Differential Expression of CD45RO and CD45RA in Bovine T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cattle
2.2. Peripheral Blood Mononuclear Cells (PBMCs) Isolation and Stimulation
2.3. Antibodies and Reagents
2.4. Flow Cytometry Analysis
2.5. Statistical Analysis
3. Results
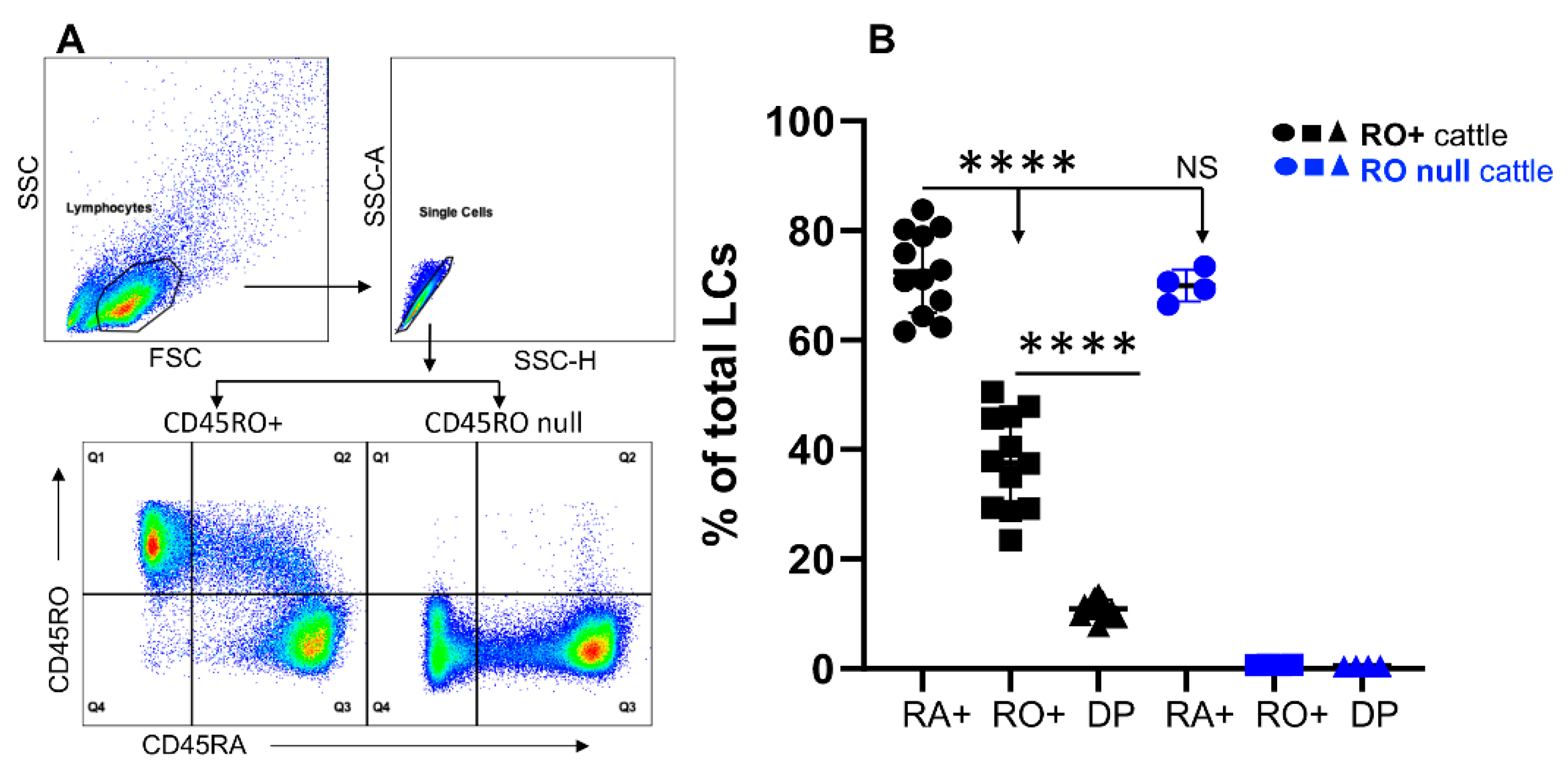
3.1. Bovine Lymphocytes Do Not Always Express CD45RO
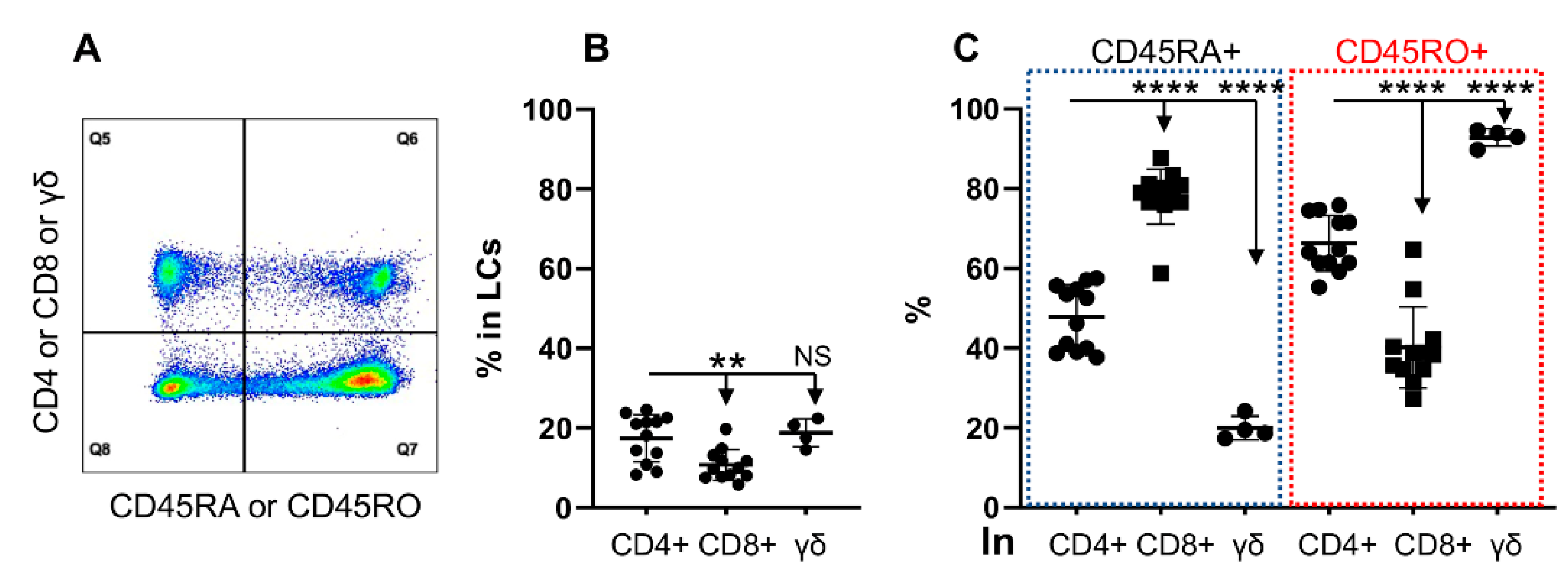
3.2. CD45RO and CD45RA Are Expressed Differentially in T Cell Subtypes
3.3. Differential Activation Status in CD45RO+ and CD45RA+ Fractions of CD4+, but Not of CD8+ T Cells
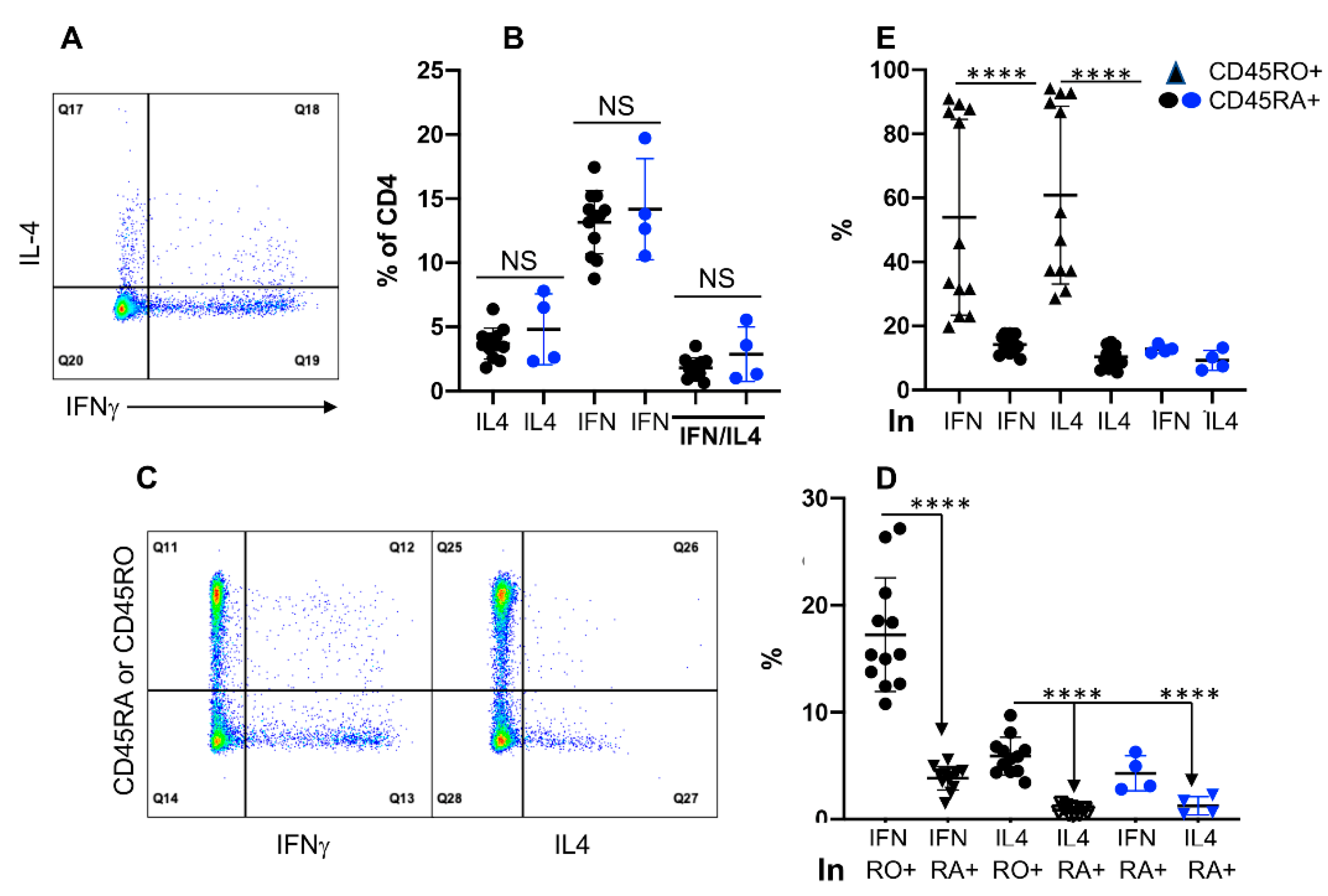
3.4. Expression of IFNγ and IL4 Is Not Associated with CD45RO in CD4+ T Cells
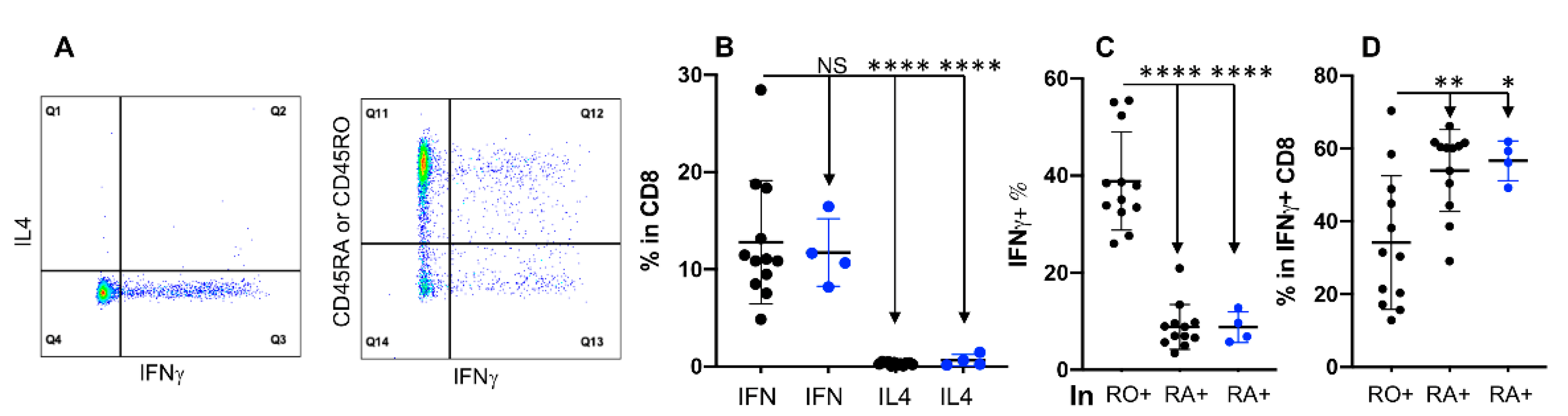
3.5. Expression of IFNγ Is Not Associated with CD45RO in CD8+ T Cells
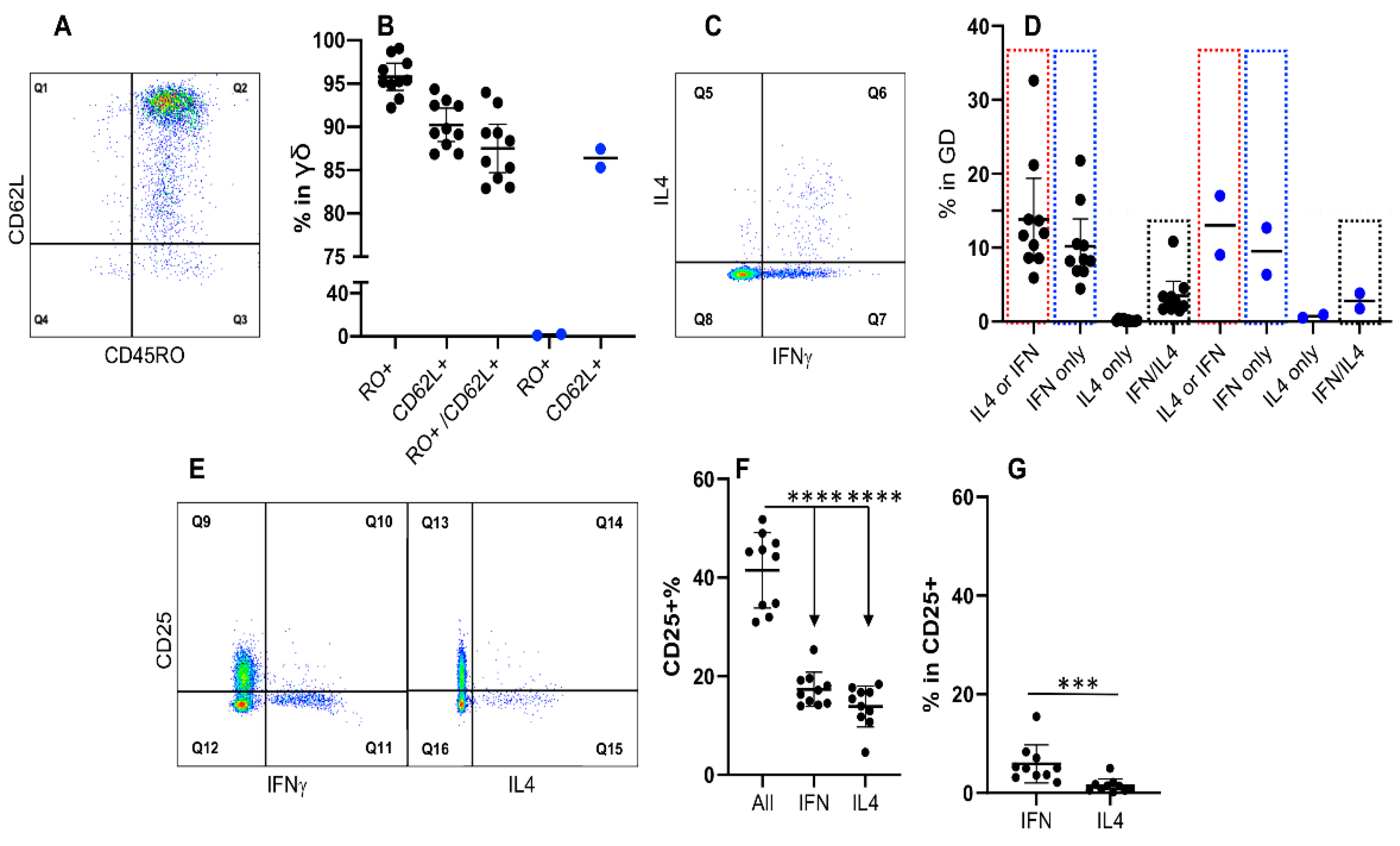
3.6. Expression of IFNγ and IL4 Is Not Associated with CD45RO in γδ T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picker, L.; Singh, M.; Zdraveski, Z.; Treer, J.; Waldrop, S.; Bergstresser, P.; Maino, V. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood 1995, 86, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Kowalczyk, D.; Ozóg, M.; Zembala, M. Three-color flow cytometry detection of intracellular cytokines in peripheral blood mononuclear cells: Comparative analysis of phorbol myristate acetate-ionomycin and phytohemagglutinin stimulation. ASM J. Clin. Diagn. Lab. Immunol. 2001, 8, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Phetsouphanh, C.; Zaunders, J.J.; Kelleher, A.D. Detecting antigen-specific T cell responses: From bulk populations to single cells. Int. J. Mol. Sci. 2015, 16, 18878–18893. [Google Scholar] [CrossRef] [PubMed]
- Suni, M.A.; Picker, L.J.; Maino, V.C. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J. Immunol. Methods 1998, 212, 89–98. [Google Scholar] [CrossRef]
- Maggioli, M.F.; Palmer, M.V.; Thacker, T.C.; Vordermeier, H.M.; Waters, W.R. Characterization of effector and memory T cell subsets in the immune response to bovine tuberculosis in cattle. PLoS ONE 2015, 10, e0122571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbar, A.; Terry, L.; Timms, A.; Beverley, P.; Janossy, G. Unidirectional phenotypic changes within the T200 complex during activation of T cells. J. Immunol. 1988, 140, 2171. [Google Scholar]
- Sanders, M.E.; Makgoba, M.W.; Shaw, S. Human naive and memory T cells: Reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol. Today 1988, 9, 195–199. [Google Scholar] [CrossRef]
- Bembridge, G.; MacHugh, N.; McKeever, D.; Awino, E.; Sopp, P.; Collins, R.; Gelder, K.; Howard, C. CD45RO expression on bovine T cells: Relation to biological function. Immunology 1995, 86, 537. [Google Scholar]
- Stabel, J.R.; Kimura, K.; Robbe-Austerman, S. Augmentation of secreted and intracellular gamma interferon following johnin purified protein derivative sensitization of cows naturally infected with Mycobacterium avium subsp. paratuberculosis. J. Vet. Diagn. Investig. 2007, 19, 43–51. [Google Scholar] [CrossRef] [Green Version]
- McInnes, E.; Sopp, P.; Howard, C.; Taylor, G. Phenotypic analysis of local cellular responses in calves infected with bovine respiratory syncytial virus. Immunology 1999, 96, 396. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Markopoulos, G.S.; Mallioras, I.; Pappas, H.; Pappas, E.; Koutlas, V.; Tzalavra, E.; Baxevanos, G.; Priska, S.; Gartzonika, K. The Humoral Immune Response to BNT162b2 Vaccine Is Associated With Circulating CD19+ B Lymphocytes and the Naïve CD45RA to Memory CD45RO CD4+ T Helper Cells Ratio in Hemodialysis Patients and Kidney Transplant Recipients. Front. Immunol. 2021, 12, 760249. [Google Scholar] [CrossRef]
- Michie, C.A.; McLean, A.; Alcock, C.; Beverley, P.C. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature 1992, 360, 264–265. [Google Scholar] [CrossRef]
- Wills, M.R.; Carmichael, A.J.; Weekes, M.P.; Mynard, K.; Okecha, G.; Hicks, R.; Sissons, J.P. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J. Immunol. 1999, 162, 7080–7087. [Google Scholar]
- Callan, M.; Tan, L.; Annels, N.; Ogg, G.; Wilson, J.; O’callaghan, C.; Steven, N.; McMichael, A.; Rickinson, A. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 1998, 187, 1395–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Shariff, N.; Cobbold, M.; Bruton, R.; Ainsworth, J.A.; Sinclair, A.J.; Nayak, L.; Moss, P.A. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 2002, 169, 1984–1992. [Google Scholar] [CrossRef] [Green Version]
- Wills, M.R.; Okecha, G.; Weekes, M.P.; Gandhi, M.K.; Sissons, P.J.; Carmichael, A.J. Identification of naive or antigen-experienced human CD8+ T cells by expression of costimulation and chemokine receptors: Analysis of the human cytomegalovirus-specific CD8+ T cell response. J. Immunol. 2002, 168, 5455–5464. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.; Covas, M.J.; Victorino, R.M. Loss of CD45RA and gain of CD45RO after in vitro activation of lymphocytes from HIV-infected patients. Immunology 1991, 73, 147–150. [Google Scholar] [PubMed]
- Gattinoni, L.; Lugli, E.; Ji, Y.; Pos, Z.; Paulos, C.M.; Quigley, M.F.; Almeida, J.R.; Gostick, E.; Yu, Z.; Carpenito, C.; et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011, 17, 1290–1297. [Google Scholar] [CrossRef]
- Ahmed, R.; Roger, L.; Del Amo, P.C.; Miners, K.L.; Jones, R.E.; Boelen, L.; Fali, T.; Elemans, M.; Zhang, Y.; Appay, V. Human stem cell-like memory T cells are maintained in a state of dynamic flux. Cell Rep. 2016, 17, 2811–2818. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Stabel, J.; Kehrli, M., Jr. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet. Immunol. Immunopathol. 2001, 82, 73–85. [Google Scholar] [CrossRef]
- Hong, H.; Gu, Y.; Sheng, S.Y.; Lu, C.G.; Zou, J.Y.; Wu, C.Y. The Distribution of Human Stem Cell–like Memory T Cell in Lung Cancer. J. Immunother. 2016, 39, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Rha, M.-S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.-H.; Jeong, H.W.; Choi, W.S. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Sopp, P.; Howard, C.J. IFN gamma and IL-4 production by CD4, CD8 and WC1 gamma delta TCR(+) T cells from cattle lymph nodes and blood. Vet. Immunol. Immunopathol. 2001, 81, 85–96. [Google Scholar] [CrossRef]
- Frie, M.C.; Sporer, K.R.; Kirkpatrick, B.W.; Coussens, P.M. T and B cell activation profiles from cows with and without Johne’s disease in response to in vitro stimulation with Mycobacterium avium subspecies paratuberculosis. Vet. Immunol. Immunopathol. 2017, 193, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Machura, E.; Mazur, B.; Pieniążek, W.; Karczewska, K. Expression of naive/memory (CD45RA/CD45RO) markers by peripheral blood CD4+ and CD8+ T cells in children with asthma. Arch. Immunol. Et Ther. Exp. 2008, 56, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermiston, M.L.; Xu, Z.; Weiss, A. CD45: A critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003, 21, 107–137. [Google Scholar] [CrossRef] [PubMed]
- Tonks, N.K.; Charbonneau, H.; Diltz, C.D.; Fischer, E.H.; Walsh, K.A. Demonstration that the leukocyte common antigen CD45 is a protein tyrosine phosphatase. Biochemistry 1988, 27, 8695–8701. [Google Scholar] [CrossRef]
- Trowbridge, I.S.; Thomas, M.L. CD45: An emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu. Rev. Immunol. 1994, 12, 85–116. [Google Scholar] [CrossRef]
- Lynch, K.W. Consequences of regulated pre-mRNA splicing in the immune system. Nat. Rev. Immunol. 2004, 4, 931–940. [Google Scholar] [CrossRef]
- Jonsson, N.N.; Cox, D.K.; Piper, E.K.; Valdivieso, E.F.M.; Constantinoiu, C.; Jackson, L.A.; Stear, M.J.; Ross, E.M.; Tabor, A.E. Allelic Variation in Protein Tyrosine Phosphatase Receptor Type-C in Cattle Influences Erythrocyte, Leukocyte and Humoral Responses to Infestation With the Cattle Tick Rhipicephalus australis. Front. Immunol. 2021, 12, 675979:1–675979:10. [Google Scholar] [CrossRef]
- Valentine M, Song K, Maresh GA, Mack H, Huaman MC, Polacino P, Ho O, Cristillo A, Kyung Chung H, Hu SL, Pincus SH. Expression of the memory marker CD45RO on helper T cells in macaques. PLoS One. 2013, 8, e73969.
- Ballingall, K.T.; Waibochi, L.; Holmes, E.C.; Woelk, C.H.; MacHugh, N.D.; Lutje, V.; McKeever, D.J. The CD45 locus in cattle: Allelic polymorphism and evidence for exceptional positive natural selection. Immunogenetics 2001, 52, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Blunt, L.; Hogarth, P.J.; Kaveh, D.A.; Webb, P.; Villarreal-Ramos, B.; Vordermeier, H.M. Phenotypic characterization of bovine memory cells responding to mycobacteria in IFNγ enzyme linked immunospot assays. Vaccine 2015, 33, 7276–7282. [Google Scholar] [CrossRef] [Green Version]
- Hogg, A.E.; Parsons, K.; Taylor, G.; Worth, A.; Beverley, P.; Howard, C.J.; Villarreal-Ramos, B. Characterization of age-related changes in bovine CD8+ T-cells. Vet. Immunol. Immunopathol. 2011, 140, 47–54. [Google Scholar] [CrossRef]
- Elnaggar, M.M.; Knowles, D.P.; Davis, W.C.; Fry, L.M. Flow Cytometric Analysis of the Cytotoxic T-Cell Recall Response to Theileria parva in Cattle Following Vaccination by the Infection and Treatment Method. Vet. Sci. 2021, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Endsley, J.J.; Endsley, M.A.; Estes, D.M. Bovine natural killer cells acquire cytotoxic/effector activity following activation with IL-12/15 and reduce Mycobacterium bovis BCG in infected macrophages. J. Leukoc. Biol. 2006, 79, 71–79. [Google Scholar] [CrossRef]
- Denis, M.; Lacy-Hulbert, S.J.; Buddle, B.M.; Williamson, J.H.; Wedlock, D.N. Streptococcus uberis-specific T cells are present in mammary gland secretions of cows and can be activated to kill S. uberis. Vet. Res. Commun. 2011, 35, 145–156. [Google Scholar] [CrossRef]
- Mitoma, S.; Carr, B.V.; Harvey, Y.; Moffat, K.; Sekiguchi, S.; Charleston, B.; Norimine, J.; Seago, J. The detection of long-lasting memory foot-and-mouth disease (FMD) virus serotype O-specific CD4+ T cells from FMD-vaccinated cattle by bovine major histocompatibility complex class II tetramer. Immunology 2021, 164, 266. [Google Scholar] [CrossRef]
- Silflow, R.M.; Degel, P.M.; Harmsen, A.G. Bronchoalveolar immune defense in cattle exposed to primary and secondary challenge with bovine viral diarrhea virus. Vet. Immunol. Immunopathol. 2005, 103, 129–139. [Google Scholar] [CrossRef]
- Hagberg, M.; Lundén, A.; Höglund, J.; Morrison, D.; Waller, K.P.; Wattrang, E. Characterization of bovine lymphocytes stimulated in vitro by Dictyocaulus viviparus homogenate. Parasite Immunol. 2008, 30, 342–353. [Google Scholar] [CrossRef]
- Hagberg, M. Immune Cell Responses to the Cattle Lungworm, Dictyocaulus Viviparus. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2008. Available online: https://pub.epsilon.slu.se/1769/1/200837_Kappan_med_bildtext.pdf (accessed on 10 March 2022).
- Hogg, A.E.; Worth, A.; Beverley, P.; Howard, C.J.; Villarreal-Ramos, B. The antigen-specific memory CD8+ T-cell response induced by BCG in cattle resides in the CD8+ γ/δTCR− CD45RO+ T-cell population. Vaccine 2009, 27, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J.; Sopp, P.; Parsons, K.R.; McKeever, D.J.; Taracha, E.L.; Jones, B.V.; MacHugh, N.D.; Morrison, W.I. Distinction of naive and memory BoCD4 lymphocytes in calves with a monoclonal antibody, CC76, to a restricted determinant of the bovine leukocyte-common antigen, CD45. Eur. J. Immunol. 1991, 21, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Maupome, M.; Palmer, M.V.; Waters, W.R.; McGill, J.L. Characterization of gammadelta T Cell Effector/Memory Subsets Based on CD27 and CD45R Expression in Response to Mycobacterium bovis Infection. Immunohorizons 2019, 3, 208–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Tian, F.; Yu, Y.; Luo, J.; Hu, Q.; Bequette, B.J.; Baldwin VI, R.L.; Liu, G.; Zan, L.; Scott Updike, M. Muscle transcriptomic analyses in Angus cattle with divergent tenderness. Mol. Biol. Rep. 2012, 39, 4185–4193. [Google Scholar] [CrossRef]
- Tuo, W.; Li, L.; Lv, Y.; Carrillo, J.; Brown, D.; Davis, W.C.; Song, J.; Zarlenga, D.; Xiao, Z. Abomasal mucosal immune responses of cattle with limited or continuous exposure to pasture-borne gastrointestinal nematode parasite infection. Vet. Parasitol. 2016, 229, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Mendez, J.; Sun, D.; Tuo, W.; Xiao, Z. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite Ostertagia ostertagi. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Si, H.; Wu, S.-W.; Mendez, J.O.; Zarlenga, D.; Tuo, W.; Xiao, Z. Characterization of IL-10-producing neutrophils in cattle infected with Ostertagia ostertagi. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, J.A.; He, Y.; Li, Y.; Liu, J.; Erdman, R.A.; Sonstegard, T.S.; Song, J. Integrated metabolomic and transcriptome analyses reveal finishing forage affects metabolic pathways related to beef quality and animal welfare. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Panagioti, E.; Klenerman, P.; Lee, L.N.; Van Der Burg, S.H.; Arens, R. Features of effective T cell-inducing vaccines against chronic viral infections. Front. Immunol. 2018, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Flaxman, A.; Ewer, K.J. Methods for measuring T-cell memory to vaccination: From mouse to man. Vaccines 2018, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.; Poppema, S.; Little, M.T.; Dragowska, W.; Lansdorp, P.M. CD45 isoform expression on human haemopoietic cells at different stages of development. Br. J. Haematol. 1994, 88, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Clement, L.T. Isoforms of the CD45 common leukocyte antigen family: Markers for human T-cell differentiation. J. Clin. Immunol. 1992, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Rao, T.K.S.; Varghese, A.; Rathor, V.S. Internal parasite management in grazing livestock. J. Parasit. Dis. 2013, 37, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Blumerman, S.L.; Herzig, C.T.; Baldwin, C.L. WC1+ γδ T cell memory population is induced by killed bacterial vaccine. Eur. J. Immunol. 2007, 37, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Kulberg, S.; Boysen, P.; Storset, A.K. Reference values for relative numbers of natural killer cells in cattle blood. Dev. Comp. Immunol. 2004, 28, 941–948. [Google Scholar] [CrossRef]
- Xiao, Z.; Kandel, A.; Li, L. Synergistic Activation of Bovine CD4+ T Cells by Neutrophils and IL-12. Pathogens 2021, 10, 694. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.-X.; Leonard, W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Nelson, B.H. IL-2, regulatory T cells, and tolerance. J. Immunol. 2004, 172, 3983–3988. [Google Scholar] [CrossRef] [Green Version]
- Schuster, K.; Gadiot, J.; Andreesen, R.; Mackensen, A.; Gajewski, T.F.; Blank, C. Homeostatic proliferation of naïve CD8+ T cells depends on CD62L/L-selectin-mediated homing to peripheral LN. Eur. J. Immunol. 2009, 39, 2981–2990. [Google Scholar] [CrossRef]
- Waldrop, S.L.; Pitcher, C.J.; Peterson, D.M.; Maino, V.C.; Picker, L.J. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: Evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 1997, 99, 1739–1750. [Google Scholar] [CrossRef] [Green Version]
- Totté, P.; Duperray, C.; Dedieu, L. CD62L defines a subset of pathogen-specific bovine CD4 with central memory cell characteristics. Dev. Comp. Immunol. 2010, 34, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Casey, K.A.; Jameson, S.C.; Curtsinger, J.M.; Mescher, M.F. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J. Immunol. 2009, 182, 2786–2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mescher, M.F.; Curtsinger, J.M.; Agarwal, P.; Casey, K.A.; Gerner, M.; Hammerbeck, C.D.; Popescu, F.; Xiao, Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006, 211, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Kandel, A.; Masello, M.; Xiao, Z. CD4+ T Cell Responses to Pathogens in Cattle; Abubakar, M., Ed.; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/chapters/78918 (accessed on 18 June 2021).
- Holtmeier, W.; Kabelitz, D. gammadelta T cells link innate and adaptive immune responses. Chem. Immunol. Allergy 2005, 86, 151–183. [Google Scholar] [CrossRef]
- Baldwin, C.L.; Yirsaw, A.; Gillespie, A.; Le Page, L.; Zhang, F.; Damani-Yokota, P.; Telfer, J.C. γδ T cells in livestock: Responses to pathogens and vaccine potential. Transbound. Emerg. Dis. 2020, 67, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.; Rahner, T.; Palmer, M.; Cheng, D.; Nonnecke, B.; Whipple, D. Expression of L-selectin (CD62L), CD44, and CD25 on activated bovine T cells. Infect. Immun. 2003, 71, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Toka, F.N.; Kenney, M.A.; Golde, W.T. Rapid and transient activation of γδ T cells to IFN-γ production, NK cell-like killing, and antigen processing during acute virus infection. J. Immunol. 2011, 186, 4853–4861. [Google Scholar] [CrossRef]
- Phalke, S.P.; Chiplunkar, S.V. Activation status of γδ T cells dictates their effect on osteoclast generation and bone resorption. Bone Rep. 2015, 3, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Babor, M.; Lane, J.; Schulten, V.; Patil, V.S.; Seumois, G.; Rosales, S.L.; Fu, Z.; Picarda, G.; Burel, J. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Carrasco, J.; Godelaine, D.; Van Pel, A.; Boon, T.; van der Bruggen, P. CD45RA on human CD8 T cells is sensitive to the time elapsed since the last antigenic stimulation. Blood 2006, 108, 2897–2905. [Google Scholar] [CrossRef]
- Faint, J.M.; Annels, N.E.; Curnow, S.J.; Shields, P.; Pilling, D.; Hislop, A.D.; Wu, L.; Akbar, A.N.; Buckley, C.D.; Moss, P.A. Memory T cells constitute a subset of the human CD8+ CD45RA+ pool with distinct phenotypic and migratory characteristics. J. Immunol. 2001, 167, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamann, D.; Baars, P.A.; Rep, M.H.; Hooibrink, B.; Kerkhof-Garde, S.R.; Klein, M.R.; Lier, R.A.v. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997, 186, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Deans, J.; Boyd, A.; Pilarski, L. Transitions from high to low molecular weight isoforms of CD45 (T200) involve rapid activation of alternate mRNA splicing and slow turnover of surface CD45R. J. Immunol. 1989, 143, 1233–1238. [Google Scholar]
- Birkeland, M.L.; Johnson, P.; Trowbridge, I.S.; Puré, E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc. Natl. Acad. Sci. USA 1989, 86, 6734–6738. [Google Scholar] [CrossRef] [Green Version]
- Akbar, A.; Terry, L.; Timms, A.; Beverley, P.; Janossy, G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J. Immunol. 1988, 140, 2171–2178. [Google Scholar] [PubMed]
- Zola, H.; Flego, L.; Macardle, P.; Donohoe, P.; Ranford, J.; Roberton, D. The CD45RO (p180, UCHL1) marker: Complexity of expression in peripheral blood. Cell. Immunol. 1992, 145, 175–186. [Google Scholar] [CrossRef]
- Prince, H.E.; York, J.; Jensen, E.R. Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell. Immunol. 1992, 145, 254–262. [Google Scholar] [CrossRef]
- Sathaliyawala, T.; Kubota, M.; Yudanin, N.; Turner, D.; Camp, P.; Thome, J.J.; Bickham, K.L.; Lerner, H.; Goldstein, M.; Sykes, M. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 2013, 38, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Jia, X.; Su, Y.; Li, Q. Immunophenotypic characterization of CD45RO+ and CD45RA+ T cell subsets in peripheral blood of peripheral T cell lymphoma patients. Cell Biochem. Biophys. 2014, 70, 993–997. [Google Scholar] [CrossRef]
- Qin, Y.; van den Noort, S.; Kurt, J.; Gupta, S. Dual expression of CD45RA and CD45RO isoforms on myelin basic protein-specific CD4+ T-cell lines in multiple sclerosis. J. Clin. Immunol. 1993, 13, 152–161. [Google Scholar] [CrossRef]
- Chowdhury, R.R.; Valainis, J.R.; Kask, O.; Ohanyan, M.; Sun, M.; Huang, H.; Dubey, M.; von Boehmer, L.; Sola, E.; Huang, X. The role of antigen recognition in the γδ T cell response at the controlled stage of M. tuberculosis infection. bioRxiv 2021, 460324:1–460324:56. [Google Scholar]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, A.; Arruda, L.; Rådestad, E.; Uhlin, M. CD8+ γδ T cells are more frequent in CMV seropositive bone marrow grafts and display phenotype of an adaptive immune response. Stem Cells Int. 2019, 2019, 6348060:1–6348060:13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecomte, O.; Fischer, A. Antigen-independent adhesion of CD45RA (naive) and CD45RO (memory) CD4 T cells to B cells. Int. Immunol. 1992, 4, 191–196. [Google Scholar] [CrossRef]
- Frolova, E.; Scott, C.; Jones, R. CD45RO+ T-cells immunoregulate spontaneous in vitro immunoglobulin production by normal and chronic lymphocytic leukaemia B-cells. Leuk. Lymphoma 1995, 18, 103–111. [Google Scholar] [CrossRef]

| Specificity | Clone | Isotype | Source |
|---|---|---|---|
| bCD3 | MM1A | IgG1 | WSUMAC |
| bCD4 | CC8 | IgG2a | Bio-Rad |
| bCD25 | LCTB2A | IgG3 | WSUMAC |
| bTCRδ | GB21A | IgG2b | WSUMAC |
| bTCRδ | CACT61A | IgM | WSUMAC |
| bCD45RA | GC6A | IgM | WSUMAC |
| bCD45RO | ILA116 | IgG3 | WSUMAC |
| bCD62L | BAQ92A | IgG1 | WSUMAC |
| bIFNγ | CC302 | IgG1 | Bio-Rad |
| bIL4 | CC303 | IgG2a | Bio-Rad |
| Specificity | Clone | Conjugated Fluorescence | Source |
| bCD4 | CC8 | PE | Bio-Rad |
| bCD8 | CC63 | FITC | Bio-Rad |
| bCD8 | CC58 | PE | Bio-Rad |
| bCD25 | IL-A111 | PE | Bio-Rad |
| bCD25 | IL-A111 | FITC | Bio-Rad |
| bCD62L | CC32 | FITC | Bio-Rad |
| bIFNγ | CC302 | PE | Bio-Rad |
| Specificity | Secondary Antibodies | Source |
|---|---|---|
| IgG1 | Anti-mouse IgG1-APC | BioLegend |
| IgG1 | Anti-mouse IgG1-Biotin | BioLegend |
| IgG2a | Anti-mouse IgG2a-APC | BioLegend |
| IgG2b | Anti-mouse IgG2b-PE | BioLegend |
| IgG2b | Anti-mouse IgG2b-Biotin | BioLegend |
| IgG2b | Anti-mouse IgG2b-FITC | BioLegend |
| IgG3 | Anti-mouse IgG3-Biotin | BioLegend |
| IgM | Anti-mouse IgM-BV421 | BioLegend |
| Biotin | Streptavidin Per-CP | BioLegend |
| Isotype Controls | Source | |
| Mouse IgG1 | BioLegend | |
| Mouse IgG2a | BioLegend | |
| Mouse IgG2b | BioLegend | |
| Mouse IgG3 | BioLegend | |
| Mouse IgM | BioLegend | |
| Mouse IgG1-FITC | BioLegend | |
| Mouse IgG1-PE | BioLegend | |
| Mouse IgG2a-FITC | BioLegend | |
| Mouse IgG2a-PE | BioLegend | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandel, A.; Li, L.; Hada, A.; Xiao, Z. Differential Expression of CD45RO and CD45RA in Bovine T Cells. Cells 2022, 11, 1844. https://doi.org/10.3390/cells11111844
Kandel A, Li L, Hada A, Xiao Z. Differential Expression of CD45RO and CD45RA in Bovine T Cells. Cells. 2022; 11(11):1844. https://doi.org/10.3390/cells11111844
Chicago/Turabian StyleKandel, Anmol, Lei Li, Akanksha Hada, and Zhengguo Xiao. 2022. "Differential Expression of CD45RO and CD45RA in Bovine T Cells" Cells 11, no. 11: 1844. https://doi.org/10.3390/cells11111844
APA StyleKandel, A., Li, L., Hada, A., & Xiao, Z. (2022). Differential Expression of CD45RO and CD45RA in Bovine T Cells. Cells, 11(11), 1844. https://doi.org/10.3390/cells11111844









