial cells”. Pediatr. Res. 2021, 89, 1036. [Google Scholar] [CrossRef]
- Huber, J.; Vales, A.; Mitulovic, G.; Blumer, M.; Schmid, R.; Witztum, J.L.; Binder, B.R.; Leitinger, N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Fink, K.; Moebes, M.; Vetter, C.; Bourgeois, N.; Schmid, B.; Bode, C.; Helbing, T.; Busch, H.J. Selenium prevents microparticle-induced endothelial inflammation in patients after cardiopulmonary resuscitation. Crit. Care 2015, 19, 58. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Han, B.; Wang, Y.; Wang, C.; Zhang, H.; Xue, J.; Wang, X.; Niu, T.; Niu, Z.; Chen, Y. Mesenchymal stem cell-secreted extracellular vesicles carrying TGF-beta1 up-regulate miR-132 and promote mouse M2 macrophage polarization. J. Cell Mol. Med. 2020, 24, 12750–12764. [Google Scholar] [CrossRef]
- Rautou, P.E.; Leroyer, A.S.; Ramkhelawon, B.; Devue, C.; Duflaut, D.; Vion, A.C.; Nalbone, G.; Castier, Y.; Leseche, G.; Lehoux, S.; et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ. Res. 2011, 108, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rodriguez, M.B.; Tellez, E.; Casulleras, M.; Borras, F.E.; Arroyo, V.; Claria, J.; Sarrias, M.R. Reduced Plasma Extracellular Vesicle CD5L Content in Patients With Acute-On-Chronic Liver Failure: Interplay With Specialized Pro-Resolving Lipid Mediators. Front. Immunol. 2022, 13, 842996. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, S.; Oggero, S.; Bruen, R.; McCarthy, C.; Strowitzki, M.J.; Mahon, N.G.; Ryan, N.; Brennan, E.P.; Barry, M.; Perretti, M.; et al. microRNA-155 Is Decreased During Atherosclerosis Regression and Is Increased in Urinary Extracellular Vesicles During Atherosclerosis Progression. Front. Immunol. 2020, 11, 576516. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Karunakaran, D.; Geoffrion, M.; Cheng, H.S.; Tandoc, K.; Perisic Matic, L.; Hedin, U.; Maegdefessel, L.; Fish, J.E.; Rayner, K.J. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.L.; Scalia, R.; Mehta, J.L.; Williams, K.J. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: Mechanisms of formation, action, and detoxification. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2113–2121. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, F.F.; Giesen, M.K.; Nunes Franca, C.; Lutjohann, D.; Nickenig, G.; Werner, N. Monocytic microparticles promote atherogenesis by modulating inflammatory cells in mice. J. Cell Mol. Med. 2012, 16, 2777–2788. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.J.; Li, Y.S.; Wu, C.C.; Wang, K.C.; Huang, T.C.; Chen, Z.; Chien, S. Extracellular MicroRNA-92a Mediates Endothelial Cell-Macrophage Communication. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2492–2504. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Q.; Fan, M.; Guo, J.; Liu, Y.; Ji, T.; Zhu, J.; Zhao, X. Platelet-derived microparticles promote phagocytosis of oxidized low-density lipoprotein by macrophages, potentially enhancing foam cell formation. Ann. Transl. Med. 2019, 7, 477. [Google Scholar] [CrossRef]
- Distler, J.H.; Huber, L.C.; Hueber, A.J.; Reich, C.F., 3rd; Gay, S.; Distler, O.; Pisetsky, D.S. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis 2005, 10, 731–741. [Google Scholar] [CrossRef]
- Pizzirani, C.; Ferrari, D.; Chiozzi, P.; Adinolfi, E.; Sandona, D.; Savaglio, E.; Di Virgilio, F. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood 2007, 109, 3856–3864. [Google Scholar] [CrossRef] [Green Version]
- Tsiantoulas, D.; Perkmann, T.; Afonyushkin, T.; Mangold, A.; Prohaska, T.A.; Papac-Milicevic, N.; Millischer, V.; Bartel, C.; Horkko, S.; Boulanger, C.M.; et al. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 2015, 56, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermayer, G.; Afonyushkin, T.; Goderle, L.; Puhm, F.; Schrottmaier, W.; Taqi, S.; Schwameis, M.; Ay, C.; Pabinger, I.; Jilma, B.; et al. Natural IgM antibodies inhibit microvesicle-driven coagulation and thrombosis. Blood 2021, 137, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Vajen, T.; Benedikter, B.J.; Heinzmann, A.C.A.; Vasina, E.M.; Henskens, Y.; Parsons, M.; Maguire, P.B.; Stassen, F.R.; Heemskerk, J.W.M.; Schurgers, L.J.; et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J. Extracell Vesicles 2017, 6, 1322454. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, X.; Zhao, M.; Cai, T.; Liu, P.; Li, J.; Willard, B.; Zu, L.; Zhou, E.; Li, Y.; et al. Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J. Am. Heart Assoc. 2016, 5, e004099. [Google Scholar] [CrossRef] [Green Version]
- Pakala, R.; Rha, S.W.; Kuchulakanti, P.K.; Cheneau, E.; Baffour, R.; Waksman, R. Peroxisome proliferator-activated receptor gamma; Its role in atherosclerosis and restenosis. Cardiovasc. Radiat. Med. 2004, 5, 44–48. [Google Scholar] [CrossRef]
- Weber, A.A.; Schror, K. The significance of platelet-derived growth factors for proliferation of vascular smooth muscle cells. Platelets 1999, 10, 77–96. [Google Scholar] [CrossRef]
- Li, S.S.; Gao, S.; Chen, Y.; Bao, H.; Li, Z.T.; Yao, Q.P.; Liu, J.T.; Wang, Y.; Qi, Y.X. Platelet-derived microvesicles induce calcium oscillations and promote VSMC migration via TRPV4. Theranostics 2021, 11, 2410–2423. [Google Scholar] [CrossRef]
- He, C.; Hu, X.; Weston, T.A.; Jung, R.S.; Sandhu, J.; Huang, S.; Heizer, P.; Kim, J.; Ellison, R.; Xu, J.; et al. Macrophages release plasma membrane-derived particles rich in accessible cholesterol. Proc. Natl. Acad. Sci. USA 2018, 115, E8499–E8508. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Dong, Y.; Wang, H. microRNA-19b-3p-containing extracellular vesicles derived from macrophages promote the development of atherosclerosis by targeting JAZF1. J. Cell Mol. Med. 2022, 26, 48–59. [Google Scholar] [CrossRef]
- Brousseau, C.; Morissette, G.; Fortin, J.P.; Marceau, F.; Petitclerc, E. Tumor cells expressing tissue factor influence the migration of smooth muscle cells in a catalytic activity-dependent way. Can. J. Physiol. Pharmacol. 2009, 87, 694–701. [Google Scholar] [CrossRef]
- Paudel, K.R.; Oak, M.H.; Kim, D.W. Smooth Muscle Cell Derived Microparticles Acts as Autocrine Activation of Smooth Muscle Cell Proliferation by Mitogen Associated Protein Kinase Upregulation. J. Nanosci. Nanotechnol. 2020, 20, 5746–5750. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, L.; Vidal-Gomez, X.; Soleti, R.; Vergori, L.; Duluc, L.; Chwastyniak, M.; Bisserier, M.; Le Lay, S.; Villard, A.; Simard, G.; et al. Large Extracellular Vesicle-Associated Rap1 Accumulates in Atherosclerotic Plaques, Correlates With Vascular Risks and Is Involved in Atherosclerosis. Circ. Res. 2020, 127, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.F.; Wang, Z.M.; Chen, A.Q.; Wang, F.; Luo, S.; Gu, Y.; Kong, X.Q.; Zuo, G.F.; Jiang, X.M.; Ding, G.W.; et al. Plasma Small Extracellular Vesicle-Carried miRNA-501-5p Promotes Vascular Smooth Muscle Cell Phenotypic Modulation-Mediated In-Stent Restenosis. Oxid. Med. Cell Longev. 2021, 2021, 6644970. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Qin, S.; Li, W.; Wu, W.; Yang, J.; Chu, M.; Li, X.; Huo, Y.; Schaer, G.L.; Wang, S.; et al. An Endocrine Genetic Signal Between Blood Cells and Vascular Smooth Muscle Cells: Role of MicroRNA-223 in Smooth Muscle Function and Atherogenesis. J. Am. Coll Cardiol. 2015, 65, 2526–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, Z.; Wang, X.; Zheng, J.; Peng, L.; Zhou, Y.; Song, Y.; Lu, Z. Extracellular-vesicle containing miRNA-503-5p released by macrophages contributes to atherosclerosis. Aging 2021, 13, 12239–12257. [Google Scholar] [CrossRef]
- Liu, R.; Shen, H.; Ma, J.; Sun, L.; Wei, M. Extracellular Vesicles Derived from Adipose Mesenchymal Stem Cells Regulate the Phenotype of Smooth Muscle Cells to Limit Intimal Hyperplasia. Cardiovasc. Drugs Ther. 2016, 30, 111–118. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Borras, C.; Alique, M. The Contribution of Extracellular Vesicles From Senescent Endothelial and Vascular Smooth Muscle Cells to Vascular Calcification. Front. Cardiovasc. Med. 2022, 9, 854726. [Google Scholar] [CrossRef]
- Alique, M.; Ruiz-Torres, M.P.; Bodega, G.; Noci, M.V.; Troyano, N.; Bohorquez, L.; Luna, C.; Luque, R.; Carmona, A.; Carracedo, J.; et al. Microvesicles from the plasma of elderly subjects and from senescent endothelial cells promote vascular calcification. Aging 2017, 9, 778–789. [Google Scholar] [CrossRef] [Green Version]
- Schurgers, L.J.; Akbulut, A.C.; Kaczor, D.M.; Halder, M.; Koenen, R.R.; Kramann, R. Initiation and Propagation of Vascular Calcification Is Regulated by a Concert of Platelet- and Smooth Muscle Cell-Derived Extracellular Vesicles. Front. Cardiovasc. Med. 2018, 5, 36. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef] [Green Version]
- New, S.E.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 2013, 113, 72–77. [Google Scholar] [CrossRef] [PubMed]
- New, S.E.; Aikawa, E. Role of extracellular vesicles in de novo mineralization: An additional novel mechanism of cardiovascular calcification. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1753–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867. [Google Scholar] [CrossRef] [Green Version]
- Buendia, P.; Montes de Oca, A.; Madueno, J.A.; Merino, A.; Martin-Malo, A.; Aljama, P.; Ramirez, R.; Rodriguez, M.; Carracedo, J. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J. 2015, 29, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Bodega, G.; Corchete, E.; Garcia-Menendez, E.; de Sequera, P.; Luque, R.; Rodriguez-Padron, D.; Marques, M.; Portoles, J.; Carracedo, J.; et al. Microvesicles from indoxyl sulfate-treated endothelial cells induce vascular calcification in vitro. Comput. Struct. Biotechnol. J. 2020, 18, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Hutcheson, J.D.; Aikawa, M.; Iwata, H.; Pham, T.; Nykjaer, A.; Kjolby, M.; Rogers, M.; Michel, T.; Shibasaki, M.; et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J. Clin. Investig. 2016, 126, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Freise, C.; Querfeld, U.; Ludwig, A.; Hamm, B.; Schnorr, J.; Taupitz, M. Uraemic extracellular vesicles augment osteogenic transdifferentiation of vascular smooth muscle cells via enhanced AKT signalling and PiT-1 expression. J. Cell Mol. Med. 2021, 25, 5602–5614. [Google Scholar] [CrossRef]
- Furmanik, M.; Chatrou, M.; van Gorp, R.; Akbulut, A.; Willems, B.; Schmidt, H.; van Eys, G.; Bochaton-Piallat, M.L.; Proudfoot, D.; Biessen, E.; et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020, 127, 911–927. [Google Scholar] [CrossRef]
- Mallat, Z.; Hugel, B.; Ohan, J.; Leseche, G.; Freyssinet, J.M.; Tedgui, A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: A role for apoptosis in plaque thrombogenicity. Circulation 1999, 99, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Lawler, P.R.; Bhatt, D.L.; Godoy, L.C.; Luscher, T.F.; Bonow, R.O.; Verma, S.; Ridker, P.M. Targeting cardiovascular inflammation: Next steps in clinical translation. Eur. Heart J. 2021, 42, 113–131. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, J.B.; Virmani, R.; Arbustini, E.; Pasterkamp, G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur. Heart J. 2011, 32, 1977–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleti, R.; Benameur, T.; Porro, C.; Panaro, M.A.; Andriantsitohaina, R.; Martinez, M.C. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 2009, 30, 580–588. [Google Scholar] [CrossRef]
- Mezentsev, A.; Merks, R.M.; O’Riordan, E.; Chen, J.; Mendelev, N.; Goligorsky, M.S.; Brodsky, S.V. Endothelial microparticles affect angiogenesis in vitro: Role of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1106–H1114. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007, 110, 2440–2448. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Niida, S.; Azuma, E.; Yanagibashi, T.; Muramatsu, M.; Huang, T.T.; Sagara, H.; Higaki, S.; Ikutani, M.; Nagai, Y.; et al. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci. Rep. 2015, 5, 8505. [Google Scholar] [CrossRef]
- Akbar, N.; Digby, J.E.; Cahill, T.J.; Tavare, A.N.; Corbin, A.L.; Saluja, S.; Dawkins, S.; Edgar, L.; Rawlings, N.; Ziberna, K.; et al. Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight 2017, 2, e93344. [Google Scholar] [CrossRef] [Green Version]
- Arderiu, G.; Pena, E.; Badimon, L. Ischemic tissue released microvesicles induce monocyte reprogramming and increase tissue repair by a tissue factor-dependent mechanism. Cardiovasc. Res. 2021. [Google Scholar] [CrossRef]
- Arderiu, G.; Pena, E.; Civit-Urgell, A.; Badimon, L. Endothelium-Released Microvesicles Transport miR-126 That Induces Proangiogenic Reprogramming in Monocytes. Front. Immunol. 2022, 13, 836662. [Google Scholar] [CrossRef]
- Arderiu, G.; Pena, E.; Badimon, L. Angiogenic microvascular endothelial cells release microparticles rich in tissue factor that promotes postischemic collateral vessel formation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 348–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroyer, A.S.; Rautou, P.E.; Silvestre, J.S.; Castier, Y.; Leseche, G.; Devue, C.; Duriez, M.; Brandes, R.P.; Lutgens, E.; Tedgui, A.; et al. CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J. Am. Coll Cardiol. 2008, 52, 1302–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroyer, A.S.; Ebrahimian, T.G.; Cochain, C.; Recalde, A.; Blanc-Brude, O.; Mees, B.; Vilar, J.; Tedgui, A.; Levy, B.I.; Chimini, G.; et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation 2009, 119, 2808–2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, R.; Sabatier, F.; Mialhe, A.; Basire, A.; Pannell, R.; Borghi, H.; Robert, S.; Lamy, E.; Plawinski, L.; Camoin-Jau, L.; et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: A mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 2007, 110, 2432–2439. [Google Scholar] [CrossRef]
- Loyer, X.; Zlatanova, I.; Devue, C.; Yin, M.; Howangyin, K.Y.; Klaihmon, P.; Guerin, C.L.; Kheloufi, M.; Vilar, J.; Zannis, K.; et al. Intra-Cardiac Release of Extracellular Vesicles Shapes Inflammation Following Myocardial Infarction. Circ. Res. 2018, 123, 100–106. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, T.M.; Laundos, T.L.; Pereira-Carvalho, R.; Batista-Almeida, D.; Pereira, R.; Coelho-Santos, V.; Silva, A.P.; Fernandes, R.; Zuzarte, M.; Enguita, F.J.; et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc. Res. 2017, 113, 1338–1350. [Google Scholar] [CrossRef] [Green Version]
- Bobryshev, Y.V.; Killingsworth, M.C.; Lord, R.S.; Grabs, A.J. Matrix vesicles in the fibrous cap of atherosclerotic plaque: Possible contribution to plaque rupture. J. Cell Mol. Med. 2008, 12, 2073–2082. [Google Scholar] [CrossRef] [Green Version]
- Lozito, T.P.; Tuan, R.S. Endothelial cell microparticles act as centers of matrix metalloproteinsase-2 (MMP-2) activation and vascular matrix remodeling. J. Cell Physiol. 2012, 227, 534–549. [Google Scholar] [CrossRef] [Green Version]
- Martinez de Lizarrondo, S.; Roncal, C.; Calvayrac, O.; Rodriguez, C.; Varo, N.; Purroy, A.; Lorente, L.; Rodriguez, J.A.; Doeuvre, L.; Hervas-Stubbs, S.; et al. Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1477–1487. [Google Scholar] [CrossRef] [Green Version]
- Taraboletti, G.; D’Ascenzo, S.; Borsotti, P.; Giavazzi, R.; Pavan, A.; Dolo, V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 2002, 160, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Li, C.J.; Liu, Y.; Chen, Y.; Yu, D.; Williams, K.J.; Liu, M.L. Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am. J. Pathol. 2013, 182, 1552–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkesson, M.; Li, C.; Frebelius, S.; Swedenborg, J.; Wagsater, D.; Williams, K.J.; Eriksson, P.; Roy, J.; Liu, M.L. Proteolytically active ADAM10 and ADAM17 carried on membrane microvesicles in human abdominal aortic aneurysms. Thromb. Haemost. 2015, 114, 1165–1174. [Google Scholar] [CrossRef]
- Gasser, O.; Hess, C.; Miot, S.; Deon, C.; Sanchez, J.C.; Schifferli, J.A. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003, 285, 243–257. [Google Scholar] [CrossRef]
- Falati, S.; Gross, P.; Merrill-Skoloff, G.; Furie, B.C.; Furie, B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 2002, 8, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Oggero, S.; de Gaetano, M.; Marcone, S.; Fitzsimons, S.; Pinto, A.L.; Ikramova, D.; Barry, M.; Burke, D.; Montero-Melendez, T.; Cooper, D.; et al. Extracellular vesicles from monocyte/platelet aggregates modulate human atherosclerotic plaque reactivity. J. Extracell Vesicles 2021, 10, 12084. [Google Scholar] [CrossRef]
- Bohm, J.K.; Schafer, N.; Maegele, M.; Stumpges, B.; Bauerfeind, U.; Caspers, M. Plasmatic and cell-based enhancement by microparticles originated from platelets and endothelial cells under simulated in vitro conditions of a dilutional coagulopathy. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 38. [Google Scholar] [CrossRef]
- Aharon, A.; Tamari, T.; Brenner, B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb. Haemost. 2008, 100, 878–885. [Google Scholar] [CrossRef]
- Lacroix, R.; Plawinski, L.; Robert, S.; Doeuvre, L.; Sabatier, F.; Martinez de Lizarrondo, S.; Mezzapesa, A.; Anfosso, F.; Leroyer, A.S.; Poullin, P.; et al. Leukocyte- and endothelial-derived microparticles: A circulating source for fibrinolysis. Haematologica 2012, 97, 1864–1872. [Google Scholar] [CrossRef]
- Steppich, B.; Mattisek, C.; Sobczyk, D.; Kastrati, A.; Schomig, A.; Ott, I. Tissue factor pathway inhibitor on circulating microparticles in acute myocardial infarction. Thromb. Haemost. 2005, 93, 35–39. [Google Scholar] [CrossRef]
- Biro, E.; Sturk-Maquelin, K.N.; Vogel, G.M.; Meuleman, D.G.; Smit, M.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J. Thromb. Haemost. 2003, 1, 2561–2568. [Google Scholar] [CrossRef] [Green Version]
- Van Der Meijden, P.E.; Van Schilfgaarde, M.; Van Oerle, R.; Renne, T.; ten Cate, H.; Spronk, H.M. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 2012, 10, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Mackman, N.; Merrill-Skoloff, G.; Pedersen, B.; Furie, B.C.; Furie, B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood 2004, 104, 3190–3197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugel, B.; Socie, G.; Vu, T.; Toti, F.; Gluckman, E.; Freyssinet, J.M.; Scrobohaci, M.L. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood 1999, 93, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Van Beers, E.J.; Schaap, M.C.; Berckmans, R.J.; Nieuwland, R.; Sturk, A.; van Doormaal, F.F.; Meijers, J.C.; Biemond, B.J.; group, C.s. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica 2009, 94, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Suades, R.; Padro, T.; Vilahur, G.; Badimon, L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb. Haemost. 2012, 108, 1208–1219. [Google Scholar] [CrossRef]
- Tripisciano, C.; Weiss, R.; Eichhorn, T.; Spittler, A.; Heuser, T.; Fischer, M.B.; Weber, V. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci. Rep. 2017, 7, 6522. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Vilahur, G.; Martin-Yuste, V.; Sabate, M.; Sans-Rosello, J.; Sionis, A.; Badimon, L. Growing thrombi release increased levels of CD235a(+) microparticles and decreased levels of activated platelet-derived microparticles. Validation in ST-elevation myocardial infarction patients. J. Thromb. Haemost. 2015, 13, 1776–1786. [Google Scholar] [CrossRef]
- Muller, I.; Klocke, A.; Alex, M.; Kotzsch, M.; Luther, T.; Morgenstern, E.; Zieseniss, S.; Zahler, S.; Preissner, K.; Engelmann, B. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003, 17, 476–478. [Google Scholar] [CrossRef] [Green Version]
- Geddings, J.E.; Hisada, Y.; Boulaftali, Y.; Getz, T.M.; Whelihan, M.; Fuentes, R.; Dee, R.; Cooley, B.C.; Key, N.S.; Wolberg, A.S.; et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J. Thromb. Haemost. 2016, 14, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Zwicker, J.I.; Trenor, C.C., 3rd; Furie, B.C.; Furie, B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Scholz, T.; Temmler, U.; Krause, S.; Heptinstall, S.; Losche, W. Transfer of tissue factor from platelets to monocytes: Role of platelet-derived microvesicles and CD62P. Thromb. Haemost. 2002, 88, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Collier, M.E.W.; Ettelaie, C.; Goult, B.T.; Maraveyas, A.; Goodall, A.H. Investigation of the Filamin A-Dependent Mechanisms of Tissue Factor Incorporation into Microvesicles. Thromb. Haemost. 2017, 117, 2034–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojkovic, S.; Thulin, A.; Hell, L.; Thaler, B.; Rauscher, S.; Baumgartner, J.; Groger, M.; Ay, C.; Demyanets, S.; Neumayer, C.; et al. IL-33 stimulates the release of procoagulant microvesicles from human monocytes and differentially increases tissue factor in human monocyte subsets. Thromb. Haemost. 2017, 117, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, F.; Roux, V.; Anfosso, F.; Camoin, L.; Sampol, J.; Dignat-George, F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 2002, 99, 3962–3970. [Google Scholar] [CrossRef]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; Lopez, J.A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef]
- Lopez-Vilchez, I.; Escolar, G.; Diaz-Ricart, M.; Fuste, B.; Galan, A.M.; White, J.G. Tissue factor-enriched vesicles are taken up by platelets and induce platelet aggregation in the presence of factor VIIa. Thromb. Haemost. 2007, 97, 202–211. [Google Scholar] [CrossRef]
- Gross, P.L.; Furie, B.C.; Merrill-Skoloff, G.; Chou, J.; Furie, B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. J. Leukoc. Biol. 2005, 78, 1318–1326. [Google Scholar] [CrossRef]
- Sinauridze, E.I.; Kireev, D.A.; Popenko, N.Y.; Pichugin, A.V.; Panteleev, M.A.; Krymskaya, O.V.; Ataullakhanov, F.I. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb. Haemost. 2007, 97, 425–434. [Google Scholar]
- Berckmans, R.J.; Nieuwland, R.; Boing, A.N.; Romijn, F.P.; Hack, C.E.; Sturk, A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 2001, 85, 639–646. [Google Scholar]
- Zhao, L.; Bi, Y.; Kou, J.; Shi, J.; Piao, D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J. Exp. Clin. Cancer Res. 2016, 35, 54. [Google Scholar] [CrossRef] [Green Version]
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 2003, 197, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Keuren, J.F.; Magdeleyns, E.J.; Bennaghmouch, A.; Bevers, E.M.; Curvers, J.; Lindhout, T. Microparticles adhere to collagen type I, fibrinogen, von Willebrand factor and surface immobilised platelets at physiological shear rates. Br. J. Haematol. 2007, 138, 527–533. [Google Scholar] [CrossRef]
- Siljander, P.; Carpen, O.; Lassila, R. Platelet-derived microparticles associate with fibrin during thrombosis. Blood 1996, 87, 4651–4663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merten, M.; Pakala, R.; Thiagarajan, P.; Benedict, C.R. Platelet microparticles promote platelet interaction with subendothelial matrix in a glycoprotein IIb/IIIa-dependent mechanism. Circulation 1999, 99, 2577–2582. [Google Scholar] [CrossRef] [Green Version]
- Raturi, A.; Miersch, S.; Hudson, J.W.; Mutus, B. Platelet microparticle-associated protein disulfide isomerase promotes platelet aggregation and inactivates insulin. Biochim. Biophys. Acta 2008, 1778, 2790–2796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, P.J.; Faioni, E.M.; Wiedmer, T.; Shattil, S.J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 1988, 263, 18205–18212. [Google Scholar] [CrossRef]
- Nomura, S.; Komiyama, Y.; Murakami, T.; Funatsu, A.; Kokawa, T.; Sugo, T.; Matsuda, M.; Yasunaga, K. Flow cytometric analysis of surface membrane proteins on activated platelets and platelet-derived microparticles from healthy and thrombasthenic individuals. Int. J. Hematol. 1993, 58, 203–212. [Google Scholar]
- Barry, O.P.; Pratico, D.; Lawson, J.A.; FitzGerald, G.A. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Investig. 1997, 99, 2118–2127. [Google Scholar] [CrossRef] [Green Version]
- Qu, M.; Zou, X.; Fang, F.; Wang, S.; Xu, L.; Zeng, Q.; Fan, Z.; Chen, L.; Yue, W.; Xie, X.; et al. Platelet-derived microparticles enhance megakaryocyte differentiation and platelet generation via miR-1915-3p. Nat. Commun. 2020, 11, 4964. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Vilahur, G.; Badimon, L. Platelet-released extracellular vesicles: The effects of thrombin activation. Cell Mol. Life Sci. 2022, 79, 190. [Google Scholar] [CrossRef]
- Banfi, C.; Brioschi, M.; Wait, R.; Begum, S.; Gianazza, E.; Pirillo, A.; Mussoni, L.; Tremoli, E. Proteome of endothelial cell-derived procoagulant microparticles. Proteomics 2005, 5, 4443–4455. [Google Scholar] [CrossRef] [PubMed]
- Landers-Ramos, R.Q.; Addison, O.A.; Beamer, B.; Katzel, L.I.; Blumenthal, J.B.; Robinson, S.; Hagberg, J.M.; Prior, S.J. Circulating microparticle concentrations across acute and chronic cardiovascular disease conditions. Physiol. Rep. 2020, 8, e14534. [Google Scholar] [CrossRef]
- Marei, I.; Chidiac, O.; Thomas, B.; Pasquier, J.; Dargham, S.; Robay, A.; Vakayil, M.; Jameesh, M.; Triggle, C.; Rafii, A.; et al. Angiogenic content of microparticles in patients with diabetes and coronary artery disease predicts networks of endothelial dysfunction. Cardiovasc. Diabetol. 2022, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.M.; Liu, M.L. Microvesicles and diabetic complications—Novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacol. Sin. 2014, 35, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Salvia, S.; Musante, L.; Lannigan, J.; Gigliotti, J.C.; Le, T.H.; Erdbrugger, U. T cell-derived extracellular vesicles are elevated in essential HTN. Am. J. Physiol. Renal Physiol. 2020, 319, F868–F875. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, N.; Badila, E.; Weiss, E.; Cochior, D.; Stepien, E.; Georgescu, A. Vascular complications in diabetes: Microparticles and microparticle associated microRNAs as active players. Biochem. Biophys. Res. Commun. 2016, 472, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Chu, K.; Lee, S.T.; Park, H.K.; Bahn, J.J.; Kim, D.H.; Kim, J.H.; Kim, M.; Kun Lee, S.; Roh, J.K. Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann. Neurol. 2009, 66, 191–199. [Google Scholar] [CrossRef]
- Bernal-Mizrachi, L.; Jy, W.; Jimenez, J.J.; Pastor, J.; Mauro, L.M.; Horstman, L.L.; de Marchena, E.; Ahn, Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 2003, 145, 962–970. [Google Scholar] [CrossRef]
- Montoro-Garcia, S.; Shantsila, E.; Tapp, L.D.; Lopez-Cuenca, A.; Romero, A.I.; Hernandez-Romero, D.; Orenes-Pinero, E.; Manzano-Fernandez, S.; Valdes, M.; Marin, F.; et al. Small-size circulating microparticles in acute coronary syndromes: Relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis 2013, 227, 313–322. [Google Scholar] [CrossRef]
- Nozaki, T.; Sugiyama, S.; Koga, H.; Sugamura, K.; Ohba, K.; Matsuzawa, Y.; Sumida, H.; Matsui, K.; Jinnouchi, H.; Ogawa, H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J. Am. Coll Cardiol. 2009, 54, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suades, R.; Padro, T.; Crespo, J.; Sionis, A.; Alonso, R.; Mata, P.; Badimon, L. Liquid Biopsy of Extracellular Microvesicles Predicts Future Major Ischemic Events in Genetically Characterized Familial Hypercholesterolemia Patients. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1172–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, S.; Krankel, N.; Nave, A.H.; Sperber, P.S.; Rohmann, J.L.; Piper, S.K.; Heuschmann, P.U.; Landmesser, U.; Endres, M.; Siegerink, B.; et al. Endothelial and Leukocyte-Derived Microvesicles and Cardiovascular Risk After Stroke: PROSCIS-B. Neurology 2021, 96, e937–e946. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Bratseth, V.; Ritschel, V.; Andersen, G.O.; Halvorsen, S.; Eritsland, J.; Arnesen, H.; Badimon, L.; Seljeflot, I. Monocyte-derived circulating microparticles (CD14(+), CD14(+)/CD11b(+) and CD14(+)/CD142(+)) are related to long-term prognosis for cardiovascular mortality in STEMI patients. Int. J. Cardiol. 2017, 227, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Suades, R.; Padro, T.; Crespo, J.; Ramaiola, I.; Martin-Yuste, V.; Sabate, M.; Sans-Rosello, J.; Sionis, A.; Badimon, L. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int. J. Cardiol. 2016, 202, 378–387. [Google Scholar] [CrossRef]
- Sarlon-Bartoli, G.; Bennis, Y.; Lacroix, R.; Piercecchi-Marti, M.D.; Bartoli, M.A.; Arnaud, L.; Mancini, J.; Boudes, A.; Sarlon, E.; Thevenin, B.; et al. Plasmatic level of leukocyte-derived microparticles is associated with unstable plaque in asymptomatic patients with high-grade carotid stenosis. J. Am. Coll Cardiol. 2013, 62, 1436–1441. [Google Scholar] [CrossRef] [Green Version]
- Morel, O.; Hugel, B.; Jesel, L.; Lanza, F.; Douchet, M.P.; Zupan, M.; Chauvin, M.; Cazenave, J.P.; Freyssinet, J.M.; Toti, F. Sustained elevated amounts of circulating procoagulant membrane microparticles and soluble GPV after acute myocardial infarction in diabetes mellitus. Thromb. Haemost. 2004, 91, 345–353. [Google Scholar] [CrossRef]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.M.; Tedgui, A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Van der Zee, P.M.; Biro, E.; Ko, Y.; de Winter, R.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin. Chem. 2006, 52, 657–664. [Google Scholar] [CrossRef]
- Stepien, E.; Stankiewicz, E.; Zalewski, J.; Godlewski, J.; Zmudka, K.; Wybranska, I. Number of microparticles generated during acute myocardial infarction and stable angina correlates with platelet activation. Arch. Med. Res. 2012, 43, 31–35. [Google Scholar] [CrossRef]
- Katopodis, J.N.; Kolodny, L.; Jy, W.; Horstman, L.L.; De Marchena, E.J.; Tao, J.G.; Haynes, D.H.; Ahn, Y.S. Platelet microparticles and calcium homeostasis in acute coronary ischemias. Am. J. Hematol. 1997, 54, 95–101. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Oudatzis, G.; Paterakis, G.; Synetos, A.; Tampaki, E.; Bouras, G.; Hahalis, G.; Alexopoulos, D.; Tousoulis, D.; Cleman, M.W.; et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int. J. Cardiol. 2014, 176, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, L.M.; Porto, I.; Di Vito, L.; De Maria, G.L.; Leone, A.M.; Tinelli, G.; Tritarelli, A.; Di Rocco, G.; Snider, F.; Capogrossi, M.C.; et al. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ. J. 2012, 76, 2174–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, P.K.; Kim, J.Y.; Chung, K.H.; Lee, B.K.; Cho, M.; Lee, D.L.; Hong, S.Y.; Choi, E.Y.; Yoon, Y.W.; Hong, B.K.; et al. Local increase in microparticles from the aspirate of culprit coronary arteries in patients with ST-segment elevation myocardial infarction. Atherosclerosis 2013, 227, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.; Biasucci, L.M.; De Maria, G.L.; Leone, A.M.; Niccoli, G.; Burzotta, F.; Trani, C.; Tritarelli, A.; Vergallo, R.; Liuzzo, G.; et al. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur. Heart J. 2012, 33, 2928–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, N.; Wassmann, S.; Ahlers, P.; Kosiol, S.; Nickenig, G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ierssel, S.H.; Hoymans, V.Y.; Van Craenenbroeck, E.M.; Van Tendeloo, V.F.; Vrints, C.J.; Jorens, P.G.; Conraads, V.M. Endothelial microparticles (EMP) for the assessment of endothelial function: An in vitro and in vivo study on possible interference of plasma lipids. PLoS ONE 2012, 7, e31496. [Google Scholar] [CrossRef] [Green Version]
- Sinning, J.M.; Losch, J.; Walenta, K.; Bohm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kim, J.M.; Moon, H.J.; Bahn, J.J.; Im, W.S.; Sunwoo, J.; Moon, J.; Kim, M.; et al. Circulating CD62E+ microparticles and cardiovascular outcomes. PLoS ONE 2012, 7, e35713. [Google Scholar] [CrossRef]
- Amabile, N.; Heiss, C.; Real, W.M.; Minasi, P.; McGlothlin, D.; Rame, E.J.; Grossman, W.; De Marco, T.; Yeghiazarians, Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 1268–1275. [Google Scholar] [CrossRef]
- Zacharia, E.; Antonopoulos, A.S.; Oikonomou, E.; Papageorgiou, N.; Pallantza, Z.; Miliou, A.; Mystakidi, V.C.; Simantiris, S.; Kriebardis, A.; Orologas, N.; et al. Plasma signature of apoptotic microvesicles is associated with endothelial dysfunction and plaque rupture in acute coronary syndromes. J. Mol. Cell Cardiol. 2020, 138, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mizrachi, L.; Jy, W.; Fierro, C.; Macdonough, R.; Velazques, H.A.; Purow, J.; Jimenez, J.J.; Horstman, L.L.; Ferreira, A.; de Marchena, E.; et al. Endothelial microparticles correlate with high-risk angiographic lesions in acute coronary syndromes. Int. J. Cardiol. 2004, 97, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Padro, T.; Alonso, R.; Crespo, J.; Perez de Isla, L.; Mata, P.; Badimon, L. Liquid Biopsy of Extracellular Microvesicles Maps Coronary Calcification and Atherosclerotic Plaque in Asymptomatic Patients With Familial Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueba, T.; Nomura, S.; Inami, N.; Nishikawa, T.; Kajiwara, M.; Iwata, R.; Yamashita, K. Plasma level of platelet-derived microparticles is associated with coronary heart disease risk score in healthy men. J. Atheroscler. Thromb. 2010, 17, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Suades, R.; Crespo, J.; Vilahur, G.; Arderiu, G.; Padro, T.; Corella, D.; Salas-Salvado, J.; Aros, F.; Martinez-Gonzalez, M.A.; et al. CD3(+)/CD45(+) and SMA-alpha(+) circulating microparticles are increased in individuals at high cardiovascular risk who will develop a major cardiovascular event. Int. J. Cardiol. 2016, 208, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Suades, R.; Padro, T.; Alonso, R.; Mata, P.; Badimon, L. High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thromb. Haemost. 2015, 114, 1310–1321. [Google Scholar] [CrossRef]
- Lazaridis, A.; Gavriilaki, E.; Nikolaidou, B.; Yiannaki, E.; Dolgyras, P.; Anyfanti, P.; Triantafyllou, A.; Koletsos, N.; Tzimos, C.; Markala, D.; et al. A study of endothelial and platelet microvesicles across different hypertension phenotypes. J. Hum. Hypertens 2021. [Google Scholar] [CrossRef]
- Csongradi, E.; Nagy, B., Jr.; Fulop, T.; Varga, Z.; Karanyi, Z.; Magyar, M.T.; Olah, L.; Papp, M.; Facsko, A.; Kappelmayer, J.; et al. Increased levels of platelet activation markers are positively associated with carotid wall thickness and other atherosclerotic risk factors in obese patients. Thromb. Haemost. 2011, 106, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Mobarrez, F.; Antoniewicz, L.; Bosson, J.A.; Kuhl, J.; Pisetsky, D.S.; Lundback, M. The effects of smoking on levels of endothelial progenitor cells and microparticles in the blood of healthy volunteers. PLoS ONE 2014, 9, e90314. [Google Scholar] [CrossRef] [Green Version]
- Wekesa, A.L.; Cross, K.S.; O’Donovan, O.; Dowdall, J.F.; O’Brien, O.; Doyle, M.; Byrne, L.; Phelan, J.P.; Ross, M.D.; Landers, R.; et al. Predicting carotid artery disease and plaque instability from cell-derived microparticles. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Sansone, R.; Baaken, M.; Horn, P.; Schuler, D.; Westenfeld, R.; Amabile, N.; Kelm, M.; Heiss, C. Endothelial microparticles and vascular parameters in subjects with and without arterial hypertension and coronary artery disease. Data Brief. 2018, 19, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, T.; Han, X.; Li, Y.; Cheng, W.; Wang, L.; Lu, Z.; Yang, J.; Zhao, M. The Level of Circulating Microparticles in Patients with Coronary Heart Disease: A Systematic Review and Meta-Analysis. J. Cardiovasc. Transl. Res. 2020, 13, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zheng, L.; Jiang, M.; Jia, R.; Zhang, X.; Quan, Q.; Du, G.; Shen, D.; Zhao, X.; Sun, W.; et al. Circulating microparticles in patients with coronary heart disease and its correlation with interleukin-6 and C-reactive protein. Mol. Biol. Rep. 2013, 40, 6437–6442. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Shan, X.F.; Wang, X.L.; Dong, W.W.; Li, Z.; Liu, X.H.; Zhang, W.; Xing, K.; Chang, F.J. Endothelial damage effects of circulating microparticles from patients with stable angina are reduced by aspirin through ERK/p38 MAPKs pathways. Cardiovasc.Ther. 2017, 35, e12273. [Google Scholar] [CrossRef]
- Vagida, M.S.; Arakelyan, A.; Lebedeva, A.M.; Grivel, J.C.; Shpektor, A.V.; Vasilieva, E.Y.; Margolis, L.B. Analysis of Extracellular Vesicles Using Magnetic Nanoparticles in Blood of Patients with Acute Coronary Syndrome. Biochemistry 2016, 81, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Wang, L.; Li, Y.; Yin, Z.; Xu, Z.; Wang, C. Quantification of endothelial microparticles on modified cytometric bead assay and prognosis in chest pain patients. Circ. J. 2014, 78, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Empana, J.P.; Boulanger, C.M.; Tafflet, M.; Renard, J.M.; Leroyer, A.S.; Varenne, O.; Prugger, C.; Silvain, J.; Tedgui, A.; Cariou, A.; et al. Microparticles and sudden cardiac death due to coronary occlusion. The TIDE (Thrombus and Inflammation in sudden DEath) study. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 28–36. [Google Scholar] [CrossRef]
- Mortberg, J.; Lundwall, K.; Mobarrez, F.; Wallen, H.; Jacobson, S.H.; Spaak, J. Increased concentrations of platelet- and endothelial-derived microparticles in patients with myocardial infarction and reduced renal function—A descriptive study. BMC Nephrol. 2019, 20, 71. [Google Scholar] [CrossRef]
- Gasecka, A.; Nieuwland, R.; Budnik, M.; Dignat-George, F.; Eyileten, C.; Harrison, P.; Lacroix, R.; Leroyer, A.; Opolski, G.; Pluta, K.; et al. Ticagrelor attenuates the increase of extracellular vesicle concentrations in plasma after acute myocardial infarction compared to clopidogrel. J. Thromb. Haemost. 2020, 18, 609–623. [Google Scholar] [CrossRef] [Green Version]
- Christersson, C.; Thulin, A.; Siegbahn, A. Microparticles during long-term follow-up after acute myocardial infarction. Association to atherosclerotic burden and risk of cardiovascular events. Thromb. Haemost. 2017, 117, 1571–1581. [Google Scholar] [CrossRef]
- Li, P.; Qin, C. Elevated circulating VE-cadherin+CD144+endothelial microparticles in ischemic cerebrovascular disease. Thromb. Res. 2015, 135, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Suades, R.; Crespo, J.; Pena, E.; Padro, T.; Jimenez-Xarrie, E.; Marti-Fabregas, J.; Badimon, L. Microparticle Shedding from Neural Progenitor Cells and Vascular Compartment Cells Is Increased in Ischemic Stroke. PLoS ONE 2016, 11, e0148176. [Google Scholar] [CrossRef] [PubMed]
- Bivard, A.; Lincz, L.F.; Maquire, J.; Parsons, M.; Levi, C. Platelet microparticles: A biomarker for recanalization in rtPA-treated ischemic stroke patients. Ann. Clin. Transl. Neurol. 2017, 4, 175–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenz-Pipaon, G.; San Martin, P.; Planell, N.; Maillo, A.; Ravassa, S.; Vilas-Zornoza, A.; Martinez-Aguilar, E.; Rodriguez, J.A.; Alameda, D.; Lara-Astiaso, D.; et al. Functional and transcriptomic analysis of extracellular vesicles identifies calprotectin as a new prognostic marker in peripheral arterial disease (PAD). J. Extracell Vesicles 2020, 9, 1729646. [Google Scholar] [CrossRef]
- Giarretta, I.; Gatto, I.; Marcantoni, M.; Lupi, G.; Tonello, D.; Gaetani, E.; Pitocco, D.; Iezzi, R.; Truma, A.; Porfidia, A.; et al. Microparticles Carrying Sonic Hedgehog Are Increased in Humans with Peripheral Artery Disease. Int. J. Mol. Sci 2018, 19, 3954. [Google Scholar] [CrossRef] [Green Version]
- Crawford, J.R.; Trial, J.; Nambi, V.; Hoogeveen, R.C.; Taffet, G.E.; Entman, M.L. Plasma Levels of Endothelial Microparticles Bearing Monomeric C-reactive Protein are Increased in Peripheral Artery Disease. J. Cardiovasc. Transl. Res. 2016, 9, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Jamaly, S.; Basavaraj, M.G.; Starikova, I.; Olsen, R.; Braekkan, S.K.; Hansen, J.B. Elevated plasma levels of P-selectin glycoprotein ligand-1-positive microvesicles in patients with unprovoked venous thromboembolism. J. Thromb. Haemost. 2018. [Google Scholar] [CrossRef]
- Thulin, A.; Lindback, J.; Granger, C.B.; Wallentin, L.; Lind, L.; Siegbahn, A. Extracellular vesicles in atrial fibrillation and stroke. Thromb. Res. 2020, 193, 180–189. [Google Scholar] [CrossRef]
- Anyfanti, P.; Gavriilaki, E.; Nikolaidou, B.; Yiannaki, E.; Lazaridis, A.; Papadopoulos, N.; Douma, S.; Doumas, M.; Gkaliagkousi, E. Patients with autoimmune chronic inflammatory diseases present increased biomarkers of thromboinflammation and endothelial dysfunction in the absence of flares and cardiovascular comorbidities. J. Thromb. Thrombolysis 2022, 53, 10–16. [Google Scholar] [CrossRef]
- Gustafson, C.M.; Shepherd, A.J.; Miller, V.M.; Jayachandran, M. Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biol. Sex. Differ. 2015, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Baek, R.; Varming, K.; Jorgensen, M.M. Does smoking, age or gender affect the protein phenotype of extracellular vesicles in plasma? Transfus Apher. Sci. 2016, 55, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condrat, C.E.; Varlas, V.N.; Duica, F.; Antoniadis, P.; Danila, C.A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. Pregnancy-Related Extracellular Vesicles Revisited. Int. J. Mol. Sci 2021, 22, 3904. [Google Scholar] [CrossRef] [PubMed]
- Aboulgheit, A.; Potz, B.A.; Scrimgeour, L.A.; Karbasiafshar, C.; Shi, G.; Zhang, Z.; Machan, J.T.; Schorl, C.; Brodsky, A.S.; Braga, K.; et al. Effects of High Fat Versus Normal Diet on Extracellular Vesicle-Induced Angiogenesis in a Swine Model of Chronic Myocardial Ischemia. J. Am. Heart Assoc. 2021, 10, e017437. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Mobius, W.; Simon, P.; Kramer-Albers, E.M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Bollati, V.; Pergoli, L.; Iodice, S.; De Col, A.; Tamini, S.; Cicolini, S.; Tringali, G.; De Micheli, R.; Cella, S.G.; et al. Effects of an acute bout of exercise on circulating extracellular vesicles: Tissue-, sex-, and BMI-related differences. Int. J. Obes. 2020, 44, 1108–1118. [Google Scholar] [CrossRef]
- Vechetti, I.J., Jr.; Valentino, T.; Mobley, C.B.; McCarthy, J.J. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J. Physiol. 2021, 599, 845–861. [Google Scholar] [CrossRef]
- Estrada, A.L.; Valenti, Z.J.; Hehn, G.; Amorese, A.J.; Williams, N.S.; Balestrieri, N.P.; Deighan, C.; Allen, C.P.; Spangenburg, E.E.; Kruh-Garcia, N.A.; et al. Extracellular vesicle secretion is tissue-dependent ex vivo and skeletal muscle myofiber extracellular vesicles reach the circulation in vivo. Am. J. Physiol. Cell Physiol. 2022, 322, C246–C259. [Google Scholar] [CrossRef]
- Morel, O.; Jesel, L.; Hugel, B.; Douchet, M.P.; Zupan, M.; Chauvin, M.; Freyssinet, J.M.; Toti, F. Protective effects of vitamin C on endothelium damage and platelet activation during myocardial infarction in patients with sustained generation of circulating microparticles. J. Thromb. Haemost. 2003, 1, 171–177. [Google Scholar] [CrossRef]
- Labios, M.; Martinez, M.; Gabriel, F.; Guiral, V.; Munoz, A.; Aznar, J. Effect of eprosartan on cytoplasmic free calcium mobilization, platelet activation, and microparticle formation in hypertension. Am. J. Hypertens 2004, 17, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Nomura, S.; Inami, N.; Kimura, Y.; Omoto, S.; Shouzu, A.; Nishikawa, M.; Iwasaka, T. Effect of nifedipine on adiponectin in hypertensive patients with type 2 diabetes mellitus. J. Hum. Hypertens 2007, 21, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Suades, R.; Padro, T.; Alonso, R.; Mata, P.; Badimon, L. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb. Haemost. 2013, 110, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lins, L.C.; Franca, C.N.; Fonseca, F.A.; Barbosa, S.P.; Matos, L.N.; Aguirre, A.C.; Bianco, H.T.; do Amaral, J.B.; Izar, M.C. Effects of ezetimibe on endothelial progenitor cells and microparticles in high-risk patients. Cell Biochem. Biophys. 2014, 70, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Bulut, D.; Becker, V.; Mugge, A. Acetylsalicylate reduces endothelial and platelet-derived microparticles in patients with coronary artery disease. Can. J. Physiol. Pharmacol. 2011, 89, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Hugel, B.; Jesel, L.; Mallat, Z.; Lanza, F.; Douchet, M.P.; Zupan, M.; Chauvin, M.; Cazenave, J.P.; Tedgui, A.; et al. Circulating procoagulant microparticles and soluble GPV in myocardial infarction treated by primary percutaneous transluminal coronary angioplasty. A possible role for GPIIb-IIIa antagonists. J. Thromb. Haemost. 2004, 2, 1118–1126. [Google Scholar] [CrossRef]
- Judge, H.M.; Buckland, R.J.; Sugidachi, A.; Jakubowski, J.A.; Storey, R.F. Relationship between degree of P2Y12 receptor blockade and inhibition of P2Y12-mediated platelet function. Thromb. Haemost. 2010, 103, 1210–1217. [Google Scholar] [CrossRef]
- Shouzu, A.; Nomura, S.; Omoto, S.; Hayakawa, T.; Nishikawa, M.; Iwasaka, T. Effect of ticlopidine on monocyte-derived microparticles and activated platelet markers in diabetes mellitus. Clin. Appl. Thromb. Hemost. 2004, 10, 167–173. [Google Scholar] [CrossRef]
- Park, S.H.; Belcastro, E.; Hasan, H.; Matsushita, K.; Marchandot, B.; Abbas, M.; Toti, F.; Auger, C.; Jesel, L.; Ohlmann, P.; et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: Protective effect of gliflozins. Cardiovasc. Diabetol. 2021, 20, 65. [Google Scholar] [CrossRef]
- Akawi, N.; Checa, A.; Antonopoulos, A.S.; Akoumianakis, I.; Daskalaki, E.; Kotanidis, C.P.; Kondo, H.; Lee, K.; Yesilyurt, D.; Badi, I.; et al. Fat-Secreted Ceramides Regulate Vascular Redox State and Influence Outcomes in Patients With Cardiovascular Disease. J. Am. Coll Cardiol. 2021, 77, 2494–2513. [Google Scholar] [CrossRef]
- Di Tomo, P.; Lanuti, P.; Di Pietro, N.; Baldassarre, M.P.A.; Marchisio, M.; Pandolfi, A.; Consoli, A.; Formoso, G. Liraglutide mitigates TNF-alpha induced pro-atherogenic changes and microvesicle release in HUVEC from diabetic women. Diabetes Metab Res. Rev. 2017, 33, e2925. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Silvis, M.J.M.; Fiolet, A.T.L.; Opstal, T.S.J.; Dekker, M.; Suquilanda, D.; Zivkovic, M.; Duyvendak, M.; The, S.H.K.; Timmers, L.; Bax, W.A.; et al. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: A LoDoCo2 biomarker substudy. Atherosclerosis 2021, 334, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.A.; Morille, M.; Piffoux, M.; Arumugam, S.; Mauduit, P.; Larghero, J.; Bianchi, A.; Aubertin, K.; Blanc-Brude, O.; Noel, D.; et al. Development of extracellular vesicle-based medicinal products: A position paper of the group “Extracellular Vesicle translatiOn to clinicaL perspectiVEs—EVOLVE France”. Adv. Drug Deliv. Rev. 2021, 179, 114001. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Fan, Q.; Han, X.; Dong, Z.; Xu, J.; Bai, J.; Tao, W.; Sun, D.; Wang, C. Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J. Control. Release 2021, 329, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Loyer, X.; Boulanger, C.M. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur. J. Pharmacol. 2015, 763, 90–103. [Google Scholar] [CrossRef]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef]
- Liu, H.; Gao, W.; Yuan, J.; Wu, C.; Yao, K.; Zhang, L.; Ma, L.; Zhu, J.; Zou, Y.; Ge, J. Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction. J. Mol. Cell Cardiol. 2016, 91, 123–133. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Liu, L.; Xi, A.; Chen, B.; Li, Y.; Du, J. Macrophage-Derived mir-155-Containing Exosomes Suppress Fibroblast Proliferation and Promote Fibroblast Inflammation during Cardiac Injury. Mol. Ther. 2017, 25, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Mayo, J.N.; Bearden, S.E. Driving the Hypoxia-Inducible Pathway in Human Pericytes Promotes Vascular Density in an Exosome-Dependent Manner. Microcirculation 2015, 22, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Kervadec, A.; Bellamy, V.; El Harane, N.; Arakelian, L.; Vanneaux, V.; Cacciapuoti, I.; Nemetalla, H.; Perier, M.C.; Toeg, H.D.; Richart, A.; et al. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J. Heart Lung Transplant. 2016, 35, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Lima Correa, B.; El Harane, N.; Gomez, I.; Rachid Hocine, H.; Vilar, J.; Desgres, M.; Bellamy, V.; Keirththana, K.; Guillas, C.; Perotto, M.; et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc. Res. 2021, 117, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Viola, M.; de Jager, S.C.A.; Sluijter, J.P.G. Targeting Inflammation after Myocardial Infarction: A Therapeutic Opportunity for Extracellular Vesicles? Int. J. Mol. Sci. 2021, 22, 7831. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Ratajczak, J. Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin. Transl. Med. 2016, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Jansen, F.; Yang, X.; Hoelscher, M.; Cattelan, A.; Schmitz, T.; Proebsting, S.; Wenzel, D.; Vosen, S.; Franklin, B.S.; Fleischmann, B.K.; et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 2013, 128, 2026–2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbar, N.; Braithwaite, A.T.; Corr, E.M.; Koelwyn, G.J.; van Solingen, C.; Cochain, C.; Saliba, A.E.; Corbin, A.; Pezzolla, D.; Moller Jorgensen, M.; et al. Rapid neutrophil mobilisation by VCAM-1+ endothelial extracellular vesicles. Cardiovasc. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Agouni, A.; Mostefai, H.A.; Porro, C.; Carusio, N.; Favre, J.; Richard, V.; Henrion, D.; Martinez, M.C.; Andriantsitohaina, R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007, 21, 2735–2741. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Porro, C.; Andriantsitohaina, R.; Martinez, M.C. Microparticles carrying Sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS ONE 2010, 5, e12688. [Google Scholar] [CrossRef] [Green Version]
- Comarita, I.K.; Vilcu, A.; Constantin, A.; Procopciuc, A.; Safciuc, F.; Alexandru, N.; Dragan, E.; Nemecz, M.; Filippi, A.; Chitoiu, L.; et al. Therapeutic Potential of Stem Cell-Derived Extracellular Vesicles on Atherosclerosis-Induced Vascular Dysfunction and Its Key Molecular Players. Front. Cell Dev. Biol. 2022, 10, 817180. [Google Scholar] [CrossRef]
- Wolf, M.; Poupardin, R.W.; Ebner-Peking, P.; Andrade, A.C.; Blochl, C.; Obermayer, A.; Gomes, F.G.; Vari, B.; Maeding, N.; Eminger, E.; et al. A functional corona around extracellular vesicles enhances angiogenesis, skin regeneration and immunomodulation. J. Extracell Vesicles 2022, 11, e12207. [Google Scholar] [CrossRef]
- Saludas, L.; Oliveira, C.C.; Roncal, C.; Ruiz-Villalba, A.; Prosper, F.; Garbayo, E.; Blanco-Prieto, M.J. Extracellular Vesicle-Based Therapeutics for Heart Repair. Nanomaterials 2021, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.A.; Lotvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, D.S.; Badawi, M.; Phelps, M.A.; Schmittgen, T.D. Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil is in Details of Therapeutic Loading. Pharm. Res. 2017, 34, 1053–1066. [Google Scholar] [CrossRef] [Green Version]
- Robson, A. Exosomes improve myocardial recovery after infarction. Nat. Rev. Cardiol. 2020, 17, 758. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Wenzel, D.; Becher, U.M.; Freitag, D.F.; Klein, A.M.; Eberbeck, D.; Schulte, M.; Zimmermann, K.; Bergemann, C.; Gleich, B.; et al. Combined targeting of lentiviral vectors and positioning of transduced cells by magnetic nanoparticles. Proc. Natl. Acad. Sci. USA 2009, 106, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosen, S.; Rieck, S.; Heidsieck, A.; Mykhaylyk, O.; Zimmermann, K.; Bloch, W.; Eberbeck, D.; Plank, C.; Gleich, B.; Pfeifer, A.; et al. Vascular Repair by Circumferential Cell Therapy Using Magnetic Nanoparticles and Tailored Magnets. ACS Nano 2016, 10, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Vosen, S.; Rieck, S.; Heidsieck, A.; Mykhaylyk, O.; Zimmermann, K.; Plank, C.; Gleich, B.; Pfeifer, A.; Fleischmann, B.K.; Wenzel, D. Improvement of vascular function by magnetic nanoparticle-assisted circumferential gene transfer into the native endothelium. J. Control. Release 2016, 241, 164–173. [Google Scholar] [CrossRef]
- Maiullari, F.; Chirivi, M.; Costantini, M.; Ferretti, A.M.; Recchia, S.; Maiullari, S.; Milan, M.; Presutti, D.; Pace, V.; Raspa, M.; et al. In vivo organized neovascularization induced by 3D bioprinted endothelial-derived extracellular vesicles. Biofabrication 2021, 13. [Google Scholar] [CrossRef]
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A.; et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res 2018, 114, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, U.D.S.; Swingle, K.; Girish, A.; Luc, N.N.; de la Fuente, M.; Alvikas, J.; Haldeman, S.; Hassoune, A.; Shah, K.; Kim, Y.; et al. Platelet-mimicking procoagulant nanoparticles augment hemostasis in animal models of bleeding. Sci. Transl. Med. 2022, 14, eabb8975. [Google Scholar] [CrossRef]
- Ryan, S.T.; Hosseini-Beheshti, E.; Afrose, D.; Ding, X.; Xia, B.; Grau, G.E.; Little, C.B.; McClements, L.; Li, J.J. Extracellular Vesicles from Mesenchymal Stromal Cells for the Treatment of Inflammation-Related Conditions. Int. J. Mol. Sci. 2021, 22, 3023. [Google Scholar] [CrossRef]
- Verweij, F.J.; Balaj, L.; Boulanger, C.M.; Carter, D.R.F.; Compeer, E.B.; D’Angelo, G.; El Andaloussi, S.; Goetz, J.G.; Gross, J.C.; Hyenne, V.; et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat. Methods 2021, 18, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Yadid, M.; Lind, J.U.; Ardona, H.A.M.; Sheehy, S.P.; Dickinson, L.E.; Eweje, F.; Bastings, M.M.C.; Pope, B.; O’Connor, B.B.; Straubhaar, J.R.; et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Paloschi, V.; Sabater-Lleal, M.; Middelkamp, H.; Vivas, A.; Johansson, S.; van der Meer, A.; Tenje, M.; Maegdefessel, L. Organ-on-a-chip technology: A novel approach to investigate cardiovascular diseases. Cardiovasc. Res. 2021, 117, 2742–2754. [Google Scholar] [CrossRef] [PubMed]
- Verweij, F.J.; Hyenne, V.; Van Niel, G.; Goetz, J.G. Extracellular Vesicles: Catching the Light in Zebrafish. Trends Cell Biol. 2019, 29, 770–776. [Google Scholar] [CrossRef] [PubMed]
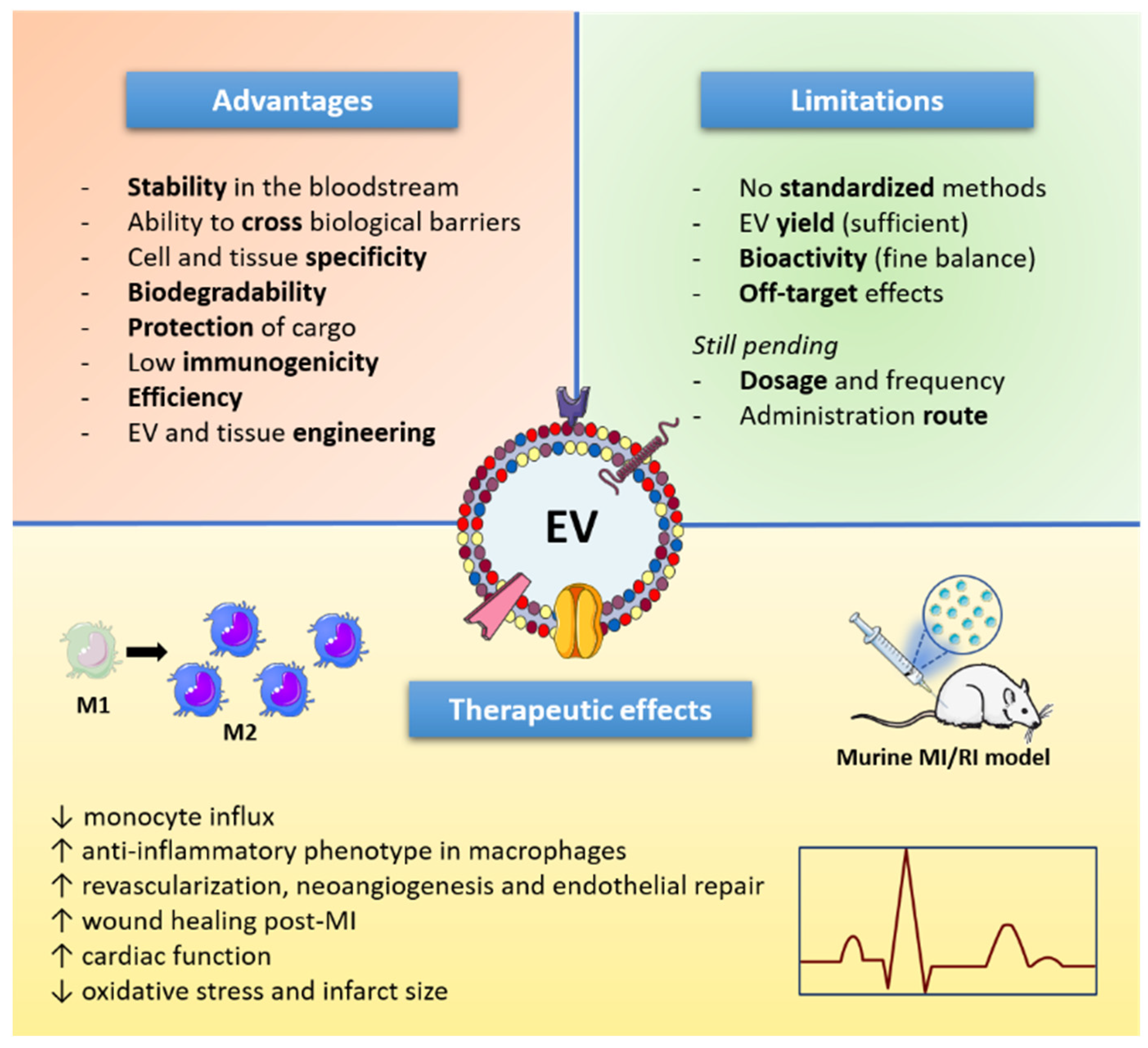
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suades, R.; Greco, M.F.; Padró, T.; Badimon, L. Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis. Cells 2022, 11, 1845. https://doi.org/10.3390/cells11111845
Suades R, Greco MF, Padró T, Badimon L. Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis. Cells. 2022; 11(11):1845. https://doi.org/10.3390/cells11111845
Chicago/Turabian StyleSuades, Rosa, Maria Francesca Greco, Teresa Padró, and Lina Badimon. 2022. "Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis" Cells 11, no. 11: 1845. https://doi.org/10.3390/cells11111845
APA StyleSuades, R., Greco, M. F., Padró, T., & Badimon, L. (2022). Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis. Cells, 11(11), 1845. https://doi.org/10.3390/cells11111845








