Osteopontin (OPN/SPP1), a Mediator of Tumor Progression, Is Regulated by the Mesenchymal Transcription Factor Slug/SNAI2 in Colorectal Cancer (CRC)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Cells
2.3. Tumor Xenogafts
2.4. Viability Assay
2.5. SiRNA
2.6. Promoter Reporter Assay
2.7. Chromatin Immunoprecipitation, ChIP Assay
2.8. Real-time RT (Reverse Transcription)-PCR
2.9. Western Blot
2.10. ELISA
2.11. Immunostaining
2.12. Migration and Invasion
2.13. RNA-Sequencing and Pathway Enrichment Analysis
2.14. Statistical Analysis
3. Results
3.1. Global Gene Expression Analysis in Slug/SNAI2-Transfected HT-29 Cells
3.2. Influence of Slug/SNAI2 on OPN Expression in CRC Cells with Different Genetic Background
3.3. Slug/SNAI2 Increases the Expression and Secretion of Osteopontin
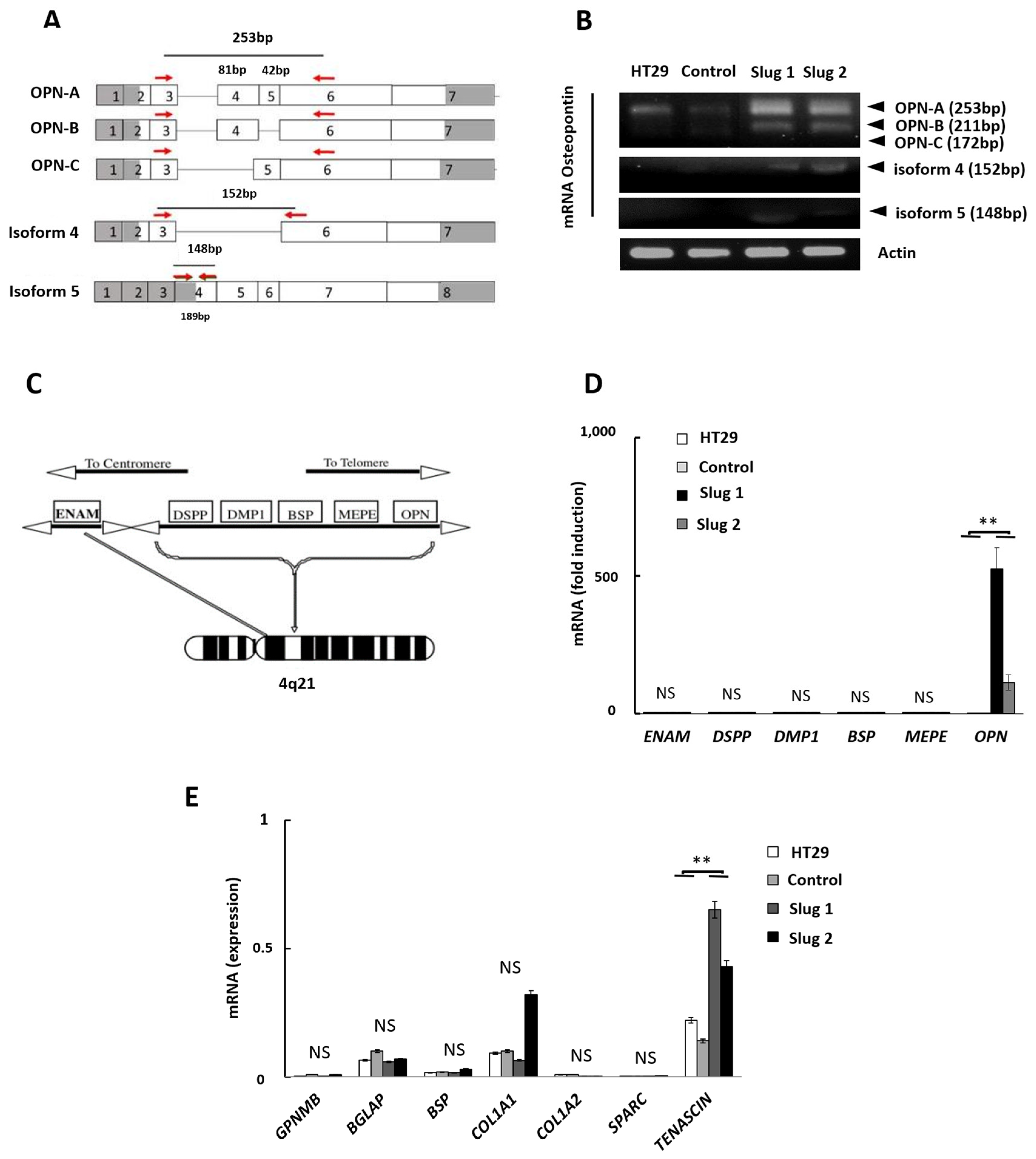
3.4. Influence of Slug/SNAI2 on the Expression of OPN Isoforms, SIBLING Genes, and other Bone-Related Genes
3.5. Slug/SNAI2 Directly Binds to the OPN Promoter and Enhances Its Transcriptional Activity
3.6. The Influence of Osteopontin on Cellular Growth, Migration and Invasion
3.7. Slug/SNAI2 Upregulates OPN in Human Tumor Xenografts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 21, 209–2479. [Google Scholar] [CrossRef]
- Basbinar, Y.; Calıbası-Kocal, G. Cancer Metastasis; IntechOpen: London, UK, 2018; ISBN 978-1-78984-633-1. [Google Scholar]
- Ortega, J.; Vigil, C.E.; Chodkiewicz, C. Current Progress in Targeted Therapy for Colorectal Cancer. Cancer Control J. Moffitt Cancer Cent. 2010, 17, 7–15. [Google Scholar] [CrossRef]
- Grassadonia, A.; Di Marino, P.; Ficorella, C.; Cortellini, A.; Cannita, K.; Parisi, A.; Gamucci, T.; Zoratto, F.; Vici, P.; Barba, M.; et al. Impact of Primary Tumor Location in Patients with RAS Wild-Type Metastatic Colon Cancer Treated with First-Line Chemotherapy plus Anti-EGFR or Anti-VEGF Monoclonal Antibodies: A Retrospective Multicenter Study. J. Cancer 2019, 10, 5926–5934. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–Mesenchymal Transitions in Development and Pathologies. Curr. Opin. Cell Biol. 2003, 15, 740–746. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.Z.; Miow, Q.H.; Miki, Y.; Noda, T.; Mori, S.; Huang, R.Y.; Thiery, J.P. Epithelial-mesenchymal Transition Spectrum Quantification and Its Efficacy in Deciphering Survival and Drug Responses of Cancer Patients. EMBO Mol. Med. 2014, 6, 1279–1293. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Nieto, M.A.; Sargent, M.G.; Wilkinson, D.G.; Cooke, J. Control of Cell Behavior during Vertebrate Development by Slug, a Zinc Finger Gene. Science 1994, 264, 835–839. [Google Scholar] [CrossRef]
- Toiyama, Y.; Yasuda, H.; Saigusa, S.; Tanaka, K.; Inoue, Y.; Goel, A.; Kusunoki, M. Increased Expression of Slug and Vimentin as Novel Predictive Biomarkers for Lymph Node Metastasis and Poor Prognosis in Colorectal Cancer. Carcinogenesis 2013, 34, 2548–2557. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Mancera, P.A.; González-Herrero, I.; Pérez-Caro, M.; Gutiérrez-Cianca, N.; Flores, T.; Gutiérrez-Adán, A.; Pintado, B.; Sánchez-Martín, M.; Sánchez-García, I. SLUG in Cancer Development. Oncogene 2005, 24, 3073–3082. [Google Scholar] [CrossRef] [Green Version]
- Shioiri, M.; Shida, T.; Koda, K.; Oda, K.; Seike, K.; Nishimura, M.; Takano, S.; Miyazaki, M. Slug Expression Is an Independent Prognostic Parameter for Poor Survival in Colorectal Carcinoma Patients. Br. J. Cancer 2006, 94, 1816–1822. [Google Scholar] [CrossRef] [Green Version]
- Amilca-Seba, K.; Sabbah, M.; Larsen, A.K.; Denis, J.A. Osteopontin as a Regulator of Colorectal Cancer Progression and Its Clinical Applications. Cancers 2021, 13, 3793. [Google Scholar] [CrossRef]
- El-Tanani, M.K.; Campbell, F.C.; Kurisetty, V.; Jin, D.; McCann, M.; Rudland, P.S. The Regulation and Role of Osteopontin in Malignant Transformation and Cancer. Cytokine Growth Factor Rev. 2006, 17, 463–474. [Google Scholar] [CrossRef]
- Zhao, M.; Liang, F.; Zhang, B.; Yan, W.; Zhang, J. The Impact of Osteopontin on Prognosis and Clinicopathology of Colorectal Cancer Patients: A Systematic Meta-Analysis. Sci. Rep. 2015, 5, 12713. [Google Scholar] [CrossRef]
- Mésange, P.; Poindessous, V.; Sabbah, M.; Escargueil, A.E.; de Gramont, A.; Larsen, A.K. Intrinsic Bevacizumab Resistance Is Associated with Prolonged Activation of Autocrine VEGF Signaling and Hypoxia Tolerance in Colorectal Cancer Cells and Can Be Overcome by Nintedanib, a Small Molecule Angiokinase Inhibitor. Oncotarget 2014, 5, 4709–4721. [Google Scholar] [CrossRef] [Green Version]
- Poindessous, V.; Koeppel, F.; Raymond, E.; Comisso, M.; Waters, S.J.; Larsen, A.K. Marked Activity of Irofulven toward Human Carcinoma Cells: Comparison with Cisplatin and Ecteinascidin. Clin. Cancer Res. 2003, 9, 2817–2825. [Google Scholar]
- Bastos, A.C.S.F.; Blunck, C.B.; Emerenciano, M.; Gimba, E.R.P. Osteopontin and Their Roles in Hematological Malignancies: Splice Variants on the New Avenues. Cancer Lett. 2017, 408, 138–143. [Google Scholar] [CrossRef]
- Bolós, V.; Peinado, H.; Pérez-Moreno, M.A.; Fraga, M.F.; Esteller, M.; Cano, A. The Transcription Factor Slug Represses E-Cadherin Expression and Induces Epithelial to Mesenchymal Transitions: A Comparison with Snail and E47 Repressors. J. Cell Sci. 2003, 116, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Sun, B.; Sun, D.; Liu, T.; Che, N.; Gu, Q.; Dong, X.; Li, R.; Liu, Y.; Li, J. Slug Promotes Hepatocellular Cancer Cell Progression by Increasing Sox2 and Nanog Expression. Oncol. Rep. 2015, 33, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Cheng, X.; Li, Y.; Guo, M.; Zhao, W.; Qiu, J.; Zheng, Y.; Meng, M.; Ping, X.; Chen, X.; et al. C/EBPδ-Slug-Lox1 Axis Promotes Metastasis of Lung Adenocarcinoma via OxLDL Uptake. Oncogene 2020, 39, 833–848. [Google Scholar] [CrossRef]
- Lambertini, E.; Lisignoli, G.; Torreggiani, E.; Manferdini, C.; Gabusi, E.; Franceschetti, T.; Penolazzi, L.; Gambari, R.; Facchini, A.; Piva, R. Slug Gene Expression Supports Human Osteoblast Maturation. Cell. Mol. Life Sci. CMLS 2009, 66, 3641–3653. [Google Scholar] [CrossRef]
- Many, G.M.; Yokosaki, Y.; Uaesoontrachoon, K.; Nghiem, P.P.; Bello, L.; Dadgar, S.; Yin, Y.; Damsker, J.M.; Cohen, H.B.; Kornegay, J.N.; et al. OPN-a Induces Muscle Inflammation by Increasing Recruitment and Activation of pro-Inflammatory Macrophages. Exp. Physiol. 2016, 101, 1285–1300. [Google Scholar] [CrossRef]
- Sun, J.; Feng, A.; Chen, S.; Zhang, Y.; Xie, Q.; Yang, M.; Shao, Q.; Liu, J.; Yang, Q.; Kong, B.; et al. Osteopontin Splice Variants Expressed by Breast Tumors Regulate Monocyte Activation via MCP-1 and TGF-Β1. Cell. Mol. Immunol. 2013, 10, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Nakahara, F.; Asada, N.; Zhang, D.; Gao, X.; Xu, C.; Alfieri, A.; Brodin, N.P.; Zimmerman, S.E.; Mar, J.C.; et al. Snai2 Maintains Bone Marrow Niche Cells by Repressing Osteopontin Expression. Dev. Cell 2020, 53, 503–513.e5. [Google Scholar] [CrossRef]
- Marin, F.; Nieto, M.A. Expression of Chicken Slug and Snail in Mesenchymal Components of the Developing Central Nervous System. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2004, 230, 144–148. [Google Scholar] [CrossRef]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and BHLH Factors in Tumour Progression: An Alliance against the Epithelial Phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef]
- Wels, C.; Joshi, S.; Koefinger, P.; Bergler, H.; Schaider, H. Transcriptional Activation of ZEB1 by Slug Leads to Cooperative Regulation of the Epithelial-Mesenchymal Transition-like Phenotype in Melanoma. J. Investig. Dermatol. 2011, 131, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Uygur, B.; Wu, W.-S. SLUG Promotes Prostate Cancer Cell Migration and Invasion via CXCR4/CXCL12 Axis. Mol. Cancer 2011, 10, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, R.; Mallets, E.; Gomez-Cambronero, J. The Transcription Factors Slug (SNAI2) and Snail (SNAI1) Regulate Phospholipase D (PLD) Promoter in Opposite Ways towards Cancer Cell Invasion. Mol. Oncol. 2016, 10, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Uppuladinne, M.V.N.; Jani, V.; Sonavane, U.; Joshi, R.R.; Bapat, S.A. Auto-Regulation of Slug Mediates Its Activity during Epithelial to Mesenchymal Transition. Biochim. Biophys. Acta 2015, 1849, 1209–1218. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wen, G.; Sun, Y.; Shen, Y.; Zeng, Y.; Du, M.; Zhu, G.; Wang, G.; Meng, X. Osteopontin Promotes Colorectal Cancer Cell Invasion and the Stem Cell-Like Properties through the PI3K-AKT-GSK/3β-β/Catenin Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3014–3025. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Lett, G.S.; Haubein, N.C. Osteopontin Is a Marker for Cancer Aggressiveness and Patient Survival. Br. J. Cancer 2010, 103, 861–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prall, F.; Maletzki, C.; Linnebacher, M. Microdensitometry of osteopontin as an immunohistochemical prognostic biomarker in colorectal carcinoma tissue microarrays: Potential and limitations of the method in ‘biomarker pathology’. Histopathology 2012, 61, 823–832. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amilca-Seba, K.; Tan, T.Z.; Thiery, J.-P.; Louadj, L.; Thouroude, S.; Bouygues, A.; Sabbah, M.; Larsen, A.K.; Denis, J.A. Osteopontin (OPN/SPP1), a Mediator of Tumor Progression, Is Regulated by the Mesenchymal Transcription Factor Slug/SNAI2 in Colorectal Cancer (CRC). Cells 2022, 11, 1808. https://doi.org/10.3390/cells11111808
Amilca-Seba K, Tan TZ, Thiery J-P, Louadj L, Thouroude S, Bouygues A, Sabbah M, Larsen AK, Denis JA. Osteopontin (OPN/SPP1), a Mediator of Tumor Progression, Is Regulated by the Mesenchymal Transcription Factor Slug/SNAI2 in Colorectal Cancer (CRC). Cells. 2022; 11(11):1808. https://doi.org/10.3390/cells11111808
Chicago/Turabian StyleAmilca-Seba, Katyana, Tuan Zea Tan, Jean-Paul Thiery, Lila Louadj, Sandrine Thouroude, Anaïs Bouygues, Michèle Sabbah, Annette K. Larsen, and Jérôme A. Denis. 2022. "Osteopontin (OPN/SPP1), a Mediator of Tumor Progression, Is Regulated by the Mesenchymal Transcription Factor Slug/SNAI2 in Colorectal Cancer (CRC)" Cells 11, no. 11: 1808. https://doi.org/10.3390/cells11111808
APA StyleAmilca-Seba, K., Tan, T. Z., Thiery, J.-P., Louadj, L., Thouroude, S., Bouygues, A., Sabbah, M., Larsen, A. K., & Denis, J. A. (2022). Osteopontin (OPN/SPP1), a Mediator of Tumor Progression, Is Regulated by the Mesenchymal Transcription Factor Slug/SNAI2 in Colorectal Cancer (CRC). Cells, 11(11), 1808. https://doi.org/10.3390/cells11111808







