Thyroid and Corticosteroid Signaling in Amphibian Metamorphosis
Abstract
1. Introduction
2. T3 and TR in Xenopus Development
2.1. Establishing a Dual Function Model for TR during Development
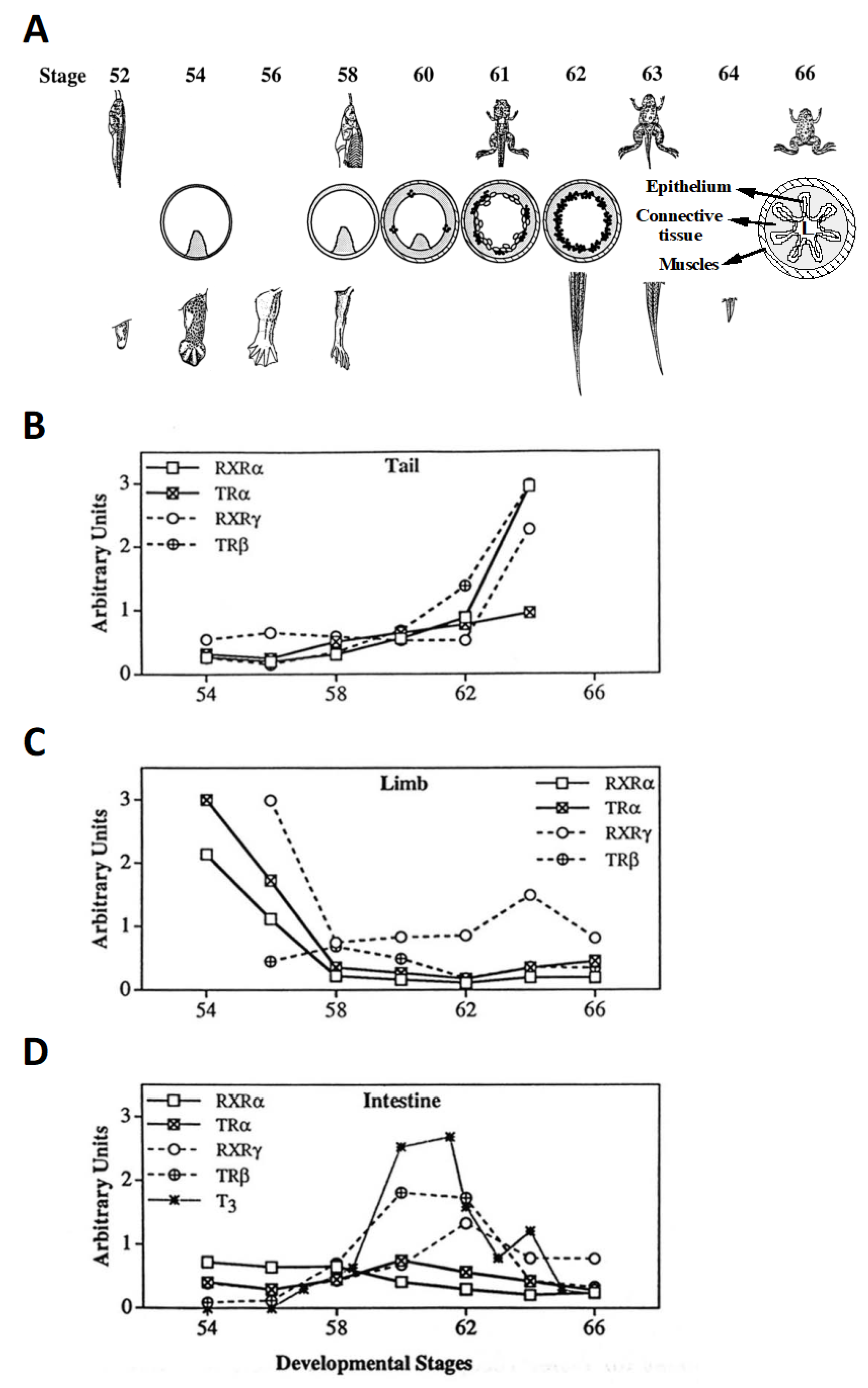
2.2. Temporal Correlation of TR Subtype Expression with Organ-Specific Metamorphosis
2.3. TR Is Essential for Completion of Metamorphosis but Derepression of Gene Expression due to TR Double-knockout Allows for Precocious Development of Many Adult Organs
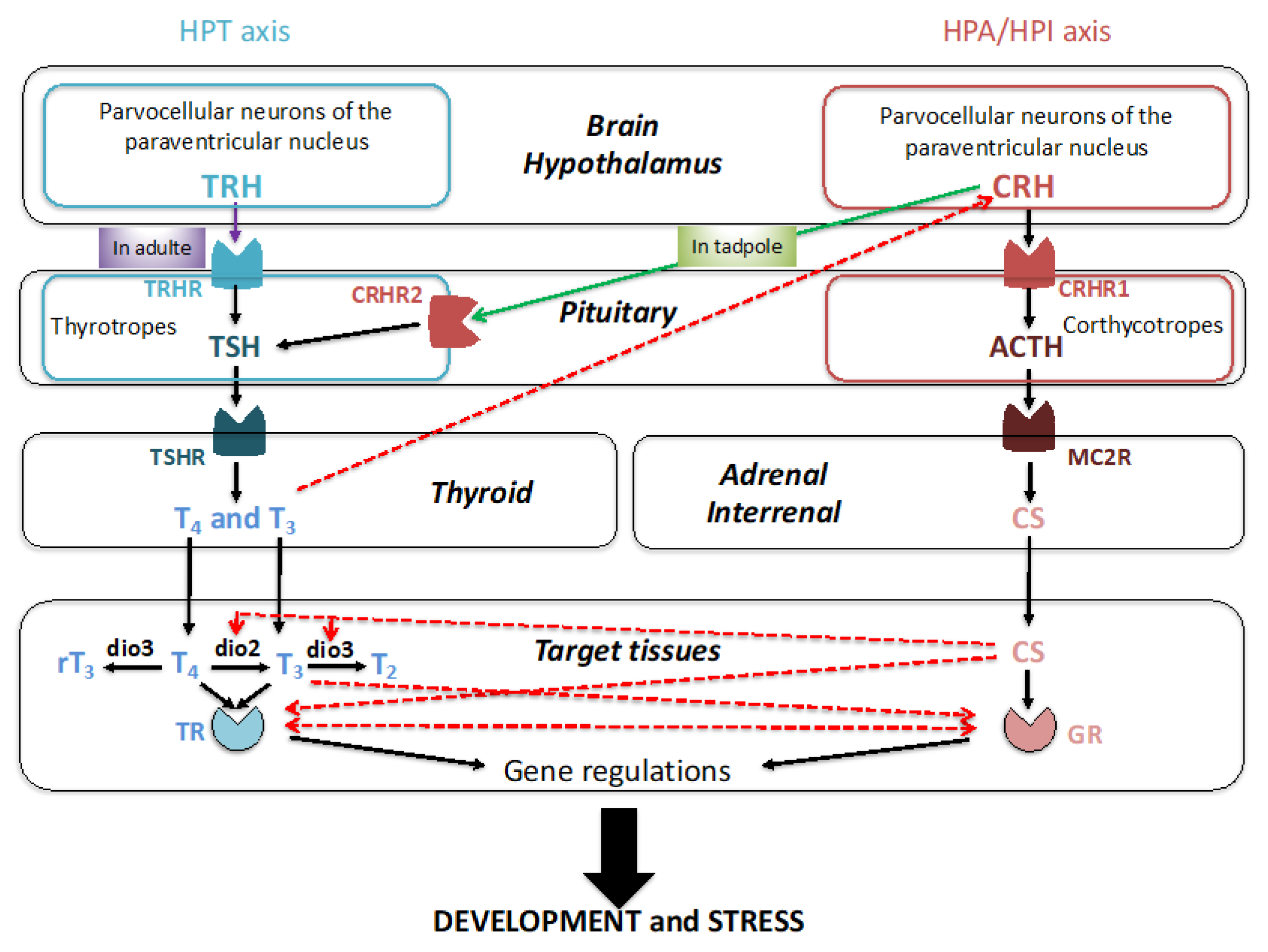
3. Corticosteroids (CSs) and Their Receptors in Xenopus Development
3.1. Production of CSs during Development
3.2. Nuclear Receptors for Corticosteroid Signaling during Development
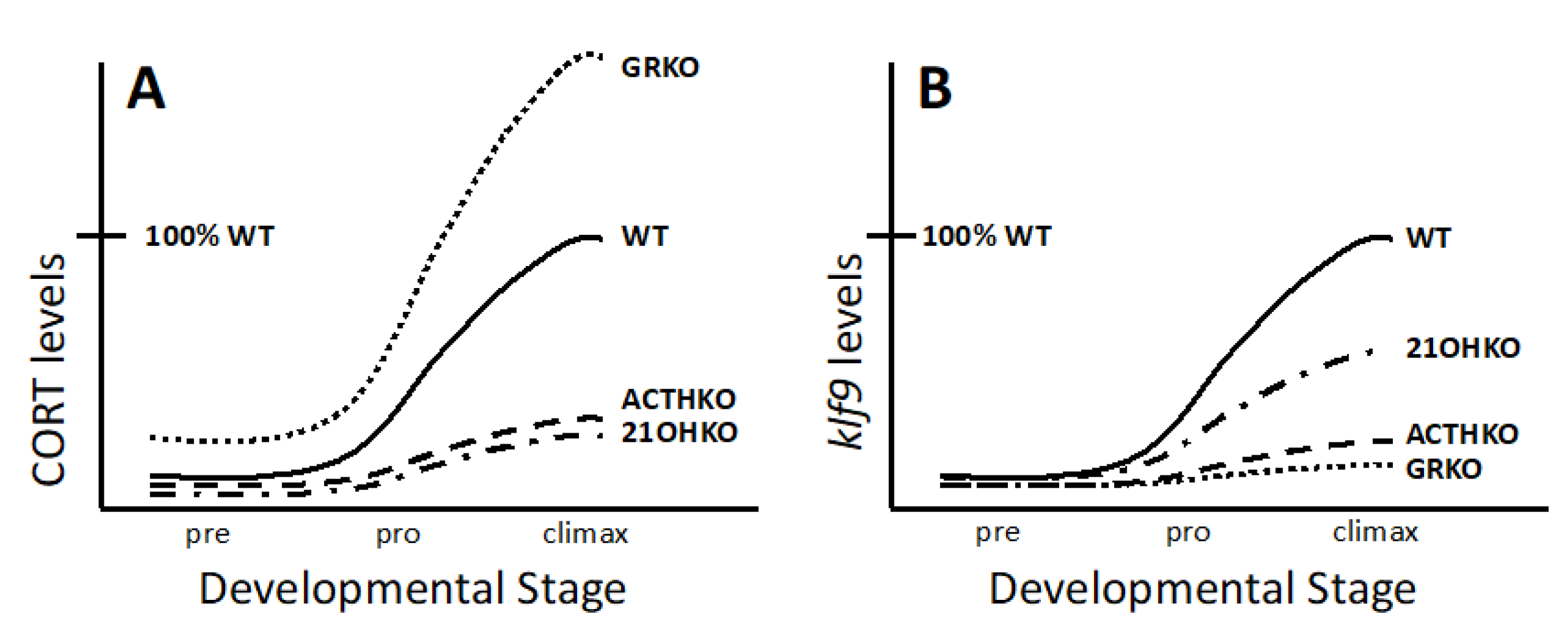
3.3. Requirement of CSs for Metamorphosis
3.4. Necessity of Corticosteroid-Mediated Enhancement of Thyroid Hormone Signaling
3.5. Developmental Roles of Corticosteroids Independent of TH
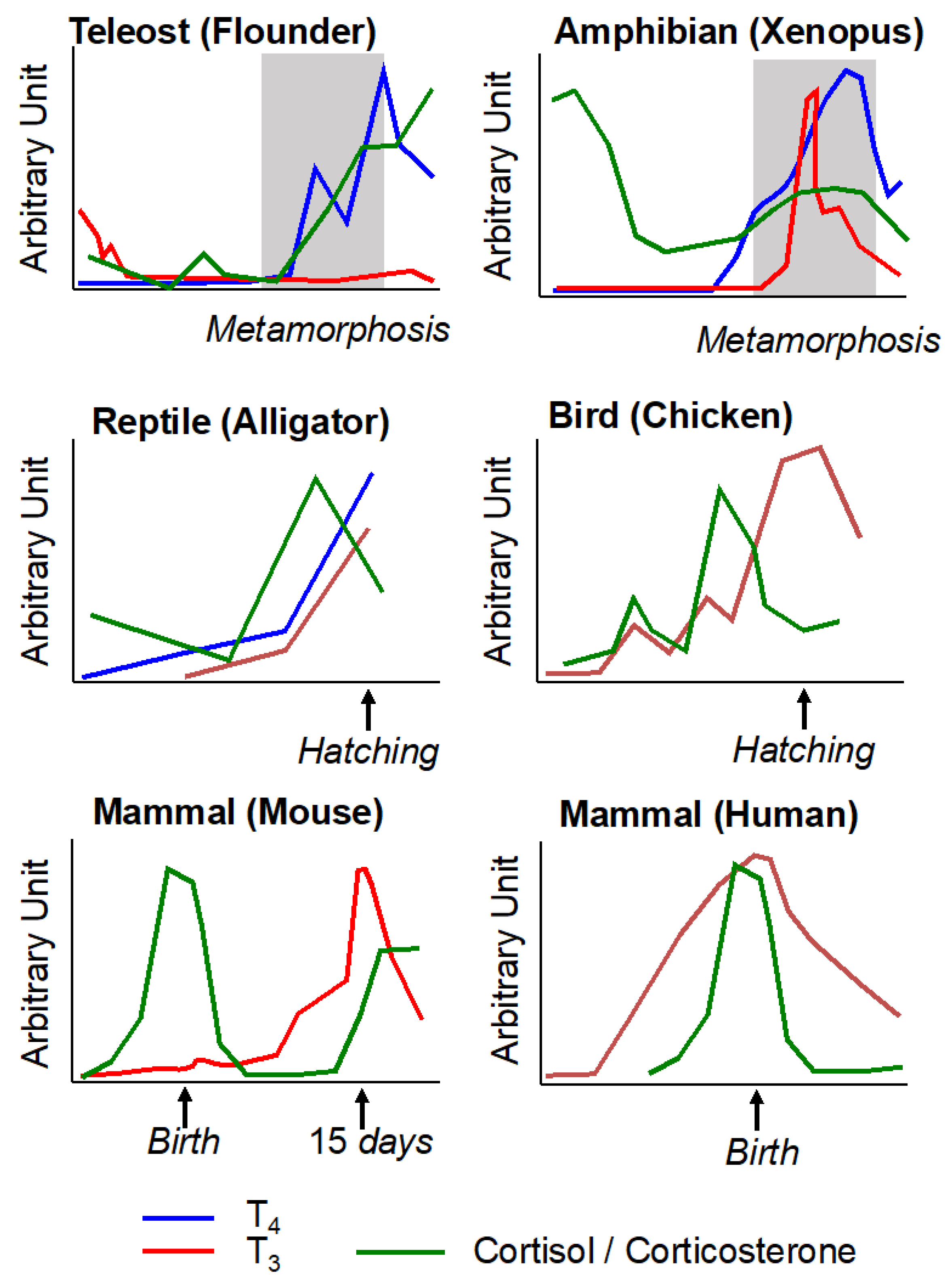
4. Conservation during Vertebrate Evolution of Thyroid Hormone and Corticosteroid Action in Post-Embryonic Development
4.1. Metamorphosis in Teleost Fishes
4.2. Egg Hatching in Sauropsids
4.3. Birth in Mammals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rousseau, K.; Dufour, S.; Sachs, L.M. Interdependence of Thyroid and Corticosteroid Signaling in Vertebrate Developmental Transitions. Front. Ecol. Evol. 2021, 9, 735487. [Google Scholar] [CrossRef]
- Shi, Y.-B. Amphibian Metamorphosis: From Morphology to Molecular Biology; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Denver, R.J. Stress hormones mediate developmental plasticity in vertebrates with complex life cycles. Neurobiol. Stress 2021, 14, 100301. [Google Scholar] [CrossRef] [PubMed]
- Glennemeier, K.A.; Denver, R.J. Small changes in whole-body corticosterone content affect larval Rana pipiens fitness components. Gen. Comp. Endocrinol. 2002, 127, 16–25. [Google Scholar] [CrossRef]
- Denver, R.J. Acceleration of anuran amphibian metamorphosis by corticotropin-releasing hormone-like peptides. Gen. Comp. Endocrinol. 1993, 91, 38–51. [Google Scholar] [CrossRef] [PubMed]
- De Groef, B.; Van Der Geyten, S.; Darras, V.M.; Kühn, E.R. Role of corticotropin-releasing hormone as a thyrotropin-releasing factor in non-mammalian vertebrates. Gen. Comp. Endocrinol. 2006, 146, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.A.; Affanni, J.M.; Paz, D.A. Corticotropin-releasing factor accelerates metamorphosis in Bufo arenarum: Effect on pituitary ACTH and TSH cells. J. Exp. Zool. 2000, 286, 473–480. [Google Scholar] [CrossRef]
- Denver, R.J. Environmental stress as a developmental cue: Corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm. Behav. 1997, 31, 169–179. [Google Scholar] [CrossRef]
- Galton, V.A. Mechanisms underlying the acceleration of thyroid hormone-induced tadpole metamorphosis by corticosterone. Endocrinology 1990, 127, 2997–3002. [Google Scholar] [CrossRef]
- Bonett, R.M.; Hoopfer, E.D.; Denver, R.J. Molecular mechanisms of corticosteroid synergy with thyroid hormone during tadpole metamorphosis. Gen. Comp. Endocrinol. 2010, 168, 209–219. [Google Scholar] [CrossRef]
- Suzuki, M.R.; Kikuyama, S. Corticoids augment nuclear binding capacity for triiodothyronine in bullfrog tadpole tail fins. Gen. Comp. Endocrinol. 1983, 52, 272–278. [Google Scholar] [CrossRef]
- Krain, L.P.; Denver, R.J. Developmental expression and hormonal regulation of glucocorticoid and thyroid hormone receptors during metamorphosis in Xenopus laevis. J. Endocrinol. 2004, 181, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Buchholz, D.R. Beyond synergy: Corticosterone andthyroid hormone have numerous interaction effects on gene regulation in Xenopus tropicalis tadpoles. Endocrinology 2012, 153, 5309–5324. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Buchholz, D.R. Corticosteroid signaling in frog metamorphosis. Gen. Comp. Endocrinol. 2014, 203, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Buisine, N.; Grimaldi, A.; Jonchere, V.; Rigolet, M.; Blugeon, C.; Hamroun, J.; Sachs, L.M. Transcriptome and Methylome Analysis Reveal Complex Cross-Talks between Thyroid Hormone and Glucocrticoid Signaling at Xenopus Metamorphosis. Cells 2021, 10, 2375. [Google Scholar] [CrossRef] [PubMed]
- Bagamasbad, P.D.; Bonett, R.M.; Sachs, L.; Buisine, N.; Raj, S.; Knoedler, J.; Kyono, Y.; Ruan, Y.; Ruan, X.; Denver, R.J. Deciphering the regulatory logic of an ancient, ultraconserved nuclear receptor enhancer module. Mol. Endocrinol. 2015, 29, 856–872. [Google Scholar] [CrossRef]
- Gudernatsch, J.F. Feeding experiments on tadpoles. I. The influence of specific organs given as food on growth and differentiation: A contribution to the knowledge of organs with internal secretion. Arch. Entwickl. Org. 1912, 35, 457–483. [Google Scholar] [CrossRef]
- Dodd, M.H.I.; Dodd, J.M. The biology of metamorphosis. In Physiology of the Amphibia; Lofts, B., Ed.; Academic Press: New York, NY, USA, 1976; pp. 467–599. [Google Scholar]
- Gilbert, L.I.; Tata, J.R.; Atkinson, B.G. Metamorphosis: Post-Embryonic Reprogramming of Gene Expression in Amphibian and Insect Cells; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Tata, J.R. Gene expression during metamorphosis: An ideal model for post-embryonic development. Bioessays 1993, 15, 239–248. [Google Scholar] [CrossRef]
- Leloup, J.; Buscaglia, M. La triiodothyronine: Hormone de la métamorphose des amphibiens. CR Acad. Sci. 1977, 284, 2261–2263. [Google Scholar]
- Tata, J.R. Early metamorphic competence of Xenopus larvae. Dev. Biol. 1968, 18, 415–440. [Google Scholar] [CrossRef]
- Tata, J.R. Requirement for RNA and protein synthesis for induced regression of the tadpole tail in organ culture. Dev. Biol. 1966, 13, 77–94. [Google Scholar] [CrossRef]
- Ishizuya-Oka, A.; Shimozawa, A. Induction of metamorphosis by thyroid hormone in anuran small intestine cultured organotypically in vitro. In Vitro Cell. Dev. Biol. 1991, 27, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Tata, J.R.; Kawahara, A.; Baker, B.S. Prolactin inhibits both thyroid hormone-induced morphogenesis and cell death in cultured amphibian larval tissues. Dev. Biol. 1991, 146, 72–80. [Google Scholar] [CrossRef]
- Flamant, F.; Cheng, S.-Y.; Hollenberg, A.N.; Moeller, L.C.; Samarut, J.; Wondisford, F.E.; Yen, P.M.; Refetoff, S. Thyroid Hormone Signaling Pathways: Time for a More Precise Nomeclature. Endocrinology 2017, 158, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-B.; Wong, J.; Puzianowska-Kuznicka, M.; Stolow, M.A. Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: Roles of thyroid hormone and its receptors. Bioessays 1996, 18, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Laudet, V.; Gronemeyer, H. The Nuclear Receptor FactsBook; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Forrest, D. The erbA/thyroid hormone receptor genes in development of the central nervous system. Semin. Cancer Biol. 1994, 5, 167–176. [Google Scholar]
- Lazar, M.A. Thyroid hormone receptors: Multiple forms, multiple possibilities. Endocr. Rev. 1993, 14, 184–193. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef]
- Davis, P.J.; Davis, F.B. Nongenomic actions of thyroid hormone. Thyroid 1996, 6, 497–504. [Google Scholar] [CrossRef]
- Davis, P.J.; Davis, F.B. Nongenomic actions of thyroid hormone on the heart. Thyroid 2002, 12, 459–466. [Google Scholar] [CrossRef]
- Parkison, C.; Ashizawa, K.; McPhie, P.; Lin, K.H.; Cheng, S.Y. The monomer of pyruvate kinase, subtype M1, is both a kinase and a cytosolic thyroid hormone binding protein. Biochem. Biophys. Res. Commun. 1991, 179, 668–674. [Google Scholar] [CrossRef]
- Shi, Y.-B.; Yaoita, Y.; Brown, D.D. Genomic organization and alternative promoter usage of the two thyroid hormone receptor ß genes in Xenopus laevis. J. Biol. Chem. 1992, 267, 733–788. [Google Scholar] [CrossRef]
- Yaoita, Y.; Shi, Y.B.; Brown, D.D. Xenopus laevis alpha and beta thyroid hormone receptors. Proc. Natl. Acad. Sci. USA 1990, 87, 7090–7094. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Matsuda, H.; Shi, Y.-B. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology 2008, 149, 5610–5618. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Tsai, M.J.; O’Malley, B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann. Rev. Biochem. 1994, 63, 451–486. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, B.W.; Malovannaya, A.; Qin, J. Minireview: Nuclear receptor and coregulator proteomics—2012 and beyond. Mol. Endocrinol. 2012, 26, 1646–1650. [Google Scholar] [CrossRef]
- Bulynko, Y.A.; O’Malley, B.W. Nuclear receptor coactivators: Structural and functional biochemistry. Biochemistry 2011, 50, 313–328. [Google Scholar] [CrossRef]
- McKenna, N.J.; Cooney, A.J.; DeMayo, F.J.; Downes, M.; Glass, C.K.; Lanz, R.B.; Lazar, M.A.; Mangelsdorf, D.J.; Moore, D.D.; Qin, J.; et al. Minireview: Evolution of NURSA, the Nuclear Receptor Signaling Atlas. Mol. Endocrinol. 2009, 23, 740–746. [Google Scholar] [CrossRef]
- Grimaldi, A.; Buisine, N.; Miller, T.; Shi, Y.B.; Sachs, L.M. Mechanisms of thyroid hormone receptor action during development: Lessons from amphibian studies. Biochim. Biophys. Acta 2013, 1830, 3882–3892. [Google Scholar] [CrossRef]
- Shi, Y.B.; Matsuura, K.; Fujimoto, K.; Wen, L.; Fu, L. Thyroid hormone receptor actions on transcription in amphibia: The roles of histone modification and chromatin disruption. Cell Biosci. 2012, 2, 42. [Google Scholar] [CrossRef]
- Perissi, V.; Jepsen, K.; Glass, C.K.; Rosenfeld, M.G. Deconstructing repression: Evolving models of co-repressor action. Nat. Rev. Genet. 2010, 11, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, Y.; Brown, D.D. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990, 4, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, A.; Brown, D.D. The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J. Biol. Chem. 1992, 267, 739–745. [Google Scholar] [CrossRef]
- Wang, Z.; Brown, D.D. Thyroid hormone-induced gene expression program for amphibian tail resorption. J. Biol. Chem. 1993, 268, 16270–16278. [Google Scholar] [CrossRef]
- Wong, J.; Shi, Y.-B. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J. Biol. Chem. 1995, 270, 18479–18483. [Google Scholar] [CrossRef]
- Sachs, L.M.; Damjanovski, S.; Jones, P.L.; Li, Q.; Amano, T.; Ueda, S.; Shi, Y.B.; Ishizuya-Oka, A. Dual functions of thyroid hormone receptors during Xenopus development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, 199–211. [Google Scholar] [CrossRef]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus Laevis (Daudin); Garland Publishing Inc.: New York, NY, USA, 1994. [Google Scholar]
- Puzianowska-Kuznicka, M.; Damjanovski, S.; Shi, Y.-B. Both thyroid Hormone and 9-cis Retinoic Acid receptors are Required to Efficiently mediate the Effects of Thyroid Hormone on Embryonic Development and Specific Gene Regulation in Xenopus laevis. Mol. Cell. Biol. 1997, 17, 4738–4749. [Google Scholar] [CrossRef]
- Nakajima, K.; Yaoita, Y. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev. Dyn. 2003, 227, 246–255. [Google Scholar] [CrossRef]
- Schreiber, A.M.; Brown, D.D. Tadpole skin dies autonomously in response to thyroid hormone at metamorphosis. Proc. Natl. Acad. Sci. USA 2003, 100, 1769–1774. [Google Scholar] [CrossRef]
- Das, B.; Schreiber, A.M.; Huang, H.; Brown, D.D. Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc. Natl. Acad. Sci. USA 2002, 99, 12230–12235. [Google Scholar] [CrossRef]
- Schreiber, A.M.; Das, B.; Huang, H.; Marsh-Armstrong, N.; Brown, D.D. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 10739–10744. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.R.; Hsia, V.S.-C.; Fu, L.; Shi, Y.-B. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol. Cell. Biol. 2003, 23, 6750–6758. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.R.; Tomita, A.; Fu, L.; Paul, B.D.; Shi, Y.-B. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol. Cell. Biol. 2004, 24, 9026–9037. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.R.; Paul, B.D.; Fu, L.; Shi, Y.B. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen. Comp. Endocrinol. 2006, 145, 1–19. [Google Scholar] [CrossRef]
- Choi, J.; Ishizuya-Oka, A.; Buchholz, D.R. Growth, development, and intestinal remodeling occurs in the absence of thyroid hormone receptor alpha in tadpoles of Xenopus tropicalis. Endocrinology 2017, 158, 1623–1633. [Google Scholar] [CrossRef]
- Choi, J.; Suzuki, K.I.; Sakuma, T.; Shewade, L.; Yamamoto, T.; Buchholz, D.R. Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression as revealed by gene disruption in Xenopus tropicalis. Endocrinology 2015, 156, 735–744. [Google Scholar] [CrossRef]
- Sakane, Y.; Iida, M.; Hasebe, T.; Fujii, S.; Buchholz, D.R.; Ishizuya-Oka, A.; Yamamoto, T.; Suzuki, K.T. Functional analysis of thyroid hormone receptor beta in Xenopus tropicalis founders using CRISPR-Cas. Biol. Open 2018, 7, bio030338. [Google Scholar] [CrossRef]
- Nakajima, K.; Tazawa, I.; Yaoita, Y. Thyroid Hormone Receptor alpha- and beta-Knockout Xenopus tropicalis Tadpoles Reveal Subtype-Specific Roles During Development. Endocrinology 2018, 159, 733–743. [Google Scholar] [CrossRef]
- Wen, L.; Shi, Y.B. Unliganded thyroid hormone receptor alpha controls developmental timing in Xenopus tropicalis. Endocrinology 2015, 156, 721–734. [Google Scholar] [CrossRef]
- Wen, L.; Shibata, Y.; Su, D.; Fu, L.; Luu, N.; Shi, Y.-B. Thyroid hormone receptor α controls developmental timing and regulates the rate and coordination of tissue specific metamorphosis in Xenopus tropicalis. Endocrinology 2017, 158, 1985–1998. [Google Scholar] [CrossRef]
- Wen, L.; Shi, Y.B. Regulation of growth rate and developmental timing by Xenopus thyroid hormone receptor alpha. Dev. Growth. Differ. 2016, 58, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.M. Unliganded thyroid hormone receptor function: Amphibian metamorphosis got TALENs. Endocrinology 2015, 156, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.M. Unliganded TRs regulate growth and developmental timing during early embryogenesis: Evidence for a dual function mechanism of TR action. Cell Biosci. 2015, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Tazawa, I.; Shi, Y.B. A unique role of thyroid hormone receptor beta in regulating notochord resorption during Xenopus metamorphosis. Gen. Comp. Endocrinol. 2019, 277, 66–72. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanizaki, Y.; Shi, Y.B. Thyroid hormone receptor beta is critical for intestinal remodeling during Xenopus tropicalis metamorphosis. Cell Biosci. 2020, 10, 46. [Google Scholar] [CrossRef]
- Shibata, Y.; Wen, L.; Okada, M.; Shi, Y.B. Organ-Specific Requirements for Thyroid Hormone Receptor Ensure Temporal Coordination of Tissue-Specific Transformations and Completion of Xenopus Metamorphosis. Thyroid 2020, 30, 300–313. [Google Scholar] [CrossRef]
- Buchholz, D.R.; Shi, Y.B. Dual function model revised by thyroid hormone receptor alpha knockout frogs. Gen. Comp. Endocrinol. 2018, 265, 214–218. [Google Scholar] [CrossRef]
- Nakajima, K.; Tanizaki, Y.; Luu, N.; Zhang, H.; Shi, Y.B. Comprehensive RNA-Seq analysis of notochord-enriched genes induced during Xenopus tropicalis tail resorption. Gen. Comp. Endocrinol. 2020, 287, 113349. [Google Scholar] [CrossRef]
- Shi, Y.B. Life without thyroid hormone receptor. Endocrinology 2021, 162, bqab028. [Google Scholar] [CrossRef]
- Sachs, L.M.; Shi, Y.-B. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl. Acad. Sci. USA 2000, 97, 13138–13143. [Google Scholar] [CrossRef]
- Tomita, A.; Buchholz, D.R.; Shi, Y.-B. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol. Cell. Biol. 2004, 24, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.M.; Jones, P.L.; Havis, E.; Rouse, N.; Demeneix, B.A.; Shi, Y.-B. N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol. Cell. Biol. 2002, 22, 8527–8538. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Buchholz, D.R.; Paul, B.D.; Shi, Y.-B. A role of unliganded thyroid hormone receptor in postembryonic development in Xenopus laevis. Mech. Dev. 2007, 124, 476–488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, H.; Paul, B.D.; Choi, C.Y.; Hasebe, T.; Shi, Y.-B. Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol. Cell. Biol. 2009, 29, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Buchholz, D.R.; Fu, L.; Shi, Y.-B. Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. J. Biol. Chem. 2005, 280, 27165–27172. [Google Scholar] [CrossRef]
- Paul, B.D.; Fu, L.; Buchholz, D.R.; Shi, Y.-B. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol. Cell. Biol. 2005, 25, 5712–5724. [Google Scholar] [CrossRef]
- Paul, B.D.; Buchholz, D.R.; Fu, L.; Shi, Y.-B. SRC-p300 coactivator complex is required for thyroid hormone induced amphibian metamorphosis. J. Biol. Chem. 2007, 282, 7472–7481. [Google Scholar] [CrossRef]
- Havis, E.; Sachs, L.M.; Demeneix, B.A. Metamorphic T3-response genes have specific co-regulator requirements. EMBO Rep. 2003, 4, 883–888. [Google Scholar] [CrossRef]
- Tanizaki, Y.; Bao, L.; Shi, B.; Shi, Y.-B. A role of endogenous histone acetyltransferase steroid hormone receptor coactivator (SRC) 3 in thyroid hormone signaling during Xenopus intestinal metamorphosis. Thyroid 2021, 31, 692–702. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanizaki, Y.; Zhang, H.; Lee, H.; Dasso, M.; Shi, Y.-B. Thyroid hormone receptor is essential for larval epithelial apoptosis and adult epithelial stem cell development but not adult intestinal morphogenesis during Xenopus tropicalis metamorphosis. Cells 2021, 10, 536. [Google Scholar] [CrossRef]
- Bock, K.A. Die einwirkung von nebennierenrinden-extract auf den ablauf der thyroxin-metamorphose bei froschlarven und beim axolotl. Klin. Woschschr. 1938, 17, 1311–1314. [Google Scholar] [CrossRef]
- Frieden, E.; Naile, B. Biochemistry of amphibian metamorphosi: 1. Enhancement of induced metamorphosis by glucocorticoids. Science 1955, 121, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Gasche, P. Effect of Desoxycorticosterone acetate on larvae of Xenopus laevis in their various stages of metamorphosis. Helv. Physiol. Pharmacol. 1945, Acta 3, C10. [Google Scholar]
- Kobayashi, H. Effects of desoxycorticosterone acetate on metamorphosis induced by thyroxine in anuran tadpoles. Endocrinology 1958, 62, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Knoedler, J.R.; Denver, R.J. A mechanism to enhance cellular responsivity to hormone action: Krüppel-like factor 9 promotes thyroid hormone receptor-β autoinduction during postembryonic brain development. Endocrinology 2016, 157, 1683–1693. [Google Scholar] [CrossRef][Green Version]
- Denver, R.J. Endocrinology of Complex Life Cycles: Amphibians. In Anonymous Hormones, Brain, and Behavior, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, Chapter 2.09; pp. 145–168. [Google Scholar]
- Hanke, W. The adrenal cortex in amphibia. In General, Comparative, and Clinical Endocrinology of the Adrenal Cortex; Jones, I.C., Henderson, I.W., Eds.; Academic Press, Inc.: London, UK, 1978; Volume 2, Chapter 5; pp. 419–495. [Google Scholar]
- Hayes, T.B.; Wu, T.H. Interdependence of corticosterone and thyroid hormones in toad larvae (Bufo boreas). II. Regulation of corticosterone and thyroid hormones. J. Exp. Zool. 1995, 271, 103–111. [Google Scholar] [CrossRef]
- Shewade, L.H.; Schoephoerster, J.A.; Patmann, M.D.; Kulkarni, S.S.; Buchholz, D.R. Corticosterone is Essential for Survival Through Frog Metamorphosis. Endocrinology 2020, 161, bqaa193. [Google Scholar] [CrossRef]
- Sterner, Z.R.; Shewade, L.H.; Mertz, K.M.; Sturgeon, S.M.; Buchholz, D.R. Glucocorticoid receptor is required to survive through metamorphosis in the frog Xenopus tropicalis. Gen. Comp. Endocrinol. 2020, 291, 113419. [Google Scholar] [CrossRef]
- Jolivet-Jaudet, G.; Leloup-Hatey, J. Interrenal function during Amphibia metamorphosis: In vitro biosynthesis of radioacdtive corticosteroids from (4-14C)-progesterone by interrenal in Xenopus laevis tadpoles. Comp. Biochem. Physiol. Part B 1984, 79, 239–244. [Google Scholar] [CrossRef]
- Leist, K.H.; Bergerhoff, K.; Pehlemann, F.W.; Hanke, W. Histopathological investigations of the development of the interrenal organ in the clawed toad (Xenopus laevis Daudin). Z. Zellforsch. Mikrosk. Anat. 1969, 93, 105–125. [Google Scholar] [CrossRef]
- Rapola, J. Development of the amphibian adrenal cortex: Morphological and physiological studies on Xenopus laevis Daudin. Ann. Acad. Sci. Fenn. Ser. 1962, A64, 1–81. [Google Scholar]
- Uchiyama, M.; Konno, N. Hormonal regulation of ion and water transport in anuran amphibians. Gen. Comp. Endocrinol. 2006, 147, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hanke, W.; Leist, K.H. The effect of ACTH and corticosteroids on carbohydrate metabolism during the metamorphosis of Xenopus laevis. Gen. Comp. Endocrinol. 1971, 16, 137–148. [Google Scholar] [CrossRef]
- Maher, J.M.; Werner, E.E.; Denver, R.J. Stress hormones mediate predator- induced phenotypic plasticity in amphibian tadpoles. Proc. Biol. Sci. 2013, 280, 20123075. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Barker, K.S.; Davis, A.T. Involvement of glucocorticoids in the reorganization of the amphibian immune system at metamorphosis. Dev. Immunol. 1997, 5, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Williams-Dautovich, J.; Cummins, C.L. Minireview: New Molecular Mediators of Glucocorticoid Receptor Activity in Metabolic Tissues. Mol. Endocrinol. 2014, 28, 999–1011. [Google Scholar] [CrossRef]
- Weikum, E.R.; Knuesel, M.T.; Ortlund, E.A.; Yamamoto, K.R. Glucocorticoid receptor control of transcription: Precision and plasticity via allostery. Nat. Rev. Mol. Cell. Bio. 2017, 18, 159–174. [Google Scholar] [CrossRef]
- Bridgham, J.T.; Carroll, S.M.; Thornton, J.W. Evolution of Hormone-Receptor Complexity by Molecular Exploitation. Science 2006, 312, 97–101. [Google Scholar] [CrossRef]
- James-Zorn, C.; Ponferrada, V.G.; Burns, K.A.; Fortriede, J.D.; Lotay, V.S.; Liu, Y.; Karpinka, B.J.; Karimi, K.; Zorn, A.M.; Vize, P.D. Xenbase: Core features, data acquisition, and data processing. Genesis 2015, 53, 486–497. [Google Scholar] [CrossRef]
- Shewade, L.H.; Schneider, K.A.; Brown, A.C.; Buchholz, D.R. In-vivo regulation of Krüppel-like factor 9 by corticosteroids and their receptors across tissues in tadpoles of Xenopus tropicalis. Gen. Comp. Endocrinol. 2017, 248, 79–86. [Google Scholar] [CrossRef]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The Multifaceted Mineralocorticoid Receptor. Compr. Physiol. 2014, 4, 965–994. [Google Scholar] [CrossRef]
- Kikuyama, S.; Niki, K.; Mayumi, M.; Kawamura, K. Retardation of thyroxine-induced metamorphosis by Amphenone B in toad tadpoles. Endocrinol. Jpn. 1982, 29, 659–662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchholz, D.R.; Hayes, T.B. Variation in thyroid hormone action and tissue content underlies species differences in the timing of metamorphosis in desert frogs. Evol. Dev. 2005, 7, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Derby, A. An in vitro quantitative analysis of the response of tadpole tissue to thyroxine. J. Exp. Zool. 1968, 168, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sterner, Z.R.; Buchholz, D.R. Glucocorticoid receptor mediates corticosterone-thyroid hormone synergy essential for metamorphosis in Xenopus tropicalis tadpoles. Gen. Comp. Endocrinol. 2022, 315, 113942. [Google Scholar] [CrossRef]
- Kolber, B.J.; Wieczorek, L.; Muglia, L.J. Hypothalamic–pituitary–adrenal axis dysregulation and behavioral analysis of mouse mutants with altered glucocorticoid or mineralocorticoid receptor function. Stress 2008, 11, 321–338. [Google Scholar] [CrossRef]
- Wada, H. Glucocorticoids: Mediators of vertebrate ontogenetic transitions. Gen. Comp. Endocrinol. 2008, 156, 441–453. [Google Scholar] [CrossRef]
- De Jesus, E.; Hirano, T.; Inui, Y. Changes in cortisol and thyroid hormone concentrations during early development and metamorphosis in the Japanese flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 1991, 82, 369–376. [Google Scholar] [CrossRef]
- Shepherdley, C.A.; Daniels, C.B.; Orgeig, S.; Richardson, S.J.; Evans, B.K.; Darras, V.M. Glucocorticoids, thyroid hormones, and iodothyronine deiodinases in embryonic saltwater crocodiles. Am. J. Physiol. 2002, 283, 1155–1163. [Google Scholar] [CrossRef]
- Reyns, G.E.; Venken, K.; De Escobar, G.M.; Kühn, E.R.; Darras, V.M. Dynamics and regulation of intracellular thyroid hormone concentrations in embryonic chicken liver, kidney, brain, and blood. Gen. Comp. Endocrinol. 2003, 134, 80–87. [Google Scholar] [CrossRef]
- Hadj-Sahraoui, N.; Seugnet, I.; Ghorbel, M.; Demeneix, B. Hypothyroidism prolongs miotic acivity in the post-natal mouse brain. Neurosci. Lett. 2000, 280, 79–82. [Google Scholar] [CrossRef]
- Brown, C.; Urbinati, E.; Zhang, W.; Brown, S.; McComb-Kobza, M. Maternal thyroid and glucocorticoid hormone interactions in larval fish development, and their applications in aquaculture. Rev. Fish. Sci. Aquac. 2014, 22, 207–220. [Google Scholar] [CrossRef]
- De Jesus, E.G.T.; Toledo, J.D.; Simpas, M.S. Thyroid hormones promote early metamorphosis in grouper (Epinephelus coioides) larvae. Gen. Comp. Endocrinol. 1998, 112, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Campinho, M.A.; Galay-Burgos, M.; Sweeney, G.E.; Power, D.M. Coordination of deiodinase and thyroid hormone receptor expression during the larval to juvenile transition in sea bream (Sparus aurata, Linnaeus). Gen. Comp. Endocrinol. 2010, 165, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Taillebois, L.; Keith, P.; Valade, P.; Torres, P.; Baloche, S.; Dufour, S.; Rousseau, K. Involvement of thyroid hormones in the control of larval metamorphosis in Sicyopterus lagocephalus (Teleostei: Gobioidei) at the time of river recruitment. Gen. Comp. Endocrinol. 2011, 173, 281–288. [Google Scholar] [CrossRef]
- Salis, P.; Roux, N.; Huang, D.; Marcionetti, A.; Mouginot, P.; Reynaud, M.; Salles, O.; Salamin, N.; Pujol, B.; Parichy, D.M.; et al. Thyroid hormones regulate the formation and environmental plasticity of white bars in clownfishes. Proc. Natl. Acad. Sci. USA 2021, 118, e2101634118. [Google Scholar] [CrossRef]
- Inui, Y.; Miwa, S. Metamorphosis of flatfish (Pleuronectiformes). In Metamorphosis in Fish; Dufour, S., Rousseau, K., Kapoor, B., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 107–153. [Google Scholar]
- Miwa, S.; Tagawa, M.; Inui, Y.; Hirano, T. Thyroxine surge in metamorphosing flounder larvae. Gen. Comp. Endocrinol. 1988, 70, 158–163. [Google Scholar] [CrossRef]
- Hotta, Y.; Aritaki, M.; Tagawa, M.; Tanaka, M. Changes in tissue thyroid hormone levels of metamorphosing spotted halibut Verasper variegatus reared at different temperatures. Fish. Sci. 2001, 67, 1119–1124. [Google Scholar] [CrossRef]
- Einarsdóttir, I.E.; Silva, N.; Power, D.M.; Smáradóttir, H.; Björnsson, B.T. Thyroid and pituitary gland development from hatching through metamorphosis of a teleost flatfish, the Atlantic halibut. Anat. Embryol. 2006, 211, 47–60. [Google Scholar] [CrossRef]
- Schreiber, A.M.; Specker, J.L. Metamorphosis in the summer flounder, Paralichthys dentatus: Stage-specific developmental response to altered thyroid status. Gen. Comp. Endocrinol. 1998, 111, 156–166. [Google Scholar] [CrossRef]
- Inui, Y.; Miwa, S. Thyroid hormone induces metamorphosis of flounder larvae. Gen. Comp. Endocrinol. 1985, 60, 450–454. [Google Scholar] [CrossRef]
- De Jesus, E.G.; Inui, Y.; Hirano, T. Cortisol enhances the stimulating action of thyroid hormones on dorsal fin-ray resorption of flounder larvae in vitro. Gen. Comp. Endocrinol. 1990, 79, 167–173. [Google Scholar] [CrossRef]
- Solbakken, J.S.; Norberg, B.; Watanabe, K.; Pittman, K. Thyroxine as a mediator of metamorphosis of Atlantic halibut, Hippoglossus hippoglossus. Environ. Biol. Fishes 1999, 56, 53–65. [Google Scholar] [CrossRef]
- Campinho, M.A.; Silva, N.; Martins, G.G.; Anjos, L.; Florindo, C.; Roman-Padilla, J.; Garcia-Cegarra, A.; Louro, B.; Manchado, M.; Power, D.M. A thyroid hormone regulated asymmetric responsive centre is correlated with eye migration during flatfish metamorphosis. Sci. Rep. 2018, 8, 12267. [Google Scholar] [CrossRef]
- Isorna, E.; Obregon, M.J.; Calvo, R.M.; Vázquez, R.; Pendón, C.; Falcón, J.; Muñoz-Cueto, J.A. Iodothyronine deiodinases and thyroid hormone receptors regulation during flatfish (solea senegalensis) metamorphosis. J. Exp. Zool. 2009, 312, 231–246. [Google Scholar] [CrossRef]
- Yamano, K.; Miwa, S. Differential gene expression of thyroid hormone receptor α and β in fish development. Gen. Comp. Endocrinol. 1998, 109, 75–85. [Google Scholar] [CrossRef]
- Marchand, O.; Duffraisse, M.; Triqueneaux, G.; Safi, R.; Laudet, V. Molecular cloning and developmental expression patterns of thyroid hormone receptors and T3 target genes in the turbot (Scophtalmus maximus) during post-embryonic development. Gen. Comp. Endocrinol. 2004, 135, 345–357. [Google Scholar] [CrossRef]
- Yamada, T.; Donai, H.; Okauchi, M.; Tagawa, M.; Araki, K. Induction of ambicoloration by exogenous cortisol during metamorphosis of spotted halibut Verasper variegatus. Comp. Biochem. Physiol. B 2011, 160, 174–180. [Google Scholar] [CrossRef]
- Arjona, F.J.; de Vroeze, E.; Visser, T.J.; Flik, G.; Klaren, P.H.M. Idenification and functional characterization of Zebrafish Solute Carrier Slc16a2 (Mct8) as a Thyroid Hormone Membrane Transporter. Endocrinology 2011, 152, 5065–5073. [Google Scholar] [CrossRef]
- Hoar, W.S. The physiology of smolting salmonids. Fish Physiol. 1988, 11, 275–343. [Google Scholar] [CrossRef]
- Rousseau, K.; Martin, P.; Boeuf, G.; Dufour, S. Salmonid secondary metamorphosis: Smoltification. In Metamorphosis in Fish; Dufour, S.K., Rousseau, K., Kappor, B., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 167–215. [Google Scholar]
- Björnsson, B.T.; Einarsdottir, I.E.; Power, D. Is salmon smoltification an example of vertebrate metamorphosis? Lessons learnt from work on flatfish larval development. Aquaculture 2012, 362–363, 264–272. [Google Scholar] [CrossRef]
- McBride, J.; Higgs, D.; Fagerlund, U.; Buckley, J. Thyroid hormones and steroid hormones: Potential for control of growth and smoltification of salmonids. Aquaculture 1982, 28, 201–210. [Google Scholar] [CrossRef]
- Fleming, M.S.; Maugars, G.; Lafont, A.-G.; Rancon, J.; Fontaine, R.; Nourizadeh-Lillabadi, R.; Weltzien, F.-A.; Yebra-Pimentel, E.S.; Dirks, R.; McCormick, S.D.; et al. Functional divergence of thyrotropin beta-subunit paralogs gives new insights into salmon smoltification metamorphosis. Sci. Rep. 2019, 9, 4561. [Google Scholar] [CrossRef] [PubMed]
- Marchand, O.; Safi, R.; Escriva, H.; Van Rompaey, E.; Prunet, P.; Laudet, V. Molecular cloning and characterization of thyroid hormone receptors in teleost fish. J. Mol. Endocrinol. 2001, 26, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.; Rogers, S.A.; Kille, P.; Sweeney, G.E. Molecular cloning and expression of thyroid hormone receptor alpha during salmonid development. Gen. Comp. Endocrinol. 2002, 125, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Raine, J.C.; Cameron, C.; Vijayan, M.M.; MacKenzie, D.S.; Leatherland, J.F. Effect of fasting on thyroid hormone levels, and TRα and TRβ mRNA accumulation in late-stage embryo and juvenile rainbow trout, Oncorhynchus mykiss. Comp. Biochem. Physiol. 2005, 140, 452–459. [Google Scholar] [CrossRef]
- Harada, M.; Yoshinaga, T.; Ojima, D.; Iwata, M. cDNA cloning and expression analysis of thyroid hormone receptor in the coho salmon Oncorhynchus kisutch during smoltification. Gen. Comp. Endocrinol. 2008, 155, 658–667. [Google Scholar] [CrossRef]
- Specker, J.; Schreck, C. Changes in plasma corticosteroids during smoltification of coho salmon Oncorhynchus kisutch. Gen. Comp. Endocrinol. 1982, 46, 53–58. [Google Scholar] [CrossRef]
- Langhorne, P.; Simpson, T.H. The interrelationship of cortisol, Gill (Na + K) ATPase, and homeostasis during the Parr-Smolt transformation of atlantic salmon (Salmo salar L.). Gen. Comp. Endocrinol. 1986, 61, 203–213. [Google Scholar] [CrossRef]
- Young, G.; Björnsson, B.; Prunet, P.; Lin, R.; Bern, H. Smoltification and seawater adaptation in coho salmon (Oncorhynchus kisutch): Plasma prolactin, growth hormone, thyroid hormones, and cortisol. Gen. Comp. Endocrinol. 1989, 74, 335–345. [Google Scholar] [CrossRef]
- Nagae, M.; Fuda, H.; Hara, A.; Saneyoshi, M.; Yamauchi, K. Changes in Serum Concentrations of Immunoglobulin M (IgM), Cortisol and Thyroxine (T4) during Smoltification in the Masu Salmon Oncorhynchus masou. Fish. Sci. 1994, 60, 241–242. [Google Scholar] [CrossRef]
- Langdon, J.S.; Thorpe, J.E.; Roberts, R.J. Effects of cortisol and acth on gill Na+/K+-ATPase, SDH and chloride cells in juvenile atlantic salmon Salmo salar L. Comp. Biochem. Physiol. 1984, 77, 9–12. [Google Scholar] [CrossRef]
- Richman, N.H.; de Diaz, S.T.; Nishioka, R.S.; Prunet, P.; Bern, H.A. Osmoregulatory and endocrine relationships with chloride cell morphology and density during smoltification in coho salmon (Oncorhynchus kisutch). Aquaculture 1987, 60, 265–285. [Google Scholar] [CrossRef]
- Tipsmark, C.K.; Jørgensen, C.; Brande-Lavridsen, N.; Engelund, M.; Olesen, J.H.; Madsen, S.S. Effects of cortisol, growth hormone and prolactin on gill claudin expression in Atlantic salmon. Gen. Comp. Endocrinol. 2009, 163, 270–277. [Google Scholar] [CrossRef]
- McLeese, J.M.; Johnsson, J.; Huntley, T.F.M.; Clarke, W.C.; Weisbart, M. Seasonal changes in osmoregulation, cortisol, and cortisol receptor activity in the gills of parr/smolt of steelhead trout and steelhead-rainbow trout hybrids, Oncorhynchus mykiss. Gen. Comp. Endocrinol. 1994, 93, 103–113. [Google Scholar] [CrossRef]
- Mazurais, D.; Ducouret, B.; Tujague, M.; Valotaire, Y.; D’Cotta, H.; Gallais, C.; Prunet, P. Regulation of the glucocorticoid receptor mRNA levels in the gills of Atlantic salmon (Salmo salar) during smoltification. Bull. Fr. Pêche Piscic. 1998, 350–351, 499–510. [Google Scholar] [CrossRef]
- Mizuno, S.; Ura, K.; Onodera, Y.; Fukada, H.; Misaka, N.; Hara, A.; Adachi, S.; Yamauchi, K. Changes in transcript levels of gill cortisol receptor during smoltification in wild masu salmon, oncorhynchus masou. Zoolog. Sci. 2001, 18, 853–860. [Google Scholar] [CrossRef][Green Version]
- Kiilerich, P.; Kristiansen, K.; Madsen, S.S. Hormone receptors in gills of smolting Atlantic salmon, Salmo salar: Expression of growth hormone, prolactin, mineralocorticoid and glucocorticoid receptors and 11β-hydroxysteroid dehydrogenase type 2. Gen. Comp. Endocrinol. 2007, 152, 295–303. [Google Scholar] [CrossRef]
- Ebbesson, L.O.E.; Nilsen, T.O.; Helvik, J.V.; Tronci, V.; Stefansson, S.O. Corticotropin-releasing factor neurogenesis during midlife development in salmon: Genetic, environmental and thyroid hormone regulation. J. Neuroendocrinol. 2011, 23, 733–741. [Google Scholar] [CrossRef]
- Redding, J.M.; Patiño, R.; Schreck, C.B. Cortisol effects on plasma electrolytes and thyroid hormones during smoltification in coho salmon Oncorhynchus kisutch. Gen. Comp. Endocrinol. 1991, 81, 373–382. [Google Scholar] [CrossRef]
- Shin, H.S.; Choi, Y.J.; Kim, N.N.; Lee, J.; Ueda, H.; Choi, C.Y. Effects of exogenous cortisol and seawater adaptation on thyroid hormone receptors in the smolt stage of the sockeye salmon, Oncorhynchus nerka. Ichthyol. Res. 2014, 61, 9–16. [Google Scholar] [CrossRef]
- McNabb, F.M.A. Avian thyroid development and adaptive plasticity. Gen. Comp. Endocrinol. 2006, 147, 93–101. [Google Scholar] [CrossRef] [PubMed]
- De Groef, B.; Grommen, S.V.H.; Darras, V.M. Hatching the cleidoic egg: The role of thyroid hormones. Front. Endocrinol. 2013, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- McNabb, F.M.A.; Stanton, F.W.; Dicken, S.G. Post-hatching thyroid development and body growth in precocial vs altricial birds. Comp. Biochem. Physiol. 1984, 78, 629–635. [Google Scholar] [CrossRef]
- Výboh, P.; Zeman, M.; Juráni, M.; Buyse, J.; Decuypere, E. Plasma thyroid hormone and growth hormone patterns in precocial Japanese quail and altricial European starlings during postnatal development. Comp. Biochem. Physiol. 1996, 114, 23–27. [Google Scholar] [CrossRef]
- Grossowicz, N. Influence of thiourea on development of the chick embryo. Proc. Soc. Exp. Biol. Med. 1946, 63, 151–152. [Google Scholar] [CrossRef]
- Romanoff, A.; Laufer, H. The effect of injected thiourea on the development of some organs of the chick embryo. Endocrinology 1956, 59, 611–619. [Google Scholar] [CrossRef]
- Haba, G.; Nishigori, H.; Tezuka, Y.; Kagami, K.; Sugiyama, T.; Nishigori, H. Effect of antithyroid drug on chick embryos during the last week of development: Delayed hatching and decreased cerebellar acetylcholinesterase activity. J. Obstet. Gynaecol. Res. 2011, 37, 1549–1556. [Google Scholar] [CrossRef]
- Galton, V.A.; Hiebert, A. The ontogeny of the enzyme systems for the 5- and 5-deiodination of thyroid hormones in chick embryo liver. Endocrinology 1987, 120, 2604–2610. [Google Scholar] [CrossRef]
- Darras, V.M.; Visser, T.J.; Berghman, L.R.; Kühn, E.R. Ontogeny of type I and type III deiodinase activities in embryonic and posthatch chicks: Relationship with changes in plasma triiodothyronine and growth hormone levels. Comp. Biochem. Physiol. 1992, 103, 131–136. [Google Scholar] [CrossRef]
- Van Herck, S.L.J.; Delbaere, J.; Bourgeois, N.M.A.; McAllan, B.M.; Richardson, S.J.; Darras, V.M. Expression of thyroid hormone transporters and deiodinases at the brain barriers in the embryonic chicken: Insights into the regulation of thyroid hormone availability during neurodevelopment. Gen. Comp. Endocrinol. 2015, 214, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D.; Sjoberg, M.; Vennstrom, B. Contrasting developmental and tissue-specific expression of α and β thyroid hormone receptor genes. EMBO J. 1990, 9, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, M.; Vennström, B.; Forrest, D. Thyroid hormone receptors in chick retinal development: Differential expression of mRNAs for α and N-terminal variant β receptors. Development 1992, 114, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Frye, B.E. Functional development of the hypothalamo-hypophyseal-adrenal cortex axis in the chick embryo, Gallus domesticus. J. Exp. Zool. 1973, 185, 277–291. [Google Scholar] [CrossRef]
- Scott, T.R.; Johnson, W.A.; Satterlee, D.G.; Gildersleeve, R.P. Circulating levels of corticosterone in the serum of developing chick embryos and newly hatched chicks. Poult. Sci. 1981, 60, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, B.C.; Hussein, M.O. Serum corticosterone levels in embryos, newly hatched, and young turkey poults. Poult. Sci. 1985, 64, 2195–2201. [Google Scholar] [CrossRef]
- Porter, T.E.; Ghavam, S.; Muchow, M.; Bossis, I.; Ellestad, L. Cloning of partial cDNAs for the chicken glucocorticoid and mineralocorticoid receptors and characterization of mRNA levels in the anterior pituitary gland during chick embryonic development. Domest. Anim. Endocrinol. 2007, 33, 226–239. [Google Scholar] [CrossRef]
- Mashaly, M.M. Effect of exogenous corticosterone on chicken embryonic development. Poult. Sci. 1991, 70, 371–374. [Google Scholar] [CrossRef]
- Tona, K.; Onagbesan, O.; Bruggeman, V.; De Smit, L.; Figueiredo, D.; Decuypere, E. Non-ventilation during early incubation in combination with dexamethasone administration during late incubation: 1. Effects on physiological hormone levels, incubation duration and hatching events. Domest. Anim. Endocrinol. 2007, 33, 32–46. [Google Scholar] [CrossRef]
- Hylka, V.W.; Doneen, B.A. Ontogeny of embryonic chicken lung: Effects of pituitary gland, corticosterone, and other hormones upon pulmonary growth and synthesis of surfactant phospholipids. Gen. Comp. Endocrinol. 1983, 52, 108–120. [Google Scholar] [CrossRef]
- Kingsbury, J.; Emery, S.; Adams, A. Effects of thiourea on the adrenal glands of chick embryos. Endocrinology 1955, 56, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, E.; Scanes, C.; Kühn, E. Effects of Glucocorticoids on Circulating Concentrations of Thyroxine (T4) and Triiodothyronine (T3) and on Peripheral Monodeiodination in Pre- and Post-Hatching Chicken. Horm. Metab. Res. 1983, 15, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Darras, V.M.; Kotanen, S.P.; Geris, K.L.; Berghman, L.R.; Kühn, E.R. Plasma thyroid hormone levels and iodothyronine deiodinase activity following an acute glucocorticoid challenge in embryonic compared with posthatch chickens. Gen. Comp. Endocrinol. 1996, 104, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Rupik, W. Structural and ultrastructural differentiation of the thyroid gland during embryogenesis in the grass snake Natrix natrix L. (Lepidosauria, Serpentes). Zoology 2011, 114, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Dimond, M.-T. The reactions of developing snapping turtles, Chelydra serpentina serpentina (Linné), to thiourea. J. Exp. Zool. 1954, 127, 93–117. [Google Scholar] [CrossRef]
- McGlashan, J.K.; Thompson, M.B.; Van Dyke, J.U.; Spencer, R.J. Thyroid hormones reduce incubation period without developmental or metabolic costs in murray river short-necked turtles (Emydura macquarii). Physiol. Biochem. Zool. 2017, 90, 34–46. [Google Scholar] [CrossRef][Green Version]
- Sullivan, L.C.; Orgeig, S.; Daniels, C.B. Control of the development of the pulmonary surfactant system in the saltwater crocodile, Crocodylus porosus. Am. J. Physiol. 2002, 283, 1164–1176. [Google Scholar] [CrossRef]
- Sullivan, L.C.; Orgeig, S.; Wood, P.G.; Daniels, C.B. The ontogeny of pulmonary surfactant secretion in the embryonic green sea turtle (Chelonia mydas). Physiol. Biochem. Zool. 2001, 74, 493–501. [Google Scholar] [CrossRef]
- Sullivan, L.C.; Orgeig, S.; Daniels, C.B. Regulation of pulmonary surfactant secretion in the developing lizard, Pogona vitticeps. Comp. Biochem. Physiol. 2002, 133, 539–546. [Google Scholar] [CrossRef]
- Medler, K.F.; Lance, V.A. Sex differences in plasma corticosterone levels in alligator (Alligator mississippiensis) embryos. J. Exp. Zool. 1998, 280, 238–244. [Google Scholar] [CrossRef]
- Jennings, D.H.; Moore, M.C.; Knapp, R.; Matthews, L.; Orchinik, M. Plasma steroid-binding globulin mediation of differences in stress reactivity in alternative male phenotypes in tree lizards, Urosaurus ornatus. Gen. Comp. Endocrinol. 2000, 120, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Morris, Y. The comparative endocrinology of sea turtles. Copeia 1985, 3, 723–735. [Google Scholar] [CrossRef]
- Weiss, S.L.; Johnston, G.; Moore, M.C. Corticosterone stimulates hatching of late-term tree lizard embryos. Comp. Biochem. Physiol. 2007, 146, 360–365. [Google Scholar] [CrossRef][Green Version]
- Izaz, A.; Pan, T.; Wang, L.; Zhang, H.; Duan, S.; Li, E.; Yan, P.; Wu, X. Molecular cloning, characterization, and gene expression behavior of glucocorticoid and mineralocorticoid receptors from the Chinese alligator (Alligator sinensis). J. Exp. Zool. 2021, 336, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.; Orgeig, S.; Daniels, C. The Role of Extrinsic and Intrinsic Factors in the Evolution of the Control of Pulmonary Surfactant Maturation during Development in the Amniotes. Physiol. Biochem. Zool. 2003, 76, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Forhead, A.J.; Fowden, A.L. Thyroid hormones in fetal growth and prepartum maturation. J. Endocrinol. 2014, 221, R87–R103. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Dussault, J. Ontogenesis of thyroid function in the neonatal rat. Thyroxine and triiodothyronine production rates. Endocrinology 1977, 101, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Lamers, W.H.; Mooren, P.G.; Griep, H.; Endert, E.; Degenhart, H.J.; Charles, R. Hormones in perinatal rat and spiny mouse: Relation to altricial and precocial timing of birth. Am. J. Physiol. 1986, 251, E78–E85. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Landers, K.; Li, H.; Mortimer, R.H.; Richard, K. Thyroid hormones and fetal neurological development. J. Endocrinol. 2011, 209, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Ayromlooi, J.; Bandyopadhyay, D.; Bandyopadhyay, S.; Neogi, A.; Steinberg, H. Potentiation of surfactant release in fetal lung by thyroid hormone action. J. Appl. Physiol. 1984, 56, 1621–1626. [Google Scholar] [CrossRef]
- Warburton, D.; Parton, L.; Buckley, S.; Cosico, L.; Enns, G.; Saluna, T. Combined effects of corticosteroid, thyroid hormones, and (β-agonist on surfactant, pulmonary mechanics, and β-receptor binding in fetal lamb lung. Pediatr. Res. 1988, 24, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Van Tuyl, M.; Blommaart, P.E.; De Boer, P.A.J.; Wert, S.E.; Ruijter, J.M.; Islam, S.; Schnitzer, J.; Ellison, A.R.; Tibboel, D.; Moorman, A.F.M.; et al. Prenatal exposure to thyroid hormone is necessary for normal postnatal development of murine heart and lungs. Dev. Biol. 2004, 272, 104–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bassett, J.H.; Williams, G.R. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr. Rev. 2016, 37, 135–187. [Google Scholar] [CrossRef]
- Sirakov, M.; Plateroti, M. The thyroid hormones and their nuclear receptors in the gut: From developmental biology to cancer. Biochim. Biophys. Acta 2011, 1812, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.P.; Britton, H.G.; James, V.H.T.; Nixon, D.A.; Parker, R.A.; Wintour, E.M.; Wright, R.D. Steroid secretion by the adrenal gland of foetal and neonatal sheep. J. Endocrinol. 1968, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.; Giannopoulos, G.; Solomon, S. Corticosteroid levels in mother and fetus of the rabbit during gestation. Endocrinology 1973, 93, 1342–1348. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Jun, J.K.; Maymon, E.; Mazor, M.; Park, J.S. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am. J. Obstet. Gynecol. 1998, 179, 1107–1114. [Google Scholar] [CrossRef]
- Van Rensburg, S.J. Gestation in sheep after foetal adrenalectomy and cortisol acetate administration. J. Endocrinol. 1967, 38, 83–84. [Google Scholar] [CrossRef]
- Liggins, G.C. Premature delivery of foetal lambs infused with glucocorticoids. J. Endocrinol. 1069, 45, 515–523. [Google Scholar] [CrossRef]
- Shanks, A.L.; Grasch, J.L.; Quinney, S.K.; Haas, D.M. Controversies in antenatal corticosteroids. Semin. Fetal Neonatal Med. 2019, 24, 182–188. [Google Scholar] [CrossRef]
- Fowden, A.L.; Forhead, A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015, 100, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Forhead, A.J.; Curtis, K.; Kaptein, E.; Visser, T.J.; Fowden, A.L. Developmental control of iodothyronine deiodinases by cortisol in the ovine fetus and placenta near term. Endocrinology 2006, 147, 5988–5994. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Forhead, A.J. Hormones as epigenetic signals in developmental programming. Exp. Physiol. 2009, 94, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Zdraveska, N.; Kocova, M. Thyroid function and dysfunction in preterm infants-Challenges in evaluation, diagnosis and therapy. Clin. Endocrinol. 2021, 95, 556–570. [Google Scholar] [CrossRef]
- LaFranchi, S.H. Thyroid Function in Preterm/Low Birth Weight Infants: Impact on Diagnosis and Management of Thyroid Dysfunction. Front. Endocrinol. 2021, 12, 666207. [Google Scholar] [CrossRef]
- Cole, T.J.; Short, K.L.; Hooper, S.B. The science of steroids. Semin. Fetal Neonatal Med. 2019, 24, 170–175. [Google Scholar] [CrossRef]

| Embryogenesis | Premetamorphosis | Prometamorphosis | Early Metamorphic Climax | Late Climax | ||
|---|---|---|---|---|---|---|
| Stages 1–45 | 45–54 | 54–58 | 58–62 | 62–66 | ||
| Rate of development | TRα KO | No effect 1 | Acceleration | Delay | No effect 1 | No effect 1 |
| TRβ KO | No effect 1 | No effect 1 | No effect 1 | Minor delay | Delay | |
| TRα + β KO | No effect 1 | Acceleration | Further Delay | Delay, then death | N/A 2 | |
| Limb | TRα KO | No effect 1 | Acceleration | Delay | No effect 1 | No effect 1 |
| TRβ KO | No effect 1 | No effect 1 | No effect 1 | No effect 1 | No effect 1 | |
| TRα + β KO | No effect 1 | Acceleration | Further Delay | No effect 1 | N/A 2 | |
| Intestine | TRα KO | No effect 1 | No effect 1 | No effect 1 | Delay | Delay |
| TRβ KO | No effect 1 | No effect 1 | No effect 1 | Delay | Delay | |
| TRα + β KO | No effect 1 | No effect 1 | No effect 1 | Complex effects 3 | N/A 2 | |
| Tail | TRα KO | No effect 1 | No effect 1 | No effect 1 | No effect 1 | No effect 1 |
| TRβ KO | No effect 1 | No effect 1 | No effect 1 | Delay | Delay 4 | |
| TRα + β KO | No effect 1 | No effect 1 | No effect 1 | Inhibition | N/A 2 |
| ACTH KO | GR KO | cyp21a2 KO | |
|---|---|---|---|
| GR | Expressed | Absent | Expressed |
| MR | Expressed | Expressed | Expressed |
| CORT | low | high | low |
| ALDO | Not measured | high | Not measured |
| steroid precursors | Not measured | high | high |
| CORT responsivity | Not measured | Absent | Not measured |
| TH responsivity | very low | very low | low |
| development rate | slow | fast then slow | slow |
| survival | no | no | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, B.; Sterner, Z.R.; Buchholz, D.R.; Shi, Y.-B.; Sachs, L.M. Thyroid and Corticosteroid Signaling in Amphibian Metamorphosis. Cells 2022, 11, 1595. https://doi.org/10.3390/cells11101595
Paul B, Sterner ZR, Buchholz DR, Shi Y-B, Sachs LM. Thyroid and Corticosteroid Signaling in Amphibian Metamorphosis. Cells. 2022; 11(10):1595. https://doi.org/10.3390/cells11101595
Chicago/Turabian StylePaul, Bidisha, Zachary R. Sterner, Daniel R. Buchholz, Yun-Bo Shi, and Laurent M. Sachs. 2022. "Thyroid and Corticosteroid Signaling in Amphibian Metamorphosis" Cells 11, no. 10: 1595. https://doi.org/10.3390/cells11101595
APA StylePaul, B., Sterner, Z. R., Buchholz, D. R., Shi, Y.-B., & Sachs, L. M. (2022). Thyroid and Corticosteroid Signaling in Amphibian Metamorphosis. Cells, 11(10), 1595. https://doi.org/10.3390/cells11101595







