Homocysteine Metabolism Pathway Is Involved in the Control of Glucose Homeostasis: A Cystathionine Beta Synthase Deficiency Study in Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Phenotyping
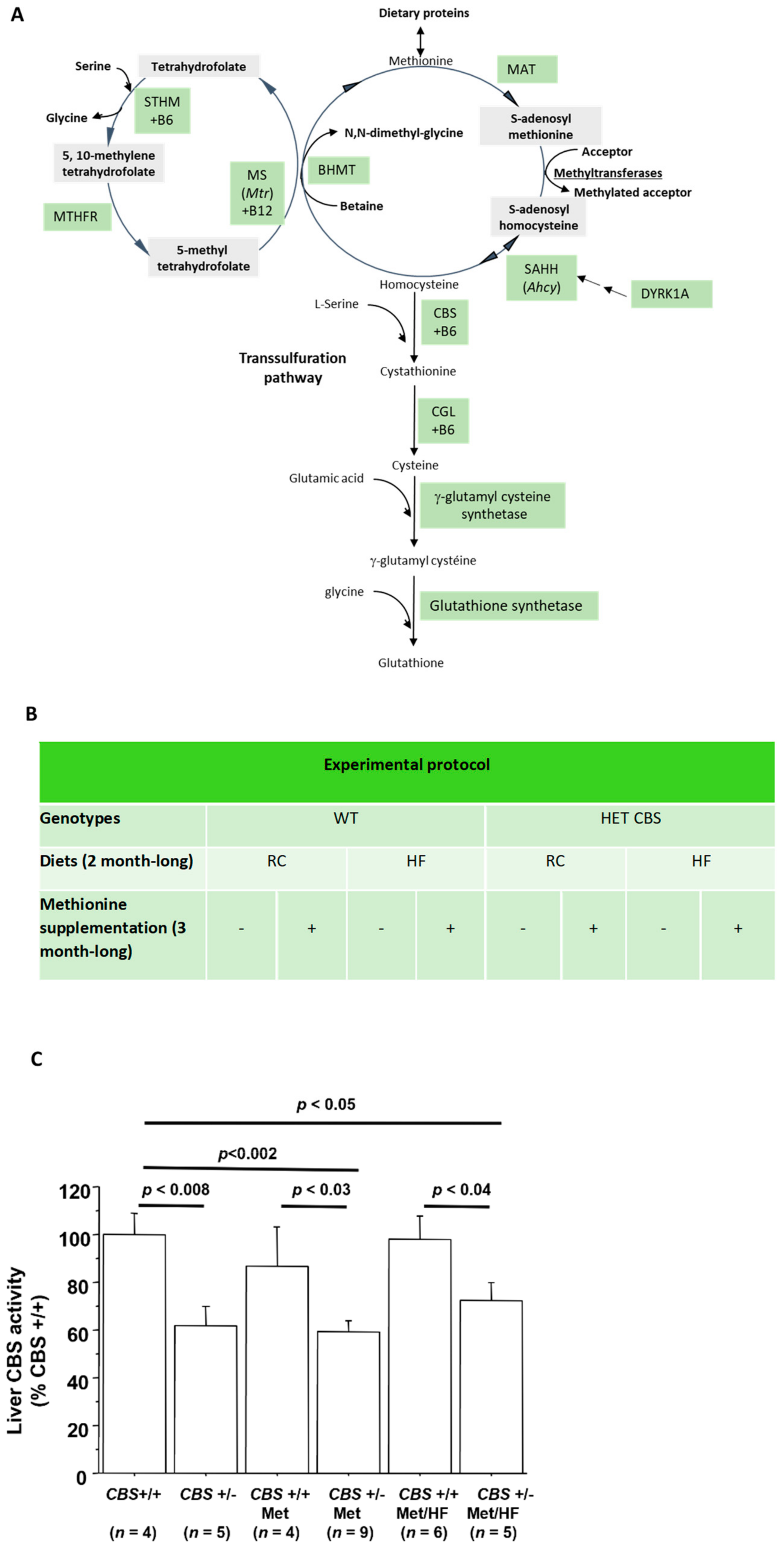
2.2. CBS Enzyme Activity Assays
2.3. Plasma Hcy Assays
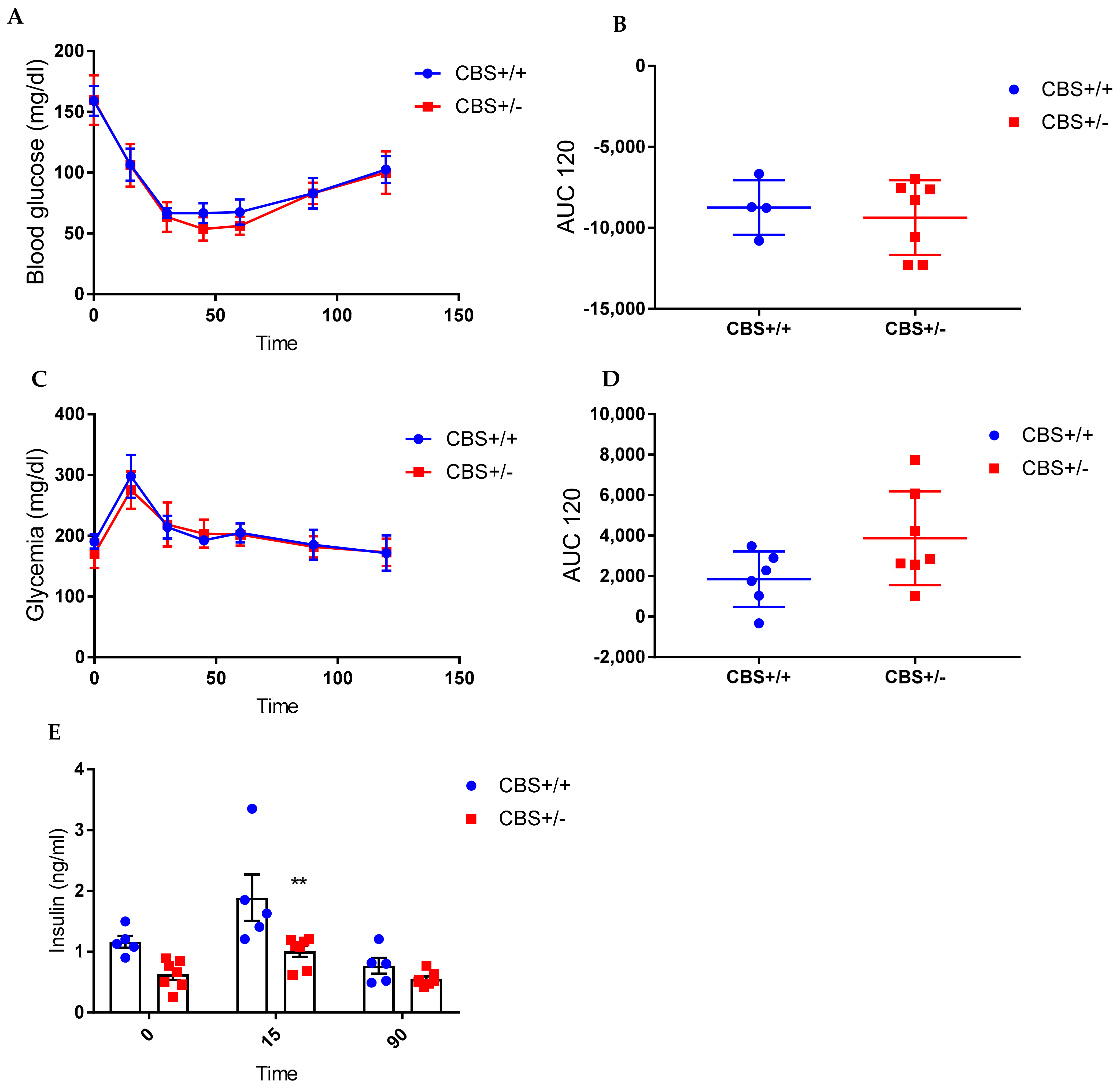
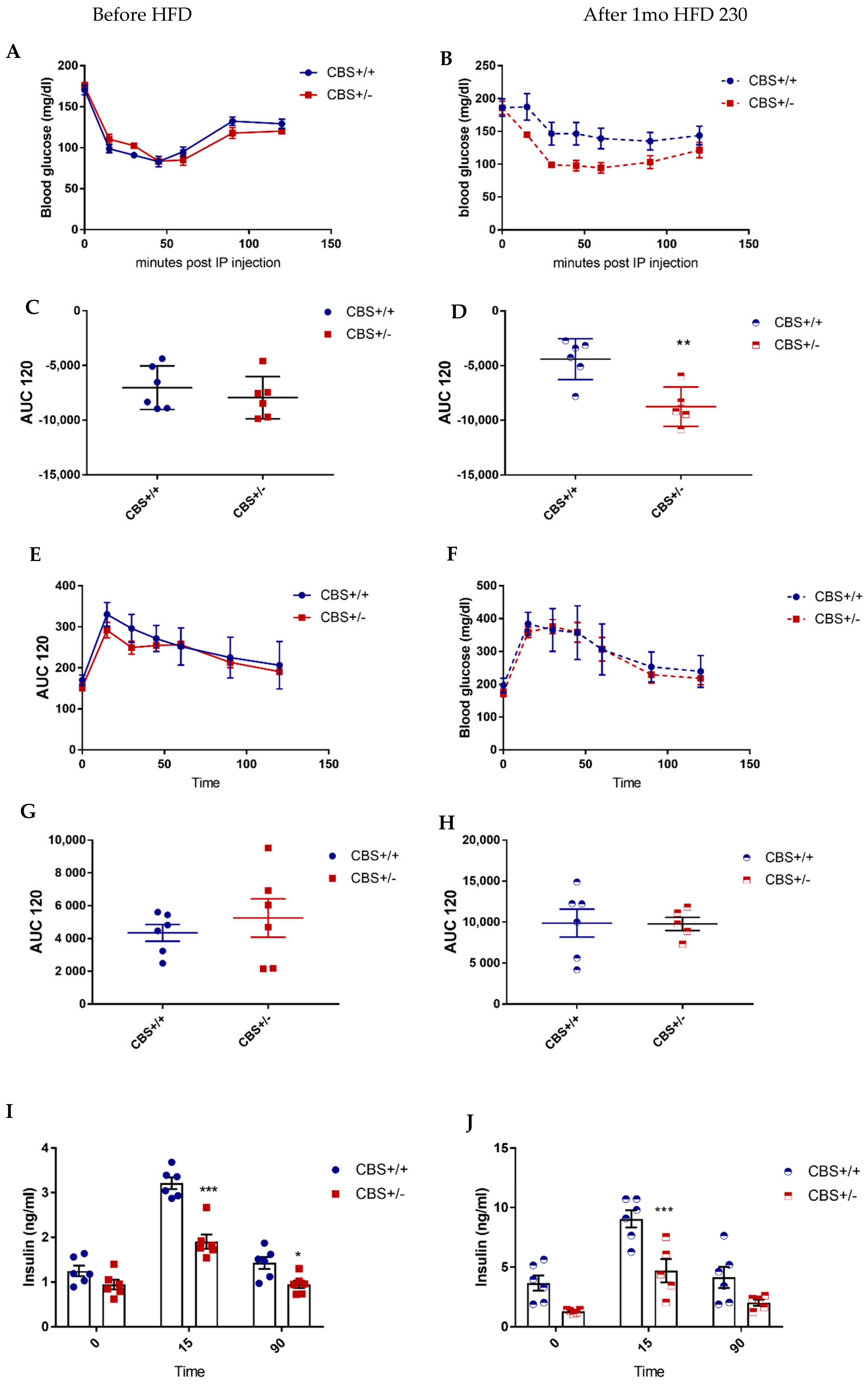
2.4. Glucose and Insulin Tolerance Tests
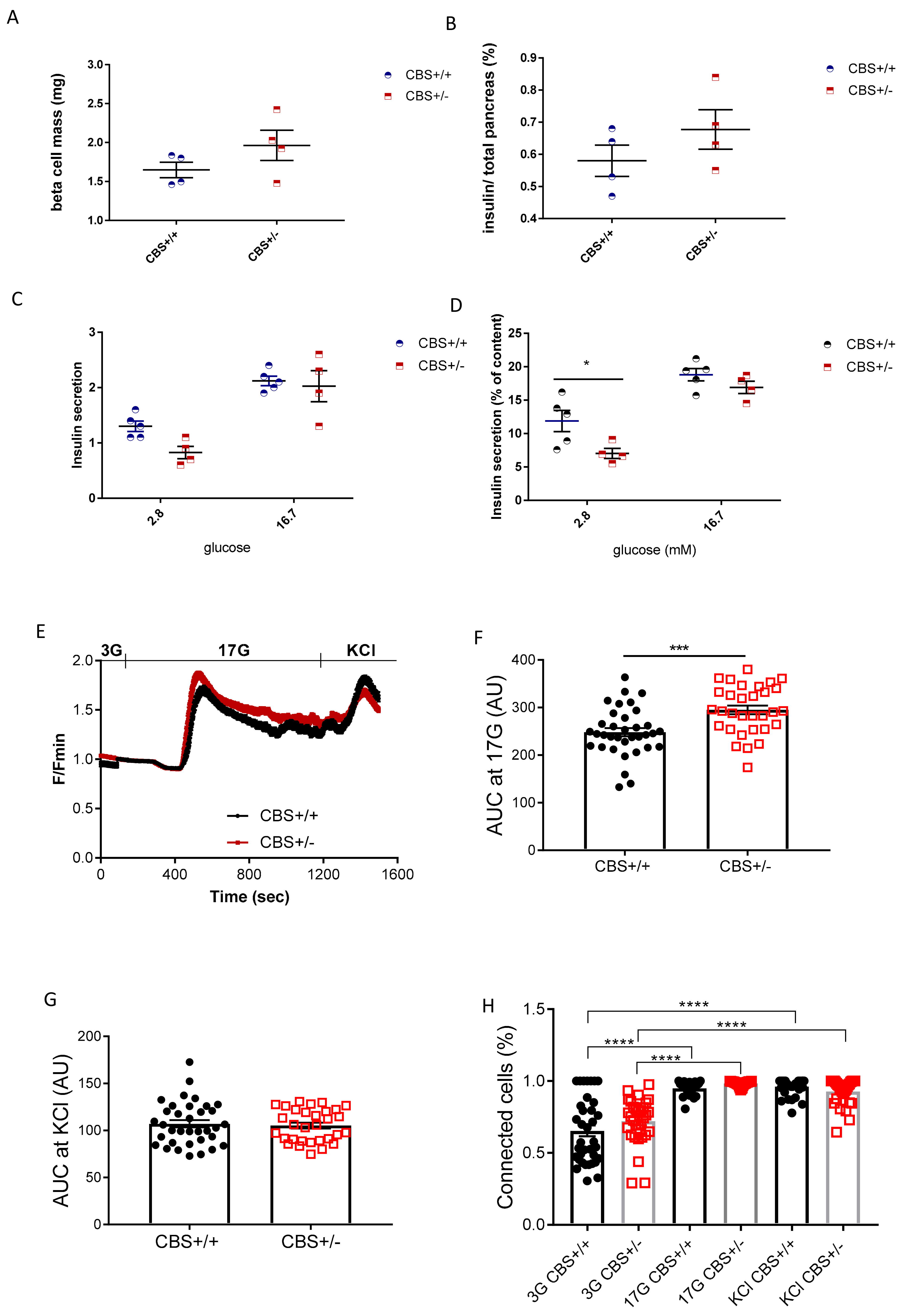
2.5. Immunohistochemistry and Quantification
2.6. Islet Isolation and Culture
2.7. Static Batch Incubation
2.8. Measurement of Intracellular Free Ca2+ and Pearson (R)-Based Connectivity and Correlation Analyses
2.9. RNA Extraction, cDNA Synthesis, and Real-Time PCR Using SYBR-Green Chemistry
2.10. Correlation Studies in Liver and Islets
2.11. Network Construction
3. Results
3.1. Determination of Moderate to Intermediate HHcy Effects on the Body Weight and Glucose Homeostasis In Vivo
3.1.1. Genotype-Only Effect (Moderate HHcy) on Glucose Homeostasis
3.1.2. Genotype and Methionine Effect (Intermediate HHcy) on Glucose Homeostasis
3.2. Determination of Islets Insulin Secretion, Calcium Imaging, and Cell Connectivity
3.3. Differential Modulation of the Autonomic Nervous System in CBS+/− Mice
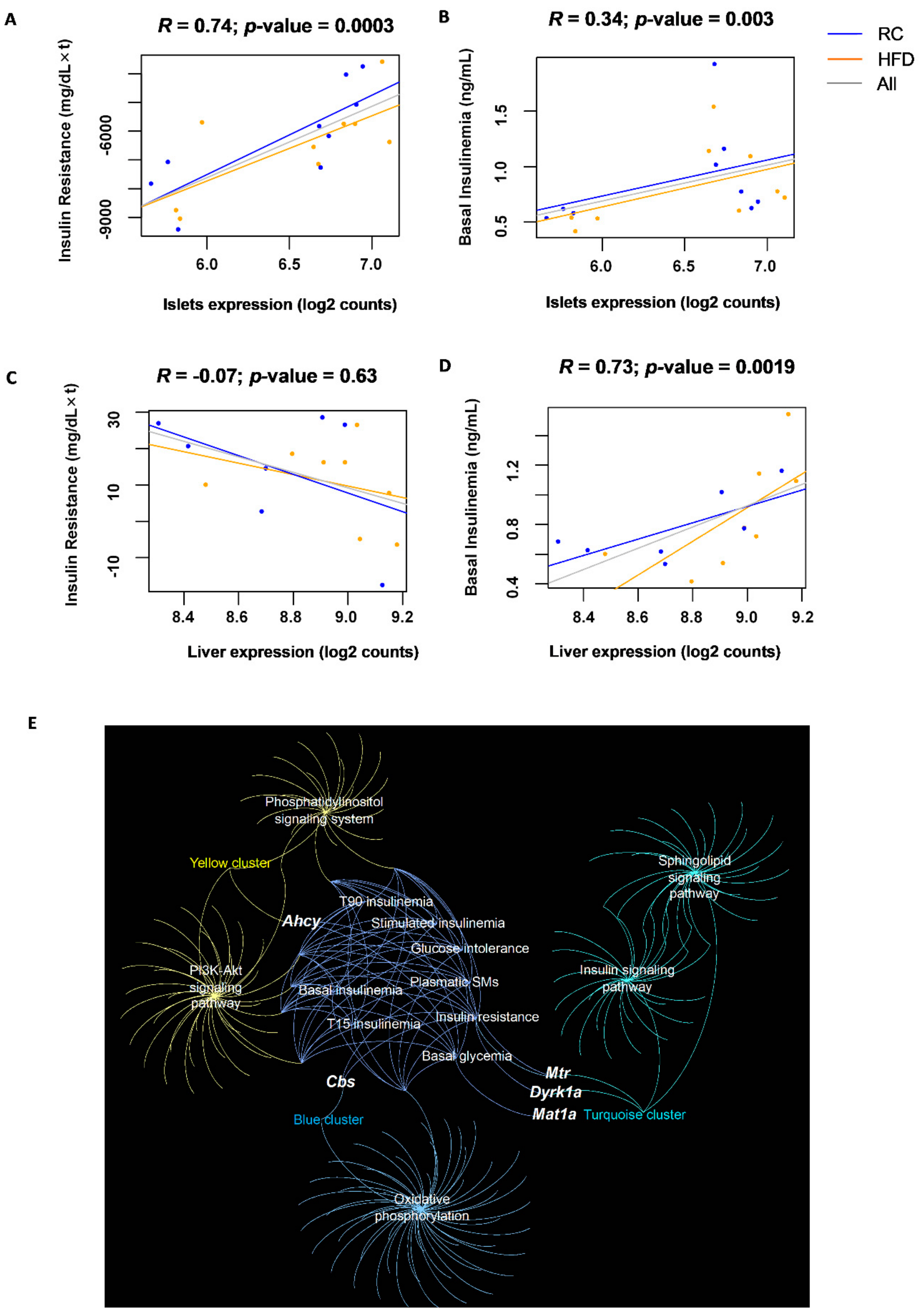
3.4. Islet Expression of CBS Pathway Genes Correlates with Glucose Homeostasis Traits in a Multi-Strain Study
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fowler, B. Disorders of homocysteine metabolism. J. Inherit. Metab. Dis. 1997, 20, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- Nieraad, H.; Pannwitz, N.; de Bruin, N.; Geisslinger, G.; Till, U. Hyperhomocysteinemia: Metabolic Role and Animal Studies with a Focus on Cognitive Performance and Decline—A Review. Biomolecules 2021, 11, 1546. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Hůlková, H.; Krijt, J.; Kožich, V.; Bublil, E.M.; Majtan, T. Long-term uninterrupted enzyme replacement therapy prevents liver disease in murine model of severe homocystinuria. Hum. Mutat. 2020, 41, 1662–1670. [Google Scholar] [CrossRef]
- Sarna, L.K.; Siow, Y.L.; Karmin, O. The CBS/CSE system: A potential therapeutic target in NAFLD? Can. J. Physiol. Pharmacol. 2015, 93, 1–11. [Google Scholar] [CrossRef]
- Sudchada, P.; Saokaew, S.; Sridetch, S.; Incampa, S.; Jaiyen, S.; Khaithong, W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2012, 98, 151–158. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Jiang, C.; Xu, M.; Pang, Y.; Feng, J.; Xiang, X.; Kong, W.; Xu, G.; Li, Y.; et al. Hyperhomocysteinemia promotes insulin resistance by inducing endoplasmic reticulum stress in adipose tissue. J. Biol. Chem. 2013, 288, 9583–9592. [Google Scholar] [CrossRef]
- Watanabe, M.; Osada, J.; Aratani, Y.; Kluckman, K.; Reddick, R.; Malinow, M.R.; Maeda, N. Mice deficient in cystathionine beta-synthase: Animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA 1995, 92, 1585–1589. [Google Scholar] [CrossRef]
- Le Stunff, H.; Véret, J.; Kassis, N.; Denom, J.; Meneyrol, K.; Paul, J.; Cruciani-Guglielmacci, C.; Magnan, C.; Janel, N. Deciphering the Link between Hyperhomocysteinemia and Ceramide Metabolism in Alzheimer-Type Neurodegeneration. Front. Neurol. 2019, 10, 807. [Google Scholar] [CrossRef]
- Baloula, V.; Fructuoso, M.; Kassis, N.; Gueddouri, D.; Paul, J.-L.; Janel, N. Homocysteine-lowering gene therapy rescues signaling pathways in brain of mice with intermediate hyperhomocysteinemia. Redox Biol. 2018, 19, 200–209. [Google Scholar] [CrossRef]
- Cruciani-Guglielmacci, C.; Bellini, L.; Denom, J.; Oshima, M.; Fernandez, N.; Normandie-Levi, P.; Berney, X.P.; Kassis, N.; Rouch, C.; Dairou, J.; et al. Molecular phenotyping of multiple mouse strains under metabolic challenge uncovers a role for Elovl2 in glucose-induced insulin secretion. Mol. Metab. 2017, 6, 340–351. [Google Scholar] [CrossRef]
- Joly-Amado, A.; Denis, R.G.; Castel, J.; Lacombe, A.; Cansell, C.; Rouch, C.; Kassis, N.; Dairou, J.; Cani, P.D.; Ventura-Clapier, R.; et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 2012, 31, 4276–4288. [Google Scholar] [CrossRef]
- Peterfi, Z.I.; Farkas, R.G.P.; Denis, E.; Farkas, M.; Uchigashima, T.; Fuzesi, M.; Watanabe, R.M.; Lechan, Z.; Liposits, S.; Fekete, C.; et al. Endocannabinoid and nitric oxide systems of the hypothalamic paraventricular nucleus mediate effects of NPY on energy expenditure. Mol. Metab. 2018, 18, 120–133. [Google Scholar] [CrossRef]
- Miller, J.W.; Nadeau, M.R.; Smith, J.; Smith, D.; Selhub, J. Folate-deficiency-induced homocysteinaemia in rats: Disruption of S-adenosylmethionine’s co-ordinate regulation of homocysteine metabolism. Biochem. J. 1994, 298, 415–419. [Google Scholar] [CrossRef]
- Ducros, V.; Demuth, K.; Sauvant, M.-P.; Quillard, M.; Caussé, E.; Candito, M.; Read, M.-H.; Drai, J.; Garcia, I.; Gerhardt, M.-F. Methods for homocysteine analysis and biological relevance of the results. J. Chromatogr. B 2002, 781, 207–226. [Google Scholar] [CrossRef]
- Rachdi, L.; Kariyawasam, D.; Aïello, V.; Herault, Y.; Janel, N.; Delaba, J.M.; Polak, M.; Scharfmann, R. Dyrk1A induces pancreatic beta cell mass expansion and improves glucose tolerance. Cell Cycle 2014, 13, 2221–2229. [Google Scholar] [CrossRef]
- Amouyal, C.; Castel, J.; Guay, C.; Lacombe, A.; Denom, J.; Migrenne-Li, S.; Rouault, C.; Marquet, F.; Georgiadou, E.; Stylianides, T.; et al. A surrogate of Roux-en-Y gastric bypass (the enterogastro anastomosis surgery) regulates multiple beta-cell pathways during resolution of diabetes in ob/ob mice. eBioMedicine 2020, 58, 102895. [Google Scholar] [CrossRef]
- Akalestou, E.; Suba, K.; Lopez-Noriega, L.; Georgiadou, E.; Chabosseau, P.; Gallie, A.; Wretlind, A.; Legido-Quigley, C.; Leclerc, I.; Salem, V.; et al. Intravital imaging of islet Ca2+ dynamics reveals enhanced beta cell connectivity after bariatric surgery in mice. Nat. Commun. 2021, 12, 5165. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Hamelet, J.; Aït-Yahya-Graison, E.; Matulewicz, E.; Noll, C.; Badel-Chagnon, A.; Camproux, A.-C.; DeMuth, K.; Paul, J.-L.; Delabar, J.M.; Janel, N. Homocysteine threshold value based on cystathionine beta synthase and paraoxonase 1 activities in mice. Eur. J. Clin. Investig. 2007, 37, 933–938. [Google Scholar] [CrossRef]
- Rutter, G.A.; Georgiadou, E.; Martinez-Sanchez, A.; Pullen, T.J. Metabolic and functional specialisations of the pancreatic beta cell: Gene disallowance, mitochondrial metabolism and intercellular connectivity. Diabetologia 2020, 63, 1990–1998. [Google Scholar] [CrossRef]
- Ferrer, J.; Gomis, R.; Alvarez, J.F.; Casamitjana, R.; Vilardell, E. Signals derived from glucose metabolism are required for glucose regulation of pancreatic islet GLUT2 mRNA and protein. Diabetes 1993, 42, 1273–1280. [Google Scholar] [CrossRef]
- Hodson, D.J.; Mitchell, R.K.; Bellomo, E.A.; Sun, G.; Vinet, L.; Meda, P.; Li, D.; Li, W.H.; Bugliani, M.; Marchetti, P.; et al. Lipotoxicity disrupts incretin-regulated human beta cell connectivity. J. Clin. Invest. 2013, 123, 4182–4194. [Google Scholar] [CrossRef]
- Johnston, N.R.; Mitchell, R.K.; Haythorne, E.; Pessoa, M.P.; Semplici, F.; Ferrer, J.; Piemonti, L.; Marchetti, P.; Bugliani, M.; Bosco, D.; et al. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell. Metab. 2016, 24, 389–401. [Google Scholar] [CrossRef]
- Sanchez-Archidona, A.R.; Cruciani-Guglielmacci, C.; Roujeau, C.; Wigger, L.; Lallement, J.; Denom, J.; Barovic, M.; Kassis, N.; Mehl, F.; Weitz, J.; et al. Plasma triacylglycerols are biomarkers of beta-cell function in mice and humans. Mol. Metab. 2021, 54, 101355. [Google Scholar] [CrossRef]
- Patterson, S.; Flatt, P.R.; McClenaghan, N.H. Homocysteine-induced impairment of insulin secretion from clonal pancreatic BRIN-BD11 beta-cells is not prevented by catalase. Pancreas 2007, 34, 144–151. [Google Scholar] [CrossRef]
- Scullion, S.M.; Gurgul-Convey, E.; Elsner, M.; Lenzen, S.; Flatt, P.R.; McClenaghan, N.H. Enhancement of homocysteine toxicity to insulin-secreting BRIN-BD11 cells in combination with alloxan. J. Endocrinol. 2012, 214, 233–238. [Google Scholar] [CrossRef]
- Kimura, H. Production and Physiological Effects of Hydrogen Sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef]
- Takahashi, N.; Wei, F.Y.; Watanabe, S.; Hirayama, M.; Ohuchi, Y.; Fujimura, A.; Kaitsuka, T.; Ishii, I.; Sawa, T.; Nakayama, H.; et al. Reactive sulfur species regulate tRNA methylthiolation and contribute to insulin secretion. Nucleic. Acids. Res. 2017, 45, 435–445. [Google Scholar] [CrossRef]
- Zheng, F.; Han, J.; Lu, H.; Cui, C.; Yang, J.; Cui, Q.; Cai, J.; Zhou, Y.; Tang, C.; Xu, G.; et al. Cystathionine beta synthase-hydrogen sulfide system in paraventricular nucleus reduced high fatty diet induced obesity and insulin resistance by brain-adipose axis. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Luquet, S.; Magnan, C. The central nervous system at the core of the regulation of energy homeostasis. Front. Biosci. 2009, 1, 448–465. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. Neural regulation of pancreatic islet cell mass and function. Diabetes Obes. Metab. 2014, 16, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rosario, W.; Singh, I.; Wautlet, A.; Patterson, C.; Flak, J.; Becker, T.C.; Ali, A.; Tamarina, N.; Philipson, L.H.; Enquist, L.W.; et al. The Brain–to–Pancreatic Islet Neuronal Map Reveals Differential Glucose Regulation From Distinct Hypothalamic Regions. Diabetes 2016, 65, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Han, S.J.; Hamdan, F.F.; Jeon, J.; Li, B.; Li, J.H.; Cui, Y.; Mears, D.; Lu, H.; Deng, C.; et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006, 3, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Peterhoff, M.; Sieg, A.; Brede, M.; Chao, C.-M.; Hein, L.; Ullrich, S. Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. Eur. J. Endocrinol. 2003, 149, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Kreier, F.; Yilmaz, A.; Kalsbeek, A.; Romijn, J.A.; Sauerwein, H.P.; Fliers, E.; Buijs, R.M. Hypothesis: Shifting the equilibrium from activity to food leads to autonomic unbalance and the metabolic syndrome. Diabetes 2003, 52, 2652–2656. [Google Scholar] [CrossRef]
- Bortolotto, L.A.; Safar, M.E.; Billaud, E.; Lacroix, C.; Asmar, R.; London, G.M.; Blacher, J. Plasma homocysteine, aortic stiffness, and renal function in hypertensive patients. Hypertension. 1999, 34, 837–842. [Google Scholar] [CrossRef]
- Mendes, R.; Mostarda, C.; Candido, G.; Moraes-Silva, I.; D’Almeida, V.; Belló-Klein, A.; Irigoyen, M.; Rigatto, K. Moderate hyperhomocysteinemia provokes dysfunction of cardiovascular autonomic system and liver oxidative stress in rats. Auton. Neurosci. 2014, 180, 43–47. [Google Scholar] [CrossRef][Green Version]
- Feng, X.; Xu, Y. Hyperhomocysteinemia as a Metabolic Risk Factor for Glucose Intolerance Among High-Risk Groups of Chinese Adults. Med. Sci. Monit. 2017, 23, 2775–2781. [Google Scholar] [CrossRef]
- Munshi, M.N.; Stone, A.; Fink, L.; Fonseca, V. Hyperhomocysteinemia following a methionine load in patients with non-insulin-dependent diabetes mellitus and macrovascular disease. Metabolism 1996, 45, 133–135. [Google Scholar] [CrossRef]
- A Hofmann, M.; Kohl, B.; Zumbach, M.S.; Borcea, V.; Bierhaus, A.; Henkels, M.; Amiral, J.; Fiehn, W.; Ziegler, R.; Wahl, P.; et al. Hyperhomocyst(e)inemia and Endothelial Dysfunction in IDDM. Diabetes Care 1997, 20, 1880–1886. [Google Scholar] [CrossRef]
- Bar-On, H.; Kidron, M.; Friedlander, Y.; Ben-Yehuda, A.; Selhub, J.; Rosenberg, I.H.; Friedman, G. Plasma total homocysteine levels in subjects with hyperinsulinemia. J. Intern. Med. 2000, 247, 287–294. [Google Scholar] [CrossRef]
- Moulle, V.S.; Tremblay, C.; Castell, A.C.; Vivot, K.; Ethier, M.; Fergusson, G.; Alquier, T.; Ghislain, J.; Poitout, V. The autonomic nervous system regulates pancreatic beta-cell proliferation in adult male rats. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E234–E243. [Google Scholar] [CrossRef]
- Gosmain, Y.; Katz, L.S.; Masson, M.H.; Cheyssac, C.; Poisson, C.; Philippe, J. Pax6 is crucial for beta-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol. Endocrinol. 2012, 26, 696–709. [Google Scholar] [CrossRef]
- Hart, A.W.; Mella, S.; Mendrychowski, J.; van Heyningen, V.; Kleinjan, D.A. The Developmental Regulator Pax6 Is Essential for Maintenance of Islet Cell Function in the Adult Mouse Pancreas. PLoS ONE 2013, 8, e54173. [Google Scholar] [CrossRef]
- Swisa, A.; Avrahami, D.; Eden, N.; Zhang, J.; Feleke, E.; Dahan, T.; Cohen-Tayar, Y.; Stolovich-Rain, M.; Kaestner, K.H.; Glaser, B.; et al. PAX6 maintains beta cell identity by repressing genes of alternative islet cell types. J. Clin. Investig. 2017, 127, 230–243. [Google Scholar] [CrossRef]
- Spaeth, J.M.; Gupte, M.; Perelis, M.; Yang, Y.P.; Cyphert, H.; Guo, S.; Liu, J.H.; Guo, M.; Bass, J.; Magnuson, M.A.; et al. Defining a Novel Role for the Pdx1 Transcription Factor in Islet beta-Cell Maturation and Proliferation During Weaning. Diabetes 2017, 66, 2830–2839. [Google Scholar] [CrossRef]
- Piccand, J.; Strasser, P.; Hodson, D.J.; Meunier, A.; Ye, T.; Keime, C.; Birling, M.C.; Rutter, G.A.; Gradwohl, G. Rfx6 maintains the functional identity of adult pancreatic beta cells. Cell Rep. 2014, 9, 2219–2232. [Google Scholar] [CrossRef]
- Henseleit, K.D.; Nelson, S.B.; Kuhlbrodt, K.; Hennings, J.C.; Ericson, J.; Sander, M. NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development 2005, 132, 3139–3149. [Google Scholar] [CrossRef]
- Noll, C.; Planque, C.; Ripoll, C.; Guedj, F.; Diez, A.; Ducros, V.; Belin, N.; Duchon, A.; Paul, J.-L.; Badel, A.; et al. DYRK1A, a Novel Determinant of the Methionine-Homocysteine Cycle in Different Mouse Models Overexpressing this Down-Syndrome-Associated Kinase. PLoS ONE 2009, 4, e7540. [Google Scholar] [CrossRef]
- Planque, C.; Dairou, J.; Noll, C.; Bui, L.; Ripoll, C.; Guedj, F.; Delabar, J.; Janel, N. Mice deficient in cystathionine beta synthase display increased Dyrk1A and SAHH activities in brain. J. Mol. Neurosci. 2013, 50, 1–6. [Google Scholar] [CrossRef]
- Rachdi, L.; Kariyawasam, D.; Guez, F.; Aïello, V.; Arbonés, M.L.; Janel, N.; Delabar, J.-M.; Polak, M.; Scharfmann, R. Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass. Diabetologia 2014, 57, 960–969. [Google Scholar] [CrossRef]

| Genotype | CBS+/+ | CBS+/− | CBS+/+ | CBS+/− | CBS+/+ | CBS+/− |
|---|---|---|---|---|---|---|
| Age | 2 mo | 2 mo | 7 mo | 7 mo | 8 mo | 8 mo |
| Treatment and diet | none | none | methionine for 5 mo | methionine for 5 mo | Meth. for 6 mo, HFD for 1 mo | Meth. for 6 mo, HFD for 1 mo |
| Hcy (µmol/L) | 3.3 ± 0.3 | 7.4 ± 1 *** | 9.6 ± 2.7 | 30.4 ± 4.6 *** | 6.4 ± 0.4 | 37.1 ± 3.2 *** |
| Body weight (g) | 28.3 ± 1.74 | 27.53 ± 0.66 | 33.50 ± 0.44 | 31.53 ± 1.09 | 42.17 ± 1.1 | 37.98 ± 1.78 |
| Fat mass (g) | 4.51 ± 0.35 | 4.56 ± 0.42 | 7.06 ± 0.45 | 6.01 ± 0.73 | 16.36 ± 1.04 | 13.34 ± 1.34 |
| Lean mass (g) | 21.51 ± 1.42 | 20.63 ± 0.64 | 23.89 ± 0.34 | 22.93 ± 0.38 | 23.43 ± 0.37 | 22.47 ± 0.45 |
| Pancreatic Islets (mRNA) | CBS+/+ | CBS+/− |
|---|---|---|
| (n = 4) | (n = 3) | |
| AdRa2a (adrenergic receptor) | 100 ± 16 | 43 ± 5 ** |
| Cbs | 100 ± 16 | 58 ± 14 * |
| Cx36 (connexin 36) | 100 ± 12 | 30 ± 10 *** |
| Gck | 100 ± 11 | 78 ± 19 |
| Glucagon | 100 ± 26 | 262 ± 17 *** |
| Glut2 | 100 ± 40 | 248 ± 87 * |
| Ins1 | 100 ± 7 | 139 ± 33 |
| Ins2 | 100 ± 4 | 76 ± 10 ** |
| Ldha | 100 ± 20 | 53 ± 22 |
| MafA | 100 ± 13 | 30 ± 7 *** |
| Mct1 | 100 ± 10 | 61 ± 16 |
| M3 (muscarinic Receptor) | 100 ± 20 | 40 ± 15 * |
| Nkx6-1 | 100 ± 15 | 68 ± 6 |
| Nkx6-2 | 100 ± 20 | 26 ± 3 ** |
| Pax6 | 100 ± 12 | 65 ± 11 * |
| Pdx1 | 100 ± 7 | 51 ± 4 *** |
| Rfx6 | 100 ± 11 | 70 ± 7 * |
| Somatostatin | 100 ± 15 | 69 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruciani-Guglielmacci, C.; Meneyrol, K.; Denom, J.; Kassis, N.; Rachdi, L.; Makaci, F.; Migrenne-Li, S.; Daubigney, F.; Georgiadou, E.; Denis, R.G.; et al. Homocysteine Metabolism Pathway Is Involved in the Control of Glucose Homeostasis: A Cystathionine Beta Synthase Deficiency Study in Mouse. Cells 2022, 11, 1737. https://doi.org/10.3390/cells11111737
Cruciani-Guglielmacci C, Meneyrol K, Denom J, Kassis N, Rachdi L, Makaci F, Migrenne-Li S, Daubigney F, Georgiadou E, Denis RG, et al. Homocysteine Metabolism Pathway Is Involved in the Control of Glucose Homeostasis: A Cystathionine Beta Synthase Deficiency Study in Mouse. Cells. 2022; 11(11):1737. https://doi.org/10.3390/cells11111737
Chicago/Turabian StyleCruciani-Guglielmacci, Céline, Kelly Meneyrol, Jessica Denom, Nadim Kassis, Latif Rachdi, Fatna Makaci, Stéphanie Migrenne-Li, Fabrice Daubigney, Eleni Georgiadou, Raphaël G. Denis, and et al. 2022. "Homocysteine Metabolism Pathway Is Involved in the Control of Glucose Homeostasis: A Cystathionine Beta Synthase Deficiency Study in Mouse" Cells 11, no. 11: 1737. https://doi.org/10.3390/cells11111737
APA StyleCruciani-Guglielmacci, C., Meneyrol, K., Denom, J., Kassis, N., Rachdi, L., Makaci, F., Migrenne-Li, S., Daubigney, F., Georgiadou, E., Denis, R. G., Rodriguez Sanchez-Archidona, A., Paul, J.-L., Thorens, B., Rutter, G. A., Magnan, C., Le Stunff, H., & Janel, N. (2022). Homocysteine Metabolism Pathway Is Involved in the Control of Glucose Homeostasis: A Cystathionine Beta Synthase Deficiency Study in Mouse. Cells, 11(11), 1737. https://doi.org/10.3390/cells11111737







