Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics
Abstract
1. Introduction
2. Cellular Distribution and Membrane Topology
3. Ligand Binding
4. Structure of the Ligand-Binding Domain
5. Signaling through G Proteins
5.1. G Protein Activation
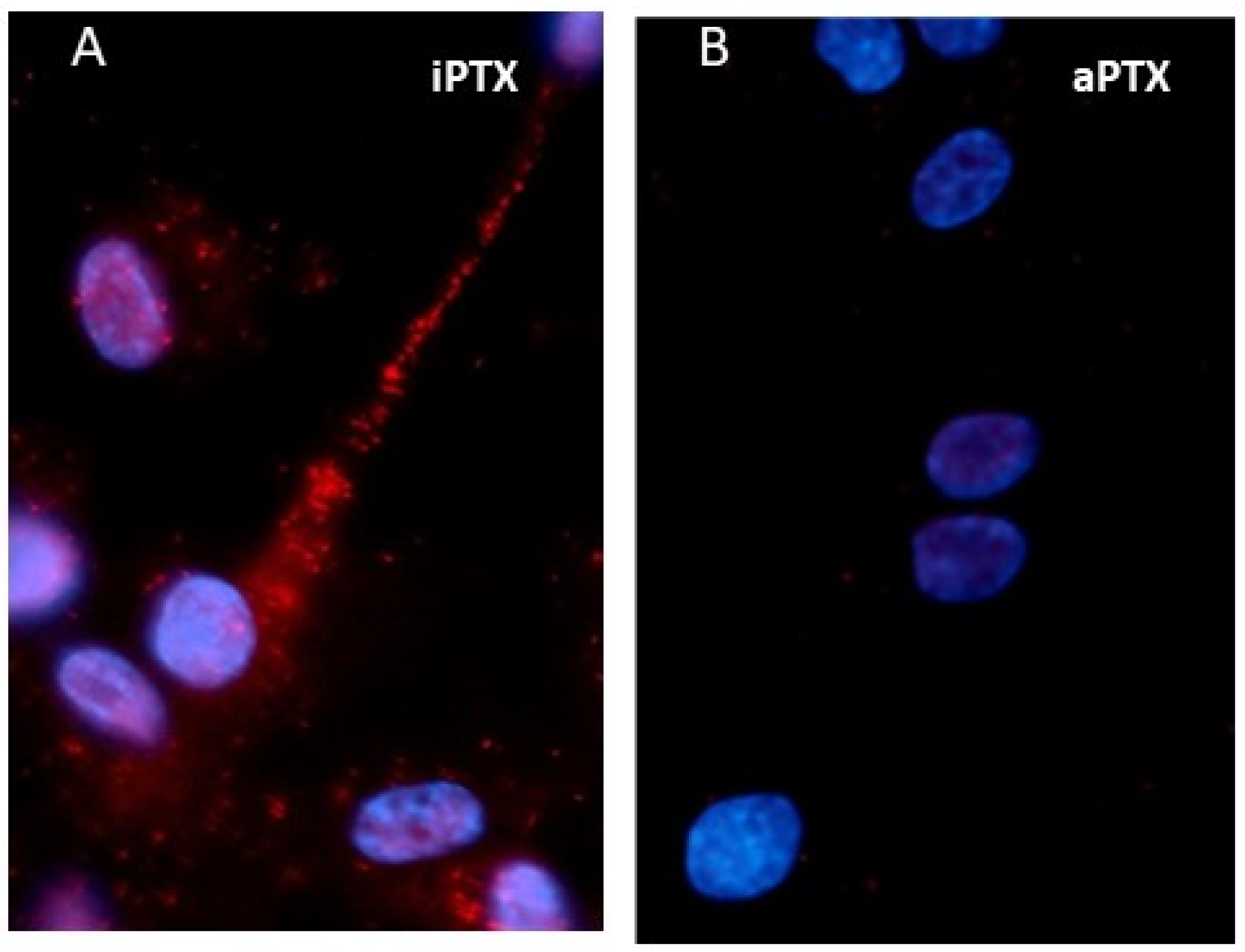
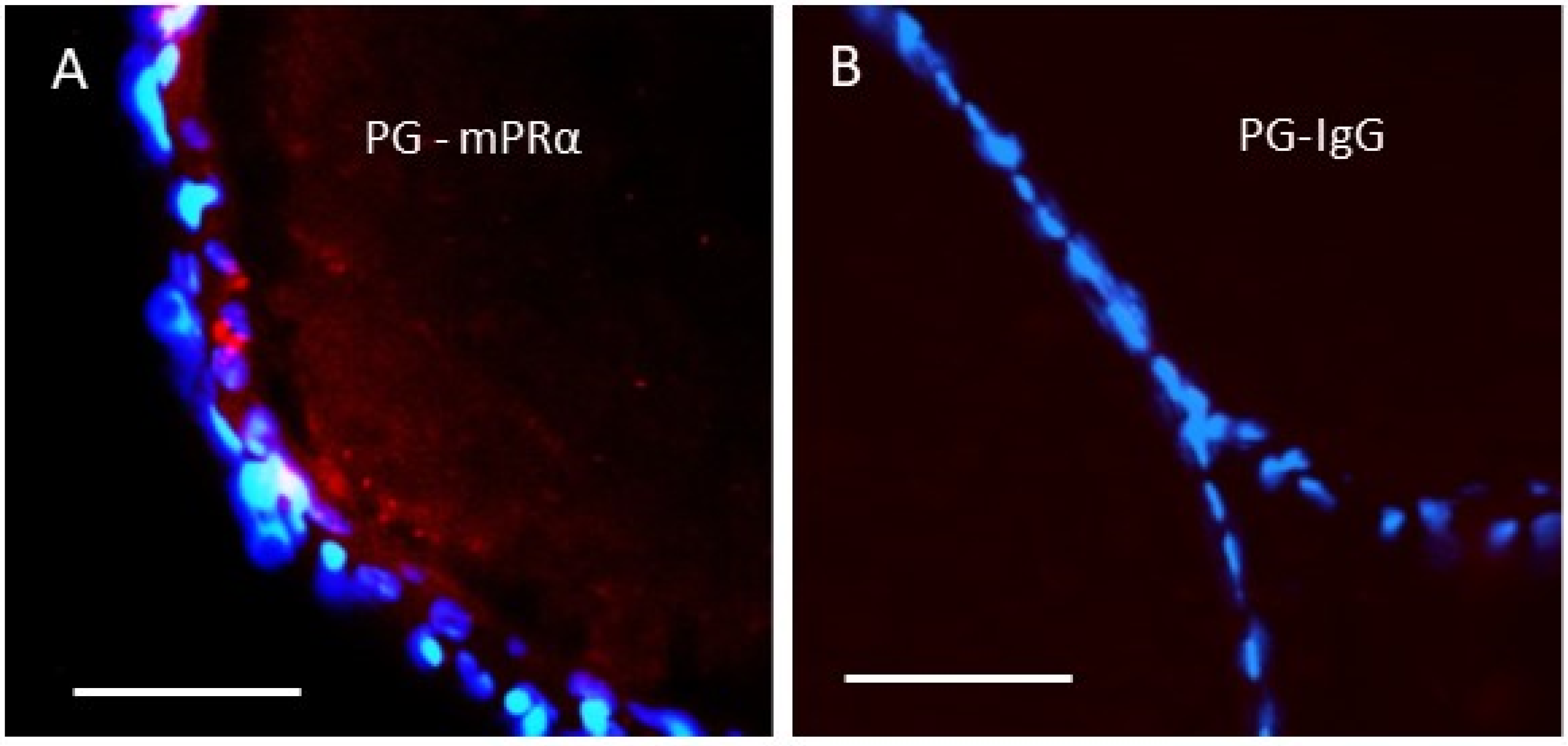
5.2. Association of mPRs with G Proteins
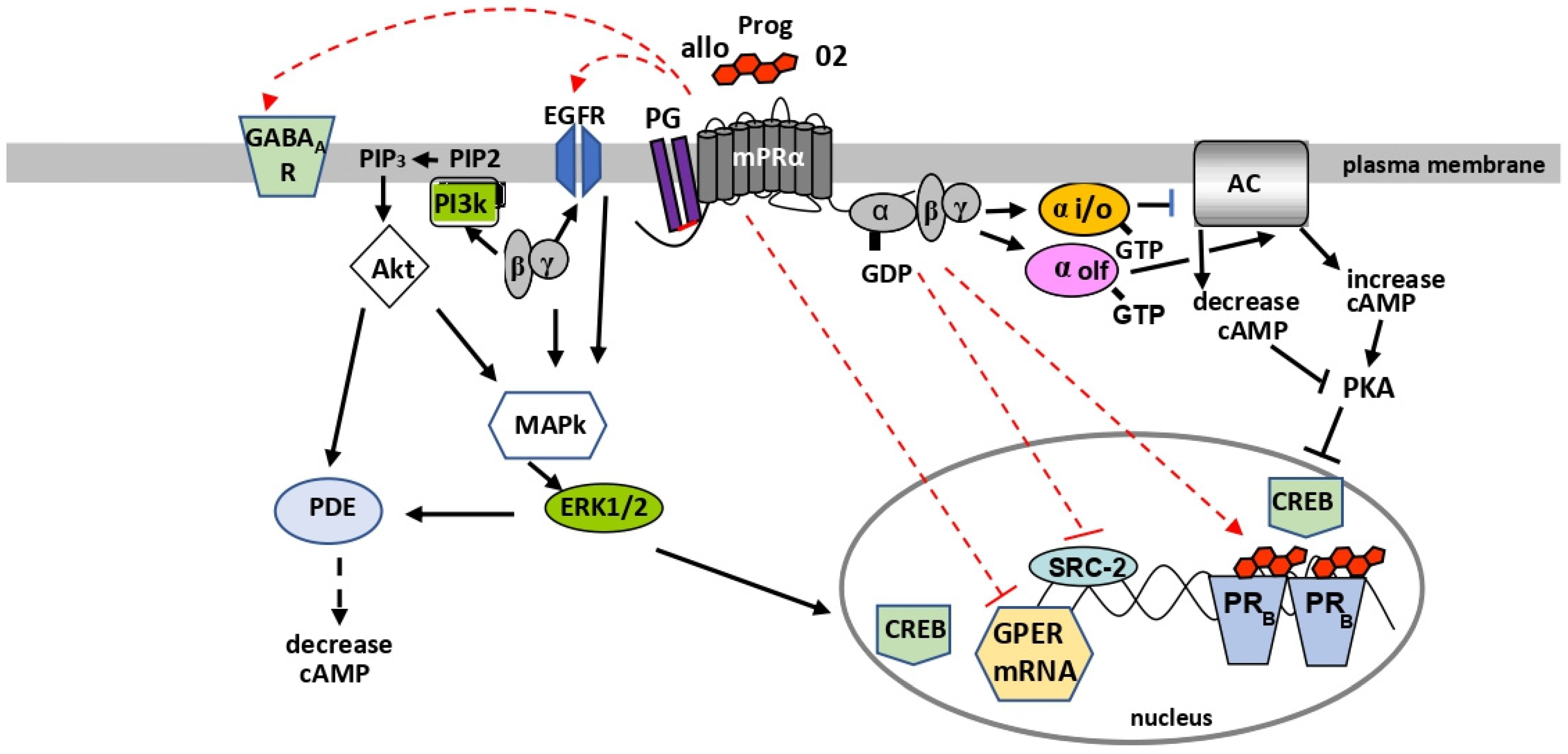
6. Second Messenger Signaling through G Proteins
6.1. cAMP/PKA Signaling
6.2. PI3K/AKT and MAPkinase/ERK1/2 Signaling
6.3. Activation of Multiple Signaling Pathways
7. Association of mPRs with Adaptor Proteins
7.1. Association of mPRα with PGRMC1
7.2. Association of mPRβ with APPL1 and VLDL
8. mPR Regulation of PRs, GABAA Receptors, and GPER
8.1. Regulation of PR Transactivation
8.2. Regulation of GABAA Receptor Phosphorylation
8.3. Regulation of GPER Expression
9. Conclusions and Future Studies
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mPRs | Membrane progesterone receptors |
| PAQR | Progestin and adipoQ receptor family |
| mPRα | Membrane progesterone receptor alpha (PAQR7) |
| mPRβ | Membrane progesterone receptor beta (PAQR8) |
| mPRγ | Membrane progesterone receptor gamma (PAQR5) |
| mPRδ | Membrane progesterone receptor delta (PAQR6) |
| mPRε | Membrane progesterone receptor epsilon (PAQR9) |
| PR | Nuclear progesterone receptor |
| PGRMC1 | Progesterone receptor component 1 |
| GPER | G protein-coupled estrogen receptor |
| EGFR | Epidermal growth factor receptor |
| GPCRs | G protein-coupled receptors |
| AdipoRs | Adiponectin receptors |
| AdipoR1 | Adiponectin receptor 1 |
| AdipoR2 | Adiponectin receptor 2 |
| GABAA | Gamma butyric acid type A |
| Gi | Inhibitory G protein |
| GS | Stimulatory G protei |
| Go | Olfactory G protein |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Serine–threonine kinase |
| MAPkinase | Mitogen-activated protein kinase |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| PKA | Protein kinase A |
| PDE | Phosphodiesterase |
| AC | Adenylyl cyclase |
| CREB | cAMP response element-binding protein |
References
- Zhu, Y.; Rice, C.D.; Pang, Y.F.; Pace, M.; Thomas, P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component one (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008, 29, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Dong, J.; Groenen, P.; Kelder, J.; de Vlieg, J.; Zhu, Y.; Tubbs, C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor α subtypes and their evolutionary origins. Endocrinology 2007, 148, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.T.; Hu, T.; Arterburn, M.; Boyle, B.; Bright, J.M.; Emtage, P.C.; Funk, W.D. PAQR proteins: A novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J. Mol. Evol. 2005, 61, 372–380. [Google Scholar] [CrossRef]
- Ren, J.; Chung-Davidson, Y.-W.; Jia, L.; Li, W. Genomic sequence analyses of classical and non-classical lamprey progesterone receptor genes and the inference of homologous gene evolution in metazoans. BMC Evol. Biol. 2019, 19, 136. [Google Scholar] [CrossRef]
- Ryu, C.S.; Klein, K.; Zanger, U.M. Membrane associated progesterone receptors: Promiscuous proteins with pleiotropic functions-focus on interactions with cytochromes P450. Front. Pharmacol. 2017, 8, 159. [Google Scholar] [CrossRef]
- Scarpin, K.M.; Grahaam, J.D.; Mote, P.A.; Clarke, C.L. Progesterone action in human tissues: Regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl. Recep. Sign. 2009, 7, e009. [Google Scholar] [CrossRef]
- Thomas, P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish model. Gen. Comp. Endocrinol. 2012, 175, 367–383. [Google Scholar] [CrossRef]
- Tan, W.; Pang, Y.; Tubbs, C.; Thomas, P. Induction of sperm hypermotility through membrane progestin receptor alpha (mPRα): A teleost model of rapid, multifaceted, nongenomic progestin signaling. Gen. Comp. Endocrinol. 2019, 279, 60–66. [Google Scholar] [CrossRef]
- Ben-Yehoshua, L.J.; Lewellyn, A.L.; Thomas, P.; Maller, J.L. The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol. Endocrinol. 2007, 21, 664–673. [Google Scholar] [CrossRef]
- Aparicio, I.M.; Garcia-Herreros, M.; O’Shea, L.C.; Hensey, C.; Lonergan, P.; Fair, T. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol. Reprod. 2011, 84, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Tubbs, C.; Garry, V.F. Progestin function in vertebrate gametes mediated by membrane progestin receptors (mPRs): Identification of mPRα on human sperm and its association with sperm motility. Steroids 2009, 74, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Cosmes, P.; Vázquez-Martínez, E.R.; Cerbόn, M.; Camacho-Arroyo, I. Membrane progesterone receptor in reproduction and cancer. Mol. Cell. Endocrinol. 2016, 434, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Medina-Laver, Y.; Rodríguez-Varela, C.; Salsano, S.; Labarta, E.; Domínguez, F. What do we know about classical and non-classical progesterone receptors in the human female reproductive tract? A review. Int. J. Mol. Sci. 2021, 22, 11278. [Google Scholar] [CrossRef]
- Mittelman-Smith, M.A.; Rudolph, L.M.; Mohr, M.A.; Micevych, P.E. Rodent models of non-classical progesterone action regulating ovulation. Front. Endocrinol. 2017, 8, 165. [Google Scholar] [CrossRef]
- Dressing, G.E.; Goldberg, J.E.; Charles, N.J.; Schwertfeger, K.L.; Lange, C.A. Membrane progesterone receptor expression in mammalian tissues: A review of regulation and physiological implications. Steroids 2011, 76, 11–17. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, T.; Zheng, L.; Huang, J.; Zheng, Y.; Li, J. PAQR7: An intermediary mediating nongenomic progesterone action in female reproductive tissue. Reprod. Biol. 2021, 21, 100529. [Google Scholar] [CrossRef]
- Pedroza, D.A.; Subramani, R.; Lakshamanaswamy, R. Classical and non-classical progesterone signaling in breast cancers. Cancers 2020, 12, 2440. [Google Scholar] [CrossRef]
- Krietsch, T.; Fernandes, M.S.; Kero, J.; Losel, R.; Heyens, M.; Lam, E.-W.F.; Huhtaniemi, I.; Brosens, J.J.; Gellersen, B. Human homologs of the putative G protein-coupled membrane progestin receptors mPR [alpha, beta, and gamma] localize to the endoplasmic reticulum and are not activated by progesterone. Mol. Endocrinol. 2006, 20, 3146–3164. [Google Scholar] [CrossRef]
- Smith, J.L.; Kupchak, B.R.; Garitaonandia, I.; Hoang, L.K.; Maina, A.S.; Regalla, L.M.; Lyons, T.J. Heterologous expression of human mPRα, mPRβ and mPRγ in yeast confirms their ability to function as membrane progesterone receptors. Steroids 2008, 73, 1160–1173. [Google Scholar] [CrossRef]
- Kelder, J.; Pang, Y.; Dong, J.; Schaftenaar, G.; Thomas, P. Molecular modeling, mutational analysis and steroid specificity of the ligand binding pocket of mPRα (PAQR7): Shared ligand binding with AdipoR1 and its structural basis. J. Steroid Biochem. Mol. Biol. 2022, 218, 106082. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bond, J.; Thomas, P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Petäjä-Repo, U.E.; Hogue, M.; Laperriere, A.; Walker, P.; Bouvier, M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human opioid receptor. J. Biol. Chem. 2000, 275, 13727–13736. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.; Reynolds, A.; Stenbeck, G.; Dong, J.; Thomas, P.; Karteris, E. Internalization of membrane progesterone receptor α (mPRα) after treatment with progesterone: Potential involvement of a clathrin-dependent pathway. Mol. Med. Rep. 2010, 3, 27–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nader, N.; Dib, M.; Hodeify, R.; Courjaret, R.; Elmi, A.; Hammad, A.S.; Dey, R.; Huang, X.-Y.; Machaca, K. Membrane progesterone receptor induces meiosis in Xenopus oocytes through endocytosis into signaling endosomes and interaction with APPL1 and Akt2. PLoS Biol. 2021, 19, e30001117. [Google Scholar] [CrossRef] [PubMed]
- Deckert, C.M.; Heiker, J.T.; Beck-Sickinger, A.G. Localization of novel adiponectin constructs. J. Recept. Signal. Transduct. 2006, 26, 647–657. [Google Scholar] [CrossRef]
- Morrill, G.E.; Kostellow, A.B.; Gupta, R.K. A computational analysis of non-genomic plasma membrane progestin binding proteins: Signaling through ion channel-linked cell surface receptors. Steroids 2013, 78, 1233–1244. [Google Scholar] [CrossRef]
- Jyoti Md, M.S.; Rana Md, R.; Ali Md, H.; Tokomoto, T. Establishment of a steroid binding assay for membrane progesterone receptor alpha (PAQR7) by using graphene quantum dots (GQDs). Biochem. Biophys. Res. Commun. 2022, 592, 1–6. [Google Scholar] [CrossRef]
- Pang, Y.; Dong, J.; Thomas, P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and ε (mPRδ and mPRε) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology 2013, 154, 283–295. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Anti-apoptotic actions of allopregnanolone and ganaxolone mediated through membrane progesterone receptors (PAQRs) in neuronal cells. Front. Endocrinol. 2020, 11, 417. [Google Scholar] [CrossRef]
- Kelder, J.; Azevedo, R.; Pang, Y.; Vlieg, J.; Dong, J.; Thomas, P. Comparison between steroid binding to membrane progesterone receptor α (mPRα) and to nuclear progesterone receptor: Correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRα-specific agonists. Steroids 2010, 75, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Polikarpova, A.V.; Maslakova, A.A.; Levina, I.S.; Kulikova, L.E.; Kuznetsov, Y.V.; Gueva, A.A.; Shchelkunova, T.A.; Zavarzin, L.V.; Smirnova, O.V. Selection of progesterone derivatives specific to membrane progesterone receptors. Biochemistry 2017, 82, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Aizen, J.; Thomas, P. Membrane progestin receptor alpha mediates flounder progestin-induced sperm hypermotility and increased fertilization success in southern (Paralichthys lethostigma). Gen. Comp. Endocrinol. 2014, 200, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Aizen, J.; Pang, Y.; Harris, C.; Converse, A.; Zhu, Y.; Aguirre, M.; Thomas, P. Roles of progesterone receptor membrane component 1 and membrane progestin receptor alpha in regulation of zebrafish oocyte maturation. Gen. Comp. Endocrinol. 2018, 263, 51–61. [Google Scholar] [CrossRef] [PubMed]
- González-Orozco, J.C.; Hansberg-Pastor, V.; Valadez-Cosmes, P.; Nicolas-Ortega, W.; Bastida-Beristain, Y.; De La Fuente-Granada, M.; González-Arenas, A.; Camacho-Arroyo, I. Activation of membrane progesterone receptor-alpha increases proliferation, migration, and invasion of human glioblastoma cells. Mol. Cell. Endocrinol. 2018, 477, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Castelnovo, L.F.; Caffino, L.; Bonalume, V.; Fumagalli, F.; Thomas, P.; Magnaghi, V. Membrane progesterone receptors (mPRs/PAQRs) differently regulate migration, proliferation and differentiation of rat Schwann cells. J. Mol. Neurosci. 2020, 70, 433–448. [Google Scholar] [CrossRef]
- Dressing, G.E.; Alyea, R.; Pang, Y.; Thomas, P. Membrane progesterone receptors (mPRs) mediate antimorbidity in breast cancer cells and are expressed in human breast tumors. Horm. Cancer 2012, 3, 101–112. [Google Scholar] [CrossRef]
- Zachariades, E.; Mparmpakas, D.; Pang, Y.; Rand-Weaver, M.; Thomas, P.; Karteris, E. Changes in placental membrane progesterone receptors in term and preterm labour. Placenta 2012, 33, 367–372. [Google Scholar] [CrossRef]
- Lu, X.; Guan, A.; Chen, X.; Xiao, J.; Xie, M.; Yang, B.; He, S.; You, S.; Li, W.; Chen, Q. mPRα mediates P4/Org OD 02-0 to improve the sensitivity of lung adenocarcinoma to EGFR-TKIs via the EGFR-SRC-ERK1/2 pathway. Mol. Carcinog. 2020, 59, 179–192. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Li, W.; Zhang, C.; Su, P.; Wang, Y.; Sun, W.; Wang, X.; Li, L.; Wu, X. Rapamycin antagonizes BCRP-mediated drug resistance through the PI3K/Akt/mTOR signaling pathway in mPRα-positive breast cancer. Front. Oncol. 2021, 11, 608570. [Google Scholar] [CrossRef]
- Vasiliauskaité-Brooks, I.; Sounier, R.; Rochaux, P.; Bellot, G.; Fortier, M.; Hoh, F.; Colibus, L.D.; Bechara, C.; Saied, E.M.; Arenz, C.; et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 2017, 544, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Letz, M.; Bringmann, P.; Mann, M.; Mueller-Fahrnow, A.; Reipert, D.; Scholz, P.; Wurtz, J.M.; Egner, U. Investigation of the binding interactions of progesterone using mutein of the human progesterone receptor ligand binding domain designed on the basis of a three-dimensional protein model. Biochim. Biophys. Acta 1999, 1429, 391–400. [Google Scholar] [CrossRef]
- Moussatche, P.; Lyons, T.J. Non-genomic progesterone signaling and its non-canonical receptor. Biochem. Soc. Trans. 2012, 40, 200–204. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Nodo, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.J.; Lee, G.Y.; Chung, J.-J.; Ahn, Y.H.; Hong, S.H.; Kim, J.B. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen activated protein kinase and peroxisome proliferator-activated receptor. Diabetes 2006, 55, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Watanabe, K.; Hirano, K.; Inoue, D.; Li, X.; Terasawa, K.; Konishi, M.; Itoh, N.; Kimura, I. Membrane progesterone receptor beta (mPRβ/Paqr8) promotes progesterone-dependent neurite outgrowth in PC12 neuronal cells via a non-G protein-coupled receptor (GPCR) signaling. Sci. Rep. 2017, 7, 5168. [Google Scholar] [CrossRef] [PubMed]
- Karteris, E.; Zervou, S.; Pang, Y.; Dong, J.; Hillhouse, E.W.; Randeva, H.S.; Thomas, P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: Potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 2006, 20, 1519–1534. [Google Scholar] [CrossRef]
- Pang, Y.J.; Dong, J.; Thomas, P. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor alpha (mPRα). Am. J. Physiol. Endocrinol. Metab. 2015, 308, E899–E911. [Google Scholar] [CrossRef]
- Sleiter, N.; Pang, Y.; Perk, C.; Horton, T.H.; Dong, J.; Thomas, P.; Levine, J.E. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology 2009, 150, 3833–3844. [Google Scholar] [CrossRef]
- Pang, Y.; Thomas, P. Progesterone induces relaxation of vascular smooth muscle cells through mPRα (PAQR7). Mol. Cell. Endocrinol. 2018, 474, 20–34. [Google Scholar] [CrossRef]
- Dressing, G.; Pang, Y.; Dong, J.; Thomas, P. Progestin signaling through mPRα in Atlantic croaker granulosa/theca cell co-cultures and its involvement in progestin inhibition of apoptosis. Endocrinology 2010, 151, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, C.; Thomas, P. Progestin signaling through an olfactory G protein and membrane progestin receptor alpha in Atlantic croaker sperm: Potential role in induction of sperm hypermotility. Endocrinology 2009, 150, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Pace, M.C.; Thomas, P. Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev. Biol. 2005, 286, 70–79. [Google Scholar] [CrossRef]
- Tubbs, C.; Tan, W.; Shi, B.; Thomas, P. Identification of 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) receptor binding and membrane progestin alpha on southern flounder sperm (Paralichthys lethostigma) and their likely role in 20β-S stimulation of sperm motility. Gen. Comp. Endocrinol. 2011, 170, 629–639. [Google Scholar] [CrossRef]
- Dosiou, C.; Hamilton, A.E.; Pang, Y.; Overgaard, M.T.; Tulac, S.; Dong, J.; Thomas, P.; Guidice, L.C. Expression of membrane progesterone receptors (mPRs) on human T lymphocytes and Jurkat cells and activation of G proteins by progesterone. J. Endocrinol. 2008, 196, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Ashley, R.L.; Clay, C.M.; Farmerie, T.A.; Niswender, G.D.; Nett, T.M. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology 2006, 147, 4151–4159. [Google Scholar] [CrossRef]
- Hawes, B.E.; Luttrell, L.M.; van Biesen, T.; Lefkowitz, R.J. Phosphatidylinositol 3-kinase an early intermediate in the Gβγ-mediated mitogen activated protein kinase signaling pathway. J. Biol Chem. 1996, 271, 12133–12136. [Google Scholar] [CrossRef]
- Hanna, R.; Pang, Y.; Thomas, P.; Zhu, Y. 2006 Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors α and β in transfected cells. J. Endocrinol. 2006, 190, 247–260. [Google Scholar] [CrossRef]
- Tan, W.; Thomas, P. Activation of the Pi3k/Akt pathway and modulation of phosphodiesterase activity via membrane progestin receptor-alpha (mPRalpha) regulate progestin-initiated sperm hypermotility in Atlantic croaker. Biol. Reprod. 2014, 90, 105. [Google Scholar] [CrossRef]
- Salazar, M.; Lerma-Ortiz, A.; Hooks, G.M.; Ashley, A.K.; Ashley, R.L. Progestin-mediated activation of MAPK and AKT in nuclear progesterone receptor negative breast cancer epithelial cells: The role of membrane progesterone receptors. Gene 2016, 591, 6–13. [Google Scholar] [CrossRef]
- Song, G.; Ouyang, G.; Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 2005, 9, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Li, W.; You, S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells via a membrane progesterone receptor mediated pathway. Breast Cancer Res. 2010, 12, R34. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Thomas, P. Involvement of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) in mPRα (PAQR7)- mediated progesterone induction of vascular smooth muscle relaxation. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E453–E466. [Google Scholar] [CrossRef] [PubMed]
- Werry, T.D.; Sexton, P.M.; Christopoulos, A. “Ins and outs” of seven transmembrane receptor signaling to ERK. Trends Endocrinol. Metab. 2005, 16, 26–33. [Google Scholar] [CrossRef]
- Tan, W.; Thomas, P. Involvement of epidermal growth factor receptors and mitogen-activated protein kinase in progestin induction of sperm hypermotility in Atlantic croaker through membrane progestin receptor-alpha. Mol. Cell. Endocrinol. 2015, 414, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Charles, N.J.; Thomas, P.; Lange, C.A. Expression of membrane progesterone receptors (mPR/PAQR) in ovarian cancer cells: Implications for progesterone-induced signaling events. Horm. Cancer 2010, 1, 167–176. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, X.; Lu, X.; Xie, M.; He, B.; He, S.; You, S.; Chen, Q. Progesterone/Org inhibits lung adenocarcinoma cell growth via membrane progesterone receptor alpha. Thorac. Cancer 2020, 11, 2209–2223. [Google Scholar] [CrossRef]
- Lu, J.; Reese, J.; Zhou, Y.; Hirsch, E. Progesterone-induced activation of membrane-bound progesterone receptors in murine macrophage cells. J. Endocrinol. 2015, 224, 183–194. [Google Scholar] [CrossRef]
- Lösel, R.M.; Besong, D.; Peluso, J.J.; Wehling, M. Progesterone receptor membrane component 1-Many tasks for a versatile protein. Steroids 2008, 73, 929–934. [Google Scholar] [CrossRef]
- Cahil, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef]
- Hampton, K.K.; Frazier, H.; Anderson, K.; Thibault, O.; Craven, R.J. Insulin receptor plasma membrane levels increased by the progesterone receptor membrane component 1. Mol. Pharmacol. 2018, 94, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Rohe, H.J.; Twist, K.E.; Craven, R.J. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J. Biol. Chem. 2010, 285, 24775–24782. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Powell, D.W.; Bard, M.; Eckstein, J.; Barbuch, R.; Link, A.J.; Espenshade, P.J. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007, 5, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.A.; Medlock, A.E. Thoughts on interactions between PGRMC1 and diverse attested and potential hydrophobic ligands. J. Steroid Biochem. Mol. Biol. 2017, 171, 11–33. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Dong, J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor α (mPRα) by progesterone receptor membrane component 1 (PGRMC1): Evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology 2014, 155, 1107–1119. [Google Scholar] [CrossRef]
- Sueldo, C.; Liu, X.; Peluso, J.J. Progestin and adipoQ Receptor 7, progesterone membrane receptor component 1 (PGRMC1), and PGRMC2 and their role in regulating progesterone’s ability to suppress human granulosa/luteal cells from entering into the cell cycle. Biol. Reprod. 2015, 93, 63. [Google Scholar] [CrossRef]
- Wu, X.-J.; Thomas, P.; Zhu, Y. Pgrmc1 knockout impairs oocyte maturation in zebrafish. Front. Endocrinol. 2018, 9, 560. [Google Scholar] [CrossRef]
- Kabe, Y.; Nakane, T.; Koike, I.; Yamamoto, T.; Sugiura, Y.; Harada, E.; Sugase, K.; Shimamura, T.; Ohmura, M.; Muraoka, K.; et al. Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat. Commun. 2016, 7, 11030. [Google Scholar] [CrossRef]
- Kabe, Y.; Handa, H.; Suematsu, M. Functional and structural regulation of the carbon monoxide (CO)-responsive membrane protein PGRMC1. J. Clin. Biochem. Nutr. 2018, 63, 12–17. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Zhu, Y.; Detweiler, C.; Doughty, K. Multiple rapid progestin actions and progestin membrane receptor subtypes in fish. Steroids 2004, 69, 567–573. [Google Scholar] [CrossRef]
- Mao, X.; Kikani, C.K.; Riojas, R.A.; Langlais, P.; Wang, L.; Ramos, F.J.; Fanng, Q.; Christ-Robert, C.Y.; Hong, J.Y.; Kim, R.-Y.; et al. APPL1 binds to adiponectin receptors and mediates adiponectin signaling and function. Nat. Cell. Biol. 2006, 8, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Nader, N.; Dib, M.; Courjaret, R.; Hodeify, R.; Machaca, R.; Graumann, J.; Machaca, K. The VDL receptor regulates membrane progesterone receptor trafficking and non-genomic signaling. J. Cell Sci. 2018, 131, 212522. [Google Scholar] [CrossRef] [PubMed]
- Paraakala, M.L.; Zhang, Y.; Modgil, A.; Chadchankar, J.; Vien, T.N.; Ackley, M.A.; Doherty, J.J.; Davies, P.A.; Moss, S.J. Metabotropic, but not allosteric, effects of neurosteroids on GABAergic inhibition depend of phosphorylation of GABAA receptors. J. Biol. Chem. 2019, 294, 12220–12230. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.F.; Thomas, P. Role of G protein estrogen receptor 1, GPER, in inhibition of spontaneous maturation of zebrafish oocytes by endogenous estrogens. Dev. Biol. 2010, 342, 194–206. [Google Scholar] [CrossRef]
- Goncharov, A.I.; Levina, I.S.; Shliapina, V.L.; Morozov, I.A.; Rubtsov, P.M.; Zavarzin, I.V.; Smirnova, O.V.; Shchelkunova, T.A. Cytotoxic effects of the selective ligands of membrane progesterone receptors in human pancreatic adenocarcinoma cells BxPC3. Biochemistry 2021, 86, 1446–1460. [Google Scholar] [CrossRef]
- El Khamlichi, C.; Reverchon-Assadi, F.; Hervouet-Coste, N.; Blot, L.; Reiter, E.; Morisset-Lopez, S. Bioluminescence resonance energy transfer as a method to study protein-protein interactions: Application to G protein coupled receptor technology. Molecules 2019, 24, 537. [Google Scholar] [CrossRef]
- Low, T.Y.; Syrafruddin, S.E.; Mohtoar, M.A.; Vellaichemy, A.; Rahman, N.S.A.; Pung, Y.-F.; Tan, C.S.H. Recent progress in mass spectrometry-based strategies for elucidating protein-protein interactions. Cell. Mol. Life Sci. 2021, 78, 5325–5339. [Google Scholar] [CrossRef]
- Kaplan, M.; Pinto, C.; Houben, K.; Baldus, M. Nuclear magnetic resonance (NMR0 applied to membrane protein complexes. Quart. Rev. Biophys. 2016, 49, e15. [Google Scholar] [CrossRef]
- Drescher Dg Selvakumar, D.; Drescher, M.J. Analysis of protein interactions by surface plasmon resonance. Adv. Chem. Struct. Biol. 2018, 110, 1–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, P. Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics. Cells 2022, 11, 1785. https://doi.org/10.3390/cells11111785
Thomas P. Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics. Cells. 2022; 11(11):1785. https://doi.org/10.3390/cells11111785
Chicago/Turabian StyleThomas, Peter. 2022. "Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics" Cells 11, no. 11: 1785. https://doi.org/10.3390/cells11111785
APA StyleThomas, P. (2022). Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics. Cells, 11(11), 1785. https://doi.org/10.3390/cells11111785




