The Anti-CGRP Antibody Fremanezumab Lowers CGRP Release from Rat Dura Mater and Meningeal Blood Flow
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Administration of Antibodies
2.3. Preparation for CGRP Release Measurements
2.4. Analysis of Released CGRP Concentration
2.5. Preparation for Meningeal Blood Flow Recordings
2.6. Meningeal Blood Flow Recordings
2.7. Data Processing and Statistics
3. Results
3.1. Tolerability of Treatments
3.2. Body Weight
3.3. CGRP Release from the Dura Mater
3.3.1. Exclusion of Antibody-Assay Interactions
3.3.2. Exclusion of Side Difference
3.3.3. Impact of Antibodies on CGRP Release
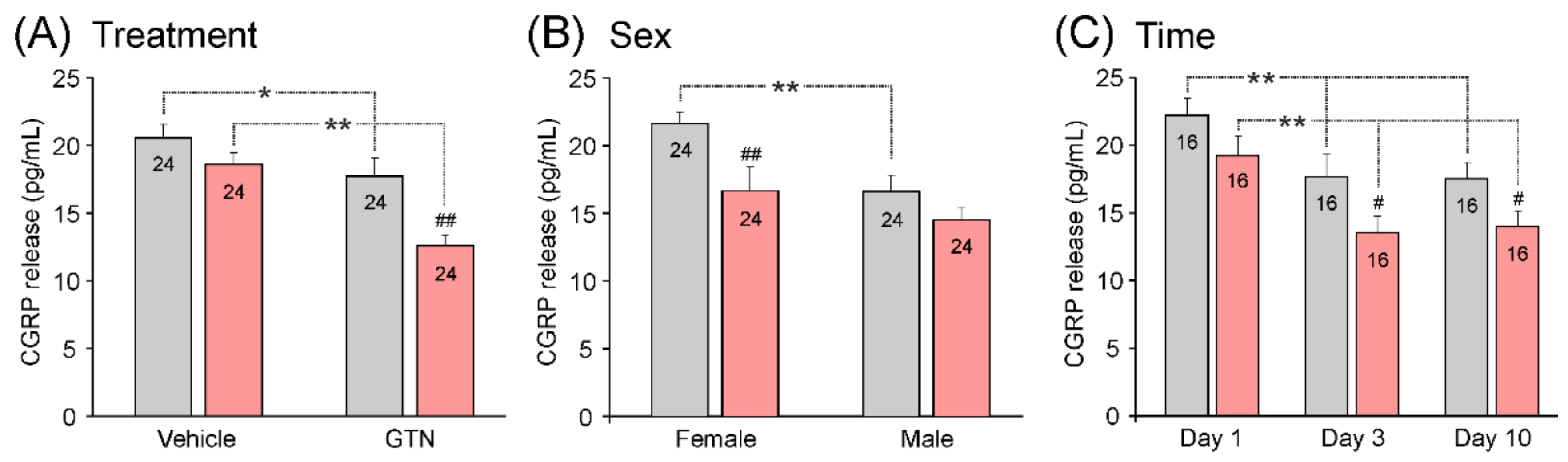
3.3.4. Impact of Treatment, Sex, and Waiting Time on Basal CGRP Release
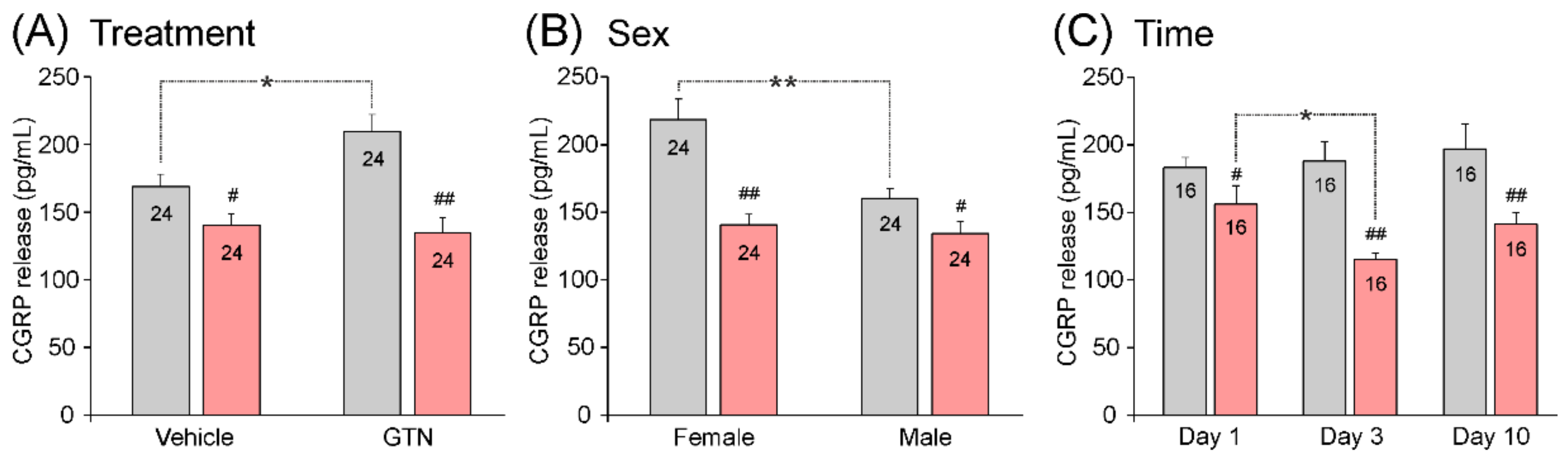
3.3.5. Impact of Treatment, Sex, and Waiting Time on Stimulated CGRP Release
3.3.6. Additional Experiments with Longer Waiting Time
3.4. Meningeal Blood Flow
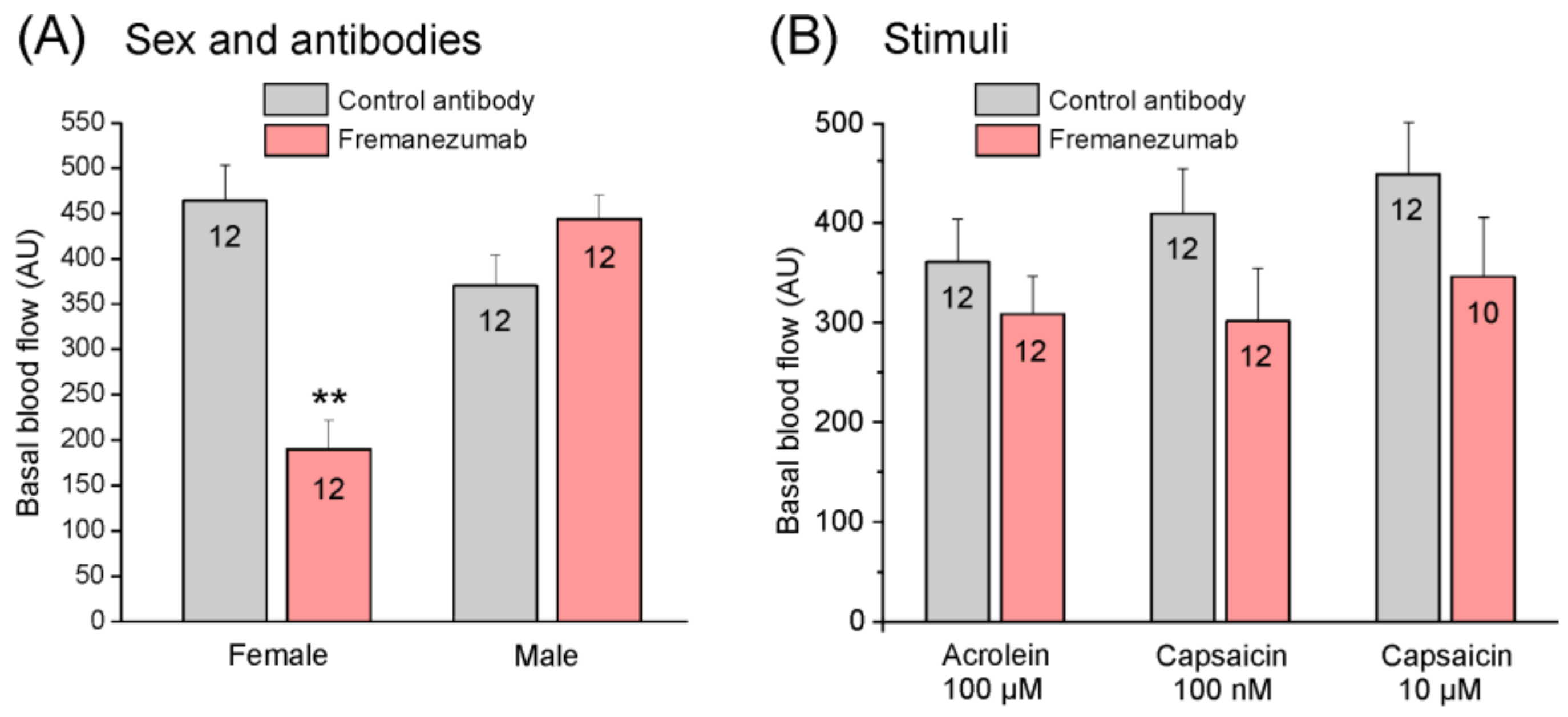
3.4.1. Basal Blood Flow
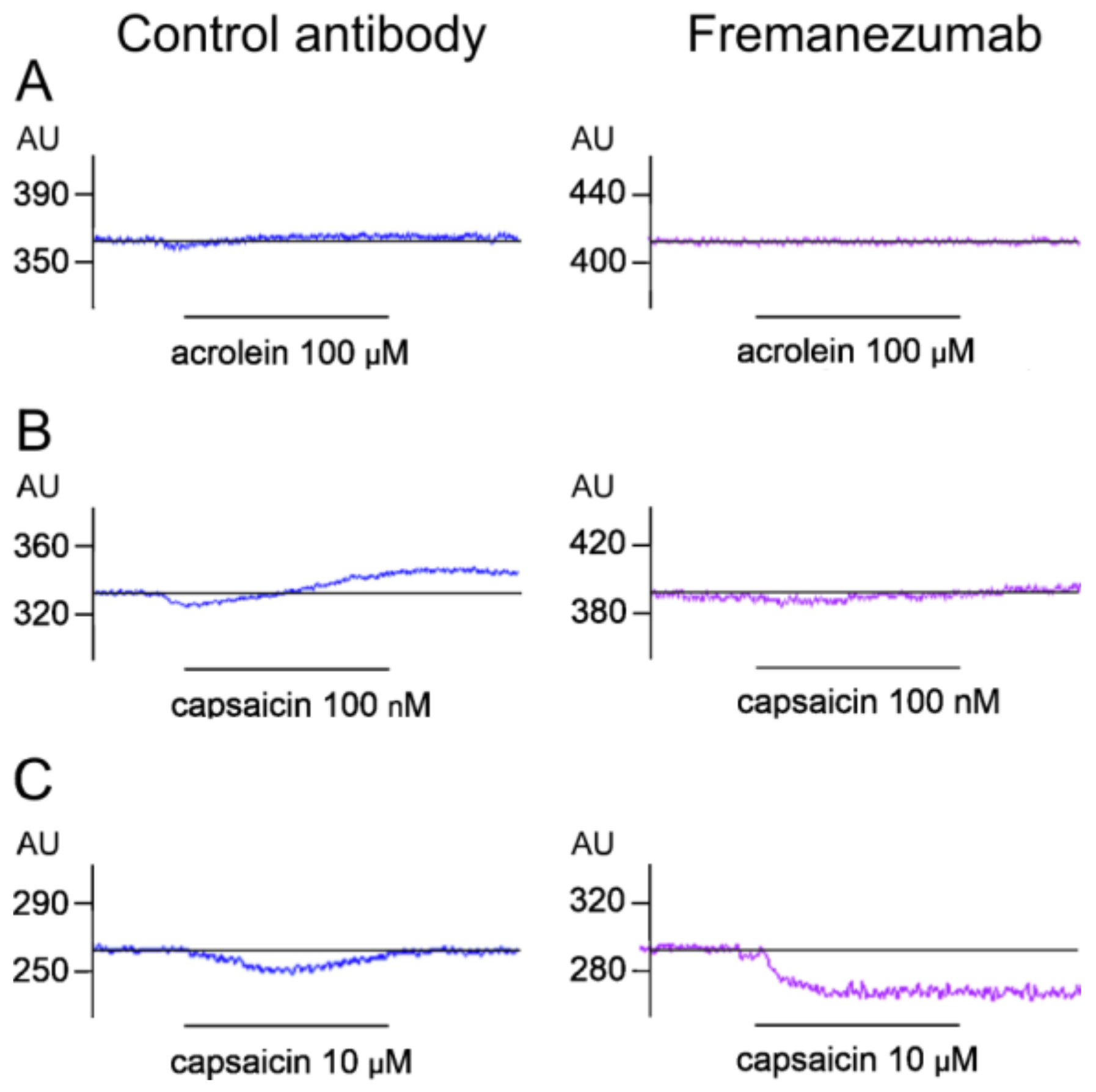
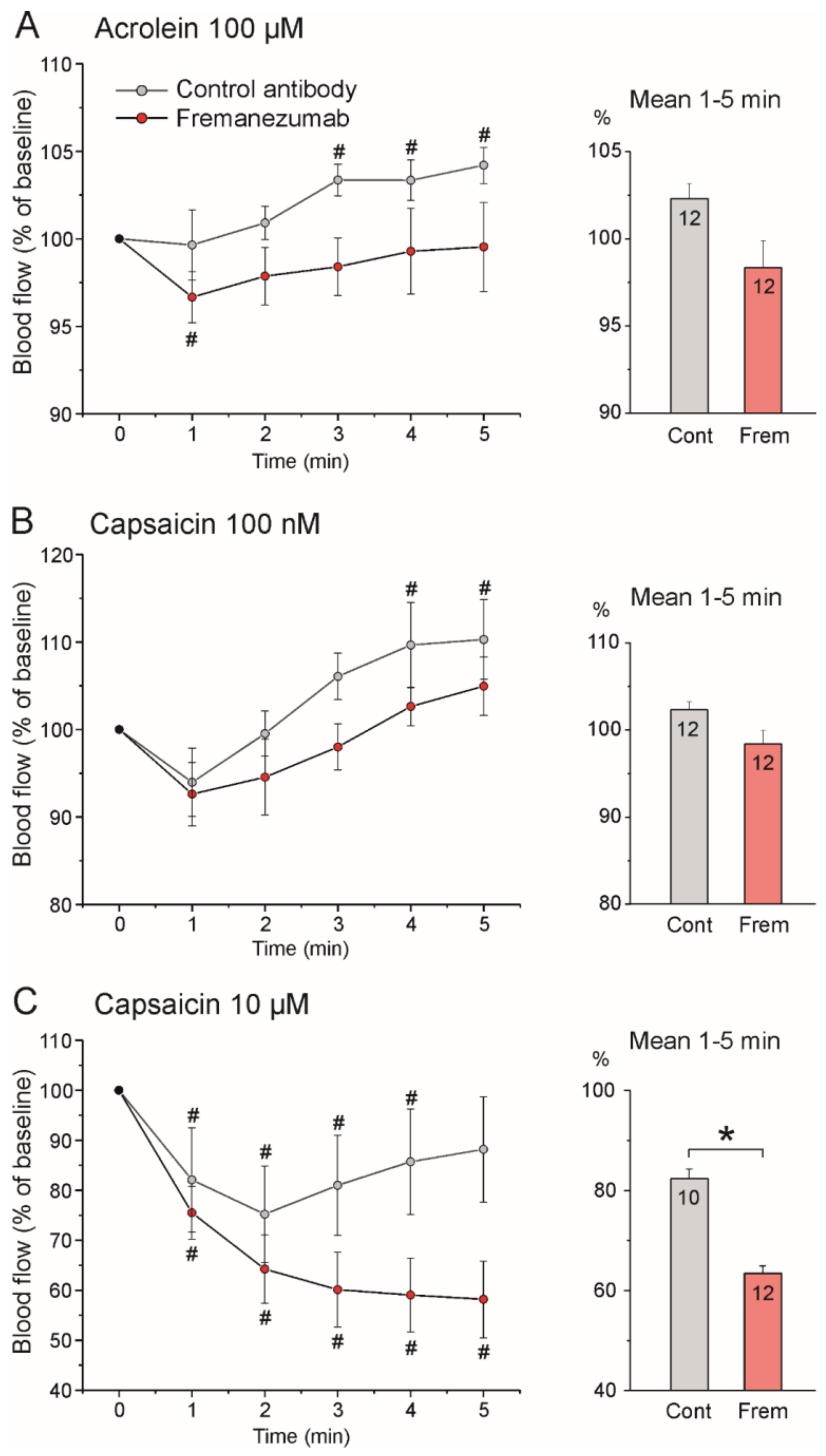
3.4.2. Stimulated Blood Flow
Stimulation with Acrolein
Stimulation with Low-Dose Capsaicin
Stimulation with High-Dose Capsaicin
4. Discussion
4.1. Sex Difference in CGRP Release
4.2. Where Do Anti-CGRP Antibodies Act?
4.3. Meningeal Blood Flow Induced by the Stimulation of TRP Receptors
4.4. Possible Effects of Anti-CGRP Antibodies beyond CGRP Neutralization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Edvinsson, L.; Goadsby, P.J. Neuropeptides in Migraine and Cluster Headache. Cephalalgia Int. J. Headache 1994, 14, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Vollesen, A.L.; Benemei, S.; Cortese, F.; Labastida-Ramírez, A.; Marchese, F.; Pellesi, L.; Romoli, M.; Ashina, M.; Lampl, C.; School of Advanced Studies of the European Headache Federation (EHF-SAS). Migraine and Cluster Headache—The Common Link. J. Headache Pain 2018, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Carmine Belin, A.; Ran, C.; Edvinsson, L. Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache. Brain Sci. 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of Vasoactive Peptides in the Extracerebral Circulation of Humans and the Cat during Activation of the Trigeminovascular System. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef]
- Ashina, M.; Bendtsen, L.; Jensen, R.; Schifter, S.; Olesen, J. Evidence for Increased Plasma Levels of Calcitonin Gene-Related Peptide in Migraine Outside of Attacks. Pain 2000, 86, 133–138. [Google Scholar] [CrossRef]
- Sarchielli, P.; Alberti, A.; Codini, M.; Floridi, A.; Gallai, V. Nitric Oxide Metabolites, Prostaglandins and Trigeminal Vasoactive Peptides in Internal Jugular Vein Blood during Spontaneous Migraine Attacks. Cephalalgia Int. J. Headache 2000, 20, 907–918. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Larrosa, D.; Ramón, C.; Vega, J.; Martínez-Camblor, P.; Pascual, J. Interictal Increase of CGRP Levels in Peripheral Blood as a Biomarker for Chronic Migraine. Neurology 2013, 81, 1191–1196. [Google Scholar] [CrossRef]
- Lassen, L.H.; Jacobsen, V.B.; Haderslev, P.A.; Sperling, B.; Iversen, H.K.; Olesen, J.; Tfelt-Hansen, P. Involvement of Calcitonin Gene-Related Peptide in Migraine: Regional Cerebral Blood Flow and Blood Flow Velocity in Migraine Patients. J. Headache Pain 2008, 9, 151–157. [Google Scholar] [CrossRef]
- Vollesen, A.L.H.; Snoer, A.; Beske, R.P.; Guo, S.; Hoffmann, J.; Jensen, R.H.; Ashina, M. Effect of Infusion of Calcitonin Gene-Related Peptide on Cluster Headache Attacks: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1187–1197. [Google Scholar] [CrossRef]
- Buzzi, M.G.; Moskowitz, M.A. Evidence for 5-HT1B/1D Receptors Mediating the Antimigraine Effect of Sumatriptan and Dihydroergotamine. Cephalalgia Int. J. Headache 1991, 11, 165–168. [Google Scholar] [CrossRef]
- Hargreaves, R.; Olesen, J. Calcitonin Gene-Related Peptide Modulators—The History and Renaissance of a New Migraine Drug Class. Headache 2019, 59, 951–970. [Google Scholar] [CrossRef]
- Dodick, D.W.; Lipton, R.B.; Ailani, J.; Lu, K.; Finnegan, M.; Trugman, J.M.; Szegedi, A. Ubrogepant for the Treatment of Migraine. N. Engl. J. Med. 2019, 381, 2230–2241. [Google Scholar] [CrossRef]
- Croop, R.; Lipton, R.B.; Kudrow, D.; Stock, D.A.; Kamen, L.; Conway, C.M.; Stock, E.G.; Coric, V.; Goadsby, P.J. Oral Rimegepant for Preventive Treatment of Migraine: A Phase 2/3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2021, 397, 51–60. [Google Scholar] [CrossRef]
- Storer, R.J.; Akerman, S.; Goadsby, P.J. Calcitonin Gene-Related Peptide (CGRP) Modulates Nociceptive Trigeminovascular Transmission in the Cat. Br. J. Pharmacol. 2004, 142, 1171–1181. [Google Scholar] [CrossRef]
- Fischer, M.J.M.; Koulchitsky, S.; Messlinger, K. The Nonpeptide Calcitonin Gene-Related Peptide Receptor Antagonist BIBN4096BS Lowers the Activity of Neurons with Meningeal Input in the Rat Spinal Trigeminal Nucleus. J. Neurosci. 2005, 25, 5877–5883. [Google Scholar] [CrossRef]
- Fischer, M.J.M.; Schmidt, J.; Koulchitsky, S.; Klussmann, S.; Vater, A.; Messlinger, K. Effect of a Calcitonin Gene-Related Peptide-Binding L-RNA Aptamer on Neuronal Activity in the Rat Spinal Trigeminal Nucleus. J. Headache Pain 2018, 19, 3. [Google Scholar] [CrossRef]
- Koulchitsky, S.; Fischer, M.J.M.; Messlinger, K. Calcitonin Gene-Related Peptide Receptor Inhibition Reduces Neuronal Activity Induced by Prolonged Increase in Nitric Oxide in the Rat Spinal Trigeminal Nucleus. Cephalalgia Int. J. Headache 2009, 29, 408–417. [Google Scholar] [CrossRef]
- Schuster, N.M.; Rapoport, A.M. New Strategies for the Treatment and Prevention of Primary Headache Disorders. Nat. Rev. Neurol. 2016, 12, 635–650. [Google Scholar] [CrossRef]
- Raffaelli, B.; Neeb, L.; Reuter, U. Monoclonal Antibodies for the Prevention of Migraine. Expert Opin. Biol. Ther. 2019, 19, 1307–1317. [Google Scholar] [CrossRef]
- Schoenen, J.; Manise, M.; Nonis, R.; Gérard, P.; Timmermans, G. Monoclonal Antibodies Blocking CGRP Transmission: An Update on Their Added Value in Migraine Prevention. Rev. Neurol. 2020, 176, 788–803. [Google Scholar] [CrossRef]
- Noseda, R.; Schain, A.J.; Melo-Carrillo, A.; Tien, J.; Stratton, J.; Mai, F.; Strassman, A.M.; Burstein, R. Fluorescently-Labeled Fremanezumab Is Distributed to Sensory and Autonomic Ganglia and the Dura but Not to the Brain of Rats with Uncompromised Blood Brain Barrier. Cephalalgia Int. J. Headache 2020, 40, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.L.; Ernstsen, C.; Olesen, J.; Kristensen, D.M. No Central Action of CGRP Antagonising Drugs in the GTN Mouse Model of Migraine. Cephalalgia Int. J. Headache 2020, 40, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Burstein, R.; Strassman, A.M. Calcitonin Gene-Related Peptide Does Not Excite or Sensitize Meningeal Nociceptors: Implications for the Pathophysiology of Migraine. Ann. Neurol. 2005, 58, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Covasala, O.; Stirn, S.L.; Albrecht, S.; De Col, R.; Messlinger, K. Calcitonin Gene-Related Peptide Receptors in Rat Trigeminal Ganglion Do Not Control Spinal Trigeminal Activity. J. Neurophysiol. 2012, 108, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.K.; Ramachandran, R.; Christensen, S.L.T.; Gupta, S.; Jansen-Olesen, I.; Olesen, J. CGRP Infusion in Unanesthetized Rats Increases Expression of C-Fos in the Nucleus Tractus Solitarius and Caudal Ventrolateral Medulla, but Not in the Trigeminal Nucleus Caudalis. Cephalalgia Int. J. Headache 2015, 35, 220–233. [Google Scholar] [CrossRef]
- Wattiez, A.-S.; Wang, M.; Russo, A.F. CGRP in Animal Models of Migraine. In Calcitonin Gene-Related Peptide (CGRP) Mechanisms (Handbook of Experimental Pharmacology); Brain, S.D., Geppetti, P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 255, pp. 85–107. ISBN 978-3-030-21453-1. [Google Scholar]
- Moehring, F.; Sadler, K.E. Female-Specific Effects of CGRP Suggest Limited Efficacy of New Migraine Treatments in Males. J. Neurosci. 2019, 39, 9062–9064. [Google Scholar] [CrossRef]
- Lassen, L.H.; Haderslev, P.A.; Jacobsen, V.B.; Iversen, H.K.; Sperling, B.; Olesen, J. CGRP May Play a Causative Role in Migraine. Cephalalgia Int. J. Headache 2002, 22, 54–61. [Google Scholar] [CrossRef]
- Hansen, J.M.; Hauge, A.W.; Olesen, J.; Ashina, M. Calcitonin Gene-Related Peptide Triggers Migraine-like Attacks in Patients with Migraine with Aura. Cephalalgia Int. J. Headache 2010, 30, 1179–1186. [Google Scholar] [CrossRef]
- Christensen, C.E.; Younis, S.; Deen, M.; Khan, S.; Ghanizada, H.; Ashina, M. Migraine Induction with Calcitonin Gene-Related Peptide in Patients from Erenumab Trials. J. Headache Pain 2018, 19, 105. [Google Scholar] [CrossRef]
- Ashina, H.; Schytz, H.W.; Ashina, M. CGRP in Human Models of Migraine. Handb. Exp. Pharmacol. 2019, 255, 109–120. [Google Scholar] [CrossRef]
- O’Connor, T.P.; van der Kooy, D. Enrichment of a Vasoactive Neuropeptide (Calcitonin Gene Related Peptide) in the Trigeminal Sensory Projection to the Intracranial Arteries. J. Neurosci. 1988, 8, 2468–2476. [Google Scholar] [CrossRef]
- Hoheisel, U.; Mense, S.; Scherotzke, R. Calcitonin Gene-Related Peptide-Immunoreactivity in Functionally Identified Primary Afferent Neurones in the Rat. Anat. Embryol. 1994, 189, 41–49. [Google Scholar] [CrossRef]
- Bennett, D.L.; Dmietrieva, N.; Priestley, J.V.; Clary, D.; McMahon, S.B. TrkA, CGRP and IB4 Expression in Retrogradely Labelled Cutaneous and Visceral Primary Sensory Neurones in the Rat. Neurosci. Lett. 1996, 206, 33–36. [Google Scholar] [CrossRef]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef]
- Jenkins, D.W.; Langmead, C.J.; Parsons, A.A.; Strijbos, P.J. Regulation of Calcitonin Gene-Related Peptide Release from Rat Trigeminal Nucleus Caudalis Slices In Vitro. Neurosci. Lett. 2004, 366, 241–244. [Google Scholar] [CrossRef]
- Kageneck, C.; Nixdorf-Bergweiler, B.E.; Messlinger, K.; Fischer, M.J. Release of CGRP from Mouse Brainstem Slices Indicates Central Inhibitory Effect of Triptans and Kynurenate. J. Headache Pain 2014, 15, 7. [Google Scholar] [CrossRef]
- Messlinger, K.; Ebersberger, A.; Schaible, H.G. Release of Immunoreactive Substance P in the Brain Stem upon Stimulation of the Cranial Dura Mater with Low PH—Inhibition by the Serotonin (5-HT1) Receptor Agonist CP 93,129. Br. J. Pharmacol. 1998, 125, 1726–1732. [Google Scholar] [CrossRef]
- Ebersberger, A.; Averbeck, B.; Messlinger, K.; Reeh, P.W. Release of Substance P, Calcitonin Gene-Related Peptide and Prostaglandin E2 from Rat Dura Mater Encephali Following Electrical and Chemical Stimulation In Vitro. Neuroscience 1999, 89, 901–907. [Google Scholar] [CrossRef]
- Eberhardt, M.; Neeb, L.; Vogel, E.-M.; Tiegs, G.; Reuter, U.; Messlinger, K.; Fischer, M.J.M. Glyceroltrinitrate Facilitates Stimulated CGRP Release but Not Gene Expression of CGRP or Its Receptor Components in Rat Trigeminal Ganglia. Neuropeptides 2009, 43, 483–489. [Google Scholar] [CrossRef]
- Hoffmann, J.; Wecker, S.; Neeb, L.; Dirnagl, U.; Reuter, U. Primary Trigeminal Afferents Are the Main Source for Stimulus-Induced CGRP Release into Jugular Vein Blood and CSF. Cephalalgia Int. J. Headache 2012, 32, 659–667. [Google Scholar] [CrossRef]
- Durham, P.L.; Masterson, C.G. Two Mechanisms Involved in Trigeminal CGRP Release: Implications for Migraine Treatment. Headache 2013, 53, 67–80. [Google Scholar] [CrossRef]
- Marics, B.; Peitl, B.; Varga, A.; Pázmándi, K.; Bácsi, A.; Németh, J.; Szilvássy, Z.; Jancsó, G.; Dux, M. Diet-Induced Obesity Alters Dural CGRP Release and Potentiates TRPA1-Mediated Trigeminovascular Responses. Cephalalgia Int. J. Headache 2016, 37, 581–591. [Google Scholar] [CrossRef]
- Dux, M.; Will, C.; Eberhardt, M.; Fischer, M.J.M.; Messlinger, K. Stimulation of Rat Cranial Dura Mater with Potassium Chloride Causes CGRP Release into the Cerebrospinal Fluid and Increases Medullary Blood Flow. Neuropeptides 2017, 64, 61–68. [Google Scholar] [CrossRef]
- Christiansen, I.; Thomsen, L.L.; Daugaard, D.; Ulrich, V.; Olesen, J. Glyceryl Trinitrate Induces Attacks of Migraine without Aura in Sufferers of Migraine with Aura. Cephalalgia Int. J. Headache 1999, 19, 660–667; discussion 626. [Google Scholar] [CrossRef]
- Juhasz, G.; Zsombok, T.; Modos, E.A.; Olajos, S.; Jakab, B.; Nemeth, J.; Szolcsanyi, J.; Vitrai, J.; Bagdy, G. NO-Induced Migraine Attack: Strong Increase in Plasma Calcitonin Gene-Related Peptide (CGRP) Concentration and Negative Correlation with Platelet Serotonin Release. Pain 2003, 106, 461–470. [Google Scholar] [CrossRef]
- Sances, G.; Tassorelli, C.; Pucci, E.; Ghiotto, N.; Sandrini, G.; Nappi, G. Reliability of the Nitroglycerin Provocative Test in the Diagnosis of Neurovascular Headaches. Cephalalgia Int. J. Headache 2004, 24, 110–119. [Google Scholar] [CrossRef]
- Tassorelli, C.; Greco, R.; Wang, D.; Sandrini, M.; Sandrini, G.; Nappi, G. Nitroglycerin Induces Hyperalgesia in Rats—A Time-Course Study. Eur. J. Pharmacol. 2003, 464, 159–162. [Google Scholar] [CrossRef]
- Ramachandran, R.; Bhatt, D.K.; Ploug, K.B.; Hay-Schmidt, A.; Jansen-Olesen, I.; Gupta, S.; Olesen, J. Nitric Oxide Synthase, Calcitonin Gene-Related Peptide and NK-1 Receptor Mechanisms Are Involved in GTN-Induced Neuronal Activation. Cephalalgia Int. J. Headache 2014, 34, 136–147. [Google Scholar] [CrossRef]
- Capuano, A.; Greco, M.C.; Navarra, P.; Tringali, G. Correlation between Algogenic Effects of Calcitonin-Gene-Related Peptide (CGRP) and Activation of Trigeminal Vascular System, in an In Vivo Experimental Model of Nitroglycerin-Induced Sensitization. Eur. J. Pharmacol. 2014, 740, 97–102. [Google Scholar] [CrossRef]
- Seiler, K.; Nusser, J.I.; Lennerz, J.K.; Neuhuber, W.L.; Messlinger, K. Changes in Calcitonin Gene-Related Peptide (CGRP) Receptor Component and Nitric Oxide Receptor (SGC) Immunoreactivity in Rat Trigeminal Ganglion Following Glyceroltrinitrate Pretreatment. J. Headache Pain 2013, 14, 74. [Google Scholar] [CrossRef]
- Kurosawa, M.; Messlinger, K.; Pawlak, M.; Schmidt, R.F. Increase of Meningeal Blood Flow after Electrical Stimulation of Rat Dura Mater Encephali: Mediation by Calcitonin Gene-Related Peptide. Br. J. Pharmacol. 1995, 114, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.J.; Hargreaves, R.J.; Hill, R.G.; Shepheard, S.L. Intravital Microscope Studies on the Effects of Neurokinin Agonists and Calcitonin Gene-Related Peptide on Dural Vessel Diameter in the Anaesthetized Rat. Cephalalgia Int. J. Headache 1997, 17, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.A.; Birk, S.; Doods, H.; Edvinsson, L.; Olesen, J. Inhibitory Effect of BIBN4096BS on Cephalic Vasodilatation Induced by CGRP or Transcranial Electrical Stimulation in the Rat. Br. J. Pharmacol. 2004, 143, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Jansen, I.; Cunha e Sa, M.; Gulbenkian, S. Demonstration of Neuropeptide Containing Nerves and Vasomotor Responses to Perivascular Peptides in Human Cerebral Arteries. Cephalalgia Int. J. Headache 1994, 14, 88–96. [Google Scholar] [CrossRef]
- Kunkler, P.E.; Ballard, C.J.; Oxford, G.S.; Hurley, J.H. TRPA1 Receptors Mediate Environmental Irritant-Induced Meningeal Vasodilatation. Pain 2011, 152, 38–44. [Google Scholar] [CrossRef]
- Dux, M.; Deák, É.; Tassi, N.; Sántha, P.; Jancsó, G. Endovanilloids Are Potential Activators of the Trigeminovascular Nocisensor Complex. J. Headache Pain 2016, 17, 53. [Google Scholar] [CrossRef]
- Dux, M.; Rosta, J.; Messlinger, K. TRP Channels in the Focus of Trigeminal Nociceptor Sensitization Contributing to Primary Headaches. Int. J. Mol. Sci. 2020, 21, 342. [Google Scholar] [CrossRef]
- Marics, B.; Peitl, B.; Pázmándi, K.; Bácsi, A.; Németh, J.; Oszlács, O.; Jancsó, G.; Dux, M. Diet-Induced Obesity Enhances TRPV1-Mediated Neurovascular Reactions in the Dura Mater. Headache 2017, 57, 441–454. [Google Scholar] [CrossRef]
- Denner, A.C.; Vogler, B.; Messlinger, K.; De Col, R. Role of Transient Receptor Potential Ankyrin 1 Receptors in Rodent Models of Meningeal Nociception—Experiments In Vitro. Eur. J. Pain Lond. Engl. 2016, 21, 843–854. [Google Scholar] [CrossRef]
- Deák, É.; Rosta, J.; Boros, K.; Kis, G.; Sántha, P.; Messlinger, K.; Jancsó, G.; Dux, M. Chronic Adriamycin Treatment Impairs CGRP-Mediated Functions of Meningeal Sensory Nerves. Neuropeptides 2018, 69, 46–52. [Google Scholar] [CrossRef]
- Dux, M.; Sántha, P.; Jancsó, G. Capsaicin-Sensitive Neurogenic Sensory Vasodilatation in the Dura Mater of the Rat. J. Physiol. 2003, 552, 859–867. [Google Scholar] [CrossRef]
- Melo-Carrillo, A.; Strassman, A.M.; Nir, R.-R.; Schain, A.J.; Noseda, R.; Stratton, J.; Burstein, R. Fremanezumab-A Humanized Monoclonal Anti-CGRP Antibody-Inhibits Thinly Myelinated (Aδ) but Not Unmyelinated (C) Meningeal Nociceptors. J. Neurosci. 2017, 37, 10587–10596. [Google Scholar] [CrossRef]
- Bigal, M.E.; Rapoport, A.M.; Silberstein, S.D.; Walter, S.; Hargreaves, R.J.; Aycardi, E. From LBR-101 to Fremanezumab for Migraine. CNS Drugs 2018, 32, 1025–1037. [Google Scholar] [CrossRef]
- Bhakta, M.; Vuong, T.; Taura, T.; Wilson, D.S.; Stratton, J.R.; Mackenzie, K.D. Migraine Therapeutics Differentially Modulate the CGRP Pathway. Cephalalgia Int. J. Headache 2021, 41, 499–514. [Google Scholar] [CrossRef]
- Melo-Carrillo, A.; Noseda, R.; Nir, R.-R.; Schain, A.J.; Stratton, J.; Strassman, A.M.; Burstein, R. Selective Inhibition of Trigeminovascular Neurons by Fremanezumab: A Humanized Monoclonal Anti-CGRP Antibody. J. Neurosci. 2017, 37, 7149–7163. [Google Scholar] [CrossRef]
- González-Hernández, A.; Marichal-Cancino, B.A.; García-Boll, E.; Villalón, C.M. The Locus of Action of CGRPergic Monoclonal Antibodies against Migraine: Peripheral over Central Mechanisms. CNS Neurol. Disord. Drug Targets 2020, 19, 344–359. [Google Scholar] [CrossRef]
- Zeller, J.; Poulsen, K.T.; Sutton, J.E.; Abdiche, Y.N.; Collier, S.; Chopra, R.; Garcia, C.A.; Pons, J.; Rosenthal, A.; Shelton, D.L. CGRP Function-Blocking Antibodies Inhibit Neurogenic Vasodilatation without Affecting Heart Rate or Arterial Blood Pressure in the Rat. Br. J. Pharmacol. 2008, 155, 1093–1103. [Google Scholar] [CrossRef]
- Lionetto, L.; Curto, M.; Cisale, G.Y.; Capi, M.; Cipolla, F.; Guglielmetti, M.; Martelletti, P. Fremanezumab for the Preventive Treatment of Migraine in Adults. Expert Rev. Clin. Pharmacol. 2019, 12, 741–748. [Google Scholar] [CrossRef]
- Gupta, S.; Amrutkar, D.V.; Mataji, A.; Salmasi, H.; Hay-Schmidt, A.; Sheykhzade, M.; Messlinger, K.; Olesen, J.; Jansen-Olesen, I. Evidence for CGRP Re-Uptake in Rat Dura Mater Encephali. Br. J. Pharmacol. 2010, 161, 1885–1898. [Google Scholar] [CrossRef][Green Version]
- Avona, A.; Burgos-Vega, C.; Burton, M.D.; Akopian, A.N.; Price, T.J.; Dussor, G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. J. Neurosci. 2019, 39, 4323–4331. [Google Scholar] [CrossRef]
- Rea, B.J.; Wattiez, A.-S.; Waite, J.S.; Castonguay, W.C.; Schmidt, C.M.; Fairbanks, A.M.; Robertson, B.R.; Brown, C.J.; Mason, B.N.; Moldovan-Loomis, M.-C.; et al. Peripherally Administered Calcitonin Gene-Related Peptide Induces Spontaneous Pain in Mice: Implications for Migraine. Pain 2018, 159, 2306–2317. [Google Scholar] [CrossRef]
- Zheng, F.; Nixdorf-Bergweiler, B.E.; van Brederode, J.; Alzheimer, C.; Messlinger, K. Excitatory Effects of Calcitonin Gene-Related Peptide (CGRP) on Superficial Sp5C Neurons in Mouse Medullary Slices. Int. J. Mol. Sci. 2021, 22, 3794. [Google Scholar] [CrossRef]
- Miller, S.; Liu, H.; Warfvinge, K.; Shi, L.; Dovlatyan, M.; Xu, C.; Edvinsson, L. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 2016, 328, 165–183. [Google Scholar] [CrossRef]
- Yamanaka, G.; Suzuki, S.; Morishita, N.; Takeshita, M.; Kanou, K.; Takamatsu, T.; Suzuki, S.; Morichi, S.; Watanabe, Y.; Ishida, Y.; et al. Role of Neuroinflammation and Blood-Brain Barrier Permutability on Migraine. Int. J. Mol. Sci. 2021, 22, 8929. [Google Scholar] [CrossRef]
- Edvinsson, J.C.A.; Warfvinge, K.; Krause, D.N.; Blixt, F.W.; Sheykhzade, M.; Edvinsson, L.; Haanes, K.A. C-Fibers May Modulate Adjacent Aδ-Fibers through Axon-Axon CGRP Signaling at Nodes of Ranvier in the Trigeminal System. J. Headache Pain 2019, 20, 105. [Google Scholar] [CrossRef]
- Hong, S.; Wiley, J.W. Altered Expression and Function of Sodium Channels in Large DRG Neurons and Myelinated A-Fibers in Early Diabetic Neuropathy in the Rat. Biochem. Biophys. Res. Commun. 2006, 339, 652–660. [Google Scholar] [CrossRef]
- Huang, Z.-J.; Song, X.-J. Differing Alterations of Sodium Currents in Small Dorsal Root Ganglion Neurons after Ganglion Compression and Peripheral Nerve Injury. Mol. Pain 2008, 4, 20. [Google Scholar] [CrossRef]
- Close, L.N.; Eftekhari, S.; Wang, M.; Charles, A.C.; Russo, A.F. Cortical Spreading Depression as a Site of Origin for Migraine: Role of CGRP. Cephalalgia Int. J. Headache 2019, 39, 428–434. [Google Scholar] [CrossRef]
- Schain, A.J.; Melo-Carrillo, A.; Stratton, J.; Strassman, A.M.; Burstein, R. CSD-Induced Arterial Dilatation and Plasma Protein Extravasation Are Unaffected by Fremanezumab: Implications for CGRP’s Role in Migraine with Aura. J. Neurosci. 2019, 39, 6001–6011. [Google Scholar] [CrossRef]
- Lennerz, J.K.; Rühle, V.; Ceppa, E.P.; Neuhuber, W.L.; Bunnett, N.W.; Grady, E.F.; Messlinger, K. Calcitonin Receptor-like Receptor (CLR), Receptor Activity-Modifying Protein 1 (RAMP1), and Calcitonin Gene-Related Peptide (CGRP) Immunoreactivity in the Rat Trigeminovascular System: Differences between Peripheral and Central CGRP Receptor Distribution. J. Comp. Neurol. 2008, 507, 1277–1299. [Google Scholar] [CrossRef]
- Eftekhari, S.; Salvatore, C.A.; Calamari, A.; Kane, S.A.; Tajti, J.; Edvinsson, L. Differential Distribution of Calcitonin Gene-Related Peptide and Its Receptor Components in the Human Trigeminal Ganglion. Neuroscience 2010, 169, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, K.; Balcziak, L.K.; Russo, A.F. Cross-Talk Signaling in the Trigeminal Ganglion: Role of Neuropeptides and Other Mediators. J. Neural Transm. 2020, 127, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. CGRP: Sensory Neuropeptide with Multiple Neurologic Implications. Neurology 2011, 77, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Vause, C.V.; Durham, P.L. Calcitonin Gene-Related Peptide Differentially Regulates Gene and Protein Expression in Trigeminal Glia Cells: Findings from Array Analysis. Neurosci. Lett. 2010, 473, 163–167. [Google Scholar] [CrossRef]
- Dieterle, A.; Fischer, M.J.M.; Link, A.S.; Neuhuber, W.L.; Messlinger, K. Increase in CGRP- and NNOS-Immunoreactive Neurons in the Rat Trigeminal Ganglion after Infusion of an NO Donor. Cephalalgia Int. J. Headache 2011, 31, 31–42. [Google Scholar] [CrossRef]
- Buldyrev, I.; Tanner, N.M.; Hsieh, H.; Dodd, E.G.; Nguyen, L.T.; Balkowiec, A. Calcitonin Gene-Related Peptide Enhances Release of Native Brain-Derived Neurotrophic Factor from Trigeminal Ganglion Neurons. J. Neurochem. 2006, 99, 1338–1350. [Google Scholar] [CrossRef]
- Beckers, H.J.; Klooster, J.; Vrensen, G.F.; Lamers, W.P. Ultrastructural Identification of Trigeminal Nerve Terminals in the Pterygopalatine Ganglion of Rats: An Anterograde Tracing and Immunohistochemical Study. Brain Res. 1991, 557, 22–30. [Google Scholar] [CrossRef]
- Motosugi, H.; Chiba, T.; Konno, A.; Kaneko, T. Distribution of Neuropeptides in Rat Pterygopalatine Ganglion: Light and Electron Microscopic Immunohistochemical Studies. Arch. Histol. Cytol. 1992, 55, 513–524. [Google Scholar] [CrossRef]
- Kirch, W.; Neuhuber, W.; Tamm, E.R. Immunohistochemical Localization of Neuropeptides in the Human Ciliary Ganglion. Brain Res. 1995, 681, 229–234. [Google Scholar] [CrossRef]
- Csati, A.; Tajti, J.; Tuka, B.; Edvinsson, L.; Warfvinge, K. Calcitonin Gene-Related Peptide and Its Receptor Components in the Human Sphenopalatine Ganglion—Interaction with the Sensory System. Brain Res. 2012, 1435, 29–39. [Google Scholar] [CrossRef]
- Gottselig, R.; Messlinger, K. Noxious Chemical Stimulation of Rat Facial Mucosa Increases Intracranial Blood Flow through a Trigemino-Parasympathetic Reflex—An Experimental Model for Vascular Dysfunctions in Cluster Headache. Cephalalgia Int. J. Headache 2004, 24, 206–214. [Google Scholar] [CrossRef]
- Leone, M.; Ferraro, S.; Proietti Cecchini, A. The Neurobiology of Cluster Headache. Handb. Clin. Neurol. 2021, 182, 401–414. [Google Scholar] [CrossRef]
- Khan, S.; Schoenen, J.; Ashina, M. Sphenopalatine Ganglion Neuromodulation in Migraine: What Is the Rationale? Cephalalgia Int. J. Headache 2014, 34, 382–391. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dux, M.; Vogler, B.; Kuhn, A.; Mackenzie, K.D.; Stratton, J.; Messlinger, K. The Anti-CGRP Antibody Fremanezumab Lowers CGRP Release from Rat Dura Mater and Meningeal Blood Flow. Cells 2022, 11, 1768. https://doi.org/10.3390/cells11111768
Dux M, Vogler B, Kuhn A, Mackenzie KD, Stratton J, Messlinger K. The Anti-CGRP Antibody Fremanezumab Lowers CGRP Release from Rat Dura Mater and Meningeal Blood Flow. Cells. 2022; 11(11):1768. https://doi.org/10.3390/cells11111768
Chicago/Turabian StyleDux, Mária, Birgit Vogler, Annette Kuhn, Kimberly D. Mackenzie, Jennifer Stratton, and Karl Messlinger. 2022. "The Anti-CGRP Antibody Fremanezumab Lowers CGRP Release from Rat Dura Mater and Meningeal Blood Flow" Cells 11, no. 11: 1768. https://doi.org/10.3390/cells11111768
APA StyleDux, M., Vogler, B., Kuhn, A., Mackenzie, K. D., Stratton, J., & Messlinger, K. (2022). The Anti-CGRP Antibody Fremanezumab Lowers CGRP Release from Rat Dura Mater and Meningeal Blood Flow. Cells, 11(11), 1768. https://doi.org/10.3390/cells11111768





